Abstract
Standard total hip arthroplasty (THA) is the established surgical treatment for patients older than 65 years with progressive osteoarthritis but survivorship curves wane in patients younger than 50. Resurfacing hip arthroplasty (RHA) is an alternative for younger, active patients reportedly providing superior range of motion. Quantitative investigation of functional recovery following arthroplasty may elucidate limitations that aid in device selection. Although limited long-term kinematic data are available, the early rate of recovery and gait compensations are not well described. This information may aid in refining rehabilitation protocols based on limitations specific to the implant. We presumed hip motion and forces for subjects receiving RHA are more similar to age-matched controls during physically demanding tasks, such as stair negotiation, at early time points than those for THA. In a pilot study, we quantified walking and stair negotiation preoperatively and 3 months postoperatively for seven patients with RHA (mean age, 49 years), seven patients with standard THA (mean age, 52 years), and seven age-matched control subjects (mean age, 56 years). Although both treatment groups demonstrated trends toward functional recovery, the RHA group had greater improvements in hip extension and abduction moment indicating typical loading of the hip. Further investigation is needed to determine if differences persist long term or are clinically meaningful.
Introduction
THA relieves pain and improves function in patients with advanced hip arthritis [23, 28, 29]. Although surgeons have a wide choice of implant types and bearing technology (metal on polyethylene, ceramic, metal on metal, and so on), the majority of the standard THA devices available today can greatly improve patients’ quality of life when implanted appropriately [21, 25, 28].
Resurfacing hip arthroplasty (RHA) has regained popularity in recent years. Initial attempts at RHA in the early years of arthroplasty frequently failed as a result of material wear, aseptic loosening, and femoral neck fracture attributable to high stresses and poor technique [3, 9, 19]. The advent of metal-on-metal (MoM) bearings has been a large factor in the early reports of success with RHA [2, 30]. Favorable results on 5- to 10-year followups regarding aseptic loosening, stability, toxicity of wear, and implant survivorship have been reported with modern resurfacing devices [6, 10, 30].
RHA is particularly attractive to the active young adult with disabling hip arthritis [24]. The MoM bearing surface reportedly has low wear in one simulation study [23] and by only resurfacing the head and neck, the femoral canal remains virgin and proximal bone stock is preserved for future revision option. The larger head sizes used in RHA may decrease dislocation rates when compared with smaller, more conventional head sizes used in THA [30]. In addition, the potential functional benefits of RHA have driven patients to request hip resurfacing as an alternative to THA [24]. To date, however, these potential functional benefits have been largely unconfirmed.
Functional efficacy of RHA devices in vivo is needed to determine performance postoperatively in comparison to standard hip replacements since many believe conservation of bone stock is crucial to younger patients who will require future revisions. If RHA devices can provide identical or superior outcomes to traditional THA devices, physicians may opt to perform the more conservative procedure. A number of studies report the functional gains in gait after THA, though functional analysis of RHA groups are limited [4, 7, 11, 13, 20, 30]. Even fewer studies have been performed on comparative hip devices for critical assessment of differences in functional performance with resurfacing or standard products. Selected gait results from previous and current work have been highlighted (Table 1). Mont et al. assessed gait in 15 patients an average of 13 months after RHA [20] and demonstrated more normal hip abduction angle and moment for the RHA group in comparison to a THA treatment and osteoarthritis group; however, preoperative data were absent. Foucher et al. concluded preoperative gait adaptations persisted 1 year postoperatively in patients with THA [11].
Table 1.
Summary of current and previously published gait variables
| Significant gait variable | ||||||
|---|---|---|---|---|---|---|
| Study | Time postoperatively | Hip abduction moment | Hip abduction angle | Velocity | Stance width | Step length |
| Current study (Shrader et al. 2009) | 3 mo | Significantly greater peak in RHA than THA group | Significantly greater peak in RHA than THA group | Higher in THA than RHA group | Greater in THA than RHA group | Greater in RHA than THA group |
| Nallegowda et al. 2003 [21] | 8.9 mo | Not reported | Not reported | Higher in control than THA group | Greater in control than THA group | Greater in control than THA group |
| Mont et al. 2007 [20] | 6–18 mo | Greater initial peak in RHA than THA group | Greater hip abduction in THA than RHA group | Significantly higher in RHA than THA group | Not reported | Not reported |
| Gore et al. 1985 [13] | 6 mo and 2 year | Not reported | Similar between THA and RHA groups | Significantly higher in RHA than THA group | Not reported | Not reported |
| Newman et al. 2008 [22] | 1 year | Not reported | Similar between control subjects and RHA | Similar between control subjects and RHA | Not reported | Not reported |
| Foucher et al. 2007 [11] | 1 year | Significantly lower peaks in THA than control group | Lower peaks in THA than control group | Similar results between THA and control group | Not reported | Not reported |
RHA = resurfacing hip arthroplasty.
