Abstract
Femoral varus osteotomy is one of the most common treatments for patients with symptomatic Legg-Calvé-Perthes disease with more severe deformities. We hypothesized knee valgus alignment at maturity in patients with Legg-Calvé-Perthes disease is an effect of the disease and not an effect of femoral varus osteotomy. We retrospectively compared matched groups of 28 patients with and without femoral varus osteotomy. The two groups were similar with respect to age at onset and classification of Herring et al. The distribution of valgus alignment among patients who had surgery and patients who did not have surgery was assessed at maturity and was similar between the operative and nonoperative groups. The data suggest valgus malalignment (genu valgum) is not a side effect of femoral varus osteotomy in patients with Legg-Calvé-Perthes disease, but rather an effect of the disease.
Level of Evidence: Level III, prognostic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
The treatment of Legg-Calvé-Perthes (LCP) disease is controversial. Nonoperative treatment is used for the majority of patients. Surgery is reserved for specific indications. As containment of the femoral head is thought to be fundamental to long-term outcome [2, 8], surgery usually is considered when proper containment cannot be achieved with nonoperative methods [2].
To achieve containment, several procedures have been described, including pelvic osteotomies and femoral varus osteotomy (FVO) [1, 2, 6]. One study suggests FVO may produce a tendency to valgus mechanical axis deviation (MAD) [5]. It is thought to be a compensatory mechanism for medial displacement of the femoral head induced by surgery. However, overall limb alignment also may be affected by hip disease. Shim et al. [9] reported three cases of genu valgum in children with coxa vara resulting from hip disease. The first case described was a boy diagnosed with LCP disease.
We therefore hypothesized knee valgus alignment at maturity in patients with LCP disease is an effect of the disease and not an effect of FVO.
Materials and Methods
We retrospectively compared two matched groups of patients with LCP disease initially classified as Herring B or C [3], a group treated nonoperatively and a group treated operatively with FVO. Between 1977 and 1997, we saw 280 patients with LCP disease. Of these, 28 with unilateral disease, initially classified as Herring B or C and operated on using FVO, were available for clinical and radiographic reviews at maturity and had radiographs available from the initial consultation. We recommended surgery for inadequate coverage of the femoral head in the fragmentation phase. Inadequate coverage does not directly relate to the Herring classification at a given time as a patient classified as having Herring Class B disease subsequently may have either adequate or inadequate coverage of the femoral head. We used Heyman’s index [4] as a quantification of the coverage of the femoral head. A Heyman’s index greater than 120% was considered inadequate coverage. We created a comparison group of 28 patients treated nonoperatively from the same time interval (1977–1997). All patients had unilateral LCP disease, initially classified as Herring B or C.
In the operative group, the mean age of onset was 7.1 years (range, 4.7–11.4 years); there were 26 classified as having Herring B and two classified as having Herring C disease. The mean age of the patients at surgery was 7.8 years (range, 5.8–11.8 years). The minimum followup was 3.9 years (mean, 9.4 years; range, 3.9–12.7 years). In the nonoperative group, the mean age of onset was 5.8 years (range, 1.8–12.5 years); there were 24 patients classified as having Herring B and four classified as having Herring C disease. Age at onset distribution between the two groups was similar (p = 0.068), as was the distribution of Herring classes (p = 0.34). The minimum followup was 3.3 years (mean, 10.7 years; range, 3.3–15.6 years).
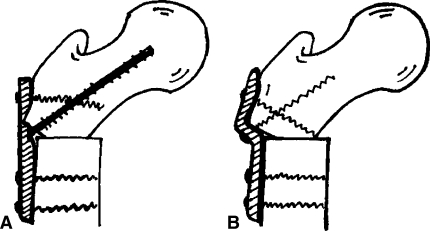
Osteotomy involved a transverse subtrochanteric osteotomy performed through a lateral incision. The proximal fragment was placed in varus and impacted into the distal fragment. The osteotomy was fixed using either an S-shaped plate or a straight plate on the lateral surface of the femur (Fig. 1).
Fig. 1A–B.
The osteotomy was fixed using either (A) a straight plate or (B) an S-shaped plate (right) on the lateral surface of the femur.
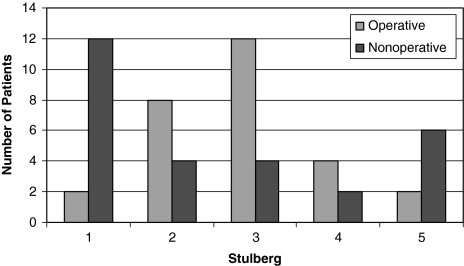
Anteroposterior (AP) and frog lateral radiographs of the pelvis were obtained at presentation in both groups. We (MJ, MK) classified the initial radiographs according to the classification of Herring et al. [3] and final radiographs using the system of Stulberg et al. [10] (Fig. 2).
