Abstract
Current trends in orthopaedic surgery have explored different forms of adjuvant treatments to minimize postoperative pain and the risk of nausea and vomiting. A small single preoperative dose of dexamethasone, as part of a comprehensive multimodal analgesic regimen in low-risk patients undergoing total hip arthroplasty (THA), provides antiemetic and opioid-sparing effects but the longer-term effects on pain, complications, or function are not known. We therefore asked whether such a routine would affect longer-term pain, complications, or function. Fifty patients undergoing elective primary THA using spinal anesthesia were initially randomized to receive either dexamethasone (40 mg intravenous) or saline placebo. The patients, anesthesiologists, nurses, and research coordinators were blinded to the study arms. The functional outcome was measured using the Harris hip score. Outcomes were assessed 6 weeks and 1 year postoperatively. We observed no difference in resting pain between the two groups at either time period. Both groups had similar functional outcome scores for the total Harris hip score and individual scoring items at each followup interval. There were no wound complications, deep infections, or osteonecrosis in the contralateral hip at 1-year followup. We recommend the addition of a small single preoperative dose of dexamethasone to a comprehensive multimodal analgesic regimen in low-risk patients given its immediate antiemetic and opioid-sparing effects, and absence of subsequent effects.
Level of Evidence: Level II, therapeutic study (prospective comparative study). See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Postoperative nausea and vomiting, as well as inadequate pain relief, can result in poor surgical outcome following total hip arthroplasty (THA). Patients have reported that postoperative nausea and vomiting and pain are the least desirable anesthesia outcome prior to undergoing surgery [16]. Postoperative nausea and vomiting and pain are also strongly associated with lower satisfaction rates [18]. Patients experiencing nausea and vomiting or moderate to severe pain on the first postoperative day were four times more likely to be dissatisfied after surgery. Patient-controlled analgesia and central neuraxial blockade have typically been used to control postoperative pain following THA. Two studies suggest comparable resting pain levels beyond 6 hours in patients receiving either patient-controlled analgesia or epidural analgesia [2, 22]. The frequency of postoperative nausea and vomiting is similar for both techniques and has been estimated to be approximately 23% with an epidural and 37% in those treated with systemic opioids [22, 26]. Current trends in perioperative care have explored other adjuvant treatments to reduce postoperative pain and the risk of nausea and vomiting.
The antiinflammatory effects of glucocorticoids in the postoperative period are well known [5, 10, 23]. A small single dose of dexamethasone reduces C-reactive protein (CRP) levels by 50% three days following total knee replacement [23]. There exist various synthetic glucocorticoids that have few mineralocorticoid properties responsible for modulating water and electrolyte balance. Dexamethasone is a high-potency and long-acting glucocorticoid with few mineralocorticoid side effects [20]. A single preoperative glucocorticoid dose has commonly been used to prevent postoperative nausea and vomiting and reduce pain and inflammation after surgery [1, 8, 12, 14, 19]. The immediate analgesic and antiemetic benefits in the perioperative period have clearly been demonstrated although glucocorticoids have yet to gain widespread acceptance because of potential side effects. The risk of femoral head osteonecrosis is negligible and has rarely been associated with a short course of corticosteroid therapy [17]. The onset of symptoms is typically within 18 months after treatment and only a few cases have been documented up until 3 years following corticosteroid therapy.
We recently reported the short-term effect on dynamic pain of a single dexamethasone dose compared to placebo after THA [11]. Dynamic pain scores at 24 hours were lower in the dexamethasone group compared to placebo. However, the functional effects of dexamethasone administration at longer term remain unknown. Lower levels of acute phase reactants, such as CRP and interleukin 6 (IL-6), reportedly independently predict pain and the ability to walk following elective THA [6]. Knowing that CRP levels usually return to normal 3 weeks after THA, we questioned whether the effect of dexamethasone on the stress response, and consequently function and analgesia, could persist at 6 weeks, and possibly one year. Perioperative dexamethasone reportedly improves short term satisfaction rates [4]. We presumed better satisfaction immediately postoperatively would result in higher outcome scores 6 weeks after total hip replacement. Although observing a persistent effect at one year is unlikely from a single dose, a longer term followup was necessary to detect possible complications.
We therefore asked whether a single dose of dexamethasone would enhance functional outcome at 6 weeks and 1 year after THA without increasing the rate of adverse events such as wound complications or osteonecrosis of the contralateral side.