Several studies describe the recovery stair ascent or descent after THA but not RHA [1, 8, 12, 18, 22]. Many of these studies were limited in power and focused only on range of motion or self-reported function scores (Table 2). In-depth critical analysis of more strenuous activities of daily living (such as stairclimbing/descent, rising from a chair, athletic maneuvers, and so on) may be required to detect subtle, but clinically important, differences between implants and/or surgical approaches. Stair negotiation places higher demands on balance, requires greater range of motion, and identifies interlimb compensations [12] in comparison to level walking. Challenging physical tasks have higher sensitivity as a tool to identify remaining functional pathology postoperatively. The earlier these antalgic preoperative adaptations can be identified, the greater likelihood of their being corrected with early postoperative physical therapies. We presumed a comprehensive analysis of ADLs, including gait and stair negotiation, would identify differences in biomechanical strategies adopted postoperatively between treatment groups.
Table 2.
Review of previously published stair negotiation results after hip arthroplasty
| Stair negotiation variables | |||||||
|---|---|---|---|---|---|---|---|
| Study | Time postoperatively | Measurement tool | Function | Abduction moment | Hip extension angle | Stance width | Pelvic obliquity |
| Current study (Shrader et al. 2009) | 3 months | Quantitative motion analysis | Foot over foot, no hand rail in THA and RHA groups | Significantly greater in RHA than THA group | RHA group had similar results as control subjects’ stair ascent | Greater than control subjects in RHA and THA groups | significantly greater in RHA and THA groups over controls stair descent |
| Newman et al. 2008 [22] | 1 year | UCLA activity score | Foot over foot, no hand rail in 87% of RHA group | Not reported | Greater at post- than preoperatively in RHA group | Not reported | Not reported |
| Foucher et al. 2008 [12] | 1 year | Quantitative motion analysis | Not reported | Greater than control subjects in THA group during stair ascent | Significantly greater than control subjects in THA group during stair ascent | Not reported | Not reported |
| Bergmann et al. 2001 [8] | 2 years | Instrumented hip | Not reported | Peak reached similar to control values in THA group | Greater at post- than preoperatively in THA group | Less post- than preoperatively in THA group | Not reported |
RHA = resurfacing hip arthroplasty.
In this preliminary study we asked whether: (1) during gait, dynamic range of motion, peak external moments, temporospatial parameters, and temporal aspects of hip muscle activation will show greater improvements at three months in the RHA population; (2) subjects receiving RHA will demonstrate greater dynamic range of motion, peak external moments, temporospatial parameters, and temporal aspects of hip muscle activation during stair negotiation; (3) serial clinical surveys will indicate higher functional score in the RHA group, correlating with the same specific gait parameters.
Materials and Methods
We recruited seven patients with a MoM hip resurfacing arthroplasty device (BHR), seven patients with an uncemented THA device (STH), and seven healthy control subjects for this study. Subjects were invited by their physician (MWS) to participate in the study, after they had elected to have hip arthroplasty treatment. The Birmingham hip resurfacing (BHR; Smith and Nephew, Memphis, TN) and Synergy total hip (STH; Smith and Nephew) implant recipients were designated to RHA and THA groups, respectively. Group demographics were noted in the osteoarthritis groups before selection of controls. The RHA group (49.7 ± 4.8 years), THA group (51.9 ± 10.1 years), and controls (50.4 ± 8.2 years) were age-matched. Height (BHR: 166 ± 11 cm, STH: 178 ± 10 cm, controls: 50.4 ± 8.2 cm), weight (BHR: 84 ± 18 kg, STH: 98 ± 18 kg, controls: 67 ± 14 kg), and gender (BHR: five male/two female, STH: four male/three female, controls: one male/six female) within groups were documented. Following preliminary data collection a power analysis was performed to determine an appropriate sample size required for clinically meaningful data. Mont et al.’s work on gait comparison between THA and RHA patients [20], using hip abduction moment results (15 RHA subjects, 0.777 (0.1) Nm/kg and 15 THA subjects, 0.632 (0.2) Nm/kg) yielded a power of 70.9% with a high effect size (1.2). Our preliminary hip abduction moment data during stair descent (7 RHA subjects, 0.84 (0.08) Nm/kg and 7 THA subjects, 0.73 (0.15) Nm/kg) returned a power of 18.2%, utilizing Cohen’s d high effect size (0.8) and a one tailed test with α = 0.05. To reach the identical power as presented in Mont et al.’s work [20] a sample size of 16 subjects in each group would be needed, assuming similar values are maintained with additional subjects. A smaller sample size was selected for preliminary assessments, with intention of further recruitment, if our presumption of functional differences between groups was supported.