Fig. 2.
The Stulberg classification is shown for patients who were treated operatively and nonoperatively.
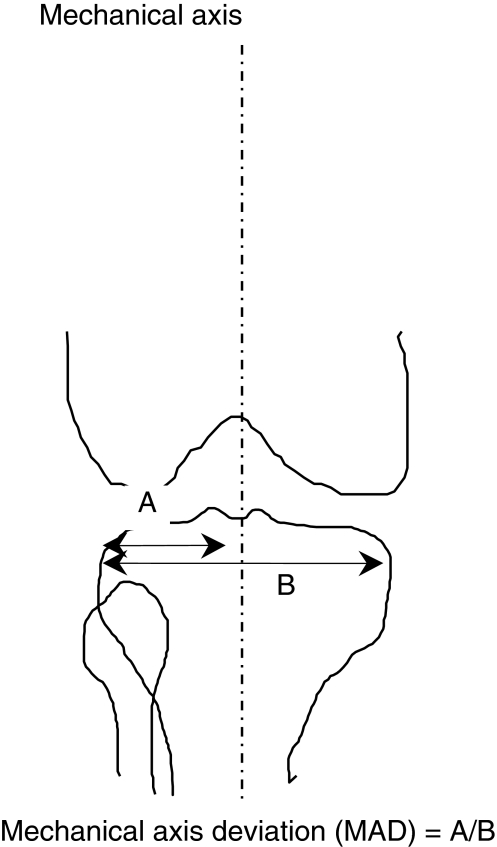
At last followup, both groups had AP radiographs of the pelvis and long-leg views in the standing position to assess limb alignment. We (MJ, MK) determined limb alignment as follows: the mechanical axis was taken from the center of the femoral head to the midpoint of the surface of the talar dome [7]. When it was not possible to locate the center of the femoral head, the axis was taken from the center of a circle obtained by tracing the shape of the acetabulum (ie, the theoretical center of the femoral head if this were a normal joint). The MAD then was determined by measuring the distance between the lateral-most part of the tibial plateau and the mechanical axis expressing this as a percentage of the width of the tibial plateau. Thus, a value less than 50% was indicative of lateral MAD or valgus and greater than 50% was indicative of medial MAD or varus [7] (Fig. 3). Patients with a difference between the diseased side and the normal side with respect to lower limb alignment were defined as having altered alignment. Among patients with altered alignment, patients with valgus alignment on the diseased side compared with the opposite side were identified and referred to as having valgus alignment. In the same way, patients with varus alignment on the diseased side compared with the opposite side were identified and referred to as having varus alignment.
Fig. 3.
Measurement of the MAD of the lower limb is shown.
Limb length discrepancy (LLD) at maturity between the diseased side and the opposite side was assessed radiographically. LLD was defined as the difference in height (expressed in millimeters) and measured on scaled long-leg views between the two highest parts of the ossified femoral heads. As a convention, negative values indicated shortening of the diseased side. The mean LLD at maturity in the operative group was compared with the mean LLD at maturity in the nonoperative group using analysis of variance.
We assessed how much varus was performed at the time of surgery and how much was remodeled with time up to skeletal maturity. The preoperative neck shaft angle (NSA) of the diseased upper femur, the immediate postoperative NSA, and the NSA at final followup were assessed radiographically. Two different paired-sample t tests were used to look for a major difference between preoperative NSA and postoperative NSA and postoperative NSA and NSA at final followup. The two groups were checked for similarity with respect to age at onset (analysis of variance) and Herring classification (chi square test). To determine the distribution of patients with valgus alignment among operative and nonoperative patients, we used a chi square test. The statistical analysis was performed using SPSS® software (SPSS Inc, Chicago, IL).
Results
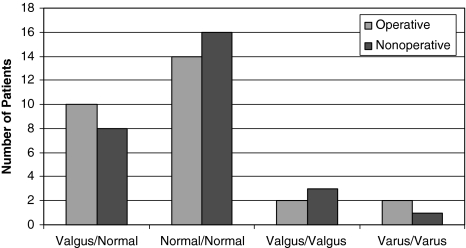
The operative and nonoperative groups had similar distributions of patients with valgus alignment (p = 0.57). In the operative group, 10 patients had valgus alignment compared with the opposite limb and 18 had similar alignment. In the nonoperative group, eight patients had valgus alignment compared with the opposite limb and 20 had similar alignment (Fig. 4).
Fig. 4.
The distribution of lower limb alignment in patients treated operatively and nonoperatively is shown.
In the operative group, the mean LLD at maturity was greater (p = 0.022) than that in the nonoperative group (−5.1 versus −0.4, respectively).
