Abstract
Synovial sarcoma generally is associated with poor prognosis. With recent advances in molecular biology, it has become apparent not all synovial sarcomas share the same tumor biology. 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) is useful for risk assessment in several types of sarcomas. We therefore assessed the clinical value of 18F-FDG-PET-derived maximum standard uptake value (SUVmax) for predicting survival in patients with synovial sarcoma. 18F-FDG-PET was performed in 44 patients with synovial sarcoma before therapy and resection. SUVmax was calculated for each tumor and then evaluated for prognostic usefulness along with metastasis at presentation, tumor grade, histopathologic subtype, age, gender, postsurgical margins, anatomic location, and tumor size for overall survival and progression-free survival. SUVmax ranged from 1.2 to 13.0 (median, 4.35). Pretherapy tumor SUVmax predicted overall survival and progression-free survival. Patients presenting with a SUVmax greater than 4.35 had a decreased disease-free survival and were therefore at high risk for having local recurrences and metastatic disease.
Level of Evidence: Level I, diagnostic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Synovial sarcoma is a rare malignant neoplasm, accounting for only 6% to 10% of all soft tissue sarcomas. There are approximately 800 new cases of synovial sarcoma per year in the United States [26]. Historically, synovial sarcoma has been associated with poor prognosis, with survival at 5 years ranging from 55% to 76% [2, 16, 20, 21, 23, 27]. However, with advances in molecular biology and classification, it has emerged all synovial sarcomas do not share the same tumor biology when it comes to local recurrence, metastasis, and survival [2, 16, 17, 19–23].
Because of the varied biologic aggressiveness of synovial sarcomas, much effort has been placed in identifying prognostic factors with clinical value to better predict survival in individual patients with synovial sarcoma. Primary tumor size [1, 2, 12, 16, 17, 20–23, 27], tumor stage [20, 23], gender [6, 23, 27], age [2, 6, 12, 17, 19, 21, 27], tumor grade [6, 23], histologic subtype [2, 17, 19, 20, 23], tumor necrosis [23], mitotic activity [23], invasion of bone and neurovascular structures [1, 16], and anatomic tumor location [6, 9, 17, 19, 23] all influence the natural history of primary synovial sarcomas. However, many of these findings have not been consistent so definitive conclusions based on them cannot be made.
During the past decade, FDG-PET has become increasingly available as a clinical tool in the outpatient cancer setting. The SUVmax in FDG-PET is a valuable parameter for risk assessment in sarcomas [8, 11]. Specifically, initial pretherapy SUVmax has been used for prediction of outcome in Ewing’s sarcoma, liposarcoma, and chondrosarcoma [4, 5, 14].
Based on these previous findings, we hypothesized pretherapy FDG-PET SUVmax reflects tumor biology and aggressiveness in synovial sarcoma. We therefore (1) prospectively assessed the prognostic value of pretherapy FDG-PET SUVmax in survival in patients with synovial sarcoma and (2) determined whether age, gender, histopathologic subtype, grade, margins at the time of resection, tumor size, anatomic location, and metastasis at presentation predicted survival.
Materials and Methods
We prospectively followed all patients presenting with synovial sarcoma using pretherapy FDG-PET SUVmax between December 1995 and April 2007. Patients were excluded from the study if they had any type of treatment of their tumor before being enrolled, had a FDG-PET performed and/or read at another institution, or were not candidates for chemotherapy. We enrolled 44 patients with histologically proven synovial sarcoma. We recorded age at presentation, gender, grade, histologic subtype, postsurgical resection margins, size, anatomic site, metastasis at entry, local recurrence, and outcome (survival) for each patient. The age range of the patients at the time of first FDG-PET scan was 8 to 70 years (median, 35 years) (Table 1). The median time from the first FDG-PET scan to last followup or death was 63.4 months (range, 0.1–116 months). Informed consent was obtained by signing Human Subjects and Radiation Safety Committee-approved forms.
