Introduction
The gastrointestinal tract represents an important entry site for pathogens. It is also home to a large number and diverse array of commensal bacteria many of which are beneficial to the host. A key feature of the intestinal immune system is its ability to protect against infection while avoiding the development of destructive inflammatory responses to the normal microbiota. Understanding the mechanisms that control intestinal homeostasis is an active area of research. It is hoped that uncovering the pathways used by the intestinal immune system to prevent immune pathology may direct therapeutic approaches to a broad range of autoimmune and inflammatory conditions. In addition, overcoming default pathways that maintain tolerance in the intestine may be beneficial to the development of oral vaccines. Of particular interest is the role played by populations of dendritic cells (DCs) in the intestine and associated lymphoid tissue. These cells have been implicated both in the maintenance of tolerance towards the commensal flora, and in the generation of protective immune responses against pathogens. This impressive flexibility in function is probably due to an ability to accurately sense their local environment and use these signals to shape the nature of the ensuing immune response. We are now beginning to understand some of the unique functional properties of populations of intestinal DCs, and also the type of signals that are required for them to mediate these functions. Here, we discuss intestinal DC function in the steady state, the requirements for a shift towards the generation of protective immune responses, and the potential role for DCs in the pathogenesis of inflammatory bowel disease (IBD).
Antigen-presenting cells of the intestine
The intestine and associated lymphoid tissues are home to an extensive network of innate immune cells with antigen presenting function, including macrophages, conventional CD11chi DCs and plasmacytoid DCs (pDCs) 1, 2. Adaptations to the intestinal environment that prevent the generation of destructive inflammatory responses have been demonstrated in all of these populations. Nevertheless, these cells also have well described roles in protection against enteric pathogens. The divergent functional properties of different populations of intestinal DCs and macrophages have now begun to be dissected.
A variety of subpopulations of DCs are present in the organized lymphoid structures of the intestinal immune system, including the Peyer's patches and mesenteric lymph nodes (MLNs), and also throughout the small intestinal and colonic lamina propria (Box 1, reviewed in 1, 3). DCs exist in all of these sites in the steady state, but can also develop from precursors in response to microbial and inflammatory stimuli.
Box 1.
DCs are often classified into subsets on the basis of cell-surface receptor expression. In the Peyer's patches, conventional DCs are predominantly of the CD11chiCD11b+CD8α−, CD11chiCD11b−CD8α+ and CD11chiCD11b−CD8α− subtypes, with unique functional properties and anatomical localisation described for each subset 6, 8. Peyer's patch DCs can also be described in terms of their expression of the chemokine receptors CX3CR1 and CCR6 21, 82. CX3CR1+ DCs were found to be closely associated with the follicle-associated epithelium (FAE) in the steady state, whereas CCR6+DCs, which fall largely into the CD11b+CD8α− and CD11b−CD8α− Peyer's patch DC populations, were recruited from the SED to the FAE during infection 8, 82.
Small intestinal lamina propria DCs have been described to be similar in subset composition to Peyer's patch DCs, although the presence of conventional CD8α+ DCs in the LP appears to be a contentious issue 3, 5, 109. DCs in the small intestinal lamina propria were also found to express CX3CR1 21. In the colon, DCs appear to be concentrated largely within isolated lymphoid follicles, with very few present in the lamina propria under steady-state conditions 21, 81, 110. A substantial proportion of both colonic and small intestinal lamina propria DCs express the integrin subunit CD103 (also known as αE) 7, 35. The migration of DCs from the intestinal lamina propria to the MLN has been studied by cannulating the thoracic duct lymph of mesenteric lymphadenectomised rats. Using this technique, DCs migrating in lymph were found to better stimulators of MLR than DC in the lamina propria, and were therefore likely to represent a more mature population 111.
The MLN is also home to populations of CD11chiCD11b+CD8α−, CD11chiCD11b−CD8α+ and CD11chiCD11b−CD8α− DC 6. It contains both migratory DC arriving from the intestinal lamina propria in the steady state, and resident DC that have developed from blood borne precursors. Expression of the integrin subunit CD103 (also known as αEβ7-integrin) has been reported on CD11chi DC isolated from the MLN and is likely to mark migratory DC arriving from the intestine 7, 35, 112, 113. Consistent with this, CCR7-deficient mice also show a reduced frequency of CD103+ DCs in the MLNs 35. Conversely CD103−DCs in the MLNs may arrive as precursors from the blood, as indicated by their expression of the lymph-node homing receptor CD62L (also known as L-selectin)35 (and our own unpublished findings).
An additional population of CD11cmed pDCs are also present in the Peyer's patches and MLNs 114, 115. pDCs cannot however be detected in the lymph, suggesting they do not participate in migration from the intestine to the MLNs 116.
In the steady state, the functional properties of DCs appear to vary according to their anatomical location. The clearest differences are observed between DCs of the intestine and spleen. For example, Peyer's patch DCs produced high levels of interleukin-10 (IL-10), compared with splenic DCs activated under similar conditions 4. Furthermore, naïve CD4+ T cells activated by Peyer's patch DC produced higher levels of IL-4 and IL-10, indicative of a Th2 phenotype, than those activated by splenic DC 4. Functional differences are also observed between DCs residing in the Peyer's patches, MLNs, small intestinal lamina propria and colonic lamina propria. For example, adoptive transfer of small intestinal lamina propria DCs, but not Peyer's patch DCs, from OVA fed mice could inhibit DTH responses 5. Functional differences between DC in these locations are likely to reflect a combination of factors, including differing developmental origins, local environmental conditions and maturation states.
Within these sites, DC can be subdivided according to cell surface receptor expression (Box 1). Important functional distinctions also exist between these subpopulations. For example, the capacity of Peyer's patch DCs to produce IL-10 and prime Th2 cells was found to enrich within the CD11b+ subset 6. On the other hand, CD8α+ and CD11b− CD8α− Peyer's patch DC produced IL-12 and drove production of IFN-γ by T cells 6. Similarly, CD103− MLN DC are superior to their CD103+ counterparts in promoting IFN-γ production by T cells 7. Again, these differences could be argued to reflect developmental differences between the DC subsets, or minor alterations in their environment. As such, CD11b+ DC are concentrated largely within the sub-epithelial dome (SED) of the Peyer's patch, whilst CD8α+ DCs are found in the inter-follicular region (IFR) 8. Furthermore, DC subsets may differ in their expression of receptors that allow them to respond to host and microbial factors, influencing their function.
DC are also recruited to the intestine in inflammation. Whether these cells represent a separate lineage to those present in the steady state, or whether their ability to drive pro-inflammatory responses is a function of their exposure to both pathogens and inflammatory cytokines upon arrival in the intestine remains unclear. Alternatively, these cells may simply represent a population of DC already present in the steady state becoming more dominant.
Intestinal macrophages also display some distinctive characteristics compared with splenic macrophages or those deriving from blood monocytes 9 10. Although human intestinal macrophages retain phagocytic and bactericidal activity, they lack CD14 expression, which is known to be required for the recognition of ligands for Toll-like receptor 4 (TLR4). Accordingly, when cultured with TLR4 ligands, and also with a range of other stimuli, these cells showed an impaired ability to produce pro-inflammatory cytokines 9. These modifications may contribute to intestinal immune homeostasis by ensuring that contact of intestinal antigen presenting cells (APC) with microbial products does not automatically result in the generation of potentially destructive inflammatory responses.
Origins of steady state and inflammatory DC
Steady state DC can be classified as migratory or LN resident. Migratory DC sample antigen in the tissues before migrating through lymph to the lymph nodes whilst lymphoid tissue resident DC are likely to arrive from the blood as precursors and develop into DC in situ 11. Lymph nodes contain a mixture of both DC types, which are thought to differ in terms of maturation status and expression of cell surface receptors 12. Development of migratory DC appears to be distinct to that of lymphoid tissue resident DC. Although both can derive from a CD117+Lin−CX3CR1+ bone marrow precursor, it has been suggested that some mucosal (migratory) DC do so via a monocyte stage whereas splenic (lymphoid tissue resident) DC do not 13, 14. A recent report demonstrated that CD117+Lin−CX3CR1+ precursor cells can differentiate into CX3CR1intGr1highCCR2+ and CX3CR1highGr1lowCCR2− monocytes in the bone marrow 14. The CX3CR1highGr1lowCCR2− subset has been proposed to migrate into the peripheral tissues under steady state conditions, and consistent with this CX3CR1highCCR2low monocytes in rat give rise to a small proportion of steady state intestinal lymph DC 15, 16. The reciprocal CX3CR1lowGr1highCCR2+ subset is suggested to give rise to DC in the peripheral tissues under inflammatory conditions, although Gr1high monocytes may also give rise to steady state intestinal DC 14, 15. It should however be appreciated that under steady state conditions Gr1high monocytes can migrate back to the bone marrow and become Gr1low, clouding the issue 14. Thus the origins of DC present in the tissues in the steady state and during inflammation remain unclear. CD117+Lin−CX3CR1+ precursor cells and their monocytic intermediaries also gave rise to intestinal macrophages 14.
