Abstract
We report the case of a 59-year-old man with Waldenstrom’s macroglobulinemia and active alcohol use who presented with bilateral knee pain 5 years after a bilateral staged TKA. Cultures of synovial fluid and periprosthetic tissue specimens from both knees yielded, after prolonged anaerobic incubation, a catalase- and oxidase-positive gram-negative bacillus, which was identified as Capnocytophaga canimorsus by 16S ribosomal RNA PCR analysis. C canimorsus, an organism that is commonly found in dog and cat saliva, is a rare cause of various infections in immunocompromised and healthy individuals. However, a review of the medical literature indicates C canimorsus has not been reported previously to cause infection after joint arthroplasty. The patient was immunocompromised by cytotoxic chemotherapy, corticosteroids, and alcohol use. The patient was managed successfully with bilateral two-stage exchange and 6 weeks of intravenous ertapenem therapy. Because of its fastidious and slow-growing characteristics, C canimorsus may be an unrecognized cause of culture-negative joint arthroplasty infections, especially in cases when dog and cat exposure is evident in the clinical history.
Introduction
Infection of prosthetic joints is a rare but catastrophic complication of joint arthroplasty. Staphylococcus spp are the most common pathogens causing prosthetic joint infections [2]. However, culture-negative prosthetic joint infections may be observed in 7% of cases [3]. We describe a novel pathogen, Capnocytophaga canimorsus, causing bilateral prosthetic knee infections in a patient with compromised immune function as a result of chemotherapy, prednisone use, and alcoholism. The microbiologic diagnosis of C canimorsus was delayed, and the patient underwent bilateral revision knee arthroplasties to treat his prolonged symptoms of pain and swelling. He later underwent bilateral resection TKAs. Cultures of synovial fluid aspirate and periprosthetic tissue specimens from the resection arthroplasties initially were reported as negative until after 7 days of anaerobic incubation. Therefore, we report this case to provide a brief review of C canimorsus, which should be considered as a rare potential cause of culture-negative prosthetic joint infection, particularly in individuals with epidemiologic exposure.
Case Report
The patient was a 59-year-old man who presented to our clinic because of a 2-year history of bilateral knee pain and swelling. Five years before his clinical presentation, he underwent bilateral staged TKAs at his local hospital for osteoarthrosis. He initially did well for 3 years but then had progressive pain and swelling develop in both knees. Four years after prosthesis implantation, he underwent bilateral staged polyethylene liner exchanges to address his pain and swelling, which were attributed to instability. However, these procedures, which were performed at his local hospital, provided no improvement of his clinical symptoms. Preoperatively, his synovial fluid culture was negative. Likewise, intraoperative cultures at the time of revision arthroplasty revealed no bacterial or fungal growth. Because of his ongoing clinical symptoms, he received several intraarticular injections of etanercept and corticosteroids after the bilateral revision knee surgeries.
The patient had underlying Waldenstrom’s macroglobinemia managed with rituximab followed by chronic chlorambucil therapy. Six months before presentation, he was initiated on prednisone therapy 10 mg orally twice daily for the knee pain and swelling, which had been attributed to inflammation from Waldenstrom’s disease. He also had a history of extensive alcohol use. He kept two dogs at his home, although he reported no history of dog bite or exposure of open wounds to dog saliva.
Physical examination revealed considerable instability in both knees with boggy knee effusions. His previous incisions were well-healed. His skin was intact with no dermatologic findings of septic embolization. His cardiac examination did not reveal any murmur. With the exception of his bilaterally swollen and tender knees, his physical examination was otherwise unremarkable.
