Abstract
Objective
To determine adherence to and effectiveness of ART in adolescents versus adults in southern Africa
Design
Observational cohort study
Setting
Aid for AIDS, a private-sector disease-management program in southern Africa
Subjects
Adolescents (age 11–19 years; n=154) and adults (n=7,622) initiating ART between 1999 and 2006 and having a viral-load measurement within one year after ART initiation
Main Outcome Measures
Primary: virologic suppression (HIV viral load ≤400 copies/mL), viral rebound and CD4+ T-cell count at 6, 12, 18, 24 months after ART initiation. Secondary: adherence assessed by pharmacy refills at 6, 12 and 24 months. Multivariate analyses: log-linear regression and Cox proportional hazards.
Results
A significantly smaller proportion of adolescents achieved 100% adherence at each time point (adolescents: 20.7% at 6 months, 14.3% at 12 months, 6.6% at 24 months; adults: 40.5%, 27.9%, and 20.6% at each time point, respectively; p<0.01). Patients achieving 100% 12-month adherence were significantly more likely to exhibit virologic suppression at 12 months, regardless of age. However, adolescents achieving virologic suppression had significantly shorter time to viral rebound (adjusted hazard ratio 2.03; 95% CI 1.31–3.13; p<0.003). Adolescents were less likely to experience long-term immunologic recovery despite initial CD4+ T-cell counts comparable to adults.
Conclusions
Compared to adults, adolescents in southern Africa are less adherent to ART and have lower rates of virologic suppression and immunologic recovery and a higher rate of virologic rebound after initial suppression. Studies must determine specific barriers to adherence in this population and develop appropriate interventions.
Keywords: HIV, Adolescents, Adults, Adherence, Antiretroviral Therapy, Sub-Saharan Africa
INTRODUCTION
The goal of combination antiretroviral therapy (ART) is to achieve the sustained suppression of human immunodeficiency virus (HIV) replication. Although large studies of efficacy of ART in HIV-infected adults [1–4] and children [5,6] have been conducted, relatively few data have been collected describing the virologic outcomes of ART in adolescents. According to the World Health Organization (WHO), the number of adolescents on ART continues to increase, reflecting successful treatment of perinatally-infected children, infections during early adolescence, and expanding worldwide access to ART [7]. Because of the unique behavioral characteristics of adolescents, they may have worse adherence to ART [8,9], which would increase their risk of both morbidity and drug resistance. As a result, measurement of adherence and virologic outcomes in this population is important.
The level of ART adherence required to achieve optimal virologic response remains controversial. While adherence rates of greater than 95% were traditionally considered to be mandatory for adequate response to non-boosted protease inhibitor-based ART regimens [10], recent findings have shown that Non-Nucleoside Reverse Transcriptase Inhibitor (NNRTI)-based ART often leads to viral suppression at moderate levels of adherence (70%–90%). However, individuals with such “moderate” adherence levels are likely to have improved outcomes with higher adherence [11,12]. Mills and colleagues have shown that, on average, 77% of African adults on ART had high levels of antiretroviral therapy adherence (>80%) compared to 55% of North American patients. [13] However, this meta-analysis did not include adolescent populations from Africa. In fact, the existing data on ART adherence and outcomes in adolescents come almost exclusively from the developed world.
Belzer et al. conducted a pilot survey of 31 youth (ages 13–24 years) from a multidisciplinary adolescent HIV clinic and reported that 61% of the subjects self-reported >90% compliance with their medications in the previous 90 days. “Too many pills” was the most common reason youth reported missing medication (46%), especially for the previous 90 days [9]. The first large-scale disease progression study in the U.S. of HIV-positive adolescents infected through sexual behavior or injection drug use, called REACH (Reaching for Excellence in Adolescent Care and Health), found that only 41% of adolescents (ages 12–19 years) on ART reported >95% adherence and that factors associated with poor adherence included depression, pill burden, advanced HIV status, alcohol use, and dropping out of school [14]. In this same cohort, Murphy et al. reported that only 28.3% of adolescents reported taking all of their prescribed antiretroviral medications in the previous month, and factor analysis revealed barriers to adherence to be medication-related adverse effects (both physical and psychological) and complications in day-to-day routines [15]. In another U.S. study, the Pediatric AIDS Clinical Trial Group (PACTG) 381, the cohort included 120 adolescents (ages 11–22 years) infected via high-risk behaviors and treated with at least two NRTIs plus either a protease inhibitor or an efavirenz-containing HAART regimen. Of these 120 subjects, 44 (37%) stayed on study treatment for the 3 years of observation. Twenty-nine (24%) subjects reached and maintained undetectable viral loads. Poorer adherence was the main predictor of virologic failure. [16]
In these studies, however, data from adolescents were not directly compared to data from adults, and it is also uncertain whether the data from these adolescents could be generalized to sub-Saharan Africa, currently home to 70% of all people living with AIDS [7]. Therefore, we compared adherence and virologic outcomes in adolescents (not perinatally infected) and in adults enrolled in Aid for AIDS, a large private-sector HIV management program in southern Africa.
METHODS
Data source
We evaluated records from HIV-1-infected adults and non-perinatally infected adolescents enrolled in Aid for AIDS, a private-sector, employer-subsidized disease-management program that operates in nine countries of southern Africa and that has been described in detail elsewhere. [17] Patients become eligible for ART with either a documented CD4+ T-cell count <350 cells/μL on two occasions or a medical confirmation of an AIDS-defining illness. Aid for AIDS does not manage clinics, but reimburses patients' private medical practitioners. Authorization for ART reimbursement is subject both to the receipt of a physician prescription and to approval by Aid for AIDS clinical staff, following pre-specified clinical guidelines. [18] ART is dispensed monthly at a pharmacy of the patient’s choice. For reimbursement, patients submit a claim containing the date ART was dispensed, the specific medication regimen followed, and the quantity supplied. Reimbursement requires no patient co-payment.