Sampling of antigen by intestinal DCs
DCs can pick up antigen transported across the intestinal epithelium through various different routes. First, specialised M cells present in the FAE of Peyer's patches can transcytose luminal antigen, which is then taken up by nearby DCs. Second, antigen may be transported directly into the intestinal lamina propria through a mechanism involving the neonatal Fc receptor for IgG, which may also be expressed by the intestinal epithelium in adults, or by M cells present in the villi 17, 18. Third, DCs can sample antigen directly from the intestinal lumen by forming tight-junction-like structures with intestinal epithelial cells (IECs) 19. This allows for the projection of dendrites through the epithelial-cell layer and into the lumen. It is possible that this process contributes to the sampling of antigen from the commensal flora, as DC extensions are readily detected under normal conditions 20, 21. Nevertheless, the presence of invasive bacterial species increases the frequency of transepithelial projections, particularly in the terminal ileum 19-21. Projection of dendrites across the intestinal epithelium is thought to require myeloid differentiation primary-response gene 88 (MyD88)-dependent signalling through TLRs and the chemokine receptor CX3CR1 20, 21. Indeed, in support of the idea that DC projections contribute to sampling of commensal flora, non-pathogenic Escherichia coli could only be cultured from the MLNs of CX3CR1-sufficient mice 21. However, although the absence of CX3CR1 abrogates the increase in transepithelial projections seen in the terminal ileum during infection, they still occur in more proximal regions of the small intestine 20. In addition to facilitating sampling of luminal content, the ability of DCs to penetrate the epithelium without destroying its integrity has also been suggested as a mechanism by which apoptotic epithelial cells could be engulfed and transported to the MLN 19.
Role of DCs in intestinal homeostasis
Through an ability to use signals received in their local environment to shape the course of an immune response, DCs are likely to be integral to ensuring that pathological immune responses to harmless antigens do not develop. Although intestinal DCs are clearly involved in the generation of active immune responses in the steady state, these responses create an overall tolerant state towards the commensal flora through the activation of immune regulatory mechanisms and the generation of low-level immune responses aimed at controlling the normal flora without causing pathology.
Lamina Propria DC that take up antigen in the intestine may perform this tolerogenic function through their constitutive migration through the lymph to the draining MLNs, where they can present antigen to T cells. Although the levels of DC migration are increased by inflammatory stimuli, lymph-borne DCs carrying antigen can be isolated in the absence of any overt inflammatory stimuli 22. In fact, it has recently been suggested that lamina propria DC are still present in MLNs in the steady state in the absence of a commensal flora or MyD88-dependent signalling 23. Constitutive DC traffic has been shown to deliver antigen from both commensal bacterial strains and apoptotic IECs to the MLNs 24, 25. The importance of this constitutive carriage of antigen to the MLN is suggested by studies in which impaired trafficking of DCs from the intestine to the MLN in Ccr7−/− mice results in defective induction of tolerance to oral antigen 26. An intriguing issue is the nature of the pathway that stimulates constitutive DC trafficking. Of interest, it has recently been demonstrated that disruption of E-cadherin-mediated DC clustering initiates a programme of maturation that is distinct from that driven by microbial products and leads to the generation of tolerogenic DCs 27. However, the factors involved in triggering the loss of E-cadherin interactions have yet to be defined. Alternatively, constitutive low level production of pro-inflammatory cytokines may be sufficient to stimulate DC migration 28 29.
DCs that have migrated to the MLN in the steady state, or are present in the Peyer's patches, can interact with B and T cells and initiate responses aimed at maintaining a non-inflammatory state in the intestine. Currently of particular interest is the ability of intestinal DCs to promote the development of forkhead box P3 (FOXP3+) regulatory T cells (TReg cells) in the periphery. It has recently been demonstrated that the gut-associated lymphoid tissue is a preferential site for the peripheral induction of FOXP3+ TReg cells 30. The ability to generate FOXP3+ TReg cells from naïve T cells may be of particular importance in the intestine, providing a mechanism by which the thymically derived pool of CD4+CD25+FOXP3+ TReg cells could be complemented with FOXP3+ TReg cells specific for the commensal bacteria or dietary antigens. Alternatively, it is possible that the TReg-cell repertoire does not need to be extended per se, but that diversion of naïve T cells that are strongly reactive with innocuous antigen into the TReg-cell lineage is a useful mechanism to prevent them inducing pathology later.
DCs present in the normal intestine and associated lymphoid tissues are capable of mediating this peripheral induction of FOXP3+ TReg cells 31 32 33 30 34. As such, small intestinal lamina propria and MLN DCs were significantly better than splenic DCs at inducing the expression of FOXP3 in naïve T cells in the presence of transforming growth factor-β (TGFβ) 30, 31. Furthermore, CD103+ DCs isolated from the MLNs are capable of mediating the conversion of naïve T cells into FOXP3+ T cells in the absence of any exogenous factors 33. This too was mediated by TGFβ as blocking antibodies to TGFβ prevented the expression of FOXP3. However, this was not a property common to all MLN DCs as CD103− DCs did not promote expression of FOXP3, and even in the presence of exogenous TGFβ only promoted FOXP3 expression in a small proportion of T cells 33. This may reflect the idea that CD103+ MLN DC derive from the intestinal lamina propria, whereas CD103− DC may not 35. Furthermore, it is unclear whether populations of Peyer's patch DC can also perform this function.
In contrast to these reports, Denning et al. suggest that it is the small intestinal lamina propria macrophages and not DCs that induce the differentiation of FOXP3+ TReg cells from naïve T cells 10. However, the site at which this interaction would occur in a physiological setting remains to be clarified.
An important question is what the source of TGFβ for intestinal DC-mediated induction of FOXP3 may be. It is possible that these DC produce active TGFβ in response to signals in the local environment, or that they are equipped for the efficient conversion of latent TGFβ into the active form 33. In this respect, a recent study has suggested that expression of αvβ8-integrin on DCs is important for the activation of TGFβ, the accumulation of FOXP3+ TReg cells in the intestine, and the prevention of colitis 36. Furthermore, loss of αv-integrin expression on myeloid cells led to the development of intestinal inflammation, probably through the combined effects of failure to remove apoptotic cells and activate TGF 37. Both studies reported an impaired ability of DCs to promote FOXP3 expression in naïve T cells, suggesting that local activation of latent TGFβ by DCs is important for the peripheral induction of FOXP3+ T cells. However, intestinal inflammation may also have resulted from a loss of TGFβ mediated control of effector T cell function 38.
DCs also have an important role in dictating the homing potential of recently activated T cells.DCs isolated from the Peyer's patches, small intestinal lamina propria and MLNs promote the expression of the gut homing receptors α4β7-integrin and CCR9 on T cells 35,39-41. In the MLN the ability to drive the expression of gut homing receptors on both CD4+ and CD8+ T cells was enriched within the CD103+ DC subset 7 35. CCR9 binds to CCL25 produced by epithelial cells of the small intestine and α4β7-integrin binds to mucosal vascular addressin cell-adhesion molecule 1 (MADCAM1), which is expressed on the vascular endothelium of the gastrointestinal tract. Both MADCAM1 and CCL25 are constitutively expressed indicating a constitutive migration of T cells into the intestinal lamina propria, that could be attributed to the high antigen load at these sites and the consequent need to attract TReg cells 42, 43. Consistent with this, DCs could also promote the expression of gut-homing receptors on thymically derived CD4+CD25+FOXP3+ TReg cells 44.
DCs have also been implicated in class switching to IgA, the predominant isotype at mucosal surfaces. Mechanisms of class switching to IgA are complex and likely to vary depending on the site at which it occurs, the type of B cell, dependence on T cells, and the presence of commensal versus pathogenic species 45 46. Nevertheless, a clear role for Peyer's Patch DC in class switching to IgA has been demonstrated 47 24. Consistent with this, populations of DCs in the intestine produce a variety of different cytokines and other mediators implicated in class switching to IgA, including IL-10, TGFβ, IL-6 and APRIL (a proliferation-inducing ligand)4, 6, 47 48 49.
Role of vitamin A in intestinal DC function
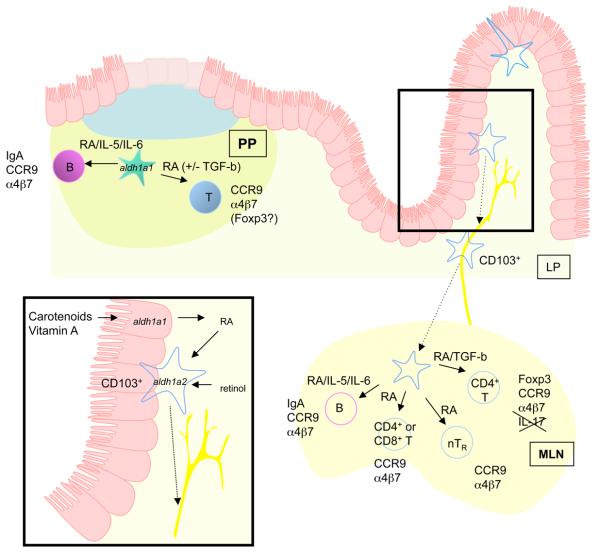
A remarkable aspect of the ability of intestinal DC to upregulate homing receptors on lymphocytes, drive the peripheral generation of FOXP3+ TReg cells and support class switching to IgA is that retinoic acid, the acid form of vitamin A, has been shown to have an important role in all of these processes (Figure 1) 10, 30, 31, 33, 34, 44, 50-52 53.