Table 1.
Characteristics of all patients and stratified by FDG-PET SUVmax
| Variable | All patients | Patients with SUVmax < 4.35 | Patients with SUVmax > 4.35 |
|---|---|---|---|
| Total | 44 | 22 | 22 |
| Age | |||
| < 18 years | 4 (9%) | 3 (14%) | 1 (5%) |
| 18–29 years | 13 (29%) | 11 (50%) | 2 (9%) |
| 30–50 years | 18 (41%) | 6 (27%) | 12 (55%) |
| > 50 years | 9 (21%) | 2 (9%) | 7 (32%) |
| Gender | |||
| Female | 28 (64%) | 15 (68%) | 13 (59%) |
| Male | 16 (36%) | 7 (32%) | 9 (41%) |
| Grade | |||
| Intermediate | 28 (64%) | 18 (82%) | 10 (45%) |
| High | 13 (30%) | 2 (9%) | 11 (50%) |
| Ungraded | 3 (6%) | 2 (9%) | 1 (5%) |
| Histologic subtype | |||
| Monophasic | 29 (66%) | 15 (68%) | 14 (64%) |
| Biphasic | 10 (23%) | 4 (18%) | 6 (27%) |
| No subtype given | 5 (11%) | 3 (14%) | 2 (9%) |
| Anatomic site | |||
| Extremity | 31 (70%) | 16 (73%) | 15 (68%) |
| Trunk | 10 (23%) | 5 (23%) | 5 (23%) |
| Pelvis | 3 (7%) | 1 (5%) | 2 (9%) |
| Size | |||
| < 5 cm | 13 (30%) | 10 (45%) | 3 (14%) |
| 5–10 cm | 21 (48%) | 10 (45%) | 11 (50%) |
| > 10 cm | 10 (23%) | 2 (9%) | 8 (36%) |
| Margins | |||
| Positive | 24 (55%) | 12 (55%) | 12 (55%) |
| Negative | 20 (45%) | 10 (45%) | 10 (45%) |
| Metastasis | |||
| Yes | 18 (41%) | 2 (9%) | 16 (73%) |
| No | 26 (59%) | 20 (91%) | 6 (27%) |
| Recurrence | |||
| Yes | 7 (16%) | 3 (14%) | 4 (18%) |
| No | 37 (84%) | 19 (86%) | 18 (82%) |
| Died of disease | 13 (30%) | 1 (5%) | 12 (55%) |
FDG-PET = 18F-fluorodeoxyglucose positron emission tomography; SUVmax = maximum standard uptake value.
Histologic features of the tumor were determined at the time of resection by an experienced sarcoma pathologist (BR, PS) and were graded according to the Fédération Nationale des Centres de Lutte Contre le Cancer system based on differentiation, mitotic index, and necrosis [7, 24]. The histopathologic subtypes of synovial sarcoma were monophasic (29) and biphasic (10). Five tumors received no histopathologic classification. Twenty-eight tumors were designated as intermediate grade, 13 as high grade, and three were not graded (Table 1). Postresection surgical margins were determined grossly by an experienced sarcoma pathologist (BR, PS). Margins were considered negative if no tumor was present at the inked margins. Twenty-four tumors had microscopically positive margins at the time of resection, whereas 20 tumors had margins that were free of tumor (Table 1).
Tumor size was defined as the maximum dimensions on gross pathology. The average size of the tumor was 8.0 cm (range, 2–30 cm) (Table 1). Anatomic site was defined as extremity (any tumor occurring in the limbs), pelvis (any tumor in the pelvis or groin), or trunk (any tumors originating in the thorax, retroperitoneum, paraspinal muscles, or axilla). The most common site of disease was the extremity (31), followed by the trunk (10), and pelvis (three).