Adherence in our analysis is estimated by pharmacy refills (the total number of months in which ART medications were claimed, divided by the total number of months during which ART was authorized). Of note, adherence based on pharmacy data has been validated with medication electronic monitor systems (MEMS caps) [19] and therapeutic drug levels [20,21] and can reliably predict virologic success [22–24], drug resistance [25], and survival [17,26,27].
This study used data from patients who had initiated ART between January 1999 and August 2006. ART is defined as taking a minimum of two nucleoside reverse transcriptase inhibitors (NRTIs) plus either one non-nucleoside reverse transcriptase inhibitor (NNRTI) or a boosted protease inhibitor (PI). To be included in the study, patients had to meet the following criteria: (1) no known prior exposure to ART; (2) age ≥11 years old at ART initiation; (3) at least 6 months of follow-up data available; (4) have a baseline (pre-ART) HIV viral load >400 copies/mL; and (5) at least one known viral load measurement after ART initiation. There were no differences in baseline characteristics for patients who did not have at least 6 months of follow-up data compared to patients meeting our eligibility criteria. Follow-up continued from initiation of ART until a) some change in ART regimen; b) loss to follow-up; c) death; or d) study end in February 2007 (six months after the last eligibility date). Patients who left their Medical Insurance Fund (MIF) or whose MIF changed to a different disease-management program were censored as “lost to follow-up” at the date of departure.
Our primary analyses compared adolescents (defined as ages 11 through 19 years, inclusive) to adults (age ≥20 years), based on age at ART initiation. The primary outcomes were virologic suppression (HIV viral load ≤400 copies/mL) and viral rebound, defined as virologic failure (viral load >400 copies/mL) after achieving virologic suppression. The cutoff value of 400 copies/mL was selected because some of the laboratories measuring HIV viral load used assays with a limit of detection of 400 copies/mL. Adherence rates were classified as ≤50%, 51–67%, 68–84%, 85–99% and 100% of possible pharmacy refills. Other covariates in the analysis included sex, race, CD4+ T-cell count and viral load at program enrollment, year of ART initiation, and number of viral-load measurements. In a secondary analysis, we divided the study population into three age group strata: adolescent (11–19 years), young adult (20–29 years), and older adult (30 years or older).
Statistical analysis
Two analytic methods to compare virologic suppression in adolescents versus adults were used. In the first method, four pre-specified time points for viral load assessment were used: 6, 12, 18 and 24 months after ART initiation. Assessments performed within 3 months of each specified time point were deemed valid for that time point. In addition, the following data were also recorded at each pre-specified time point: (a) pharmacy refill adherence to that point, and (b) last available post-ART viral load measurement. We then used virologic suppression at each time point as the dependent variable in a log-linear model, with adolescent status as an independent variable. Both univariate and multivariate models (including all covariates listed above) were analyzed. The imputation by chained equations (ICE) technique was used in <4% of adults and <10% of adolescents; in this technique, a missing value in a variable is replaced by its predicted value, as determined by multiple regression with the rest of the full model predictors [28]. The Pearson χ2 Goodness-of-Fit statistic was used to assess model fit. The second analysis employed a Cox proportional hazards model to evaluate the association between adolescent status and time from virologic suppression to virologic rebound. The assumption of proportional hazards was assessed by the model-based test for the time-by-log(t) interaction.
All p values reported are 2-tailed, with a value of <0.05 considered statistically significant. Fisher’s exact test and the Wilcoxon rank-sum test were used in two-way comparisons of binary and continuous variables, respectively. Statistical analyses were performed using STATA Release 8.2 (Stata Corporation, College Station, TX, USA).
Ethical Approvals
This study was approved by the University of Cape Town Research Ethics Committee and by the Aid for AIDS Clinical Advisory Board, Cape Town, South Africa.
RESULTS
7,776 eligible patients (97% on NNRTI-based ART versus <3% on PI-based ART) were included, of whom 154 were adolescents (11–19 years) per our definition, 1,380 were young adults (20–29 years), and 6,242 were adults (30 years and older), for a total adult (20.1–76.7 years) sample size of 7,622. Characteristics of the study cohort are shown in Table 1. Adolescents were more likely than adults to be female (72.7% vs. 62.3%, P =0.01) and to initiate ART in 2003 or later (50.1% vs. 40.3%, P =0.02). The adolescents were less likely to get NNRTI-based ART (92% vs. 97.2%; P<0.001) and more likely to have shorter follow-up duration (median 27 months; inter-quartile range [IQR] of 18.1–43.7 vs. 36.9 [IQR: 23.6–54.5], P <0.001).
Table 1.