Figure 1.
Role of Vitamin A metabolites in intestinal DC function. Intestinal DCs promoted expression of gut homing receptors on lymphocytes, peripheral generation of Foxp3+ Treg and class switching to IgA. Retinoic acid plays an important role in all of these processes. Peyer's patch DCs and MLN DCs arriving from the intestine express enzymes that allow them to metabolise retinoic acid, perhaps from retinol carried in the serum or stored in the intestine. Alternatively DCs may transport retinoic acid metabolised from dietary carotenoids or other vitamin A derivatives by IEC to lymphoid tissues. Retinoic acid induces expression of the gut homing receptors, CCR9 and α4β7, on T cells. It has an enhancing effect on TGFβ mediated induction of Foxp3 and synergises with IL-6 or IL-5 to mediate class switching to IgA.
Early studies showed that cells isolated from the MLN of vitamin-A-deficient rats were impaired in their ability to home to the intestine following adoptive transfer into naïve recipients 54. Mechanistically, this was subsequently found to be due to the ability of retinoic acid to promote the expression of gut-homing receptors on T cells 52. Indeed, the ability of MLN and Peyer's patch DCs to imprint T cells with gut-homing potential was dependent on retinoic acid. Consistent with this finding, retinoic acid has also been shown to promote the expression of gut-homing receptors on B cells and on thymically derived CD4+CD25+ TReg cells53 44.
It has been appreciated for some time that administration of retinoic acid has beneficial effects in autoimmune disease settings. It is possible that some of these effects are attributable to the reported function of retinoic acid in regulating Th1- and Th2-cell differentiation, such that in vitamin-A deficiency the balance is shifted in favour of the generation of Th1-cell responses 55. Indeed, the propensity of CD11b+ Peyer's patch DCs to drive Th2-cell responses may be mediated by retinoic acid.
Another possible explanation for the beneficial effect of retinoic acid in autoimmune disease comes from a cluster of recently published studies demonstrating that retinoic acid enhances the TGFβ-mediated generation of FOXP3+ TReg cells from naïve periperhal T cells 10, 30, 31, 33, 34, 50, 51 56. Notably, the induction of FOXP3 expression observed in the presence of small intestinal lamina propria DC and CD103+ MLN DCs could be inhibited by a retinoic acid receptor antagonist 33 30. In addition, the inclusion of both TGFβ and retinoic acid to cultures containing splenic or CD103− MLN DCs enhanced the generation of FOXP3+ T cells 33, 30, 31. Retinoic acid was also identified as an important cofactor for the differentiation of FOXP3+ TReg cells in the presence of intestinal macrophages 10 Importantly, retinoic acid has a positive effect on FOXP3 expression by human CD4+ T cells, suggesting that it may be an important therapeutic target 34. Related to this, retinoic acid has also been shown to inhibit the generation of Th17 cells 31, 34, 51 56. As a result, MLN DCs were impaired in their ability to drive IL-17 secretion by T cells in the presence of TGF-β and IL-6 31. These findings suggest that therapeutic manipulation of retinoic acid levels has the potential not only to enhance regulatory pathways, but to directly inhibit the generation of inflammatory T-cell populations.
Vitamin A levels are closely linked to the magnitude of IgA responses in the intestine, with vitamin A deficiency leading to reduced levels of IgA 55. Consistent with these findings, it has recently been demonstrated that the ability of PP DC to promote T cell independent class switching to IgA can be attributed to the combined effects of RA and IL-5 or IL-6 53.
Synthesis of retinoic acid from stored or dietary retinol occurs in a two-step reaction consisting of the oxidation of retinol to retinal, and the subsequent oxidation of retinal to retinoic acid 57. The ability of a cell to catalyse this reaction depends on expression of the appropriate enzymes, with the final step being catalysed by aldehyde dehyrogenases such as the retinal dehydrogenases, aldehyde dehydrogenase family 1, subfamily A1 (Aldh1a1) and ALDH1, subfamily A2 (Aldh1a2). Although some of the functional properties of intestinal DCs have been shown to be retinoic-acid dependent, it remains unclear whether the DCs themselves are responsible for the production of retinoic acid from retinol. In support of this idea Peyer's patches and MLN DCs have been shown to express Aldh1a1 and Aldh1a2, respectively 33, 52. Furthermore, consistent with their functional properties, MLN CD103+ DC express higher levels of Aldh1a2 than CD103− DCs taken from the same tissue 33. Most importantly, Peyer's patch and MLN DCs could convert retinol to retinoic acid in culture, particularly in the presence of T cells 52. Accordingly, the MLN and Peyer's patch DC-mediated imprinting of gut homing receptors on T cells can be partially inhibited by the retinal dehydrogenase inhibitor, citral 52. Induction of FOXP3 by pooled small intestinal lamina propria and Peyer's patch DCs was also inhibited by a retinal dehydrogenase inhibitor 34. However, similar experiments examining the induction of FOXP3 expression by intestinal lamina propria DCs showed that citral had no effect on FOXP3 expression 30. This could be explained by a contribution of other cell type to the synthesis of retinoic acid, and by the ability of DCs to store retinoic acid 58. In fact, small IECs also express ALDH1a1, indicating that they may contribute RA which can be taken up and transported by DC 52 59.
The functional properties of retinoic acid are mediated through ligation of heterodimers of the retinoic acid receptor (RAR) and retinoid X receptor families. Accordingly, CD103+ DCs were better than their CD103− counterparts at inducing early RAR signalling that led to the expression of gut homing receptors in T cells 60. RARs function as ligand-dependent transcription factors and bind RAR elements (RAREs) or retinoid X response elements (RXREs) in the promoter regions of target genes 57. Hundreds of different genes have been suggested to be either direct or indirect targets of receptor-bound retinoic acid , suggesting how it may initiate, augment or inhibit the diverse programmes of differentiation in which it has been implicated. However, retinoic acid has little effect on Foxp3 expression in isolation, making the question of how it can act to enhance the TGFβ-mediated induction of FOXP3 a more complex one. In this regard, cooperation between the TGFβ and retinoic acid pathways has been documented, with retinoic acid increasing expression of TGFβ receptor subunits (117). Furthermore, a direct interaction between RAR family members and Smad3 has been proposed, although it remains unclear whether this interaction positively or negatively regulates TGFβ signalling (118,119). Retinoic acid treatment also leads to a reduction in IFNγ production by T cells 55,61. This is consistent with the finding that T cells produce less IFNγ when cultured with CD103+ DCs 7. Since IFNγ is known to induce SMAD7, which inhibits TGFβ signalling, one possible consequence of reduced IFNγ production may be an increase in TGFβ signalling and ultimately in expression of FOXP3. Perhaps complimentary to this, RA is thought to induce production of TGFβ in DCs 58.
Among the genes directly regulated by retinoic acid, Il2ra (also known as Cd25) is a possible candidate by enhancing the proportion of FOXP3+ T cells generated 62. However, although one report suggested that IL-2 signalling was required for the induction of FOXP3 in the presence of both TGFβ and retinoic acid, a subsequent study demonstrated that TGFβ and retinoic acid could still induce FOXP3 in T cells lacking the IL-2 signalling component signal transducer and activator of transcription 5 (STAT5) or in the presence of blocking antibodies to IL-2 31 56.
Finally, costimulation through CD28 impairs the TGF-β mediated induction of FOXP3 expression in naive T cells, whilst inclusion of retinoic acid can overcome this effect 50. CD28 signals synergise with TCR signals to activate the transcription factor, activator protein 1 (AP-1) 63. Complexes of NFAT and AP1 regulate expression of genes associated with T cell activation, including IL-2. However, NFAT can also interact with Foxp3, inhibiting expression of genes normally regulated by AP1:NFAT complexes, and inducing expression of genes important for regulatory T cell function 64. Interestingly, ligand-bound RARs can inhibit the transcriptional activity of AP-1, perhaps by forming complexes with AP-1 subunits 65. In doing so, retinoic acid may directly interfere with the negative effects of costimulation on Foxp3 induction. Furthermore, retinoic acid may promote the formation of Foxp3:NFAT complexes by limiting competition by AP1. Foxp3 has been proposed to act in an autoregulatory loop by downregulating smad7 expression and allowing for enhanced TGF-β signalling 66.