For primary staging, all patients underwent MRI of the tumor region for local resection planning and CT of the lungs to establish the presence of metastasis. All patients received neoadjuvant chemotherapy. Postsurgical resection chemotherapy was given if the tumor had a good histologic response to neoadjuvant chemotherapy. External beam radiation therapy was given postoperatively if adequate surgical margins were not obtained (margins were not free of tumor). Patients initially were followed for 2 years postoperatively at 3-month intervals. Patients then were followed every 6 months for 3 years. Finally, patients were followed annually for 5 years. Physical examination, MRI of the tumor site, and CT of the lungs were performed at each followup for surveillance for local recurrence and metastasis. Although PET imaging currently is considered an excellent study in detecting tumor recurrence, it was not included for routine surveillance in this study. At the study’s inception in 1996 and until recently, PET imaging was not used routinely in imaging for sarcomas and was not routinely covered by insurance. Because only pretherapy PET imaging was funded through the senior author’s (JFE) NIH funding, posttherapy PET surveillance was not included in this study.
Detailed methods for PET imaging of patients with sarcoma have been described [10, 13]. PET imaging was performed before surgical resection or neoadjuvant chemotherapy in all patients. All PET scans were performed on one scanner and interpreted by one reviewer (JFE). Imaging studies were performed on an Advance Tomograph (General Electric Medical Systems, Waukesha, WI) operating in a two-dimensional high-sensitivity mode with 35 imaging planes per axial field of view of 15 cm (plane thickness 4.25 mm) and an in-plane resolution of 4 to 5 mm. All patients fasted for at least 12 hours before intravenous injection of 370 MBq 18F-FDG. After the patients were positioned in the tomograph, we acquired a 15-minute attenuation scan over the tumor site followed by an emission scan of the tumor site at 45 minutes after tracer injection. Subsequently, additional adjoining 15-cm fields of view of the greater tumor area were acquired. The FDG-PET scan was performed in limited views of the tumor only to assess the tumor for risk of aggressive biologic behavior. We performed these research studies only to evaluate tumor biology, not to stage the patient for cancer.
Circular or elliptic regions of interest (ROIs) were placed over the tumor site on transaxial images. We performed sagittal and coronal image reconstruction to ensure correct ROI placement. The SUVmax for each ROI was calculated automatically by the tomograph software according to the following expression:
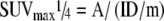 |
where A is the maximum tissue activity in the ROI, ID is the injected dose, and m is the patient’s body weight. Tumor SUVmax ranged from 1.2 to 13.0 with a median of 4.35 (Table 2).
Table 2.
Data summary for FDG-PET SUVmax
| Patients | Number | SUVmax | |||
|---|---|---|---|---|---|
| Median | Standard deviation | Minimum | Maximum | ||
| All patients | 44 | 4.35 | 2.5 | 1.2 | 13.0 |
| Patients who died of disease | 13 | 6.6 | 1.7 | 4.0 | 13.0 |
| Patients with metastasis at presentation | 5 | 6.5 | 1.1 | 6.0 | 9.6 |
| Patients who had metastasis develop | 13 | 5.9 | 1.8 | 4.0 | 9.1 |
| Patients with local recurrence | 7 | 6.6 | 2.4 | 3.1 | 7.9 |
FDG-PET = 18F-fluorodeoxyglucose positron emission tomography; SUVmax = maximum standard uptake value.
Survival was used as the primary end point in the outcome analysis [15]. All analyses were performed with standard censoring procedures for survival analysis. Cox regression was used to assess the significance of individual variables. The relationship between the time to death and the full set of measured prognostic factors was evaluated using the standard multivariate Cox proportional hazards regression analysis. This analysis permits an examination of the influence of the PET measures and allows control for other variables’ impacts. All variables were included in the initial model for multivariate analyses for overall and progression-free survival. Variables then were deleted, individually, based on their contribution (least important variable was deleted) until all remaining variables were noteworthy. Progression-free survival was defined as the time until progression of disease (local recurrence or metastasis). Patients with metastasis at presentation were removed from analysis. For the final multivariate model for progression-free survival, we included only SUVmax because no other variables were associated with progression-free survival. To assess the comparability of this data set with historical data, a multivariate analysis for overall survival without FDG-PET SUVmax data also was considered. In this analysis, all variables, with the exception of pretherapy FDG-PET SUVmax, were included in the initial model and a backward elimination procedure applied.