All surgeries were performed through a posterolateral approach by a single surgeon (MWS) who had prior training and experience with both the BHR and STH devices. All patients undergoing THA received a 36-mm Oxinium head on a highly crosslinked polyethylene bearing surface with a high offset STH stem.
One of us (MBS) determined the Harris hip score [14], Lower Extremity Activity Scale (LEAS) [27], and SF-12 (Quality Metrics) [25] health survey immediately before motion analysis at each visit.
Gait and stair negotiation were evaluated preoperatively and 3 months postoperatively. A 10-camera passive marker system using CMOS Micron Corporation MI-MV40 sensors collecting data at 120 frames per second (Eagle-4; Motion Analysis Corp, Santa Rosa, CA) and four floor-embedded force plates (OR6; Advanced Mechanical Technology Inc, Watertown, MA) were used to collect three-dimensional kinetic and kinematic data. The skeletal model was created using a modified Helen Hayes marker system [15]. Marker identification, gap fitting, and dual-pass third-order Butterworth data smoothing were performed by EVaRT postprocessing software (Motion Analysis). Events during movement cycles were manually marked using Orthotrak 6.2.8 (Motion Analysis); then multiple trials were normalized and ensemble averaged. Custom Matlab software (Matlab 8.0; Mathworks Inc, Natick, MA) was designed to plot kinematic and kinetic results. Selected parameters from kinematic, kinetic, temporal, and spatial results were recorded for the sound and injured limb. A 10 channel streaming surface electromyography (EMG) system (MA-300-10; Motion Lab Systems, Baton Rouge, LA) was used to record muscle potentials from gluteus maximus (GMax), gluteus medius (GMed), tensor fascia lata (TFL), rectus femoris (RF), and hamstrings (Ham) during each trial. Data were sampled at 1200 Hz and conditioned using MA-411-003 skin surface preamplifiers with ×20 gain factor. Raw EMG signals were rectified then filtered using a double recursive second order low pass filter with 50 Hz cutoff frequency. Signals were then time normalized to representative movement cycles determined in Orthotrak. A 100 ms window was used to determine the minimum standard deviation of the signal to set a baseline. A 50 ms moving average set at 3 standard deviations above the baseline was used to determine onset. EMG data were processed using Motion Lab System Analysis package and custom Matlab software.
Subjects walked at a self-selected speed along an 8-meter walkway during gait trials. Stair trials were executed by means of an instrumented three-step and landing platform staircase. Subjects were instructed to ascend and descend the staircase in a foot-over-foot manner [5]. Five clean foot strikes from each side were collected for validity during gait and stair tests.
Means and standard deviations of kinematic, kinetic, temporal, spatial motion data, and clinical surveys were evaluated using custom Microsoft Excel (Redmond, WA) macros. A correlation coefficient (Pearson product-moment) was generated in Microsoft Excel to determine relationships between both peak hip moment and maximum hip extension with the clinical scores (Harris hip score, LEAS, and SF-12 physical component score). All other analyses were performed using SPSS 14.0 (SPSS Inc, Chicago, IL) except where noted. One-way analysis of variance (ANOVA) was applied to compare key gait and stair negotiation parameters, respectively, within subjects and between the RHA, THA, and control groups for all study time points. Key parameters for both gait and stair tasks included: the sagittal and frontal plane pelvic and hip moments and angles; movement velocity; cadence; stance width; step length; stride length; support time; and body weight support. Homogeneity of variances were calculated to ensure the assumptions of the ANOVA and post hoc tests were met. Post hoc testing using Tukey HSD was performed when homogeneity was met while Dunnett’s T3 was used for variables that did not demonstrate homogeneity of variance. These post hoc tests determined differences between RHA, THA and controls, and within groups at different time points. Peak angles, peak moments, temporal and spatial parameters of the gait and stairs were compared between treatment groups to determine if either group approached control-like results postoperatively. Preoperatively, outliers within groups were determined using the Dixon test and removed from further analysis. Non-paired, 2-tailed Students t-tests for samples with equal variance (Microsoft Excel) were applied to the clinical survey data to compare treatment groups. Equal variance was confirmed using an F-test in Microsoft Excel.