Influence of the environment on intestinal DC function
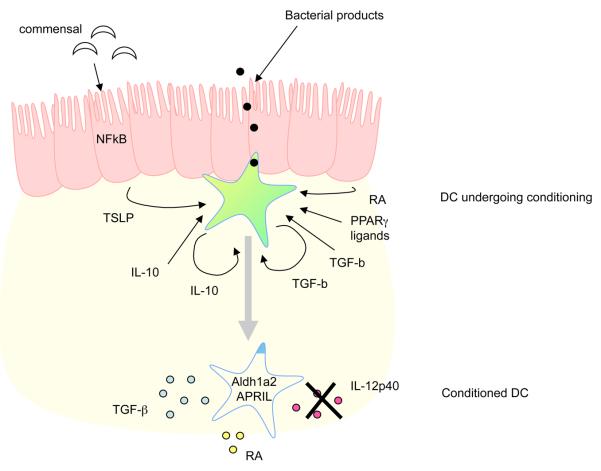
In light of the unique functional properties described for intestinal DCs, an important question is how they are acquired. Recent evidence suggests that conditioning of DCs in their local tissue environment, rather that the existence of functionally distinct DC subsets, has an important role in shaping their function (Figure 2). Communication between the intestinal epithelium and DCs is likely to be integral to this type of 67. IECs show qualitatively distinct responsiveness to commensal and pathogenic bacterial species, and therefore the epithelial-cell layer may act as a sensor for current environmental conditions and instruct nearby DCs accordingly. In fact, following in vitro co-culture with an epithelial-cell line, human DCs preferentially induced non-inflammatory Th2-cell responses 68. This effect was mediated in part by production of thymic stromal lymphopoietin (TSLP) by the epithelial cells, which can be increased in response to bacterial stimulation and NFκB activation 48, 68, 69. The finding that epithelial cell derived products can condition human DC to drive Th2 responses is consistent with the fact that isolated murine CD11b+ Peyer's patch DCs also promote the differentiation of Th2 cells 6. However, how these cells behave in vivo will be influenced by a wide range of different factors. Nevertheless, an important role for TSLP in dictating the quality of the immune response in vivo has recently been suggested. An increased number of CD11b+ and CD11b−CD8α− DCs expressing IL-12/23p40 are present in the MLNs of mice deficient in the TSLP receptor, and these mice display defective Th2 mediated immunity following infection with Trichuris muris 69. In addition to the effects noted above, TSLP can enhance production of APRIL by DCs, which is involved in class switching to IgA 48, 68, 69.
Figure 2.
Conditioning of DCs in the intestine. The functional properties of intestinal DCs are altered by factors present in the local environment. Activation of NFκB in IEC, perhaps as a result of the commensal flora signalling through TLR, enhances their production of APRIL and TSLP. TSLP and other epithelial cell derived factors can act on DC to downregulate IL-12/23p40 production in response to bacterial stimulation. DC conditioned in this way preferentially drive classical Th2 responses. IL-10 and TGFβ may also play a role in limiting the responsiveness of intestinal DCs to bacterial or other activation signals. These cytokines may derive from multiple sources, though there may be an autocrine effect of TGFβ produced by DC in response to epithelial derived signals, including retinoic acid. Bacterial products may also act directly on DCs to alter their function, for example through induction of enzymes involved in the metabolism of vitamin A. Defective conditioning of DCs in the intestine may contribute to the pathogenesis of IBD.
TSLP has also been shown to confer human thymic DCs with the ability to induce the differentiation of CD4+CD25− thymocytes into FOXP3+CD4+CD25+ TReg cells 70. It is therefore possible that the ability of CD103+ MLN DCs or small intestinal lamina propria DCs to induced FOXP3 is a result of exposure to TSLP in vivo. However, TSLP-conditioned human DCs could not induce FOXP3 expression in naïve peripheral T cells and murine TSLPR-deficient lamina propria DCs were not impaired in their ability to induce FOXP3 expression 30, 70. It is therefore unlikely that TSLP has a non-redundant role in conditioning DC for the peripheral generation of murine FOXP3+ TReg cells.
Production of retinoic acid by DCs themselves may be important for enhancing the TGF-β mediated generation of Foxp3+ TReg cells. Therefore, signals that induce expression of enzymes involved in the production of RA may be a good starting point in deciphering what type of conditioning is necessary to generate DCs capable of promoting Foxp3 expression. In this respect, The lipid-activated transcription factor, PPAR-γ, has recently been demonstrated to increase expression of retinoid metabolising enzymes in human DC, augmenting RA production 71.
Analogous to the conditioning of DCs by IEC supernatants, blood-derived monocytes conditioned with intestinal stromal cell supernatants took on a similar phenotype to that described for intestinal lamina propria macrophages, with TGFβ having a key role in this process 9. This suggests that the distinctive properties of intestinal macrophages may also be a result of environmental conditioning. In fact, the development of spontaneous intestinal pathology in both IL-10-deficient and TGFβ-deficient mice suggests roles for these cytokines in the conditioning of intestinal DCs and macrophages. At least in the case of IL-10 deficiency, intestinal pathology is thought to be due to a loss of IL-10-mediated control of myeloid cells. A myeloid-cell-specific deletion of STAT3, through which IL-10 signals, led to enhanced TLR-driven production of IL-12p40 and the development of chronic IL-12p40 driven enterocolitis 72 73. Indeed, it is possible that one of the key functions of both IL-10 and TGFβ is to control TLR-mediated activation of epithelial cells, DCs and macrophages that are continually exposed to components of the commensal flora. Consistent with this, DC co-cultured with IECs produced TGFβ, which inhibited TLR-mediated activation of DCs 74. Although some TLR signalling is important for protection from intestinal injury, IL-10 seems to have an important role in inhibiting inflammatory responses induced by commensal bacteria through the MyD88-signalling pathway 75, 76. As such, IL-10-deficient mice that also lack MyD88 fail to develop intestinal pathology, have reduced levels of IL-12p40 in the colon, and generate fewer IFNγ-producing T cells. IL-10 and TGFβ have also been implicated in the conditioning of plasmacytoid DCs in the Peyer's patches and may inhibit their production of type 1 interferons 77. Thus, many of the unique properties of intestinal APC appear to be a result of conditioning in their local environment.
Initiation of protective immunity to pathogens
We have discussed the unique functional properties of intestinal DCs that allow them to initiate responses that create an overall tolerant state towards harmless intestinal antigens. However, intestinal DCs are also implicated in the generation of protective immune responses aimed at the clearance of enteric pathogens.
The question of how intestinal DCs mediate these seemingly distinct functional roles is intriguing, and remains unresolved. It was initially postulated that the commensal flora was retained in the gut lumen through the combined actions of secreted IgA, the mucus layer and the tight junctions between epithelial cells, whereas only pathogenic species were equipped to cross the epithelial-cell layer and initiate immune responses. Consistent with this, the expression of some TLRs, including TLR5, have been suggested to be restricted to the basolateral surface of IECs, preventing their engagement by luminal bacteria. However, other studies have shown that non-invasive Salmonella typhimurium can engage TLR5 expressed by IECs when applied to the apical surface of IEC monolayers in vitro78, 79. In fact, TLR signalling and NF-κB activation in IECs in the steady state may have an important role in intestinal immune homeostasis 69, 76, 80.
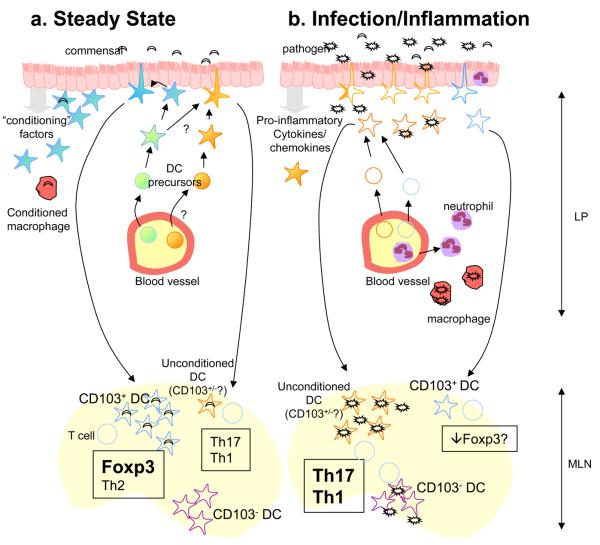
Regardless of the expression patterns of TLRs on IECs, there is also potential for TLRs expressed by DCs to be engaged by commensal species following projection of dendrites across the epithelial layer, or following their M-cell-mediated translocation into the PP and uptake by DC. In fact, commensals are routinely transported to the MLNs within DCs 24. It is however important to note that commensal bacteria are only transported to the MLNs within DCs, and do not appear to penetrate any further 24. Together, this leaves us with a scenario whereby the commensal flora can be sensed by IECs, which communicate with resident DCs to limit the generation of destructive immune responses. This allows DC to come into contact with the commensal flora and initiate host protective responses to the commensal flora, such as the differentiation of Foxp3+ TR, Th2 cells and IgA secreting B cells. In addition, we speculate there may be constitutive low level recruitment of DC from blood precursors that would be capable of driving Th1 or Th17 responses. These DC may act either as sentinels for the presence of pathogenic species, or may constitutively initiate cell mediated immune responses against the commensal flora to ensure it is kept under control (Figure 3). They may escape conditioning by chance encounter of microbial products or other inflammatory stimuli before conditioning can occur or as a result of a lineage related lack of expression of the appropriate receptors. In fact, it is possible that these DCs share their origins with DCs arriving in the tissues under inflammatory conditions. Nevertheless, under normal circumstances the balance between tolerogenic responses induced by conditioned DC, and responses induced by unconditioned DC should be such that no pathology develops. Indeed, IL-23 producing DC can be found in the terminal ileum of normal mice, where a high bacterial load is present 81
Figure 3.