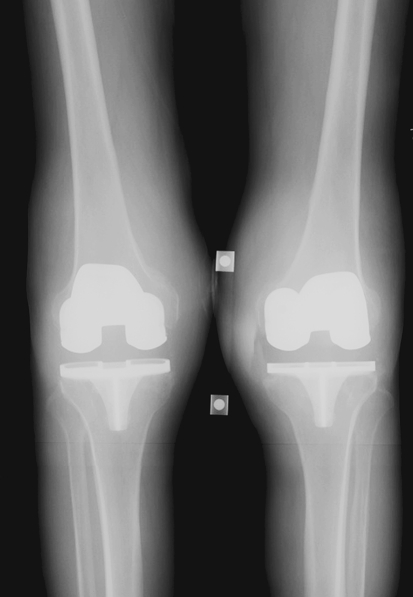
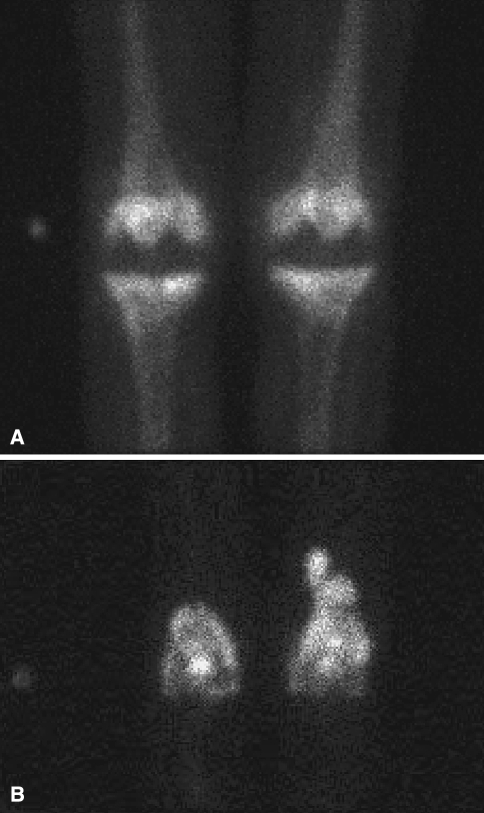
Plain radiographs revealed bilateral TKAs with some evidence of osteolysis on the left medial tibial plateau (Fig. 1). Indium-labeled leukocyte scan showed increased uptake over the bilateral distal femurs consistent with infection (Fig. 2). Peripheral leukocyte count was within normal limits (5.7 × 109/L). The erythrocyte sedimentation rate was elevated at 67 mm/hour (normal range, 0–22 mm/hour) and C-reactive protein was elevated at 5.6 mg/dL (normal range, < 0.8 mg/dL). Waldenstrom’s macroglobulinemia has been associated with elevated sedimentation rate and C-reactive protein in as much as 50% of untreated patients at the time of diagnosis [30]. This was less likely in our patient, however, as he had been receiving treatment for macroglobulinemia for 18 months before these laboratory findings.
Fig. 1.
Preoperative imaging of the both knees reveals knee effusions and some evidence of osteolysis about the right tibial component.
Fig. 2A–B.
(A) A preoperative delayed 3-hour bone scan image shows moderate uptake at the bilateral TKA prostheses. (B) A preoperative indium-labeled leukocyte scan shows discordant areas of increased uptake over the distal femurs, left greater than right, suspicious for bilateral periprosthetic infections.
Bilateral knee aspirates revealed purulent-appearing material, with elevated total nucleated cell counts in both knees. The right knee synovial fluid had 18,564 total nucleated cells/dL with 92% polymorphonucleocytes, and the left knee synovial fluid had 25,064 total nucleated cells/dL with 86% polymorphonucleocytes. Initial culture of both synovial fluid specimens from both knees showed no growth at 5 days.