Results
The median SUVmax value of 4.35 was determined as a cutoff to identify patients at high risk for not surviving their disease. Thirteen patients died of their disease. Twelve (92%) of these patients presented with a pretherapy SUVmax greater than 4.35 (range, 4.8–13). The remaining patient presented with a SUVmax of 4.0 (Table 2). Twenty-two patients presented with a SUVmax greater than 4.35. More than ½ (12) of these patients did not survive their disease, six (27%) are currently alive with disease (metastasis and/or local recurrence), and four (18%) are currently without evidence of disease (Table 1). Twenty-two patients presented with a SUVmax less than 4.35. Of these patients, one died of disease (pretherapy SUVmax = 4.0), three (14%) are alive with disease (metastasis and/or local recurrence), and 18 (82%) are alive without evidence of disease (Table 1). Five patients had American Joint Commission on Cancer [25] Stage IV disease (pulmonary metastasis) and had a median SUVmax of 6.5 (range, 6.0–9.6) (Table 2). An additional 13 patients with a median SUVmax of 5.9 (range, 4.0–9.1) had pulmonary metastasis develop at a median of 20.4 months (range, 1–56 months) after beginning treatment. Seven patients with a median SUVmax of 6.6 (range, 3.1–7.9) had local recurrence of their disease, which occurred at a median of 21.3 months (range, 12–34 months) after diagnosis (Table 2).
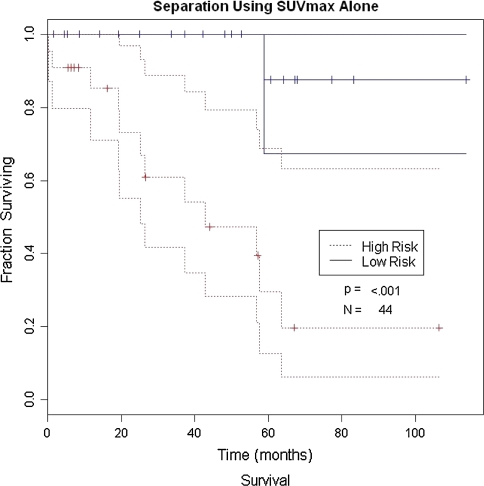
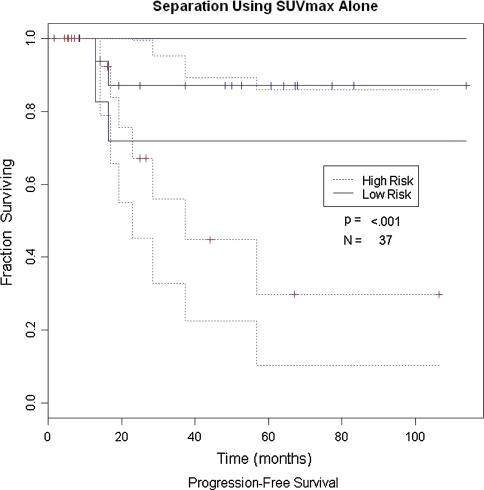
Pretherapy SUVmax, metastasis at presentation, and gender were associated with overall survival (p = 0.005, 0.0008, and 0.004, respectively) (Table 3; Fig. 1). Age, grade, histology, surgical margins, size, and anatomic site did not predict progression-free survival (Table 4; Fig. 2). When pretherapy FDG-PET SUVmax was eliminated from the analysis to compare all other data (age, gender, metastasis at presentation, size, location, histology, grade, and margins) with previously reported risk factors, metastatic status at presentation, gender, and size were associated with decreased overall survival (p = 0.0057, 0.036, and 0.05, respectively), whereas age, location, histology, margins, and grade were not (Table 5).