Materials and Methods
We randomized 50 patients undergoing elective primary THA using spinal anesthesia to receive either dexamethasone (40 mg intravenous, n = 25) or saline placebo (n = 25) between October 2006 and March 2007 [11]. The power analysis was determined by setting a statistical power of 80% with an α of 0.05 using the results of a previous study involving lower extremity orthopaedic surgery in which the mean resting pain score was 7/10 and the standard deviation approximately 2 [19]. Using a conservative standard deviation of 2.5, we previously described that a sample size of 23 subjects was sufficient to measure a decrease of 30% in early postoperative pain [11]. Patients were included if they were 18 years or older, fluent in English or French, and awaiting primary hip arthroplasty. We excluded 17 patients with contraindications to spinal anesthesia, steroid or immunosuppressive drug use within 6 months of surgery, renal failure, peptic ulcer disease, diabetes mellitus, or hypersensitivity to opioids, local anesthetics, and NSAIDs. A research collaborator, who did not participate in data collection, prepared an IV infusion bag containing 40 mg of dexamethasone or an equal volume of saline diluted into 40 mL of normal saline according to group allocation. Dexamethasone is colorless and causes no pain when given intravenously. The patients, anesthesiologists, nurses, and research coordinators gathering the data were blinded to the study arms. Both groups were similar in age, gender, and body mass index (BMI) [11]. Patients in the dexamethasone group had lower preoperative pain scores (Fig. 1) and lower total Harris hip score (HHS) (Fig. 2) than the placebo group. The baseline function and ROM scores were similar. We lost 10 patients to followup for nonmedical reasons due to a large referral network outside our province where patients are routinely followed radiographically and by phone interview.
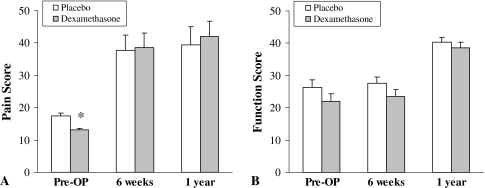
Fig. 1A–B.
(A) Pain and (B) function scores are shown. Patients were evaluated preoperatively and at 6 weeks and 1 year postoperatively. * p < 0.05 versus placebo.
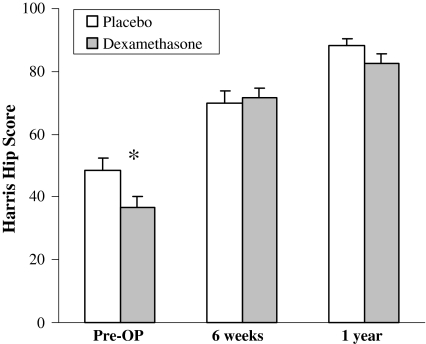
Fig. 2.
Total Harris hip scores are shown. Patients were evaluated preoperatively and at 6 weeks and 1 year postoperatively. * p < 0.05 versus placebo.
Standard lumbar spinal anesthesia at the L2-3 level was performed using 15 mg of 0.5% bupivacaine. The dexamethasone solution or placebo control was given over 10 minutes after onset of paresthesia over the operative site. In the postoperative period, each patient received a patient-controlled analgesia system that was set to deliver 1 mg of morphine every 7 minutes as needed until discontinuation at 48 hours. Acetaminophen (650 mg four times daily) and ibuprofen (400 mg four times daily) were also given orally when the patient-controlled analgesia was started and regularly thereafter.
The functional outcome was measured by clinical assistants using the Harris hip score [7]. This questionnaire is a validated outcome tool that evaluates global function, pain, and range of motion (ROM). We believed this functional outcome measure would best reflect any physiologic changes anticipated from a perioperative dose of dexamethasone. We analyzed the total score and each of the above subcategories separately to determine whether a single dose of dexamethasone had any effect on individual scoring items. The questionnaires were administered to patients preoperatively, and at 6 weeks (or earliest outpatient followup) and 1 year postoperatively. The questionnaires were collected for 40 of the 50 (80%) patients (20 of 25 in each group) at 6 weeks and 31 patients (17 dexamethasone, 14 placebo) at the 1-year followup. Complications following surgery, such as wound infections or osteonecrosis of the contralateral hip, were recorded for each patient. The orthopaedic surgeons and research assistant responsible for the questionnaires and measuring the ROM remained blinded throughout the study period.
Baseline continuous data were analyzed between treatment groups using one-way analysis of variance (ANOVA). Categorical data were compared using chi square or Fisher’s exact test when appropriate using StatView (Abacus software, Burlington, MA). Secondary analysis was performed by repeated-measures analysis. Results are expressed as mean ± standard deviation.