Because of the elevated total nucleated cell counts in the synovial fluid, elevated inflammatory markers, and indium-labeled leukocyte scan suggestive of infection, the patient underwent bilateral resection arthroplasties with the plan for two-staged reimplantation. Cultures from the knee aspirates at the time of the resection arthroplasties showed no growth. We noted severe chronic-appearing synovitis and gross purulence on exposure of the joints. The left knee had severe cavitary bone loss of the lateral femoral condyle and discontinuity of the lateral collateral ligament. The components in the right knee were well-fixed. Intraoperative frozen sections of synovial tissue were positive for acute inflammation. Fluid and tissue cultures and ultrasonicate culture specimens from the bilateral prosthetic implants were sent to the microbiology laboratory. Cement dowels impregnated with vancomycin, tobramycin, and amphotericin B were placed in the freshly reamed femoral and tibial canals. Resection arthroplasties of both knees typically would result in severe difficulty with mobilization and likely confinement to a skilled nursing facility. Using antibiotic-impregnated cement, we implanted new femoral implants and cemented new polyethylene components above tibial antibiotic spacers to serve as an articulation to allow the patient some degree of mobilization.
After 6 to 7 days incubation (Postoperative Day 2), the presurgical synovial fluid aspirate culture was reported as growing a gram-negative bacillus from the anaerobic broth. The organism was catalase- and oxidase-positive. Subsequently, all intraoperative tissue and fluid specimens yielded the same gram-negative bacillus from the anaerobic culture. The ultrasonicate culture specimens also yielded greater than 100 colony-forming units of the same organism. Using 16S ribosomal RNA PCR testing, and confirmed by DNA sequencing, the gram-negative organism later was speciated as C canimorsus. Because of its poor growth pattern, full antimicrobial susceptibility testing could not be performed, although the organism was tested β-lactamase-negative. Blood cultures were negative. Transesophageal echocardiography did not reveal a vegetative lesion suggestive of bacterial endocarditis.
The patient was treated with a 6-week course of intravenous ertapenem (1 g every 24 hours). He subsequently underwent left knee reimplantation arthroplasty at 3 months and right knee reimplantation 7 months after resection. In both instances, no acute inflammation was noted on histologic sections from the synovial lining and the intraoperative cultures showed no growth of the organism. One year after presentation and 3 months after his right knee reimplantation, the patient was doing well with no major clinical symptoms related to his knees.
Discussion
Our review of the literature indicates C canimorsus has not been reported previously to cause infection after joint arthroplasty. To treat persistent knee effusions, our patient underwent bilateral revision knee surgery. Despite negative preoperative and intraoperative cultures, the effusions may have been attributable to chronic infections with C canimorsus. Thus, the diagnosis and treatment of this infection may have been delayed owing to the difficulty in isolating the organism. C canimorsus is characteristically fastidious and grows slowly; therefore, it potentially could account for some of the culture-negative prosthetic joint infections seen in orthopaedic practice. We report this case to increase awareness in the orthopaedic community of C canimorsus as a possible pathogen causing prosthetic joint infection, especially with exposure to cats and dogs.
C canimorsus is a slender, fusiform, gram-negative bacillus that commonly is found in dog and cat saliva [15, 24]. It is facultative and capnophilic, and it grows slowly under anaerobic conditions [4]. In numerous reports, C canimorsus was identified only through PCR targeting of the bacterial 16S rRNA [6, 16, 23, 28, 29]. In our case, the organism was not detected until the seventh day of anaerobic incubation, and its identity was made through molecular 16S rRNA PCR and confirmed by DNA sequencing.
The slow-growth characteristics of the organism also may result in difficulties performing antimicrobial susceptibility testing, as in our case. Generally, however, C canimorsus is susceptible to most antibiotics, excluding aztreonam, trimotheprim-sulfamethoxazole, and aminoglycosides [15, 17]. Capnocytophaga spp are reportedly universally susceptible to carbapenems such as imipenem [1]. Ertapenem is a synthetic bactericidal carbapenem that binds penicillin-binding proteins and inhibits cell wall synthesis. It is active against bacteria that have developed penicillin resistance through penicillin-binding proteins and β-lactamase production. The organism in this case did not produce β-lactamase, and therefore common β-lactam antibiotics such as cefazolin and ceftriaxone would have sufficed for treatment. Ertapenem initially was chosen for treatment in this case because of the initial description of a gram-negative bacillus in anaerobic cultures. Since the patient tolerated the drug, which has good tissue penetration and adequate coverage against C canimorsus, it was decided to complete the 6-week treatment course with once daily intravenous ertapenem.