Demographic and clinical characteristics of study population. (Adolescent: 10–19 yrs of age; Young adult: 20–30 yrs of age; and Adult: 30 and up yrs of age)
| Variable | Adolescent (n = 154) | Young Adult (n = 1,380) | P† | Adult (n = 6,242) | P† | All Adult (n = 7,622) | P† |
|---|---|---|---|---|---|---|---|
| Age, yrs* | 16.4 (11.9–18.8) | 27.7 (25.6–29.1) | <0.001 | 37.9 (34.0–43.3) | <0.001 | 36.1 (31.5–42.0) | <0.001 |
|
| |||||||
| Female, n (%) | 112 (72.7) | 1,099 (79.6) | 0.05 | 3,650 (58.5) | <0.001 | 4,749 (62.3) | 0.01 |
|
| |||||||
| Black, n (%) | 125 (94.0) | 1,315 (95.3) | 0.52 | 5,986 (95.9) | 0.27 | 7,301 (95.8) | 0.28 |
|
| |||||||
| Crude Mortality, n (%) | 5 (3.3) | 72 (5.2) | 0.43 | 470 (7.5) | 0.04 | 542 (7.1) | 0.08 |
|
| |||||||
| Baseline‡ CD4+ T-cell count, cells/μL* | 144 (27–246) | 175 (78–278) | 0.003 | 140 (62–234) | 0.44 | 146 (64–242) | 0.24 |
|
| |||||||
| Baseline‡ viral load, log10− copies/mL, n (%) | 5.1 (4.5–5.6) | 4.9 (4.4–5.4) | 0.01 | 5.1 (4.6–5.6) | 0.96 | 5.1 (4.6–5.5) | 0.59 |
|
| |||||||
| Follow-up time, mos.* | 27.0 (18.1–43.7) | 38.1 (24.3–55.8) | <0.001 | 36.6 (23.5–54.1) | <0.001 | 36.9 (23.6–54.4) | <0.001 |
|
| |||||||
| NNRTI-based regimen, n (%) | 137 (92.0) | 1,225 (94.7) | 0.18 | 5,858 (97.7) | <0.001 | 7,083 (97.2) | 0.001 |
|
| |||||||
| # of viral loads per patient, n* | 2 (1–3) | 2 (1–4) | 0.005 | 2 (1–4) | 0.01 | 2 (1–4) | 0.01 |
|
| |||||||
| CD4+ T-cell count, cell/μL* | |||||||
| 6 months | 295 (135–482) | 281 (167–402) | 0.69 | 238 (138–369) | 0.21 | 246 (142–377) | 0.28 |
| 12 months | 281 (154–538) | 316 (173–444) | 0.59 | 268 (157–402) | 0.85 | 276 (159–412) | 0.96 |
| 18 months | 263 (157–439) | 339 (189–486) | 0.42 | 305 (172–459) | 0.80 | 308 (177–464) | 0.72 |
| 24 months | 172 (44–451) | 348 (195–506) | 0.01 | 337 (186–494) | 0.03 | 339 (187–496) | 0.02 |
|
| |||||||
| Pharmacy-claim adherence at specified times post-ART, %* | |||||||
| 6 months | 66.7 (50.0–83.3) | 83.3 (50.0–100) | 0.004 | 83.3 (66.7–100) | <0.001 | 83.3 (50.0–100) | <0.001 |
| 12 months | 66.7 (41.7–83.3) | 83.3 (50.0–100) | 0.005 | 83.3 (50.0–100) | <0.001 | 83.3 (50.0–100) | 0.001 |
| 24 months | 62.5 (33.3–80.0) | 79.2 (45.8–95.8) | 0.001 | 83.3 (53.1–95.8) | <0.001 | 80.0 (50.0–95.8) | <0.001 |
| Total | 72.7 (36.5–95.8) | 76.5 (46.7–94.6) | 0.64 | 81.8 (51.5–96.2) | 0.10 | 81.0 (50.0–95.8) | 0.15 |
|
| |||||||
| 100% Adherence, n (%) | |||||||
| 6 months | 17 (20.7) | 476 (39.2) | 0.001 | 2,359 (40.7) | <0.001 | 2,835 (40.5) | <0.001 |
| 12 months | 11 (14.3) | 316 (27.0) | 0.02 | 1,552 (28.1) | 0.01 | 1,868 (27.9) | 0.01 |
| 24 months | 4 (6.6) | 200 (19.3) | 0.01 | 973 (20.9) | 0.004 | 1,173 (20.6) | 0.004 |
|
| |||||||
| Viral suppression, n (%) | |||||||
| 6 months | 58 (63.0) | 444 (63.7) | 0.91 | 2,267 (70.5) | 0.13 | 2,711 (69.3) | 0.21 |
| 12 months | 32 (45.7) | 323 (56.0) | 0.13 | 1,659 (63.5) | 0.01 | 1,982 (62.1) | 0.01 |
| 18 months | 24 (45.3) | 276 (54.2) | 0.25 | 1,375 (61.6) | 0.02 | 1,651 (60.2) | 0.03 |
| 24 months | 17 (43.6) | 246 (55.8) | 0.18 | 1,210 (63.8) | 0.01 | 1,456 (62.3) | 0.02 |
|
| |||||||
| Viral rebound, n (%)§ | |||||||
| 6 months | 14 (31.1) | 84 (19.9) | 0.09 | 312 (15.9) | 0.01 | 396 (16.6) | 0.02 |
| 12 months | 14 (42.4) | 72 (21.2) | 0.01 | 312 (19.9) | 0.004 | 384 (20.2) | 0.004 |
| 18 months | 7 (38.9) | 67 (24.4) | 0.17 | 268 (20.9) | 0.08 | 335 (21.5) | 0.09 |
| 24 months | 6 (37.5) | 53 (25.0) | 0.37 | 244 (24.0) | 0.24 | 297 (24.2) | 0.24 |
|
| |||||||
| Ever-suppressed, n (%) | 90 (58.4) | 922 (66.8) | 0.04 | 4,557 (73.0) | <0.001 | 5,479 (71.9) | <0.001 |
Data are given as median (inter-quartile range).
P-values are all compared to adolescents and calculated using Wilcoxon rank-sum test for continuous variables and Fisher’s exact test for binary variables.
Data collected at program enrollment.
The denominator consists only of patients who had initially suppressed viral load.