Results
Preoperatively, both THA and RHA patient groups displayed slower walking velocity (0.8–0.9 m/s), decreased cadence (96.3–106.3 steps/min), and increased step width (15 cm) compared to control subjects (Table 3); both patient groups also had decreased (p = 0.04) maximum hip abduction angle compared with control subjects. Postoperatively, patients in both groups had improvements in hip abduction moments (Fig. 1), but the RHA group demonstrated a more normal pattern than the THA group. Improvements in temporal spatial parameters occurred postoperatively in both groups and were similar to control subjects (Table 3). Stride length, step width, limb velocity, and double support time were more symmetric between sound and injured limbs after surgery with the RHA group displaying slightly greater injured limb loading (injured limb: 48% body weight [BW], sound side: 52% BW) over the THA group (injured limb: 47% BW, sound side: 53% BW). Preoperative EMG indicated premature firing of GMax and GMed in midstance of gait (20%–45% of cycle) and prolonged duration in the sound limb. Surgically corrected limbs demonstrated continuous, although dysphasic, activity of the hip abductors preoperatively. At 3 months, we observed typical phasic activity during gait with GMed, GMax, and Hams firing during hip extension in midstance for both groups. Signal duration was longer in the THA group (offset 65% of gait cycle) during stance-to-swing transitions than in control subjects (offset 62% of gait cycle). (This is indicative of a phase transition, in which the THA group showed longer muscle firing time and double support time.) We identified no changes in firing patterns for the RF or TFL.
Table 3.
Results from gait analysis*
| Comparison with controls | Maximum hip abduction (degrees) | Maximum hip abduction moment (Nm/kg) | Step width (cm) | Walking speed (cm/s) | Stride length (cm) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SE) | p value | Mean (SE) | p value | Mean (SE) | p value | Mean (SE) | p value | Mean (SE) | p value | |
| BHR preoperatively | 4.67 (1.23) | 0.04 | 0.79 (0.08) | 0.83 | 15.1 (1.59) | 0.03 | 82.6 (6.84) | < 0.01 | 106.9 (5.55) | 0.04 |
| STH preoperatively | 5.3 (1.77) | 0.05 | 0.71 (0.80) | 0.31 | 15.1 (1.33) | 0.03 | 92.5 (10.33) | 0.04 | 103.8 (9.65) | 0.17 |
| BHR postoperatively | 7.12 (1.52) | 0.67 | 0.73 (0.07) | 0.21 | 12 (1.5) | 0.29 | 100.17 (9.9) | 0.06 | 118.2 (4.9) | 0.46 |
| STH postoperatively | 5.33 (1.46) | 0.04 | 0.69 (0.06) | 0.07 | 13.8 (1.33) | 0.08 | 110.4 (3.76) | 0.5 | 117.1 (4.87) | 0.35 |
| CONT | 9.21 (0.72) | NA | 0.85 (0.04) | NA | 10 (0.78) | NA | 120.5 (5.17) | NA | 126.48 (3.26) | NA |
* Mean (standard error [SE]) and p values indicating difference between treatment and control groups are noted. Values of p < 0.05 are bold; NA = Not applicable.
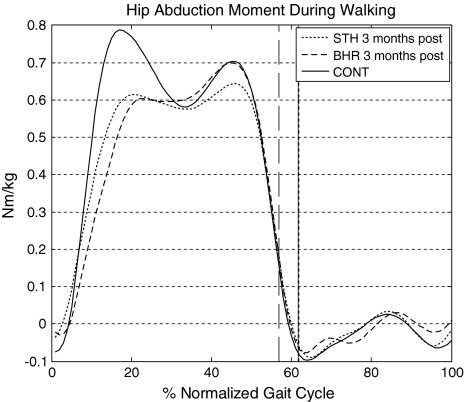
Fig. 1.
Hip abduction moment during walking displayed a more typical profile in the BHR group in comparison to the STH group 3 months postoperatively. The BHR group received a resurfacing hip arthroplasty device, the STH group received a THA device, and the controls group were healthy age-matched control subjects. Toe offs for each group are indicated by vertical lines.