Results
A single dose of dexamethasone did not provide any persistent analgesic effect at 6 weeks or 1 year: the individual scoring items, such as pain and function, were similar between the two groups at each followup interval (Fig. 1). The dexamethasone did not increase wound complications, deep infections, or osteonecrosis in the contralateral hip at 1-year followup. The total HHS scores were similar (p = 0.1) in both groups at 6 weeks and 1 year (Fig. 2).
Discussion
A small single preoperative dose of dexamethasone to a comprehensive multimodal analgesic regimen in low-risk patients provides antiemetic and opioid-sparing effects but the longer-term effects on pain, complications, or function are not known. Improved satisfaction rates and inhibitory effects of acute phase reactants following perioperative dexamethasone administration raised the question whether this would result in higher outcome scores at 6 weeks; while we suspected the scores would not differ at 1 year, we followed the patients to identify possible complications. The purpose of this study was to determine if a single dose of dexamethasone prior to THA had any persistent effect on functional outcome without increasing the risk of complications.
There are several limitations to our study. The questionnaires from out-of-province patients could not be completed, given the inability to evaluate range of motion, resulting in a lower followup rate than expected. The Harris hip score may also lack enough sensitivity to detect subtle differences in patient satisfaction and surgical outcome following a preoperative dose of dexamethasone in THA.
Glucocorticoids have potent antiinflammatory and immune-modulating properties. They have been used extensively to reduce the stress response following surgery [10]. A single preoperative dose of glucocorticoid limits complement activation and the acute phase response subsequent to hip and knee arthroplasty [5, 23]. Patients who were given dexamethasone (16 mg) prior to undergoing knee arthroplasty reportedly had a 50% decrease in peak CRP concentration on the third postoperative day [23]. In a study comparing 102 patients after elective THA for osteoarthritis, a lower interleukin-6 peak concentration accurately predicted the ability to walk 10 m and 25 m more rapidly, while a lower CRP level was associated with less pain on discharge [6]. This suggests lower concentrations of acute phase reactants directly correlate with better pain relief and functional recovery in the absence of other confounding factors, such as corticosteroids that alter a number of physiologic variables. The inhibitory action of a single glucocorticoid dose on the inflammatory response after joint arthroplasty could theoretically further improve surgical outcomes through this mechanism.
There is strong evidence to support the analgesic efficacy of a single perioperative dose of glucocorticoid. The site of action along the pain pathway has recently been reviewed [20]. Glucocorticoids act by inhibiting peripheral nerve terminals at the area of mechanical tissue injury, as well as preventing pain transmission at the synapses of afferent nociceptive pain fibers in the dorsal horn that are responsible for central sensitization. An intraoperative dose of dexamethasone reduces patient-controlled analgesia and narcotic use while providing better pain relief after lumbar discectomy [1, 12]. Another randomized controlled trial compared the effect of 125 mg methylprednisolone (equivalent to 25 mg dexamethasone), 30 mg ketorolac, and placebo administered on the first day after orthopaedic surgery [19]. The methylprednisolone and ketorolac groups had less pain compared to placebo with a number needed to treat of 3.6 and 3.1, respectively. The glucocorticoid group, however, consumed less opioids for 72 hours than the other two groups underscoring the prolonged half-life of methylprednisolone. It is unclear whether the analgesic benefit on dynamic pain of a single dose of dexamethasone in our previous study is the result of an antiinflammatory effect or the inhibition of central and peripheral nociceptors along the pain pathway.
The antiemetic effect of glucocorticoids is also well documented. Patients who received a prophylactic dose of 8 mg dexamethasone prior to undergoing major orthopaedic surgery required less rescue antiemetics, and had fewer episodes of nausea and severe vomiting compared to placebo [14]. In a large systematic review, a single dose of dexamethasone was found to markedly reduce the incidence of early and late postoperative nausea and vomiting without evidence of clinically relevant toxicity [8]. The exact dose and timing of prophylactic glucocorticoid remain unknown. It appears as though dexamethasone is most effective when given immediately before induction of anesthesia rather than at the end of surgery [25]. Dose-ranging studies support a minimum effective dose of approximately 5 mg dexamethasone in nonorthopaedic surgeries to decrease the incidence of postoperative nausea and vomiting [9, 15, 24]. The prophylactic dose in orthopaedic surgeries has typically been much higher, between 25 mg and 40 mg of dexamethasone [1, 12, 19]. However, several recent studies report smaller doses (8 mg dexamethasone) are equally as effective in preventing postoperative nausea and vomiting after orthopaedic surgery [4, 13, 14].