Response of intestinal DCs to infection. (a) In the steady state, DCs resident in the intestine are conditioned by epithelial cell derived factors and promote the differentiation of Foxp3+ Treg and IgA secreting B cells upon migration to the MLN. This may occur following sampling of the commensal flora or in response to self-antigens derived from the intestinal epithelium. A small number of DC may also be recruited that escape conditioning and drive Th1 or Th17 responses. These DC may share a precursor with other DC resident in the intestine in the steady state, but encounter bacterial products or other stimuli before conditioning can take place, or they may derive from distinct precursors and be refractory to conditioning. These cells could act as sentinels for the presence of pathogenic species, or mount responses aimed at controlling the commensal flora. (b) In contrast to the commensal flora, some pathogenic species possess virulence factors that allow them to invade the intestinal epithelium and subvert immune responses to enhance their replication. Invasion of the epithelium leads to activation of cytosolic PRR and enhanced production of chemokines and pro-inflammatory cytokines. Neutrophils, macrophages and DC precursors are recruited to the site and become activated by a combination of signals from pathogens and pro-inflammatory cytokines and chemokines. Whether these DC precursors are shared with populations of DC present in the steady state remains unclear. Although DCs resident in the tissues prior to infection may not take on pro-inflammatory function, it is possible that their ability to promote regulatory T cell differentiation may be impeded. Pathogenic microbes may also reach CD103− DC resident in the LN which are capable of driving Th1 responses, likely as a result of not being conditioned in the intestine.
A fundamental difference between the steady state and infection with pathogenic species may lie in the greater propensity of pathogens to invade the epithelial-cell layer and to penetrate beneath. Following this, their capacity to subvert immune responses and replicate may be greater than that of commensal species, resulting in an inability to contain the pathogen within intestinal antigen-presenting cells. Invasion of IECs would allow for the activation of cytosolic PRRs and both quantitative and qualitative changes in the secretion of pro-inflammatory cytokines and chemokines. Consistent with this, IEC only produced IL-8 when confronted with strains of salmonella that were both invasive and flagellated 78. This IL-8 may serve to attract neutrophils to the site of infection, which will further contribute to the inflammatory milieu. As a result, the rate of blood borne DC precursors migrating into the tissues and becoming DCs will increase. These cells will not have been subject to conditioning and can be directly activated by a combination of pathogens that have breached the epithelial-cell barrier and the pro-inflammatory cytokine milieu (Figure 3).
It is likely that the recruitment of DC that have not been subject to conditioning, rather than the activation of pre-existing DC, is essential for the generation of protective immune responses. In support of this, human monocyte derived DCs conditioned with epithelial-cell supernatants are still impaired in their ability to secrete IL-12 and drive Th1-cell responses following exposure to pathogenic Salmonella species 68. However, these DCs can drive Th1-cell responses if they encounter bacteria prior to conditioning by IEC-derived factors 68. In addition, the CCR6 mediated recruitment of DC from SED of the Peyer's patch to the FAE is required for the generation of protective T-cell responses during S. typhimurium infection 82. This recruitment was necessary despite the constitutive presence of a distinct CX3CR1+ DC subset in the FAE. DC also accumulate in the lamina propria under inflammatory conditions. However, the specific factors driving this recruitment remain to be determined.
One other possible route for the generation of protective immunity to pathogens may be the presentation of bacterial antigen by DCs normally resident in the MLNs. In this respect, CD103− MLN DCs have been shown to produce higher levels of pro-inflammatory cytokines than their intestine-derived CD103+ counterparts and drive IFNγ production by T cells 7, 33. Finally, although conditioned DCs do not regain their ability to drive Th1-cell responses following exposure to pathogenic species, it remains unclear whether aspects of their ability to induce tolerogenic responses will be impaired. For example, increased production of pro-inflammatory cytokines such as IL-6 by other cells in the microenvironment could impede the generation of FOXP3+ T cells 83.
Intestinal DCs in inflammatory diseases of the intestine
IBD is thought to be driven by dysregulated relationship between the immune system and the commensal flora that results in a chronic and destructive inflammatory response. Just as DCs have been implicated in maintaining tolerance in the intestine, inappropriate or aberrant DC function may be one factor in the pathogenesis of IBD.
Protective and pathogenic roles of DCs in intestinal inflammation
Several studies have drawn comparisons between the phenotype and function of DCs isolated from normal and inflamed intestinal tissue. For example, in Crohn's disease, gut DCs have been shown to express higher levels of TLR2 and TLR4 and produce more IL-6 and IL-12 84. Although these changes could be secondary to the ongoing inflammatory response, these results also raise the possibility that changes in DC function may directly contribute to the pathogenesis of IBD. Further support for this hypothesis comes from a T-cell-independent model of colitis in which direct activation of DCs through CD40 leads to the development of intestinal inflammation 85.
More recently, mice expressing the diphtheria toxin receptor (DTR) under the control of the CD11c promoter have been used to selectively deplete DCs to study their role in the development of intestinal inflammation. Using this system, DC ablation was shown to ameliorate dextran-sulphate sodium salt (DSS)-induced colitis 86. However, if TLR9 ligands were administered prior to colitis induction, DC ablation actually exacerbated disease 87. The authors propose that in the control mice activation of DCs with TLR9 ligands led to production of IFNβ, which in turn inhibited the production of pro-inflammatory cytokines by macrophages. Consequently, ablation of the DCs reversed this inhibitory effect. However, since pathology in this model of colitis is driven by a breach in the integrity of the epithelial cell layer, the protective effect of the DC may also have been mediated through stimulating repair of the epithelial cell layer, rather than modulation of the immune response. The role of intestinal APC in a spontaneous model of colitis has also been investigated. Here, depletion of CD11b+ cells ameliorated colitis in IL-10-deficient mice 88. This result is consistent with the idea that a failure to properly condition APCs in the intestines of IL-10-deficient mice results in a propensity for them to contribute to destructive inflammatory responses. Although the effects seen in this study were thought to relate to the depletion of macrophages, it will be interesting to determine if specific depletion of DC has a similar effect. In addition, it is possible that the impaired production of TSLP by IECs observed in Crohn's disease may alter DC conditioning and predispose them to the development of intestinal inflammation 68. Overall, it can be seen that the way in which a DC is activated or conditioned changes whether it plays a protective or pro-inflammatory role in intestinal inflammation.
Relationship between IBD susceptibility genes and DC function
Susceptibility to Crohn's disease has been associated with mutations in the gene encoding the cytosolic PRR, nucleotide-binding oligomerization domain 2 (NOD2) 89. NOD2 recognises muramy dipeptide (MDP), a component of bacterial peptidoglycan, and is expressed by DC, macrophages and paneth cells. However, neither the mechanism by which NOD2 mutations predispose to Crohn's disease, nor the cell type these mutations primarily effect are currently known. Nevertheless, there is now clear evidence to suggest that NOD2 mutations influence DC and macrophage function, raising the possibility that chronic intestinal inflammation may result from aberrant responses of DC or macrophages to the intestinal flora.
Ligand binding by NOD proteins leads to activation of NFκB and the production of pro-inflammatory cytokines. Mononuclear cells or macrophages taken from patients with a Crohn's associated NOD2 mutation show impaired NFκB activation and cytokine production following stimulation with MDP, indicating that chronic intestinal inflammation may be associated with the loss of NOD2 function 90 91. Signalling through NOD2 has also been shown to enhance the cytokine response to TLR ligation 92 93 94. In a recent study, stimulation of NOD2 in DCs enhanced the TLR-mediated induction of IL-23 and IL-1, generating DCs that can promote IL-17 production by T cells 95. However, DCs from Crohn's disease patients with NOD2 mutations lacked this IL-17 inducing capacity, which was again indicative of a loss of NOD2 function. One possible explanation for these findings is that impaired responsiveness to the commensal flora leads to a loss of immune homeostasis, a higher bacterial load and ultimately to the development of intestinal inflammation. Consistent with this, the IL-23–Th17-cell axis has been implicated in the generation of protective immunity to extracellular bacterial infection, and both IL-23+ DCs and Th17 cells can be found in the lamina propria of normal mice 81, 96-98. Furthermore, NOD2-deficient mice, in which macrophages also show impaired NF-κB activation in response to MDP, are susceptible to oral bacterial infection 99.
Conversely, other studies have demonstrated that NOD2 signalling can inhibit TLR2-mediated NF-κB activation and cytokine production in murine macrophages and DCs 100, 101. Furthermore, pre-treatment of human monocyte derived DCs with MDP inhibited cytokine responses to ligands for a variety of different TLR 100. Of particular interest was a reduction in IL-12p40 production. According to this hypothesis, the impaired responsiveness to MDP observed in monocytes from patients with Crohn's associated NOD2 mutations would actually result in exacerbated TLR driven responses to the commensal flora, and enhanced production of IL-12p40, driving intestinal inflammation. In support of this, administration of MDP protected mice from experimental colitis driven by damage to the intestinal epithelium and this protection was associated with a reduced responsiveness to multiple TLR ligands 100.
Finally, it remains possible that NOD2 mutations associated with Crohn's disease actually result in enhanced NOD2 signalling in response to MDP. Consistent with this, murine macrophages expressing a truncated form of the NOD2 protein similar to that associated with Crohn's disease actually showed enhanced NF-κB activation and IL-1β production in response to the MDP 102. However, as noted above, this was not observed in human cells harbouring the same mutation. Nevertheless, if correct, this result would also be consistent with the notion that mutations in NOD2 predispose to Crohn's through the generation of exacerbated inflammatory responses to the commensal flora.