The surgery reduced (p < 0.0001) the NSA by a mean of 25.8° (mean preoperative NSA 136.1° versus mean postoperative NSA 110.3°), although at final followup the reduction (p < 0.0001) was only 13.4°.
Discussion
The fundamental principle in the management of LCP disease is containing the femoral head in the acetabulum [2, 8]. FVO has been one method proposed to achieve containment of the femoral head but may produce a tendency to valgus MAD, perhaps as a compensatory mechanism for medial displacement of the femoral head induced by surgery [5]. We hypothesized valgus mechanical axis at maturity is not an effect of FVO, but an effect of the hip disease.
The principle limitation in this work is the small number of patients and we ensure our two groups are representative of the entire LPC population. The operative group was selected and the comparison group created, retrospectively; although we cannot ensure they were entirely comparable, they were comparable by age of onset and Herring classifications.
We found no greater incidence of valgus alignment at maturity between patients treated operatively and nonoperatively. Although there was no observation in this study, our hypothesis regarding development of genu valgum is biomechanical. In patients with LPC, there is shortening of the femoral neck, and a certain degree of varization of the proximal femur, attributable to the disease. This produces a LLD. To accommodate this shortening in the stance phase, there is a pelvic tilt and an abduction of the limb, producing a shift of the center of mass of the body outward, beyond the middle of the knee. According to the Hueter-Volkmann law, this lateral compression force through the physis of distal femur and proximal tibia produces a growth suppression on the overloaded area and a progressive genu valgum. A prospective study using gait laboratory analysis may be helpful to check this hypothesis.
To date, only two studies focused on lower limb alignment in LPC. Kitakoji et al. [5] suggested genu valgum at maturity is a consequence of FVO. They retrospectively reviewed 30 patients, all with LPC and operated on with FVO. There was no control group. They compared the mean femorotibial angle between their patients and the general population, and concluded there was a tendency to valgus alignment in patients with LCP disease treated with FVO. Additionally, they cited the work of Suda et al. [11], who reported in a long-term followup study, 19 cases with FVO for persistent dysplasia in congenital dislocation of the hip and noted a tendency to valgus in these patients. Kitakoji et al. [5] concluded the tendency to valgus was the result of the surgery (FVO), not the disease (LPC). Shim et al. [9] reported one case of LCP disease associated with progressive ipsilateral genu valgum. This patient did not have any proximal femur procedure. Shim et al. concluded the changes in the hip resulting from LPC disease were responsible for the genu valgum. Our results therefore support the hypothesis raised by Shim et al. [9].
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Axer A. Subtrochanteric osteotomy in the treatment of Perthes’ disease: a preliminary report. J Bone Joint Surg Br. 1965;47:489–499. [PubMed]
- 2.Herring JA. The treatment of Legg-Calvé-Perthes disease: a critical review of the literature. J Bone Joint Surg Am. 1994;76:448–458. [DOI] [PubMed]
- 3.Herring JA, Neustadt JB, Williams JJ, Early JS, Browne RH. The lateral pillar classification of Legg-Calvé-Perthes disease. J Pediatr Orthop. 1992;12:143–150. [DOI] [PubMed]
- 4.Heyman CH, Herndon CH. Legg-Perthes disease; a method for the measurement of the roentgenographic result. J Bone Joint Surg Am. 1950;32:767–778. [PubMed]
- 5.Kitakoji T, Hattori T, Iwata H. Femoral varus osteotomy in Legg-Calvé-Perthes disease: points at operation to prevent residual problems. J Pediatr Orthop. 1999;19:76–81. [DOI] [PubMed]
- 6.Lloyd-Roberts GC, Catterall A, Salamon PB. A controlled study of the indications for and the results of femoral osteotomy in Perthes’ disease. J Bone Joint Surg Br. 1976;58:31–36. [DOI] [PubMed]
- 7.Paley D, Tetsworth K. Mechanical axis deviation of the lower limbs: preoperative planning of uniapical angular deformities of the tibia or femur. Clin Orthop Relat Res. 1992;280:48–64. [PubMed]
- 8.Salter RB. The present status of surgical treatment for Legg-Perthes disease. J Bone Joint Surg Am. 1984;66:961–966. [DOI] [PubMed]
- 9.Shim JS, Kim HT, Mubarak SJ, Wenger DR. Genu valgum in children with coxa vara resulting from hip disease. J Pediatr Orthop. 1997;17:225–229. [DOI] [PubMed]
- 10.Stulberg SD, Cooperman DR, Wallensten R. The natural history of Legg-Calvé-Perthes disease. J Bone Joint Surg Am. 1981;63:1095–1108. [PubMed]
- 11.Suda H, Hattori T, Iwata H. Varus derotation osteotomy for persistent dysplasia in congenital dislocation of the hip: proximal femoral growth and alignment changes in the leg. J Bone Joint Surg Br. 1995;77:756–761. [PubMed]