In vitro, Capnocytophaga spp elicit little inflammatory response, thus resulting in considerable multiplication before activating the host’s immune response [27]. However, our patient had purulent fluid aspirated preoperatively, and gross purulence and severe synovitis found at the time of resection arthroplasty, arguing against these in vitro findings. It is possible, however, the chronicity of this infection eventually led to the substantial purulence observed in the tissues. However, this immunocompromised patient had chronic bilateral knee infections with no development of systemic illness or overwhelming infection necessitating emergent surgical treatment.
Clinically, C canimorsus has been described to cause sepsis and peripheral gangrene after dog bite or dog exposure in patients who are immunocompromised or immunocompetent. Reported exposures include dog or cat bite, licking of ulcers, burns, or wounds, dog ownership, and even casual contact with dogs [8, 11, 16, 22]. In one study of human C canimorsus infections [16], approximately 80% of patients had animal bite or animal contact with open wounds, and 20% had no known exposure.
C canimorsus also has been reported as a cause of endocarditis, mycotic aneurysm, and meningitis [6, 7, 12, 14, 19, 23, 25, 28]. The mortality rate is reportedly high (30%) [5, 8, 9, 13, 20]. In our case, the simultaneous clinical presentation of bilateral prosthetic knee infections would suggest a potentially disseminated disease, especially because the patient was immunocompromised. However, blood cultures and transesophageal echocardiogram did not reveal intravascular or systemic infection. Although C canimorsus has not been reported previously as a cause of prosthetic joint infection, it rarely has been reported as a cause of bone or joint infection. Capnocytophaga spp have been associated with osteomyelitis in children [11]. A report from California’s Microbial Diseases Laboratory of 56 isolates of C canimorsus revealed only one isolate obtained from a septic knee aspiration [16]; however, no additional clinical details were provided in this report [16]. There is a single case report of vertebral osteomyelitis after C canimorsus bacteremia [21] and a report of two cases of Capnocytophaga spp resulting in vertebral osteomyelitis [10].
As much as 40% of affected patients are healthy with no known immunocompromise; thus, C canimorsus often is not considered an opportunistic bacterium [18]. Common forms of immunocompromise associated with C canimorsus infection include splenectomy, alcoholism, immunosuppressive drugs, and neutropenia [8, 16, 26]. Our patient fits this profile of immunocompromise because of his alcoholism (which results in nutritional deficiency and bone marrow suppression that leads to leukopenia and neutropenia), use of prednisone (which causes profound inhibitory effects on a broad range of specific immune responses mediated by T and B lymphocytes, and potent suppression of effector functions of neutrophils, monocytes, and macrophages) and other immunosuppressive drugs such as rituximab (which is a monoclonal antibody directed against the CD20 of B lymphocytes), and underlying Waldenstrom’s macroglobulinemia (which can lead to leukocyte deficiencies and functional defects attributable to bone marrow infiltration). The specific immune defect that predisposed our patient to have C canimorsus infection develop is not entirely clear; however, all of these immune-compromising clinical factors, especially neutrophil defects, together with the history of dog exposure, would make our patient at particular risk of C canimorsus infection. Although this patient does not recall a dog bite or dog exposure to an open wound, he is a dog owner and may not remember an incidental bite or lick since he presented 2 years after his initial symptoms. Unless his pet licked his open bilateral knee wounds, his exposure likely would have been through hematogenous spread to the knees, as is common for delayed periprosthetic infections.
We report a description of prosthetic joint infection resulting from C canimorsus. Once the infection was identified, the patient was treated with two-stage exchange and appropriate antibiotic therapy. This fastidious organism grows very slowly and is difficult to isolate, although specialized PCR testing can be performed if there is high clinical suspicion. Patients undergoing workup for possible prosthetic joint infections should be questioned regarding their exposure to animals, particularly if the patient has a history of immunocompromise. The long-term effects of animal exposure for patients undergoing joint arthroplasty, however, are unknown. This patient certainly had several risk factors for C canimorsus infection, including chronic prednisone use and alcohol use. Because as much as 7% of prosthetic infections may be culture-negative [3], this organism should be considered as a potential cause when there is evident epidemiologic exposure.