In a subset of patients with adherence data available through 6, 12, and 24 months of follow-up, adolescents had consistently and significantly lower adherence than adults. Adolescents claimed medication for a median of 4/6 (IQR 3–5), 8/12 (IQR 5–10), and 15/24 (IQR 8–19) months, versus 5/6 (IQR 3–6), 10/12 (IQR 6–12), and 19/24 (IQR 12–23) months for adults (p≤0.001 at all time points). Similarly, the percentage of adolescents achieving 100% adherence was 20.7% at 6 months, 14.3% at 12 months, and 6.6% at 24 months, compared to 40.5%, 27.9%, and 20.6% for adults (p<0.01 at all time points, Table 1). For patients started on the NNRTI-based regimen, the proportion of adolescents with 100% adherence at 6, 12, and 24 months was 23.9%, 14.9% and 7.6%, compared to 99.4%, 28.8% and 21.5% for adults (p<0.01 at all time points).
The proportion of adolescents achieving viral suppression was lower than that of adults, although the differences were significant only at 12, 18, and 24 months after ART initiation (Table 1). Patients achieving 100% 12-month adherence were significantly more likely to exhibit virologic suppression at 12 months, whether adolescent (91% of perfect adherers suppressed at 12 months vs. 45% of others, p=0.007) or adult (86% vs. 59%, p<0.001). The association between adolescent status and lower rates of virologic suppression persisted despite adjustment for potential confounders, although adjustment for adherence did weaken the measured association (Table 2).
Table 2.
Relative risks for virologic suppression in adolescents compared to adults
| Time of follow-up | Unadjusted | Adjusted for all variables other than adherence † | Completely adjusted ‡ | |||
|---|---|---|---|---|---|---|
| RR (95% CI) | P | RR (95% CI) | P | RR (95% CI) | P | |
| At 6 months* | 0.91 (0.78–1.07) | 0.24 | 0.88 (0.74–1.05) | 0.16 | 0.96 (0.79–1.16) | 0.65 |
| At 12 months* | 0.74 (0.57–0.95) | 0.02 | 0.65 (0.48–0.90) | 0.01 | 0.74 (0.53–1.03) | 0.08 |
| At 18 months* | 0.75 (0.56–1.01) | 0.06 | 0.84 (0.60–1.17) | 0.31 | - | - |
| At 24 months* | 0.70 (0.49–1) | 0.05 | 0.72 (0.49–1.06) | 0.10 | 0.78 (0.53–1.15) | 0.22 |
Time is measured in months after HAART initiation.
Includes gender, race, baseline CD4, baseline viral load, ART regimen (NNRTI- vs. PI-based), ART initiation before 2003, and number of viral load measurement per patient-months.
Adherence categorized in strata of ≤50%, 51–67%, 67–84%, 85–99% and 100% and baseline variables as described in (†). No adherence data at 18 months.
In the subset of patients who achieved initial virologic suppression (N = 5504 adults and 93 adolescents, of which 3805 adults and 62 adolescents had at least one viral load measurement after initial suppression), the proportion of adolescents with viral rebound was greater than for adults (31.3% vs. 16.6%, p=0.02 at 6 months; 42.4% vs. 20.2% at 12 months, p=0.004; 38.9% vs. 21.5% at 18 months, p=0.09; and 37.5% vs. 24.2%, p=0.24 at 24 months) (Table 1). This association between adolescent status and higher rate of viral rebound was sustained in both unadjusted and adjusted models (Table 3). Furthermore, adolescents were less likely than adults to experience immunologic recovery on ART as evidenced by their median CD4+ T-cell count (IQR): 295 (135–482) vs. 246 (142–377), p=0.26 at 6 months; 281 (154–538) vs. 276 (159–412) at 12 months, p=0.96; 263 (157–439) vs. 308 (177–464) at 18 months, p=0.72; and 172 (44–451) vs. 339 (187–496), p=0.02 at 24 months) (Table 1).
Table 3.
Relative risks for viral rebound in adolescents compared to adults
| Model | Unadjusted | Adjusted for all variables other than adherence† | Completely adjusted ‡ | |||
|---|---|---|---|---|---|---|
| RR (95% CI) | P | RR (95% CI) | P | RR (95% CI) | P | |
| At 6 months* | 1.88 (1.20–2.93) | 0.005 | 2.04 (1.25–3.33) | 0.004 | 2.33 (1.71–3.17) | <0.001 |
| At 12 months* | 2.10 (1.40–3.16) | <0.001 | 1.84 (1.11–3.07) | 0.02 | 1.77 (1.23–2.54) | 0.002 |
| At 18 months* | 1.81 (1.01–3.26) | 0.05 | 2.16 (1.27–3.66) | 0.004 | - | - |
| At 24 months* | 1.55 (0.82–2.94) | 0.18 | 1.72 (0.96–3.06) | 0.07 | 1.65 (1.03–2.63) | 0.04 |
Time is measured in months after HAART initiation.
Includes gender, race, baseline CD4, baseline viral load, ART regimen (NNRTI- vs. PI-based), ART initiation before 2003, and number of viral load measurement per patient-months.
Adherence categorized in strata of ≤50%, 51–67%, 67–84%, 85–99% and 100% and baseline variables as described in (†). No adherence data up at 18 months.