Stair negotiation demonstrated larger differences between treatment groups. The RHA group achieved greater hip extension (Fig. 2) through the movement cycle compared to the THA group, which maintained reduced (p = 0.01) hip extension angle (Table 4). Both patient groups were able to perform stair ascent in a foot-over-foot manner at 3 months, although greater forward lean and increased dorsiflexion were noted in the THA group. Step width, movement velocity, and support time highlighted remaining deficits in both groups. Stair descent results also suggested differences between treatment groups and controls postoperatively (Table 5). Stair descent also illustrated dissimilarities between patient groups in hip abduction moment (Fig. 3). The maximum hip abduction moment was similar (p = 0.58) in the RHA group and control subjects (0.86 Nm/kg versus 0.99 Nm/kg, respectively) but lower (p = 0.02) in the THA group than control subjects (0.65 Nm/kg). Abnormalities of EMG timing patterns observed during stair climbing were similar to those discussed for gait.
Fig. 2.
Hip flexion angle during stair ascent indicated maximum hip extension was comparable to the control group (CONT) in the BHR (resurfacing hip arthroplasty) group. The THA group received a STH device and indicated greater hip flexion and forward tilt during the swing phase of the movement cycle. Toe offs are indicated as vertical lines along the normalized movement cycle.
Table 4.
Postoperative results of stair ascent*
| Variable | BHR | STH | CONT | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Standard error | p value | Mean | Standard error | p value | Mean | Standard error | p value | |
| Minimum hip flexion angle (degrees) | 7.39 | 3.70 | 0.80 | 15.03 | 3.50 | < 0.01 | 7.62 | 1.55 | NA |
| Step width (cm) | 12.00 | 1.50 | 0.04 | 13.80 | 1.33 | 0.03 | 10.00 | 0.78 | NA |
| Walking speed (cm/s) | 100.17 | 9.90 | < 0.01 | 110.40 | 3.76 | < 0.01 | 120.50 | 5.17 | NA |
* Statistical comparison between control subjects and treatment groups are reported with p values < 0.05 noted in bold; NA = Not applicable.
Table 5.
Postoperative stair descent results*
| Variable | BHR | STH | CONT | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Standard error | p value | Mean | Standard error | p value | Mean | Standard error | p value | |
| Maximum hip abduction moment (Nm/kg) | 0.86 | 0.04 | 0.58 | 0.65 | 0.11 | 0.02 | 0.99 | 0.07 | NA |
| Step width (cm) | 14.84 | 0.65 | 0.01 | 14.12 | 0.84 | 0.03 | 11.17 | 0.70 | NA |
| Pelvic obliquity (degrees) | 8.77 | 0.86 | 0.01 | 7.82 | 0.62 | 0.04 | 4.80 | 0.95 | NA |
* Statistical significance, p < 0.05 in bold, between treatment group and control subjects are noted; NA = Not applicable.
Fig. 3.
Hip abduction moment during stair descent indicated greater improvements in the BHR (resurfacing hip arthroplasty) group in generation of maximum moment in comparison to the THA group who received a STH device. The BHR group had similar peak moments as the age-matched control group (CONT), although toe off time noted by vertical plot lines suggests temporal shifts in support time.
Clinical scales identified improvements in pain, range of motion, and physical health for both patient groups postoperatively, but mean values were similar for both groups. Average LEAS scores did not differ between the BHR group (12.6) and STH group (11.5) as noted by a t-test (p = 0.38). Harris hip scores in the BHR group averaged 92.4 while the STH group averaged 90.4 (p = 0.74). The difference in scores was isolated to the range of motion component of the total score. Correlation analysis indicates a weak positive correlation (R2 = 0.35) between the physical health component score of the SF-12 and functional capability of hip moment generation during gait. Subjects in both treatment groups scored lower (p = 0.00) on the mental health component score of the SF-12 survey (39 ± 6 points) in comparison with the control group (48 ± 1.9 points), noting decreased social and recreational activity during recovery.
Discussion
Hip arthritis has become more prevalent in younger, middle-aged populations [26] as a result of consequences of an earlier active lifestyle or from sequelae of pediatric hip disease, requiring clinicians to have a thorough understanding of functional recovery after RHA. Until recently, the primary surgical treatment option for patients requiring hip arthroplasty was THA [20, 24, 27]. Traditionally, patients undergoing THA have been limited in postoperative activities as a result of concerns about dislocation, wear, and possible loosening [32]. RHA is an attractive alternative to THA with the reported benefits of better, more normal function in activities of daily living and in athletic endeavors [2, 10, 20, 25, 29]. However, there is limited literature supporting the notion that RHA provides better functional results than THA (Tables 2, 3). Therefore in our preliminary study we questioned whether: (1) during gait, dynamic range of motion, peak external moments, temporospatial parameters, and temporal aspects of hip muscle activation will show greater improvements at three months in the RHA population; (2) subjects receiving RHA will demonstrate greater dynamic range of motion, peak external moments, temporospatial parameters, and temporal aspects of hip muscle activation during stair negotiation; (3) serial clinical surveys will indicate higher functional score in the RHA group correlating with the same specific gait parameters.