Table 3.
Multivariate analysis for overall survival
| Variable | Hazard | p Value |
|---|---|---|
| FDG-PET SUVmax | 6.52 | 0.005 |
| Metastasis at presentation | 18.04 | 0.0008 |
| Gender | 19.42 | 0.004 |
| Size | 1.07 | 0.16 |
| Age | 2.24 | 0.076 |
| Location | 1.76 | 0.43 |
| Histology | 7.95 | 0.066 |
| Margins | 2.09 | 0.31 |
| Grade | 2.07 | 0.25 |
FDG-PET = 18F-fluorodeoxyglucose positron emission tomography; SUVmax = maximum standard uptake value.
Fig. 1.
Overall survival for patients using SUVmax alone is shown. High risk is defined as a SUVmax greater than 4.35 and low risk is defined as a SUVmax less than 4.35.
Table 4.
Multivariate analysis for progression-free survival
| Variable | Hazard | p Value |
|---|---|---|
| FDG-PET SUVmax | 2.54 | 0.006 |
| Gender | 2.53 | 0.17 |
| Size | 1.16 | 0.08 |
| Age | 1.76 | 0.08 |
| Location | 1.65 | 0.46 |
| Histology | 0.71 | 0.63 |
| Margins | 1.03 | 0.97 |
| Grade | 0.864 | 0.86 |
FDG-PET = 18F-fluorodeoxyglucose positron emission tomography; SUVmax = maximum standard uptake value.
Fig. 2.
Progression-free survival for patients using SUVmax is shown. High risk is defined as a SUVmax greater than 4.35 and low risk is defined as a SUVmax less than 4.35.
Table 5.
Multivariate analysis for overall survival without FDG-PET SUVmax data
| Variable | Hazard | p Value |
|---|---|---|
| Metastasis at presentation | 195.004 | 0.0057 |
| Gender | 38.948 | 0.036 |
| Size | 1.35 | 0.05 |
| Age | 1.059 | 0.079 |
| Location | 3.81 | 0.24 |
| Histology | 3.92 | 0.22 |
| Margins | 0.337 | 0.33 |
| Grade | 1.764 | 0.7 |
FDG-PET = 18F-fluorodeoxyglucose positron emission tomography; SUVmax = maximum standard uptake value.
Discussion
Pretherapy tumor FDG-PET SUVmax predicts survival in patients with several types of sarcoma, including liposarcoma, chondrosarcoma, and Ewing’s sarcoma [4, 5, 14]. Synovial sarcoma represents a biologically diverse subtype of soft tissue sarcomas. Currently, there is no reproducible clinical, histologic, or radiographic indictor that has proven useful in assessing patient prognosis. We therefore prospectively assessed the prognostic value of pretherapy FDG-PET SUVmax in overall survival of patients with synovial sarcoma and compared these results with age, gender, histopathologic subtype, grade, size, site, and metastasis at presentation.
One of the major limitations of this study is the differences in postresection treatment received by patients. If resection resulted in contaminated surgical margins, patients received adjuvant radiotherapy. If patients had a good histologic response to neoadjuvant chemotherapy, they received adjuvant chemotherapy as well; however, the number of patients in this subgroup was too small to be statistically analyzed. Nonetheless, given that synovial sarcomas are a rare subtype in an already uncommon group of soft tissue sarcomas and that FDG-PET has been clinically available only for the past 10 years, we have one of the largest groups of patients with synovial sarcoma that have undergone pretherapy FDG-PET imaging. Perhaps additional analysis on a larger homogenous data set could take into account the fraction of patients who had additional adjuvant therapy. Another limitation of this analysis may be the inclusion of only patients with tumors we judged at high risk: tumor size greater than 5 cm, tumor palpated deep to the fascia and firmness of the tumor by clinical examination, and MRI findings of tumor greater than 5 cm, deep to the fascia with heterogeneous signal, and peripheral edema. However, the data analysis suggested a substantial survival difference for patients in this high-risk group based on a pretherapy FDG-PET tumor SUVmax greater than 4.35. Tumor grade and histologic type (monophasic or biphasic) did not predict survival. As expected, the presence or absence of metastases at presentation was strongly predictive for decreased overall survival.