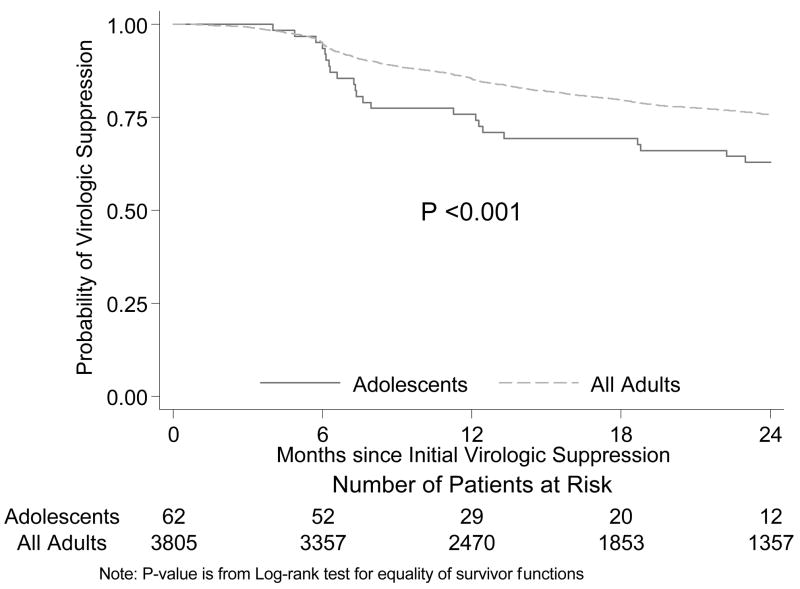
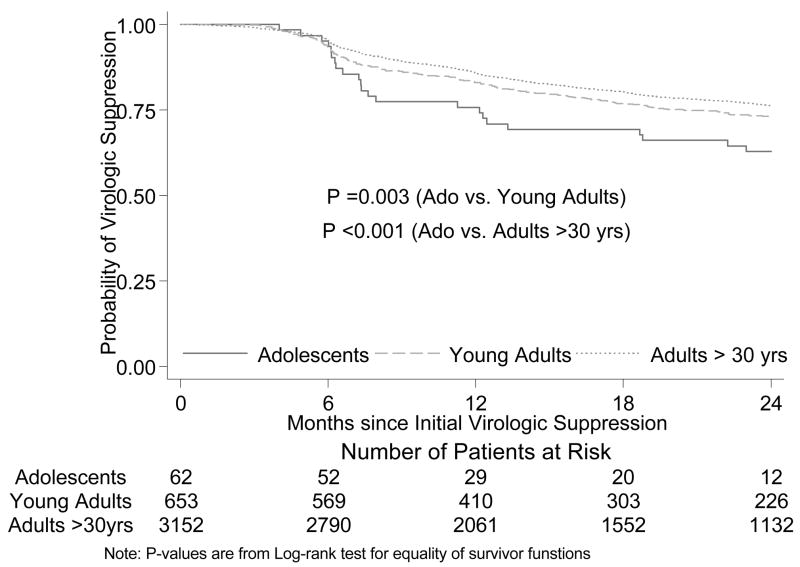
In Cox proportional hazards analysis in the subset of patients who achieved initial virologic suppression, adolescents compared with adults had a significantly shorter time to viral rebound when unadjusted (HR 2.10 [1.43–3.08]; p<0.001) and adjusted for both baseline characteristics and adherence (HR 2.18 [1.41–3.38]; p<0.001) (Fig. 1). In our secondary analyses, we found that young adults have virologic outcomes which are intermediate between those of adolescents and adults ≥30years (Table 1 and Figure 2). In the Cox proportional hazards analysis, adolescents compared to adults age 20–29 years had a significantly shorter time to viral rebound when unadjusted (HR 1.82 [1.22–2.73]; p=0.003) and adjusted for both baseline characteristics and adherence (HR 1.79 [1.12–2.86]; p=0.02). In addition, younger adults compared with adults ≥30 years had also a significantly shorter time to viral rebound when unadjusted (HR 1.18 [1.02–1.36]; p=0.02) and adjusted for both baseline characteristics and adherence (HR 1.20 [1.03–1.39]; p=0.02) (Figure 2).
Figure 1.
Times to rebound, adolescents versus adults. P for log-rank test <0.001
Figure 2.
Times to rebound, comparing adolescents to young adults (20–29 years old) and adults (≥30 years old).
DISCUSSION
Our results suggest that HIV-infected adolescents and young adults on ART in southern Africa have poorer adherence rates and poorer virologic outcomes than their adult counterparts. In this study, adolescents were approximately 50% less likely than adults to maintain perfect adherence at all time points and 70–75% less likely to be virologically suppressed (≤400 copies/mL) at 1 and 2 years after ART initiation. At six months, rates of virologic suppression among adolescents and adults were similar; thus, lower rates of long-term suppression among adolescents were largely explained by more rapid viral rebound. Interestingly, we found that young adults (age 19–20 years) have virologic outcomes which are intermediate between those of adolescents and adults ≥30years (Fig. 2). Furthermore, adolescents were less likely than young adults, adults >30 years and all adults to experience long-term immunologic recovery; despite having nearly identical initial CD4+ T-cell counts, adolescents experienced very small increases in CD4+ T-cell counts after two years, from a median of 144/mm3 to 172/mm3 vs. increases in young adults, adults >30 years and all adults from a median of 75/mm3 to 348/mm3 (p = 0.01), 140/mm3 to 337/mm3 (p = 0.03), and 146/mm3 to 339/mm3 (p = 0.02), respectively (Table 1). Our data are in agreement with studies from the developed world. Flynn and colleagues also reported that adolescents infected with HIV via high-risk behaviors have less than optimal responses to HAART therapy, with only 24% achieving and maintaining undetectable viral loads over 3 years. [13] Also in this study, CD4+ T-cell count measurements improved from entry to the end of follow-up only in the subjects with sustained undetectable viral loads.