Despite the clear analgesic and antiemetic benefits of glucocorticoids, their use has yet to become routine in the perioperative management of orthopaedic patients because of concerns over associated side effects. The risks of impaired wound healing, osteonecrosis, and adrenal suppression are daily dose-dependent and unlikely to occur following a small single dose of glucocorticoid. One study evaluating the association between steroids and osteonecrosis of bone reported a correlation between daily total and oral dose with osteonecrosis [3]. Bolus steroids, defined as ≥ 500 mg/day prednisone (125 mg dexamethasone), were not associated with osteonecrosis. A meta-analysis assessing the perioperative risks of short-term and single high-dose methylprednisolone in surgical patients found no increase in the rate of adverse events [21]. There were no differences in the incidence of wound complications or gastrointestinal bleeding, nor was there one single reported case of osteonecrosis. Methylprednisolone did, however, reduce the risk of pulmonary complications in trauma patients with multiple skeletal injuries.
We previously reported a single preoperative dose of dexamethasone effectively decreased dynamic pain at 24 hours [11]. The dexamethasone group had lower preoperative total HHS scores and more pain (lower score) compared to placebo although both groups were comparable at the 6-week and 1-year followup. The disproportionate improvement seen in the study group is likely the effect of an actual hip arthroplasty surgery where two small and relatively homogenous groups would be expected to obtain similar postoperative results. It would be erroneous to conclude the functional improvement in the study group is the result of dexamethasone administration alone. The current literature clearly documents a single glucocorticoid dose is safe and effective in providing pain relief and reducing postoperative nausea and vomiting in the perioperative care of surgical patients [3, 8, 14, 19, 21]. Nevertheless, clinical practice in orthopaedic surgery has fallen behind scientific evidence because of biased and unwarranted concerns over potential side effects.
We were unable to show an improvement in functional outcome at either followup period. However, there were no additional complications in the dexamethasone group compared to placebo. We therefore recommend the addition of a small single preoperative dose of dexamethasone to a comprehensive multimodal analgesic regimen in low-risk patients given its antiemetic and opioid-sparing effects and absence of subsequent effects on followup.
Acknowledgments
We thank Alain Petit for his help in preparing the manuscript, and Maricar Alminia and Laura Des Rosiers for their help in administration of the questionnaires and recording the clinical data.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Aminmansour B, Khalili HA, Ahmadi J, Nourian M. Effect of high-dose intravenous dexamethasone on postlumbar discectomy pain. Spine. 2006;31:2415–2417. [DOI] [PubMed]
- 2.Choi PT, Bhandari M, Scott J, Douketis J. Epidural analgesia for pain relief following hip or knee replacement. Cochrane Database Syst Rev. 2003;34. [DOI] [PMC free article] [PubMed]
- 3.Felson DT, Anderson JJ. Across-study evaluation of association between steroid dose and bolus steroids and avascular necrosis of bone. Lancet. 1987;1:902–906. [DOI] [PubMed]
- 4.Fujii Y, Nakayama M. Effects of dexamethasone in preventing postoperative emetic symptoms after total knee replacement surgery: a prospective, randomized, double-blind, vehicle-controlled trial in adult Japanese patients. Clin Ther. 2005;27:740–745. [DOI] [PubMed]
- 5.Gammer W, Bengtson A, Heideman M. Inhibition of complement activation by high-dose corticosteroids in total hip arthroplasty. Clin Orthop Relat Res. 1988;236:205–209. [PubMed]
- 6.Hall GM, Peerbhoy D, Shenkin A, Parker CJ, Salmon P. Relationship of the functional recovery after hip arthroplasty to the neuroendocrine and inflammatory responses. Br J Anaesth. 2001;87:537–542. [DOI] [PubMed]