We found tumor SUVmax ranged from 1.2 to 13.0, reflecting the wide range of tumor metabolism in synovial sarcomas. Numerous studies have shown tumor metabolism measured by FDG-PET SUVmax reflects biologic aggressiveness [3–5, 10, 11, 13, 14]. Previous data for a group of patients with sarcoma showed this value is correlated with tumor cellularity and mitosis rate [13].
The median pretherapy SUVmax of 4.35 in our study predicted overall patient survival. Patients presenting with a pretherapy SUVmax greater than 4.35 had an overall decreased (p = 0.005) survival when compared with all other variables examined in this study. Conversely, only one patient presenting with a pretherapy SUVmax less than 4.35 died of disease. Different sarcoma histologic subtypes exhibit specific FDG-PET SUVmax ranges [3–5, 8, 10, 11, 13, 14]. Our group of synovial sarcomas reportedly had a slightly lower median value than other soft tissue tumor types [10]. Our group also showed SUVmax varies over a wide range. This finding is exemplified by the subset of synovial sarcomas in this study group with a pretherapy PET SUVmax of 2.0 or less (Table 6). These relatively low SUVmax values reflect the less aggressive nature of this subtype of synovial sarcoma. Consistent with the findings reported in this study, patients who presented with a lower SUVmax of 2.0 or less had no recurrence and no metastasis and are all alive without disease at the time of this study. This highlights the fact that FDG-PET SUVmax reflects tumor biology and aggressiveness and may be an underlying reason why the SUVmax predicts survival. Although some benign processes, such as fractures, myositis ossificans, and extraabdominal fibromatosis, may show a high FDG-PET SUVmax, this reflects only the lesions ability to be locally aggressive and metabolically active. By definition, however, these processes lack the cellular biology to metastasize.
Table 6.
Characteristics of patients with initial pretherapy FDG-PET SUVmax of 2.0 or less
| SUVmax | Recurrence | Metastasis | Death | Gender | Size (cm) | Location | Grade | Histology | Age (years) |
|---|---|---|---|---|---|---|---|---|---|
| 1.2 | No | No | No | Female | 2.0 | Extremity | Intermediate | Monophasic | 8 |
| 1.9 | No | No | No | Female | 5.5 | Extremity | Intermediate | Monophasic | 17 |
| 2.0 | No | No | No | Male | 4.3 | Extremity | Intermediate | Monophasic | 36 |
| 2.0 | No | No | No | Male | 8.0 | Trunk | Intermediate | Monophasic | 16 |
| 2.0 | No | No | No | Male | 8.3 | Extremity | Intermediate | Biphasic | 10 |
FDG-PET = 18F-fluorodeoxyglucose positron emission tomography; SUVmax = maximum standard uptake value.
During the last several decades, much effort has been invested in determining prognostic factors affecting overall survival in patients with synovial sarcoma. Histologic subtype, tumor grade, anatomic location, age, gender, tumor size, and surgical margins have been reported to have prognostic implication for a patient’s overall survival [1, 2, 6, 9, 12, 16, 17, 19–24]. In our study, histologic subtype, grade, anatomic location, size, age, and surgical margins did not predict overall survival. Furthermore, only pretherapy PET-FDG SUVmax predicted progression-free survival.