We also found in the present study that HIV-infected adolescents were more likely than adults to be female (72.7% vs. 62.3%, P =0.01), a finding consistent with previous epidemiological studies in South Africa which found higher HIV seroprevalence in adolescent females that was explained by greater high-risk sexual behavior, earlier sexual debut, greater likelihood of older sexual partners, and additional sociological issues, such as gender-power imbalance. [29,30]
Low medication adherence in adolescents, the very population most likely to benefit from optimal adherence (i.e., those who would have the longest life expectancy on successful ART), underscores the urgent need to identify risk factors that contribute to poor adherence in HIV-infected adolescents in sub-Saharan Africa. Such knowledge would help guide the design of targeted interventions to achieve or maintain high adherence rates in this population. Given the limited availability of second-line and salvage antiretroviral therapy regimens in this region, preserving long-term success of first-line ART is critical, particularly in adolescents, who would be expected to live longer than HIV-infected adults by virtue of their younger age, if both groups are able to achieve equivalent treatment success.
Barriers to risk factors for non-adherence to HIV medication in adults from sub-Saharan Africa have been reported and include non-disclosure to a loved one or fear of being stigmatized [31,32]; substance abuse (mostly alcohol) [31]; cost [33–35] in countries where ART is not free of charge; and the complexity of the drug regimen [31]. While some of these factors may be specific to this setting, others overlap with factors identified in adolescents with HIV-infection or other chronic diseases from industrialized countries. Indeed, as mentioned earlier, factors associated with poor adherence in the REACH cohort included depression, pill burden, advanced HIV status, alcohol use, dropping out of school, side effects and complications of day-today routine [8, 9, 14, 15]. Furthermore, medication non-compliance for other chronic conditions appeared to be associated with a restriction of independence in daily life, lack of harmony in family relations, and low self-esteem in teenage epileptics [36 ], as well as forgetfulness, busy schedules, and non availability of medication in adolescents with cancer[ 37]. In a qualitative study in Uganda, Bikaako-Kajural et al. found that structural factors including poverty and stigma were barriers to both ART and cotrimoxazole adherence, even in children who had complete disclosure and a supportive relationship with their parents. [38] If these factors are shared by adolescents, then interventions to encourage voluntary testing and disclosure of HIV status, or to reduce the cost and complexity of antiretroviral therapy, might also improve adherence rates in this age group. Further research on barriers to ART adherence in adolescents is critically needed.
Our study has certain limitations. First, although our study population is among the largest cohorts on ART under observation in sub-Saharan Africa, our sample size for this analysis was limited by the small proportion of these patients who were adolescents. Adolescents are under-represented in the Aid for AIDS database because many HIV-infected adolescents may be newly-infected and therefore not at a sufficiently advanced disease stage to qualify for ART (CD4+ T-cell count <350 cells/μL). Furthermore, infected adolescents who are eligible to begin ART are less likely to be previously employed and therefore less likely to qualify for private health insurance -- unless they are children of a qualifying adult, since only adolescent dependents of adult employees in medical insurance schemes participating in the Aid for AIDS program are eligible. Finally, our dataset was not originally designed as a comprehensive research tool and so is limited in certain data elements and is not structured to capture the reasons for non-adherence. As a result of these limitations, further studies on ART adherence in African adolescents are needed to determine whether the results from this study are fully generalizable (e.g., to the public sector), and to describe relationships that could not be measured with the limited data in the current database. Ultimately, studies of interventions to improve adherence in this vulnerable population will be essential to maximize the number of HIV-infected infants who successfully survive into adulthood.
In conclusion, compared with adults, adolescents in southern Africa are less adherent to ART, have lower rates of virologic suppression at all time points after ART initiation, and experience more rapid viral rebound. Studies to determine barriers to adherence in adolescents, as well as to develop interventions to address them are sorely needed in this setting.
Acknowledgments
We are grateful to Joanna Downer, Ph.D., and Roderick Graham, M.A., for critical reading of this manuscript. This paper was given in part as a poster presentation at the 15th Conference on Retroviruses and Opportunistic Infections, February 3–6, 2008, Boston, MA, USA (MonPosterAb#821).
Drs. J. B. Nachega, R.E. Chaisson, and G. Maartens acknowledge research support from the National Institute of Allergy and Infectious Diseases (NIAID), United States National Institutes of Health (NIH), AI 5535901 and AI 016137. Dr. J. B. Nachega is the recipient of an NIAID/NIH Mentored Patient-Oriented Research Career Award K23 AI068582-01. Mr. D. Dowdy is supported by the NIH Medical Scientist Training Program Award 5 T32 GMO7309. The funders had no input into the results or presentation of the results reported in this manuscript.
Footnotes
Conflict of interest
consultant: R.E. Chaisson (Bristol-Myers Squibb). Honoraria: J.B. Nachega (GlaxoSmithKline, Merck-Sharp-Dohme for continuing medical education lectures) and G. Maartens (Merck-Sharp-Dohme). Grants received: G. Maartens (Merck-Sharp-Dohme). Other: J.B. Nachega (Aspen Pharmaceuticals for conferences and travel grants).
Author contributions: Conception and design: J.B. Nachega, G. Maartens and M. Cotton. Interpretation of the data: J.B. Nachega, M. Hislop, L. Regensberg, H. Nguyen, M. Cotton, G. Maartens, D.W. Dowdy, R.E. Chaisson. Drafting of the article: J.B. Nachega, D.W. Dowdy, G. Maartens. Critical revision of the article for important intellectual content: J.B. Nachega, D.W. Dowdy, R.E. Chaisson, M. Cotton, G. Maartens. Final approval of the article: J.B. Nachega, M. Hislop, D.W. Dowdy, H. Nguyen, R.E. Chaisson, L. Regensberg, G. Maartens, M. Cotton. Provision of study materials or patients: M. Hislop, L. Regensberg. Statistical expertise: J.B. Nachega, D.W. Dowdy, H. Nguyen. Administrative, technical, or logistic support: R.E. Chaisson, L. Regensberg.