The power of our preliminary data is low due to small sample sizes. Despite the small number of subjects, we found differences in some indicators of hip function with only seven patients in each group. Since none of the differences were detected between treatment groups preoperatively, these data highlight trends that may become significant with greater sample size. Functional outcomes are a criterion considered by orthopedic surgeons before prescribing specific arthroplasty devices. If the RHA group outperforms the THA group by producing biomechanical strategies more similar to healthy controls during ADLs, the device may be preferred for the appropriate patient population. Radiographic assessment prior to surgery indicated all subjects were candidates for RHA, hence gross radiographic differences were not apparent between groups. There may be, however, bias in the patients who received RHA; those patients may have been convinced of improved function with hip resurfacing preoperatively and may have had a positive bias that allowed them to recover faster. The only way to mitigate those types of factors would be a blinded, randomized, prospective trial, which would be extremely difficult to perform in this patient population. The demographics within and between treatment groups did indicate unequal male-to-female ratios and greater weight in patient groups compared to controls. All kinetic data were normalized to body weight to reduce variability, although the effects of gender on functional recovery after hip arthroplasty are unknown.
Our first question suggested greater improvements in gait parameters in the RHA group than THA group in comparison to controls. At 3 months postoperatively, our BHR patients had a higher peak hip abduction angle and moment compared with patients receiving THA during gait. When studying the complete abduction moment profile (Fig. 1) deficits were evident in both groups, in comparison to controls, in initial peak during early stance. Reduction in abduction angle and transverse forces on the hip results in the deficit seen during the limb loading component of stance. This protective mechanism may linger from antalgic behaviors adopted prior to surgery. Maximum hip extension is a key parameter in level and stair walking [31] to identify irregularities in movement. The RHA group achieved peak hip extension similar to the controls group evident through kinematic and EMG results. Delayed offset of the GMax/GMed and reduction of peak abduction and extension moments suggest an inability in force production in the THA group. Although this study was performed to identify the existence of differences between groups, the mechanism for differences was also briefly investigated. Analysis of the hip joint center (HJC) was performed to determine if change in offset was the cause of differences in moment results. Pre- and postoperative radiographs and static motion analysis trials were used to calculate the three-dimensional change in HJC relative to the pelvis [17]. Change in HJC appears slightly more lateral in the THA group postoperatively in comparison to the RHA group and further investigation is needed to determine if this has an influence on moments and function. At 1 year postoperatively, gait patterns retain protective adaptations in a THA group [11], whereas RHA groups [20] have suggested control-like gait patterns in abduction moments. If reduced hip abduction angle and moment seen during gait are not addressed, as seen in the THA group, long-term Trendelenburg gait causes bilateral joint wear as a result of high shear stresses and greater stance time on the sound hip [16] and muscle compensations for abductor weakness.
The rationale for the second question was based on the understanding that the function of normal, level-ground gait may not be a challenging enough task to detect certain differences between surgical techniques for total joint arthroplasty [1, 7, 11]. More rigorous activities of daily living such as stair negotiation may be required to identify these differences. Stair negotiation results for all patient groups indicated remaining deficits in stance width and pelvic obliquity that may be used to compensate for instability or remaining hip abductor weakness. Hip extension capability during stair ascent was greatly reduced in the THA group in comparison to BHR and the control group. This may be explained by the dysphasic activity of the GMax combined with slumped upper body posture through the movement cycle. Hip abduction moment during stair descent also remained substantially less than the BHR and control values, suggesting more difficulty in task completion in the THA group. To date, there have been few investigations of stair negotiation or other challenging tasks in patients with THA (Table 2). Our data suggest differences in functional capability do exist between THA and BHR groups. This may be the result of maintenance of hip offset, better proprioception, or some other unknown mechanism.
The third question addressed possible correlations between patient perception of function and quantitative results. Although clinical outcome surveys (Harris hip score, SF-12, LEAS) indicated no difference between groups at preoperative or 3 month time points, motion analysis highlighted dissimilarities between groups and in comparison to control subjects. Our correlation analysis suggested a weak positive relationship between subjective patient forms and quantitative moment data. As presumed, the BHR group’s average HHS, LEAS, and SF-12 results were slightly higher than seen in the THA group, though further investigation is needed to determine if trends continue. The fact that differences were not readily apparent through survey results highlights the strength and resolution of comprehensive motion analysis techniques for clinical applications in determining functional efficacy of medical devices.