IL-23 has been implicated in driving intestinal pathology in several animal models, and variants of the gene encoding the IL-23 receptor have been associated with Crohn's disease 85, 103-106. Therefore, enhanced production of IL-23 by intestinal DCs might be expected to contribute to the pathogenesis of IBD. This could be viewed as somewhat at odds with the idea that impaired generation of Th17 responses by DCs harbouring NOD2 mutations leads to the development of intestinal inflammation. However, it should be noted that it it is unclear whether the pathological role of IL-23 in the intestine is related to the activity of Th17 cells. Indeed, IL-23 has direct pro-inflammatory effects on innate cells and can also suppress Treg differentiation in the intestine 85, 105, 107. Furthermore, Th17 cells themselves may also contribute to the healing process in the intestine through the production of IL-22 108.
Conclusions
Here, we have discussed an integral role for intestinal DCs in shaping the nature of the immune response in the gut. Depending on the population of DC and the environmental conditions, these cells are capable of mounting distinct but appropriate immune responses to commensal and pathogenic microbial species, ultimately resulting in the protection of intestinal tissue from damage. In recent years the molecular pathways involved in both the conditioning and functional properties of DCs in the steady state have begun to be resolved. In particular, retinoic acid seems to be integral to several distinct functional properties ascribed to intestinal DCs. We are also beginning to gain a better understanding of how the aberrant function of DCs may contribute to the pathogenesis of IBD. In the future it will be important to understand how the functional properties of tolerogenic steady state DCs change under inflammatory conditions, and whether this process is similar in infection and in IBD. In addition, a better understanding of the importance of the generation of Th1 or Th17 responses to the commensal flora for intestinal immune homeostasis is required. In particular, what are the features of the DC that perform this function, and are they related to those mediating protective immunity to pathogens? In the future, it is hoped that these pathways can be manipulated not only for the prevention of intestinal inflammation, but for the development of better oral vaccination strategies.
References
- 1.Iwasaki A. Mucosal dendritic cells. Annu Rev Immunol. 2007;25:381–418. doi: 10.1146/annurev.immunol.25.022106.141634. [DOI] [PubMed] [Google Scholar]
- 2.Smith PD, Ochsenbauer-Jambor C, Smythies LE. Intestinal macrophages: unique effector cells of the innate immune system. Immunol Rev. 2005;206:149–159. doi: 10.1111/j.0105-2896.2005.00288.x. [DOI] [PubMed] [Google Scholar]
- 3.Johansson C, Kelsall BL. Phenotype and function of intestinal dendritic cells. Semin Immunol. 2005;17:284–294. doi: 10.1016/j.smim.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 4.Iwasaki A, Kelsall BL. Freshly isolated Peyer's patch, but not spleen, dendritic cells produce interleukin 10 and induce the differentiation of T helper type 2 cells. J Exp Med. 1999;190:229–239. doi: 10.1084/jem.190.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chirdo FG, Millington OR, Beacock-Sharp H, Mowat AM. Immunomodulatory dendritic cells in intestinal lamina propria. Eur J Immunol. 2005;35:1831–1840. doi: 10.1002/eji.200425882. [DOI] [PubMed] [Google Scholar]
- 6.Iwasaki A, Kelsall BL. Unique functions of CD11b+, CD8 alpha+, and double-negative Peyer's patch dendritic cells. J Immunol. 2001;166:4884–4890. doi: 10.4049/jimmunol.166.8.4884. [DOI] [PubMed] [Google Scholar]
- 7.Annacker O, et al. Essential role for CD103 in the T cell-mediated regulation of experimental colitis. J Exp Med. 2005;202:1051–1061. doi: 10.1084/jem.20040662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iwasaki A, Kelsall BL. Localization of distinct Peyer's patch dendritic cell subsets and their recruitment by chemokines macrophage inflammatory protein (MIP)-3alpha, MIP-3beta, and secondary lymphoid organ chemokine. J Exp Med. 2000;191:1381–1394. doi: 10.1084/jem.191.8.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smythies LE, et al. Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J Clin Invest. 2005;115:66–75. doi: 10.1172/JCI19229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat Immunol. 2007;8:1086–1094. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- 11.Naik SH, et al. Intrasplenic steady-state dendritic cell precursors that are distinct from monocytes. Nat Immunol. 2006;7:663–671. doi: 10.1038/ni1340. [DOI] [PubMed] [Google Scholar]
- 12.Wilson NS, et al. Most lymphoid organ dendritic cell types are phenotypically and functionally immature. Blood. 2003;102:2187–2194. doi: 10.1182/blood-2003-02-0513. [DOI] [PubMed] [Google Scholar]
- 13.Fogg DK, et al. A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science. 2006;311:83–87. doi: 10.1126/science.1117729. [DOI] [PubMed] [Google Scholar]
- 14.Varol C, et al. Monocytes give rise to mucosal, but not splenic, conventional dendritic cells. J Exp Med. 2007;204:171–180. doi: 10.1084/jem.20061011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 16.Yrlid U, Jenkins CD, MacPherson GG. Relationships between distinct blood monocyte subsets and migrating intestinal lymph dendritic cells in vivo under steady-state conditions. J Immunol. 2006;176:4155–4162. doi: 10.4049/jimmunol.176.7.4155. [DOI] [PubMed] [Google Scholar]
- 17.Yoshida M, et al. Human neonatal Fc receptor mediates transport of IgG into luminal secretions for delivery of antigens to mucosal dendritic cells. Immunity. 2004;20:769–783. doi: 10.1016/j.immuni.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 18.Jang MH, et al. Intestinal villous M cells: an antigen entry site in the mucosal epithelium. Proc Natl Acad Sci U S A. 2004;101:6110–6115. doi: 10.1073/pnas.0400969101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rescigno M, et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2:361–367. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- 20.Chieppa M, Rescigno M, Huang AY, Germain RN. Dynamic imaging of dendritic cell extension into the small bowel lumen in response to epithelial cell TLR engagement. J Exp Med. 2006;203:2841–2852. doi: 10.1084/jem.20061884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niess JH, et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307:254–258. doi: 10.1126/science.1102901. [DOI] [PubMed] [Google Scholar]
- 22.Turnbull EL, Yrlid U, Jenkins CD, Macpherson GG. Intestinal dendritic cell subsets: differential effects of systemic TLR4 stimulation on migratory fate and activation in vivo. J Immunol. 2005;174:1374–1384. doi: 10.4049/jimmunol.174.3.1374. [DOI] [PubMed] [Google Scholar]
- 23.Wilson NS, et al. Normal proportion and expression of maturation markers in migratory dendritic cells in the absence of germs or Toll-like receptor signaling. Immunol Cell Biol. 2008;86:200–205. doi: 10.1038/sj.icb.7100125. [DOI] [PubMed] [Google Scholar]
- 24.Macpherson AJ, Uhr T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science. 2004;303:1662–1665. doi: 10.1126/science.1091334. [DOI] [PubMed] [Google Scholar]
- 25.Huang FP, et al. A discrete subpopulation of dendritic cells transports apoptotic intestinal epithelial cells to T cell areas of mesenteric lymph nodes. J Exp Med. 2000;191:435–444. doi: 10.1084/jem.191.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Worbs T, et al. Oral tolerance originates in the intestinal immune system and relies on antigen carriage by dendritic cells. J Exp Med. 2006;203:519–527. doi: 10.1084/jem.20052016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang A, et al. Disruption of E-cadherin-mediated adhesion induces a functionally distinct pathway of dendritic cell maturation. Immunity. 2007;27:610–624. doi: 10.1016/j.immuni.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wendland M, et al. CCR9 is a homing receptor for plasmacytoid dendritic cells to the small intestine. Proc Natl Acad Sci U S A. 2007;104:6347–6352. doi: 10.1073/pnas.0609180104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yrlid U, et al. Regulation of intestinal dendritic cell migration and activation by plasmacytoid dendritic cells, TNF-alpha and type 1 IFNs after feeding a TLR7/8 ligand. J Immunol. 2006;176:5205–5212. doi: 10.4049/jimmunol.176.9.5205. [DOI] [PubMed] [Google Scholar]
- 30.Sun CM, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mucida D, et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 32.Yamazaki S, et al. Dendritic cells are specialized accessory cells along with TGF-for the differentiation of Foxp3+ CD4+ regulatory T cells from peripheral Foxp3 precursors. Blood. 2007;110:4293–4302. doi: 10.1182/blood-2007-05-088831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coombes JL, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kang SG, Lim HW, Andrisani OM, Broxmeyer HE, Kim CH. Vitamin A Metabolites Induce Gut-Homing FoxP3+ Regulatory T Cells. J Immunol. 2007;179:3724–3733. doi: 10.4049/jimmunol.179.6.3724. [DOI] [PubMed] [Google Scholar]