References
- 1.Markowitz M, Conant M, Hurley A, et al. A preliminary evaluation of nelfinavir mesylate, an inhibitor of human immunodeficiency virus (HIV)–1 protease, to treat HIV infection. J Infect Dis. 1998;177:1533–1540. doi: 10.1086/515312. [DOI] [PubMed] [Google Scholar]
- 2.Markowitz M, Saag M, Powderly WG, et al. A preliminary study of ritonavir, an inhibitor of HIV-1 protease, to treat HIV-1 infection. N Engl J Med. 1995;333:1534–1539. doi: 10.1056/NEJM199512073332204. [DOI] [PubMed] [Google Scholar]
- 3.Hirsch M, Steigbigel R, Staszewski S, et al. A randomized, controlled trial of indinavir, zidovudine, and lamivudine in adults with advanced human immunodeficiency virus type 1 infection and prior antiretroviral therapy. J Infect Dis. 1999;180:659–665. doi: 10.1086/314948. [DOI] [PubMed] [Google Scholar]
- 4.Staszewski S, Morales-Ramirez J, Tashima KT, et al. for the Study 006 Team. Efavirenz plus zidovudine and lamivudine, efavirenz plus indinavir, and indinavir plus zidovudine and lamivudine in the treatment of HIV-1 infection in adults. N Engl J Med. 1999;341:1865–1873. doi: 10.1056/NEJM199912163412501. [DOI] [PubMed] [Google Scholar]
- 5.Starr SE, Fletcher CV, Spector SA, et al. for the Pediatric AIDS Clinical Trials Group 382 Team. Combination therapy with efavirenz, nelfinavir, and nucleoside reverse-transcriptase inhibitors in children infected with human immunodeficiency virus type 1. N Engl J Med. 1999;341:1874–1881. doi: 10.1056/NEJM199912163412502. [DOI] [PubMed] [Google Scholar]
- 6.Krogstad P, Lee S, Johnson G, et al. Nucleoside-analogue reverse-transcriptase inhibitors plus nevirapine, nelfinavir, or ritonavir for pretreated children infected with human immunodeficiency virus type 1. Clin Infect Dis. 2002;34:991–1001. doi: 10.1086/338814. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. Towards Universal Access: Scaling Up Priority HIV/AIDS Interventions in the Health Sector. Progress Report April 2007. Geneva: WHO Press; 2007. [Google Scholar]
- 8.Murphy DA, Wilson CM, Durako SJ, et al. Antiretroviral medication adherence among the REACH HIV-infected adolescent cohort in the USA. AIDS Care. 2001;13:27–40. doi: 10.1080/09540120020018161. [DOI] [PubMed] [Google Scholar]
- 9.Belzer ME, Fuchs DN, Luftman GS, et al. Antiretroviral adherence issues among HIV-positive adolescents and young adults. J Adolesc Health. 1999;25:316–319. doi: 10.1016/s1054-139x(99)00052-x. [DOI] [PubMed] [Google Scholar]
- 10.Paterson DL, Swindells S, Mohr J, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med. 2000;133:21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- 11.Bangsberg DR. Less than 95% adherence to nonnucleoside reverse-transcriptase inhibitor therapy can lead to viral suppression. Clin Infect Dis. 2006;43:939–941. doi: 10.1086/507526. [DOI] [PubMed] [Google Scholar]
- 12.Nachega JB, Hislop M, Dowdy D, et al. Adherence to non-nucleoside reverse transcriptase-based HIV therapy and virologic outcomes. Ann Intern Med. 2007;146:564–573. doi: 10.7326/0003-4819-146-8-200704170-00007. [DOI] [PubMed] [Google Scholar]
- 13.Mills EJ, Nachega JB, Buchan I, et al. Adherence to antiretroviral therapy in sub-Saharan Africa and North America: a meta-analysis. JAMA. 2006;296:679–690. doi: 10.1001/jama.296.6.679. [DOI] [PubMed] [Google Scholar]
- 14.Murphy DA, Belzer M, Durako SJ, et al. Adolescent Medicine HIV/AIDS Research Network. Longitudinal antiretroviral adherence among adolescents infected with human immunodeficiency virus. Arch Pediatr Adolesc Med. 2005;159:764–770. doi: 10.1001/archpedi.159.8.764. [DOI] [PubMed] [Google Scholar]
- 15.Murphy DA, Sarr M, Durako SJ, et al. for the Adolescent Medicine HIV/AIDS Research Network. Barriers to HAART adherence among human immunodeficiency virus-infected adolescents. Arch Pediatr Adolesc Med. 2003;157:249–55. doi: 10.1001/archpedi.157.3.249. [DOI] [PubMed] [Google Scholar]
- 16.Flynn PM, Rudy BJ, Lindsey JC, et al. PACTG 381 Study Team. Long-term observation of adolescents initiating HAART therapy: three-year follow-up. AIDS Res Hum Retroviruses. 2007;23:1208–1214. doi: 10.1089/aid.2006.0290. [DOI] [PubMed] [Google Scholar]
- 17.Nachega JB, Hislop M, Dowdy DW, et al. Adherence to highly active antiretroviral therapy assessed by pharmacy claims predicts survival in HIV-infected South African adults. J Acquir Immune Defic Syndr. 2006;43:78–84. doi: 10.1097/01.qai.0000225015.43266.46. [DOI] [PubMed] [Google Scholar]
- 18.Aid for AIDS. [Accessed October 29, 2006];AfA Clinical Guidelines. Available: http://www.aidforaids.co.za.