In summary, patients who underwent BHR had greater hip abduction moments, higher clinical survey scores, and greater symmetry in muscular activation at 3 months postoperatively in comparison to those who had undergone THA. Furthermore, patients with BHR were able to perform more demanding activities of daily living such as stair negotiation with more normal mechanics compared with those who had THA. These differences during functional recovery would not have been clinically noted. Although correlation between biomechanical assessment and clinical survey was seen, quantitative motion analysis and EMG results provided an in-depth understanding between patient group results. The claims that the BHR may result in improved function may be valid, but additional testing is needed. Understanding the full mechanistic extent of inequalities between surgical devices should be investigated, since it may influence device choice. THA has been long accepted as the standard treatment in hip replacement surgery. Understanding functional outcomes following implantation of RHA devices will provide physicians the ability to make informative decisions when prescribing replacement devices. We will continue to track these and additional patients after RHA and THA until functional recovery has reached a plateau in both groups. The clinical implications of fine grain differences detectable using modern motion analysis techniques require further investigation.
Acknowledgments
We thank the staff and physicians at The SHRI-CORE Orthopedic Research Laboratories who contributed to this work as well as the patients who donated their time for this study.
Footnotes
One or more of the authors (MWS, MBS, MCJ, DJJ) have received funding from Smith and Nephew.
Each author certifies that his or her institution has approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained. This study received IRB approval from the Sun Health Institutional Review Board and all participants provided signatures upon the approved Informed Consent and Health Insurance Portability and Accountability Act forms.
This work was performed at The Sun Health Research Institute and Center for Orthopedic Research and Education.
References
- 1.Aminian K, Rezakhanlou K, De Andres E, Fritsch C, Leyvraz PF, Robert P. Temporal feature estimation during walking using miniature accelerometers: an analysis of gait improvement after hip arthroplasty. Med Biol Eng Comput. 1999;37:686–691. [DOI] [PubMed]
- 2.Amstutz HC, Campbell P, McKellop H, Schmalzreid TP, Gillespie WJ, Howie D, Jacobs J, Medley J, Merritt K. Metal on metal total hip replacement workshop consensus document. Clin Orthop Relat Res. 1996;329(Suppl):S297–303. [DOI] [PubMed]
- 3.Amstutz HC, Dorey F, O’Carroll PF. Tharies resurfacing arthroplasty: evolution and long-term results. Clin Orthop Relat Res. 1986;213:92–114. [PubMed]
- 4.Andersson L, Wesslau A, Boden H, Dalen N. Immediate or late weight bearing after uncemented total hip arthroplasty: a study of functional recovery. JArthroplasty. 2001;16:1063–1065. [DOI] [PubMed]
- 5.Andriacchi TP, Andersson GB, Fermier RW, Stern D, Galante JO. A study of lower-limb mechanics during stair-climbing. J Bone Joint Surg Am. 1980;62:749–757. [PubMed]
- 6.Australian Orthopaedic Association. National Joint Replacement Registry Annual Report, 2007. Adelaide, South Australia, Australia: AOA; 2007.