Footnotes
One of the authors (ADH) has received funding from Stryker Orthopaedics, Mahway, NJ, DePuy Orthopaedics, Inc, Warsaw, IN, and Zimmer, Inc, Warsaw, IN.
References
- 1.Arlet G, Sanson-Le Pors MJ, Casin IM, Ortenberg M, Perol Y. In vitro susceptibility of 96 Capnocytophaga strains, including a beta-lactamase producer, to new beta-lactam antibiotics and six quinolones. Antimicrob Agents Chemother. 1987;31:1283–1284. [DOI] [PMC free article] [PubMed]
- 2.Berbari EF, Hanssen AD, Duffy MC, Steckelberg JM, Ilstrup DM, Harmsen WS, Osmon DR. Risk factors for prosthetic joint infection: case-control study. Clin Infect Dis. 1998;27:1247–1254. [DOI] [PubMed]
- 3.Berbari EF, Marculescu C, Sia I, Lahr BD, Hanssen AD, Steckelberg JM, Gullerud R, Osmon DR. Culture-negative prosthetic joint infection. Clin Infect Dis. 2007;45:1113–1119. [DOI] [PubMed]
- 4.Bonatti H, Rossboth DW, Nachbaur D, Fille M, Aspock C, Hend I, Hourmont K, White L, Malnick H, Allerberger FJ. A series of infections due to Capnocytophaga spp in immunosuppressed and immunocompetent patients. Clin Microbiol Infect. 2003;9:380–387. [DOI] [PubMed]
- 5.Bryson MS, Neilly I, Rodger S, Soutar RL. Purpura fulminans associated with Capnocytophaga canimorsus infection. Br J Haematol. 2003;121:1. [DOI] [PubMed]
- 6.Chu P, Howden BP, Jones S, Fell G, Roberts AK. Once bitten, twice shy: an unusual case report of a mycotic aortic aneurysm. ANZ J Surg. 2005;75:1024–1026. [DOI] [PubMed]
- 7.de Boer MG, Lambregts PC, van Dam AP, van ‘t Wout JW. Meningitis caused by Capnocytophaga canimorsus: when to expect the unexpected. Clin Neurol Neurosurg. 2007;109:393–398. [DOI] [PubMed]
- 8.Depres-Brummer P, Buijs J, van Engelenburg KC, Oosten HR. Capnocytophaga canimorsus sepsis presenting as an acute abdomen in an asplenic patient. Neth J Med. 2001;59:213–217. [DOI] [PubMed]
- 9.Deshmukh PM, Camp CJ, Rose FB, Narayanan S. Capnocytophaga canimorsus sepsis with purpura fulminans and symmetrical gangrene following a dog bite in a shelter employee. Am J Med Sci. 2004;327:369–372. [DOI] [PubMed]
- 10.Duong M, Besancenot JF, Neuwirth C, Buisson M, Chavanet P, Portier H. Vertebral osteomyelitis due to Capnocytophaga species in immunocompetent patients: report of two cases and review. Clin Infect Dis. 1996;22:1099–1101. [DOI] [PubMed]
- 11.Elster AD, Macone AB, Kasser JR. Osteomyelitis caused by Capnocytophaga ochracea. J Pediatr Orthop. 1983;3:613–615. [DOI] [PubMed]
- 12.Gibou D, Kassiotis P, Pouedras P, Ambroselli C, Cochery T, Tattevin P. [Capnocytophaga canimorsus meningitis] [in French]. Med Mal Infect. 2008;38:32–33. [DOI] [PubMed]
- 13.Gouin P, Veber B, Collange O, Frebourg N, Dureuil B. [An unusual aetiology for septic shock: Capnocytophaga canimorsus. Is always dog man’s best friend?] [in French]. Ann Fr Anesth Reanim. 2004;23:1185–1188. [DOI] [PubMed]