- 35.Johansson-Lindbom B, et al. Functional specialization of gut CD103+ dendritic cells in the regulation of tissue-selective T cell homing. J Exp Med. 2005;202:1063–1073. doi: 10.1084/jem.20051100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Travis MA, et al. Loss of integrin alpha(v)beta8 on dendritic cells causes autoimmunity and colitis in mice. Nature. 2007;449:361–365. doi: 10.1038/nature06110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lacy-Hulbert A, et al. Ulcerative colitis and autoimmunity induced by loss of myeloid alphav integrins. Proc Natl Acad Sci U S A. 2007;104:15823–15828. doi: 10.1073/pnas.0707421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fahlen L, et al. T cells that cannot respond to TGF-beta escape control by CD4(+)CD25(+) regulatory T cells. J Exp Med. 2005;201:737–746. doi: 10.1084/jem.20040685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stagg AJ, Kamm MA, Knight SC. Intestinal dendritic cells increase T cell expression of alpha4beta7 integrin. Eur J Immunol. 2002;32:1445–1454. doi: 10.1002/1521-4141(200205)32:5<1445::AID-IMMU1445>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 40.Johansson-Lindbom B, et al. Selective generation of gut tropic T cells in gut-associated lymphoid tissue (GALT): requirement for GALT dendritic cells and adjuvant. J Exp Med. 2003;198:963–969. doi: 10.1084/jem.20031244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mora JR, et al. Selective imprinting of gut-homing T cells by Peyer's patch dendritic cells. Nature. 2003;424:88–93. doi: 10.1038/nature01726. [DOI] [PubMed] [Google Scholar]
- 42.Briskin M, et al. Human mucosal addressin cell adhesion molecule-1 is preferentially expressed in intestinal tract and associated lymphoid tissue. Am J Pathol. 1997;151:97–110. [PMC free article] [PubMed] [Google Scholar]
- 43.Kunkel EJ, et al. Lymphocyte CC chemokine receptor 9 and epithelial thymus-expressed chemokine (TECK) expression distinguish the small intestinal immune compartment: Epithelial expression of tissue-specific chemokines as an organizing principle in regional immunity. J Exp Med. 2000;192:761–768. doi: 10.1084/jem.192.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Siewert C, et al. Induction of organ-selective CD4+ regulatory T cell homing. Eur J Immunol. 2007;37:978–989. doi: 10.1002/eji.200636575. [DOI] [PubMed] [Google Scholar]
- 45.Macpherson AJ, Slack E. The functional interactions of commensal bacteria with intestinal secretory IgA. Curr Opin Gastroenterol. 2007;23:673–678. doi: 10.1097/MOG.0b013e3282f0d012. [DOI] [PubMed] [Google Scholar]
- 46.Martinoli C, Chiavelli A, Rescigno M. Entry route of Salmonella typhimurium directs the type of induced immune response. Immunity. 2007;27:975–984. doi: 10.1016/j.immuni.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 47.Sato A, et al. CD11b+ Peyer's patch dendritic cells secrete IL-6 and induce IgA secretion from naive B cells. J Immunol. 2003;171:3684–3690. doi: 10.4049/jimmunol.171.7.3684. [DOI] [PubMed] [Google Scholar]
- 48.He B, et al. Intestinal bacteria trigger T cell-independent immunoglobulin A(2) class switching by inducing epithelial-cell secretion of the cytokine APRIL. Immunity. 2007;26:812–826. doi: 10.1016/j.immuni.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 49.Cerutti A. The regulation of IgA class switching. Nat Rev Immunol. 2008 doi: 10.1038/nri2322. In the Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Benson MJ, Pino-Lagos K, Rosemblatt M, Noelle RJ. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J Exp Med. 2007;204:1765–1774. doi: 10.1084/jem.20070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schambach F, Schupp M, Lazar MA, Reiner SL. Activation of retinoic acid receptor-alpha favours regulatory T cell induction at the expense of IL-17-secreting T helper cell differentiation. Eur J Immunol. 2007;37:2396–2399. doi: 10.1002/eji.200737621. [DOI] [PubMed] [Google Scholar]
- 52.Iwata M, et al. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–538. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 53.Mora JR, et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314:1157–1160. doi: 10.1126/science.1132742. [DOI] [PubMed] [Google Scholar]
- 54.McDermott MR, et al. Impaired intestinal localization of mesenteric lymphoblasts associated with vitamin A deficiency and protein-calorie malnutrition. Immunology. 1982;45:1–5. [PMC free article] [PubMed] [Google Scholar]
- 55.Stephensen CB. Vitamin A, infection, and immune function. Annu Rev Nutr. 2001;21:167–192. doi: 10.1146/annurev.nutr.21.1.167. [DOI] [PubMed] [Google Scholar]
- 56.Elias KM, et al. Retinoic acid inhibits Th17 polarization and enhances FoxP3 expression through a Stat-3/Stat-5 independent signaling pathway. Blood. 2008;111:1013–1020. doi: 10.1182/blood-2007-06-096438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Blomhoff R, Blomhoff HK. Overview of retinoid metabolism and function. J Neurobiol. 2006;66:606–630. doi: 10.1002/neu.20242. [DOI] [PubMed] [Google Scholar]
- 58.Saurer L, McCullough KC, Summerfield A. In vitro induction of mucosa-type dendritic cells by all-trans retinoic acid. J Immunol. 2007;179:3504–3514. doi: 10.4049/jimmunol.179.6.3504. [DOI] [PubMed] [Google Scholar]
- 59.Lampen A, Meyer S, Arnhold T, Nau H. Metabolism of vitamin A and its active metabolite all-trans-retinoic acid in small intestinal enterocytes. J Pharmacol Exp Ther. 2000;295:979–985. [PubMed] [Google Scholar]
- 60.Svensson M. e. a. Retinoic acid receptor signaling levels and antigen dose regulate gut homing receptor expression on CD8+ T cells. mucosal immunol. 2008;1:38–48. doi: 10.1038/mi.2007.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cantorna MT, Nashold FE, Hayes CE. In vitamin A deficiency multiple mechanisms establish a regulatory T helper cell imbalance with excess Th1 and insufficient Th2 function. J Immunol. 1994;152:1515–1522. [PubMed] [Google Scholar]
- 62.Sidell N, Chang B, Bhatti L. Upregulation by retinoic acid of interleukin-2-receptor mRNA in human T lymphocytes. Cell Immunol. 1993;146:28–37. doi: 10.1006/cimm.1993.1003. [DOI] [PubMed] [Google Scholar]
- 63.Rincon M, Flavell RA. AP-1 transcriptional activity requires both T-cell receptor-mediated and co-stimulatory signals in primary T lymphocytes. Embo J. 1994;13:4370–4381. doi: 10.1002/j.1460-2075.1994.tb06757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu Y, et al. FOXP3 controls regulatory T cell function through cooperation with NFAT. Cell. 2006;126:375–387. doi: 10.1016/j.cell.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 65.Schule R, et al. Retinoic acid is a negative regulator of AP-1-responsive genes. Proc Natl Acad Sci U S A. 1991;88:6092–6096. doi: 10.1073/pnas.88.14.6092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fantini MC, et al. Cutting edge: TGF-beta induces a regulatory phenotype in CD4+CD25− T cells through Foxp3 induction and down-regulation of Smad7. J Immunol. 2004;172:5149–5153. doi: 10.4049/jimmunol.172.9.5149. [DOI] [PubMed] [Google Scholar]
- 67.Artis D. Epithelial-cell recognition of commensal flora and maintenance of immune homeostasis in the gut. Nat Rev Immunol. 2008 doi: 10.1038/nri2316. In the Press. [DOI] [PubMed] [Google Scholar]
- 68.Rimoldi M, et al. Intestinal immune homeostasis is regulated by the crosstalk between epithelial cells and dendritic cells. Nat Immunol. 2005;6:507–514. doi: 10.1038/ni1192. [DOI] [PubMed] [Google Scholar]
- 69.Zaph C, et al. Epithelial-cell-intrinsic IKK-beta expression regulates intestinal immune homeostasis. Nature. 2007;446:552–556. doi: 10.1038/nature05590. [DOI] [PubMed] [Google Scholar]
- 70.Watanabe N, et al. Hassall's corpuscles instruct dendritic cells to induce CD4+CD25+ regulatory T cells in human thymus. Nature. 2005;436:1181–1185. doi: 10.1038/nature03886. [DOI] [PubMed] [Google Scholar]
- 71.Szatmari I, et al. PPARgamma controls CD1d expression by turning on retinoic acid synthesis in developing human dendritic cells. J Exp Med. 2006;203:2351–2362. doi: 10.1084/jem.20060141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kobayashi M, et al. Toll-like receptor-dependent production of IL-12p40 causes chronic enterocolitis in myeloid cell-specific Stat3-deficient mice. J Clin Invest. 2003;111:1297–1308. doi: 10.1172/JCI17085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Takeda K, et al. Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity. 1999;10:39–49. doi: 10.1016/s1074-7613(00)80005-9. [DOI] [PubMed] [Google Scholar]
- 74.Butler M, et al. Modulation of dendritic cell phenotype and function in an in vitro model of the intestinal epithelium. Eur J Immunol. 2006;36:864–874. doi: 10.1002/eji.200535497. [DOI] [PubMed] [Google Scholar]
- 75.Rakoff-Nahoum S, Hao L, Medzhitov R. Role of toll-like receptors in spontaneous commensal-dependent colitis. Immunity. 2006;25:319–329. doi: 10.1016/j.immuni.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 76.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 77.Contractor N, Louten J, Kim L, Biron CA, Kelsall BL. Cutting edge: Peyer's patch plasmacytoid dendritic cells (pDCs) produce low levels of type I interferons: possible role for IL-10, TGFbeta, and prostaglandin E2 in conditioning a unique mucosal pDC phenotype. J Immunol. 2007;179:2690–2694. doi: 10.4049/jimmunol.179.5.2690. [DOI] [PubMed] [Google Scholar]