- 7.Bach CM, Winter P, Nogler M, Gobel G, Wimmer C, Ogon M. No functional impairment after Robodoc total hip arthroplasty: gait analysis in 25 patients. Acta Orthop Scand. 2002;73:386. [DOI] [PubMed]
- 8.Bergmann G, Deuretzbacher G, Heller M. Hip contact forces and gait patterns from routine activities. J Biomech. 2001;34:859–871. [DOI] [PubMed]
- 9.Bierbaum BE, Sweet R. Complications of resurfacing arthroplasty. Orthop Clin North Am. 1982;13:761–775. [PubMed]
- 10.Daniel J, Pynsent PB, McMinn DJ. Metal-on-metal resurfacing of the hip in patients under the age of 55 years with osteoarthritis. J Bone Joint Surg Br. 2004;86:177–184. [DOI] [PubMed]
- 11.Foucher KC, Hurwitz DE, Wimmer MA. Preoperative gait adaptations persist 1 year after surgery in clinically well functioning total hip replacement patients. J Biomech. 2007;40:3432–3437. [DOI] [PubMed]
- 12.Foucher KC, Hurwitz DE, Wimmer MA. Do gait adaptations during stair climbing result in changes in implant forces in subjects with total hip replacements compared to normal controls? Clin Biomech. 2008;23:754–761. [DOI] [PubMed]
- 13.Gore DR, Murray MP, Gardner GM, Sepic SB. Hip function after total vs surface replacement. Acta Orthop Scand. 1985;56:386–390. [DOI] [PubMed]
- 14.Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty; an end result study using a new method of results evaluation. J Bone Joint Surg Am. 1969;51:737. [PubMed]
- 15.Kabada MP, Ramakrishnan HK, Wootten ME. Measurement of lower extremity kinematics during level walking. J Orthop Res. 1990;8:383–392. [DOI] [PubMed]
- 16.Kanai A, Kiyama T, Genda E, Suzuki Y. Biomechanical investigation of ambulatory training in patients with acetabular dysplasia. Gait Posture. 2008;28:52–57. [DOI] [PubMed]
- 17.Kirkwood R, Culham E, Costigan P. Radiographic and noninvasive determination of hip joint center location: effect on hip joint moments. Clin Biomech. 1999;14:227–235. [DOI] [PubMed]
- 18.McCrory JL, White SC, Lifeso RM. Vertical ground reaction forces: objective measures of gait following hip arthroplasty. Gait Posture. 2001;14:104–109. [DOI] [PubMed]
- 19.McMinn D, Daniel J. History and modern concepts in surface replacement. Proc Inst Mech Eng [H]. 2006;220:239–251. [DOI] [PubMed]
- 20.Mont MA, Seyler TM, Ragland PS, Starr R, Erhart J, Bhave A. Gait analysis of patients with resurfacing hip arthroplasty compared with hip osteoarthritis and standard total hip arthroplasty. J Arthroplasty. 2007;22:100–108. [DOI] [PubMed]
- 21.Nallegowda M, Singh U, Bhan S. Balance and gait in total hip replacement. Am J Phys Med Rehabil. 2003;82:669–677. [DOI] [PubMed]
- 22.Newman M, Barker K, Pandit H, Murray D. Outcomes after metal-on-metal hip resurfacing: could we have achieved better function? Arch Phys Med Rehabil. 2008;89:660–666. [DOI] [PubMed]
- 23.Ong KL, Kurtz SM, Manley MT, Rushton N, Mohammed NA, Field RE. Biomechanics of the Birmingham hip resurfacing arthroplasty. J Bone Joint Surg Br. 2006;88:1110–1115. [DOI] [PubMed]
- 24.Pollard TC, Baker RP, Eastaugh-Waring SJ, Banniester GC. Treatment of the young active patient with osteoarthritis of the hip. A five to seven year comparison of hybrid total hip arthroplasty and metal on metal resurfacing. J Bone Joint Surg Br. 2006;88:592–600. [DOI] [PubMed]
- 25.Quality Metrics. SF-12 V2 Reference Scoring Manual. Lincoln, RI: Quality Metrics; 2005.
- 26.Report Buyers Market Report. New technologies and demographics driving the hip implant market. Vaishali UP, India: Koncept Analytics; Nov 2007.
- 27.Saleh KJ, Bershadsky B, Cheng E, Kane R. Lessons learned from the hip and knee musculoskeletal outcomes data evaluation and management system. Clin Orthop Relat Res. 2004;429:272–278. [DOI] [PubMed]
- 28.Schmalzried TP, Peters PC, Maurer BT, Bragdon CR, Harris WH. Long-duration metal-on-metal total hip arthroplasties with low wear of the articulating surfacing. J Arthroplasty. 1996;11:322–331. [DOI] [PubMed]
- 29.Schmalzried TP, Szuszczewicz ES, Northfield MR, Akizuki KH, Frankle RE, Belcher G, Amstutz HC. Quantitative assessment of walking activity after total hip or knee replacement. J Bone Joint Surg Am.. 1998;80:54–59. [DOI] [PubMed]
- 30.Treacy RB, McBryde CW, Pynsent PB. Birmingham hip resurfacing arthroplasty. A minimum follow-up of five years. J Bone Joint Surg Br. 2005;87:167–170. [DOI] [PubMed]
- 31.Winter D. Biomechanics and Motor Control of Human Movement. 3rd Ed. Waterloo, NJ: Waterloo Press; 2005.
- 32.Woo RY, Morrey BF. Dislocation after total hip arthroplasty. J Bone Joint Surg Am. 1982;64:1295–1306. [PubMed]