- 14.Gutierrez-Martin MA, Araji OA, Barquero JM, Velazquez C, Garcia-Borbolla M, Gallego P, Infantes CA. Aortic valve endocarditis by Capnocytophaga haemolytica. Ann Thorac Surg. 2007;84:1008–1010. [DOI] [PubMed]
- 15.Hore C. Important unusual infections in Australia: a critical care perspective. Crit Care Resusc. 2001;3:262–272. [PubMed]
- 16.Janda JM, Graves MH, Lindquist D, Probert WS. Diagnosing Capnocytophaga canimorsus infections. Emerg Infect Dis. 2006;12:340–342. [DOI] [PMC free article] [PubMed]
- 17.Jolivet-Gougeon A, Sixou JL, Tamanai-Shacoori Z, Bonnaure-Mallet M. Antimicrobial treatment of Capnocytophaga infections. Int J Antimicrob Agents. 2007;29:367–373. [DOI] [PubMed]
- 18.Lion C, Escande F, Burdin JC. Capnocytophaga canimorsus infections in human: review of the literature and cases report. Eur J Epidemiol. 1996;12:521–533. [DOI] [PubMed]
- 19.Lipsker D, Kara F. Images in clinical medicine: retiform purpura. N Engl J Med. 2008;358:e1. [DOI] [PubMed]
- 20.Low SC, Greenwood JE. Capnocytophaga canimorsus: infection, septicaemia, recovery and reconstruction. J Med Microbiol. 2008;57:901–903. [DOI] [PubMed]
- 21.Nelson MJ, Westfal RE. Case report: vertebral osteomyelitis/discitis as a complication of Capnocytophaga canimorsus bacteremia. J Emerg Med. 2008;35:269–271. [DOI] [PubMed]
- 22.Pers C, Gahrn-Hansen B, Frederiksen W. Capnocytophaga canimorsus septicemia in Denmark, 1982–1995: review of 39 cases. Clin Infect Dis. 1996;23:71–75. [DOI] [PubMed]
- 23.Risi GF Jr, Spangler CA. Capnocytophaga canimorsus meningitis after routine myelography: a sentinel event identifies multiple opportunities for improvement of standard practices in radiology. Am J Infect Control. 2006;34:540–542. [DOI] [PubMed]
- 24.Sabbatani S, Manfredi R, Frank G, Chiodo F. Capnocytophaga spp. brain abscess in an immunocompetent host: problems in antimicrobial chemotherapy and literature review. J Chemother. 2004;16:497–501. [DOI] [PubMed]
- 25.Sandoe JA. Capnocytophaga canimorsus endocarditis. J Med Microbiol. 2004;53:245–248. [DOI] [PubMed]
- 26.Sawmiller CJ, Dudrick SJ, Hamzi M. Postsplenectomy Capnocytophaga canimorsus sepsis presenting as an acute abdomen. Arch Surg. 1998;133:1362–1365. [DOI] [PubMed]
- 27.Shin H, Mally M, Kuhn M, Paroz C, Cornelis GR. Escape from immune surveillance by Capnocytophaga canimorsus. J Infect Dis. 2007;195:375–386. [DOI] [PubMed]
- 28.Tierney DM, Strauss LP, Sanchez JL. Capnocytophaga canimorsus mycotic abdominal aortic aneurysm: why the mailman is afraid of dogs. J Clin Microbiol. 2006;44:649–651. [DOI] [PMC free article] [PubMed]
- 29.Wareham DW, Michael JS, Warwick S, Whitlock P, Wood A, Das SS. The dangers of dog bites. J Clin Pathol. 2007;60:328–329. [DOI] [PMC free article] [PubMed]
- 30.Wiedermann D, Wiedermann B, Cidl K, Kodouskova V. Individual serum proteins and acute phase reactants in monoclonal immunoglobulinopathies: a study in patients with macroglobulinemia. Neoplasma. 1980;27:473–481. [PubMed]