- 78.Rimoldi M, et al. Monocyte-derived dendritic cells activated by bacteria or by bacteria-stimulated epithelial cells are functionally different. Blood. 2005;106:2818–2826. doi: 10.1182/blood-2004-11-4321. [DOI] [PubMed] [Google Scholar]
- 79.Sierro F, et al. Flagellin stimulation of intestinal epithelial cells triggers CCL20-mediated migration of dendritic cells. Proc Natl Acad Sci U S A. 2001;98:13722–13727. doi: 10.1073/pnas.241308598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nenci A, et al. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature. 2007;446:557–561. doi: 10.1038/nature05698. [DOI] [PubMed] [Google Scholar]
- 81.Becker C, et al. Constitutive p40 promoter activation and IL-23 production in the terminal ileum mediated by dendritic cells. J Clin Invest. 2003;112:693–706. doi: 10.1172/JCI17464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Salazar-Gonzalez RM, et al. CCR6-mediated dendritic cell activation of pathogen-specific T cells in Peyer's patches. Immunity. 2006;24:623–632. doi: 10.1016/j.immuni.2006.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bettelli E, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 84.Hart AL, et al. Characteristics of intestinal dendritic cells in inflammatory bowel diseases. Gastroenterology. 2005;129:50–65. doi: 10.1053/j.gastro.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 85.Uhlig HH, et al. Differential activity of IL-12 and IL-23 in mucosal and systemic innate immune pathology. Immunity. 2006;25:309–318. doi: 10.1016/j.immuni.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 86.Berndt BE, Zhang M, Chen GH, Huffnagle GB, Kao JY. The role of dendritic cells in the development of acute dextran sulfate sodium colitis. J Immunol. 2007;179:6255–6262. doi: 10.4049/jimmunol.179.9.6255. [DOI] [PubMed] [Google Scholar]
- 87.Abe K, et al. Conventional dendritic cells regulate the outcome of colonic inflammation independently of T cells. Proc Natl Acad Sci U S A. 2007;104:17022–17027. doi: 10.1073/pnas.0708469104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Watanabe N, et al. Elimination of local macrophages in intestine prevents chronic colitis in interleukin-10-deficient mice. Dig Dis Sci. 2003;48:408–414. doi: 10.1023/a:1021960401290. [DOI] [PubMed] [Google Scholar]
- 89.Strober W, Murray PJ, Kitani A, Watanabe T. Signalling pathways and molecular interactions of NOD1 and NOD2. Nat Rev Immunol. 2006;6:9–20. doi: 10.1038/nri1747. [DOI] [PubMed] [Google Scholar]
- 90.Inohara N, et al. Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn's disease. J Biol Chem. 2003;278:5509–5512. doi: 10.1074/jbc.C200673200. [DOI] [PubMed] [Google Scholar]
- 91.Li J, et al. Regulation of IL-8 and IL-1{beta} expression in Crohn's disease associated NOD2/CARD15 mutations 10.1093/hmg/ddh182. Hum. Mol. Genet. 2004;13:1715–1725. doi: 10.1093/hmg/ddh182. [DOI] [PubMed] [Google Scholar]
- 92.Uehara A, et al. Muramyldipeptide and diaminopimelic acid-containing desmuramylpeptides in combination with chemically synthesized Toll-like receptor agonists synergistically induced production of interleukin-8 in a NOD2- and NOD1-dependent manner, respectively, in human monocytic cells in culture. Cell Microbiol. 2005;7:53–61. doi: 10.1111/j.1462-5822.2004.00433.x. [DOI] [PubMed] [Google Scholar]
- 93.Netea MG, et al. Nucleotide-binding oligomerization domain-2 modulates specific TLR pathways for the induction of cytokine release. J Immunol. 2005;174:6518–6523. doi: 10.4049/jimmunol.174.10.6518. [DOI] [PubMed] [Google Scholar]
- 94.van Heel DA, et al. Muramyl dipeptide and toll-like receptor sensitivity in NOD2-associated Crohn's disease. Lancet. 2005;365:1794–1796. doi: 10.1016/S0140-6736(05)66582-8. [DOI] [PubMed] [Google Scholar]
- 95.van Beelen AJ, et al. Stimulation of the intracellular bacterial sensor NOD2 programs dendritic cells to promote interleukin-17 production in human memory T cells. Immunity. 2007;27:660–669. doi: 10.1016/j.immuni.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 96.Happel KI, et al. Divergent roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae. J Exp Med. 2005;202:761–769. doi: 10.1084/jem.20050193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ivanov, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 98.Mangan PR, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 99.Kobayashi KS, et al. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005;307:731–734. doi: 10.1126/science.1104911. [DOI] [PubMed] [Google Scholar]
- 100.Watanabe T, et al. Muramyl dipeptide activation of nucleotide-binding oligomerization domain 2 protects mice from experimental colitis. J Clin Invest. 2008;118:545–559. doi: 10.1172/JCI33145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Watanabe T, Kitani A, Murray PJ, Strober W. NOD2 is a negative regulator of Toll-like receptor 2-mediated T helper type 1 responses. Nat Immunol. 2004;5:800–808. doi: 10.1038/ni1092. [DOI] [PubMed] [Google Scholar]
- 102.Maeda S, et al. Nod2 mutation in Crohn's disease potentiates NF-kappaB activity and IL-1beta processing. Science. 2005;307:734–738. doi: 10.1126/science.1103685. [DOI] [PubMed] [Google Scholar]
- 103.Duerr RH, et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314:1461–1463. doi: 10.1126/science.1135245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yen D, et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116:1310–1316. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hue S, et al. Interleukin-23 drives innate and T cell-mediated intestinal inflammation. J Exp Med. 2006;203:2473–2483. doi: 10.1084/jem.20061099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kullberg MC, et al. IL-23 plays a key role in Helicobacter hepaticus-induced T cell-dependent colitis. J Exp Med. 2006;203:2485–2494. doi: 10.1084/jem.20061082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Izcue A, et al. Interleukin-23 restrains regulatory T cell activity to drive T cell-dependent colitis. Immunity. 2008;28:559–570. doi: 10.1016/j.immuni.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sugimoto K, et al. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J Clin Invest. 2008;118:534–544. doi: 10.1172/JCI33194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jang MH, et al. CCR7 is critically important for migration of dendritic cells in intestinal lamina propria to mesenteric lymph nodes. J Immunol. 2006;176:803–810. doi: 10.4049/jimmunol.176.2.803. [DOI] [PubMed] [Google Scholar]
- 110.Karlis J, et al. Characterization of colonic and mesenteric lymph node dendritic cell subpopulations in a murine adoptive transfer model of inflammatory bowel disease. Inflamm Bowel Dis. 2004;10:834–847. doi: 10.1097/00054725-200411000-00018. [DOI] [PubMed] [Google Scholar]
- 111.Liu LM, MacPherson GG. Rat intestinal dendritic cells: immunostimulatory potency and phenotypic characterization. Immunology. 1995;85:88–93. [PMC free article] [PubMed] [Google Scholar]
- 112.Kilshaw PJ. Expression of the mucosal T cell integrin alpha M290 beta 7 by a major subpopulation of dendritic cells in mice. Eur J Immunol. 1993;23:3365–3368. doi: 10.1002/eji.1830231246. [DOI] [PubMed] [Google Scholar]
- 113.Brenan M, Puklavec M. The MRC OX-62 antigen: a useful marker in the purification of rat veiled cells with the biochemical properties of an integrin. J Exp Med. 1992;175:1457–1465. doi: 10.1084/jem.175.6.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bilsborough J, George TC, Norment A, Viney JL. Mucosal CD8alpha+ DC, with a plasmacytoid phenotype, induce differentiation and support function of T cells with regulatory properties. Immunology. 2003;108:481–492. doi: 10.1046/j.1365-2567.2003.01606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Asselin-Paturel C, Brizard G, Pin JJ, Briere F, Trinchieri G. Mouse strain differences in plasmacytoid dendritic cell frequency and function revealed by a novel monoclonal antibody. J Immunol. 2003;171:6466–6477. doi: 10.4049/jimmunol.171.12.6466. [DOI] [PubMed] [Google Scholar]
- 116.Yrlid U, et al. Plasmacytoid dendritic cells do not migrate in intestinal or hepatic lymph. J Immunol. 2006;177:6115–6121. doi: 10.4049/jimmunol.177.9.6115. [DOI] [PubMed] [Google Scholar]
- 117.Balmer JE, Blomhoff R. Gene expression regulation by retinoic acid. J Lipid Res. 2002;43:1773–1808. doi: 10.1194/jlr.r100015-jlr200. [DOI] [PubMed] [Google Scholar]
- 118.La P, et al. Fusion proteins of retinoid receptors antagonize TGF-beta-induced growth inhibition of lung epithelial cells. Oncogene. 2003;22:198–210. doi: 10.1038/sj.onc.1206100. [DOI] [PubMed] [Google Scholar]
- 119.Pendaries V, Verrecchia F, Michel S, Mauviel A. Retinoic acid receptors interfere with the TGF-beta/Smad signaling pathway in a ligand-specific manner. Oncogene. 2003;22:8212–8220. doi: 10.1038/sj.onc.1206913. [DOI] [PubMed] [Google Scholar]