- 19.Choo PW, Rand CS, Inui TS, et al. Validation of patient reports, automated pharmacy records, and pill counts with electronic monitoring of adherence to antihypertensive therapy. Med Care. 1999;37:846–857. doi: 10.1097/00005650-199909000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Steiner JF, Koepsell TD, Fihn SD, et al. A general method of compliance assessment using centralized pharmacy records: description and validation. Med Care. 1988;26:814–823. doi: 10.1097/00005650-198808000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Steiner JF, Prochazka AV. The assessment of refill compliance using pharmacy records: methods, validity, and applications. J Clin Epidemiol. 1997;50:105–116. doi: 10.1016/s0895-4356(96)00268-5. [DOI] [PubMed] [Google Scholar]
- 22.Low-Beer S, Yip B, O’Shaughnessy MV, et al. Adherence to triple therapy and viral load response. J Acquir Immune Defic Syndr. 2000;23:360–361. doi: 10.1097/00126334-200004010-00016. [DOI] [PubMed] [Google Scholar]
- 23.Gross R, Yip B, Lo Re V, 3rd, et al. A simple, dynamic measure of antiretroviral therapy adherence predicts failure to maintain HIV-1 suppression. J Infect Dis. 2006;194:1108–1114. doi: 10.1086/507680. [DOI] [PubMed] [Google Scholar]
- 24.Grossberg R, Zhang YW, Gross R. A time-to-prescription-refill measure of antiretroviral adherence predicted changes in viral load in HIV. J Clin Epidemiol. 2004;57:1107–1110. doi: 10.1016/j.jclinepi.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 25.Harrigan PR, Hogg RS, Dong WW, et al. Predictors of HIV drug-resistance mutations in a large antiretroviral-naive cohort initiating triple antiretroviral therapy. J Infect Dis. 2005;191:339–347. doi: 10.1086/427192. [DOI] [PubMed] [Google Scholar]
- 26.Hogg RS, Heath K, Bangsberg DR, et al. Intermittent use of triple-combination therapy is predictive of mortality at baseline and after 1 year of follow-up. AIDS. 2002;16:1051–1058. doi: 10.1097/00002030-200205030-00012. [DOI] [PubMed] [Google Scholar]
- 27.Wood E, Hogg RS, Yip B, et al. Impact of baseline viral load and adherence on survival of HIV-infected adults with baseline CD4 cell counts = 200 cells/~L. AIDS. 2006;20:1117–1123. doi: 10.1097/01.aids.0000226951.49353.ed. [DOI] [PubMed] [Google Scholar]
- 28.Royston P. [Accessed July 4, 2008];Imputation by Chained Equations. Available: http://www.statajournal.com/article.html?article=st0067_3.
- 29.Connolly C, Colvin M, Shishana O, et al. Epidemiology of HIV in South Africa –results of a national, community-based survey. S Afr Med J. 2004;94:776–781. [PubMed] [Google Scholar]
- 30.Singh JA, Karim SS, Karim QA, et al. Enrolling adolescents in research on HIV and other sensitive issues: lessons from South Africa. PLoS Med. 2006;3:e180. doi: 10.1371/journal.pmed.0030180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mills EJ, Nachega JB, Bangsberg DR, et al. Adherence to HAART: a systematic review of developed and developing nation patient-reported barriers and facilitators. PLoS Med. 2006;3:e438. doi: 10.1371/journal.pmed.0030438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nachega JB, Stein DM, Lehman DA, et al. Adherence to antiretroviral therapy in HIV-infected adults in Soweto, South Africa. AIDS Res Hum Retroviruses. 2004;20:1053–1056. doi: 10.1089/aid.2004.20.1053. [DOI] [PubMed] [Google Scholar]
- 33.Weiser S, Wolfe W, Bangsberg D, et al. Barriers to antiretroviral adherence for patients living with HIV infection and AIDS in Botswana. J Acquir Immune Defic Syndr. 2003;34:281–288. doi: 10.1097/00126334-200311010-00004. [DOI] [PubMed] [Google Scholar]
- 34.Crane JT, Kawuma A, Oyugi JH, et al. The price of adherence: Qualitative findings from HIV positive individuals purchasing fixed-dose combination generic HIV antiretroviral therapy in Kampala, Uganda. AIDS Behav. 2006;10:437–442. doi: 10.1007/s10461-006-9080-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laniece I, Ciss M, Desclaux A, et al. Adherence to HAART and its principal determinants in a cohort of Senegalese adults. AIDS. 2003;17(Suppl 3):103–108. doi: 10.1097/00002030-200317003-00014. [DOI] [PubMed] [Google Scholar]
- 36.Friedman IM, Litt IF. Adolescents’ compliance with therapeutic regimens: psychological and social aspects and intervention. J Adolesc Health Care. 1987;8:52–67. doi: 10.1016/0197-0070(87)90246-4. [DOI] [PubMed] [Google Scholar]
- 37.Tebbi CK, Cummings KM, Zevon MA, et al. Compliance of pediatric and adolescent cancer patients. Cancer. 1986;58:1179–1184. doi: 10.1002/1097-0142(19860901)58:5<1179::aid-cncr2820580534>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 38.Bikaako-Kajura W, Luyirika E, Purcell DW, et al. Disclosure of HIV status and adherence to daily drug regimens among HIV-infected children in Uganda. AIDS Behav. 2006;10(Suppl 4):85–93. doi: 10.1007/s10461-006-9141-3. [DOI] [PubMed] [Google Scholar]