The use of FDG-PET in cancer risk assessment continues to be explored. Its many advantages for evaluation of patients with sarcomas include ability to provide quantitative, objective tumor metabolic information noninvasively, three-dimensional high-resolution images, and standardization in imaging techniques for tumor restaging and treatment response situations [18]. We used FDG-PET imaging uptake data for synovial sarcomas in a relatively large group. With this experience, we will begin to consider the use of FDG-PET imaging as an important predictor for patient prognosis and treatment planning. The pretherapy tumor SUVmax of synovial sarcoma may be useful as a means to identify patients at high risk for poor outcome. In this setting, the tumor SUVmax of synovial sarcoma might function as an objective measure of therapy effectiveness for how multimodality treatment affects protocols and surgical approaches.
Acknowledgments
We acknowledge Brian Rubin, MD, and Paul Swanson, MD, for review of the pathology. We thank Neha Patel and Marie Janes for assistance in data preparation.
Footnotes
One or more of the authors (JFE) have received funding from National Institutes of Health/National Cancer Institute Grant RO1 CA 65537.
Each author certifies that his or her institution has approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Andrassy RJ, Okcu MF, Despa S, Raney RB. Synovial sarcoma in children: surgical lessons from a single institution and review of the literature. J Am Coll Surg. 2001;192:305–313. [DOI] [PubMed]
- 2.Bergh P, Meis-Kindblom JM, Gherlinzoni F, Berlin O, Bacchini P, Bertoni F, Gunterberg B, Kindblom LG. Synovial sarcoma: identification of low and high risk groups. Cancer. 1999;85:2596–2607. [DOI] [PubMed]
- 3.Brenner W, Bohuslavizki KH, Eary JF. PET imaging of osteosarcoma. J Nucl Med. 2003;44:930–942. [PubMed]
- 4.Brenner W, Conrad EU, Eary JF. FDG PET imaging for grading and prediction of outcome in chondrosarcoma patients. Eur J Nucl Med Mol Imaging. 2004;31:189–195. [DOI] [PubMed]
- 5.Brenner W, Eary JF, Hwang W, Vernon C, Conrad EU. Risk assessment in liposarcoma patients based on FDG PET imaging. Eur J Nucl Med Mol Imaging. 2006;33:1290–1295. [DOI] [PubMed]
- 6.Campbell C, Gallagher J, Dickinson I. Synovial sarcoma: towards a simplified approach to prognosis. ANZ J Surg. 2004;74:727–731. [DOI] [PubMed]
- 7.Coindre JM, Nguyen BB, Bonichon F, de Mascarel I, Trojani M. Histopathologic grading in spindle cell soft tissue sarcomas. Cancer. 1988;61:2305–2309. [DOI] [PubMed]
- 8.Conrad EU 3rd, Morgan HD, Vernon C, Schuetze SM, Eary JF. Fluorodeoxyglucose positron emission tomography scanning: basic principles and imaging of adult soft-tissue sarcomas. J Bone Joint Surg Am. 2004;86(suppl 2):98–104. [PubMed]
- 9.Deshmukh R, Mankin HJ, Singer S. Synovial sarcoma: the importance of size and location for survival. Clin Orthop Relat Res. 2004;419:155–161. [DOI] [PubMed]
- 10.Eary JF, Conrad EU, Bruckner JD, Folpe A, Hunt KJ, Mankoff DA, Howlett AT. Quantitative [F-18]fluorodeoxyglucose positron emission tomography in pretreatment and grading of sarcoma. Clin Cancer Res. 1998;4:1215–1220. [PubMed]
- 11.Eary JF, O’Sullivan F, Powitan Y, Chandhury KR, Vernon C, Bruckner JD, Conrad EU. Sarcoma tumor FDG uptake measured by PET and patient outcome: a retrospective analysis. Eur J Nucl Med Mol Imaging. 2002;29:1149–1154. [DOI] [PubMed]
- 12.Ferrari A, Gronchi A, Casanova M, Meazza C, Gandola L, Collini P, Lozza L, Bertulli R, Olmi P, Casali PG. Synovial sarcoma: a retrospective analysis of 271 patients of all ages treated at a single institution. Cancer. 2004;101:627–634. [DOI] [PubMed]
- 13.Folpe AL, Lyles RH, Sprouse JT, Conrad EU 3rd, Eary JF. (F-18)Fluorodeoxyglucose positron emission tomography as a predictor of pathologic grade and other prognostic variables in bone and soft tissue sarcoma. Clin Cancer Res. 2000;6:1279–1287. [PubMed]
- 14.Hawkins DS, Schuetze SM, Butrynski JE, Rajendran JG, Vernon CB, Conrad EU 3rd, Eary JF. [18F]Fluorodeoxyglucose positron emission tomography predicts outcome for Ewing sarcoma family of tumors. J Clin Oncol. 2005;23:8828–8834. [DOI] [PubMed]
- 15.Kalbfleisch J. The Statistical Analysis of Failure Time Data. 2nd ed. New York, NY: John Wiley and Sons; 2002.