- 7.Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am. 1969;51:737–755. [PubMed]
- 8.Henzi I, Walder B, Tramer MR. Dexamethasone for the prevention of postoperative nausea and vomiting: a quantitative systematic review. Anesth Analg. 2000;90:186–194. [DOI] [PubMed]
- 9.Ho ST, Wang JJ, Tzeng JI, Liu HS, Ger LP, Liaw WJ. Dexamethasone for preventing nausea and vomiting associated with epidural morphine: a dose-ranging study. Anesth Analg. 2001;92:745–748. [DOI] [PubMed]
- 10.Holte K, Kehlet H. Perioperative single-dose glucocorticoid administration: pathophysiologic effects and clinical implications. J Am Coll Surg. 2002;195:694–712. [DOI] [PubMed]
- 11.Kardash KJ, Sarrazin F, Tessler MJ, Velly AM. Single-dose dexamethasone reduces dynamic pain after total hip arthroplasty. Anesth Analg. 2008;106:1253–1257. [DOI] [PubMed]
- 12.Karst M, Kegel T, Lukas A, Ludemann W, Hussein S, Piepenbrock S. Effect of celecoxib and dexamethasone on postoperative pain after lumbar disc surgery. Neurosurgery. 2003;53:331–336; discussion 336–337. [DOI] [PubMed]
- 13.Lee Y, Lai HY, Lin PC, Lin YS, Huang SJ, Shyr MH. A dose ranging study of dexamethasone for preventing patient-controlled analgesia-related nausea and vomiting: a comparison of droperidol with saline. Anesth Analg. 2004;98:1066–1071. [DOI] [PubMed]
- 14.Lee Y, Lin YS, Chen YH. The effect of dexamethasone upon patient-controlled analgesia-related nausea and vomiting. Anaesthesia. 2002;57:705–709. [DOI] [PubMed]
- 15.Liu K, Hsu CC, Chia YY. The effect of dose of dexamethasone for antiemesis after major gynecological surgery. Anesth Analg. 1999;89:1316–1318. [PubMed]
- 16.Macario A, Weinger M, Carney S, Kim A. Which clinical anesthesia outcomes are important to avoid? The perspective of patients. Anesth Analg. 1999;89:652–658. [DOI] [PubMed]
- 17.McKee MD, Waddell JP, Kudo PA, Schemitsch EH, Richards RR. Osteonecrosis of the femoral head in men following short-course corticosteroid therapy: a report of 15 cases. CMAJ. 2001;164:205–206. [PMC free article] [PubMed]
- 18.Myles PS, Williams DL, Hendrata M, Anderson H, Weeks AM. Patient satisfaction after anaesthesia and surgery: results of a prospective survey of 10,811 patients [see comment]. Br J Anaesth. 2000;84:6–10. [DOI] [PubMed]
- 19.Romundstad L, Breivik H, Niemi G, Helle A, Stubhaug A. Methylprednisolone intravenously 1 day after surgery has sustained analgesic and opioid-sparing effects. Acta Anaesthesiol Scand. 2004;48:1223–1231. [DOI] [PubMed]
- 20.Salerno A, Hermann R. Efficacy and safety of steroid use for postoperative pain relief. Update and review of the medical literature. J Bone Joint Surg Am. 2006;88:1361–1372. [DOI] [PubMed]
- 21.Sauerland S, Nagelschmidt M, Mallmann P, Neugebauer EA. Risks and benefits of preoperative high dose methylprednisolone in surgical patients: a systematic review. Drug Saf. 2000;23:449–461. [DOI] [PubMed]
- 22.Singelyn FJ, Gouverneur JM. Postoperative analgesia after total hip arthroplasty: iv PCA with morphine, patient-controlled epidural analgesia, or continuous “3-in–1” block?: a prospective evaluation by our acute pain service in more than 1,300 patients. J Clin Anesth. 1999;11:550–554. [DOI] [PubMed]
- 23.Smith C, Erasmus PJ, Myburgh KH. Endocrine and immune effects of dexamethasone in unilateral total knee replacement. J Int Med Res. 2006;34:603–611. [DOI] [PubMed]
- 24.Wang JJ, Ho ST, Lee SC, Liu YC, Ho CM. The use of dexamethasone for preventing postoperative nausea and vomiting in females undergoing thyroidectomy: a dose-ranging study. Anesth Analg. 2000;91:1404–1407. [DOI] [PubMed]
- 25.Wang JJ, Ho ST, Tzeng JI, Tang CS. The effect of timing of dexamethasone administration on its efficacy as a prophylactic antiemetic for postoperative nausea and vomiting. Anesth Analg. 2000;91:136–139. [DOI] [PubMed]
- 26.Wheeler M, Oderda GM, Ashburn MA, Lipman AG. Adverse events associated with postoperative opioid analgesia: a systematic review. J Pain. 2002;3:159–180. [DOI] [PubMed]