- 16.Lewis JJ, Antonescu CR, Leung DH, Blumberg D, Healey JH, Woodruff JM, Brennan MF. Synovial sarcoma: a multivariate analysis of prognostic factors in 112 patients with primary localized tumors of the extremity. J Clin Oncol. 2000;18:2087–2094. [DOI] [PubMed]
- 17.Machen SK, Easley KA, Goldblum JR. Synovial sarcoma of the extremities: a clinicopathologic study of 34 cases, including semi-quantitative analysis of spindled, epithelial, and poorly differentiated areas. Am J Surg Pathol. 1999;23:268–275. [DOI] [PubMed]
- 18.O’Sullivan F, Roy S, O’Sullivan J, Vernon C, Eary J. Incorporation of tumor shape into an assessment of spatial heterogeneity for human sarcomas imaged with FDG-PET. Biostatistics. 2005;6:293–301. [DOI] [PubMed]
- 19.Paulino AC. Synovial sarcoma prognostic factors and patterns of failure. Am J Clin Oncol. 2004;27:122–127. [DOI] [PubMed]
- 20.Skytting B. Synovial sarcoma: a Scandinavian Sarcoma Group project. Acta Orthop Scand Suppl. 2000;291:1–28. [DOI] [PubMed]
- 21.Spillane AJ, A’Hern R, Judson IR, Fisher C, Thomas JM. Synovial sarcoma: a clinicopathologic, staging, and prognostic assessment. J Clin Oncol. 2000;18:3794–3803. [DOI] [PubMed]
- 22.Thompson RC Jr, Garg A, Goswitz J, Cheng EY, Clohisy DR, Dusenbery K. Synovial sarcoma: large size predicts poor outcome. Clin Orthop Relat Res. 2000;373:18–24. [DOI] [PubMed]
- 23.Trassard M, Le Doussal V, Hacene K, Terrier P, Ranchere D, Guillou L, Fiche M, Collin F, Vilain MO, Bertrand G, Jacquemier J, Sastre-Garau X, Bui NB, Bonichon F, Coindre JM. Prognostic factors in localized primary synovial sarcoma: a multicenter study of 128 adult patients. J Clin Oncol. 2001;19:525–534. [DOI] [PubMed]
- 24.Trojani M, Contesso G, Coindre JM, Rouesse J, Bui NB, de Mascarel A, Goussot JF, David M, Bonichon F, Lagarde C. Soft-tissue sarcomas of adults: study of pathological prognostic variables and definition of a histopathological grading system. Int J Cancer. 1984;33:37–42. [DOI] [PubMed]
- 25.UICC: TNM Classification of Malignant Tumors. 4th ed. New York, NY: Wiley-Liss; 1992.
- 26.Weiss SW, Goldblum JR, eds. Enzinger and Weiss’s Soft Tissue Tumors. St Louis, MO: CV Mosby; 2001.
- 27.Wright PH, Sim FH, Soule EH, Taylor WF. Synovial sarcoma. J Bone Joint Surg Am. 1982;64:112–122. [PubMed]