Abstract
A rapid technique for multiple organ harvesting is described which allows removal of all the major organs within 30 to 60 minutes after beginning the donor operation. No preliminary dissection of the liver or kidneys is required or necessary since these organs are cooled by infusion of cold solutions in situ and with subsequent rapid dissection in a bloodless field. The incidence of well functioning kidneys, livers and hearts has been better than with the previous methods. The acceptance of this procedure by other personnel has been almost universal.
A method of multiple organ procurement was previously described in which the kidneys, liver, heart or heart and lungs or various combinations of these organs could be removed without jeopardizing any of the individual organs (1). Preliminary dissection of the great vessels of the abdomen and chest was carried out so that the organs to be removed could be core cooled in situ with cold intra-aortic and intraportal infusates once the aorta had been cross clamped at pre-planned levels.
Although the technique has been adopted as a worldwide standard, it has certain disadvantages when the liver is one of the grafts or when the kidney team insists upon extensive preparatory activities. Dissection of the hepatic hilar structures tends to be time consuming, particularly if an inexperienced surgeon is at work. During this dissection, unintentional occlusion of the portal vein or hepatic artery can occur intermittently. Finally, a deterrent to collaboration among different transplantation teams has been the waiting period imposed on the cardiac surgeons during the preparatory maneuvers of surgeons specializing in operations upon the kidney or liver.
We report herein a simplified modification of the original procurement procedure. This method entails virtually no preliminary dissection of the kidneys or liver. A primitive version of this new method was first used for unstable donors (2) and later refined to its present elective form. The preparatory steps necessary for the hepatic and renal transplant teams require no more than 15 or 20 minutes. Quality of organs and degree of acceptance of the method by the hospitals and collaborating teams involved were assessed.
METHODS
Material and follow-up period
The new technique was used in 48 consecutive multiple organ procurements between 16 April 1986 and 3 July 1986. For hearts and livers, the patient survival and early adequate graft function were considered to be synonymous. For kidneys, the incidence of inadequate function was defined as the necessity for dialysis in the first postoperative week. A poll was taken in each instance to determine the degree of acceptance of the so-called “rapid technique” by the operating room personnel in the various hospitals, the transplant coordinators and the collaborating surgeons from local or distant institutions.
Surgical technique
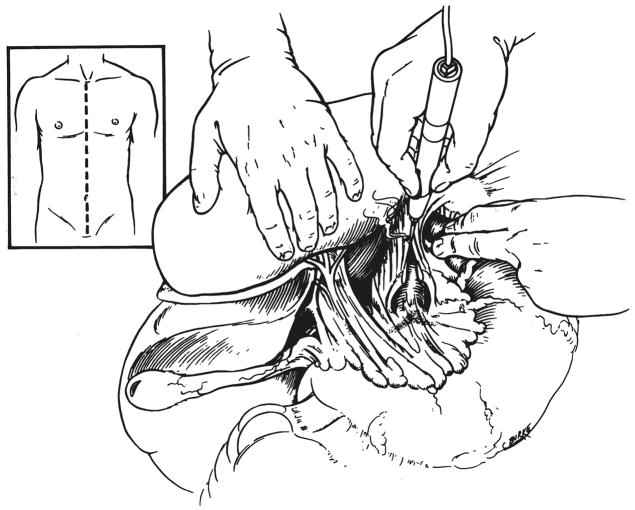
The principles of the new procedure are the same as with the previous technique (1). A complete midline incision is made from the suprasternal notch to the pubis (Fig. 1, insert). The gallbladder is incised and washed free of bile to prevent later autolysis. The left triangular ligament of the liver is incised, the esophagus is held to the left with a retracting finger and a longitudinal incision is made in the diaphragmatic crura between the retrohepatic inferior vena cava and the esophagus (Fig. 1). The aorta is encircled with a tape. Intercostal or lumbar branches are ordinarily not encountered in this location.
Fig. 1.
Incision (inset) and encirclement of aorta at the diaphragm.
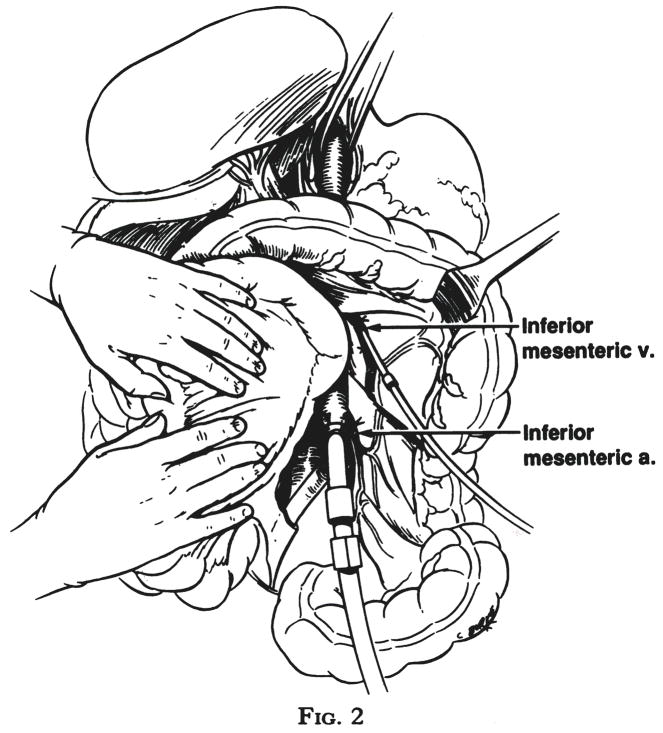
Turning more distally, the inferior mesenteric artery is ligated and divided and the aorta is encircled with a tape at the same level. The final preparatory step is isolation, ligation and cannulation of the inferior mesenteric vein (Fig. 2). The cannula is advanced superiorly for approximately 5 centimeters in adults and for lesser distances in children, so that the tip is in or just entering the portal vein.
Fig. 2.
Insertion of catheter into inferior mesenteric vein and cannulation of the distal aorta after ligation of the inferior mesenteric artery.
These preparatory maneuvers require no more than ten or 15 minutes. No further dissection is necessary or desirable for removal of either the kidneys or liver. The cardiac team is then requested to proceed as expeditiously as possible. A good cardiac team can be ready to remove the heart in an additional five or ten minutes using a variety of core cooling techniques as described previously in principle (1).
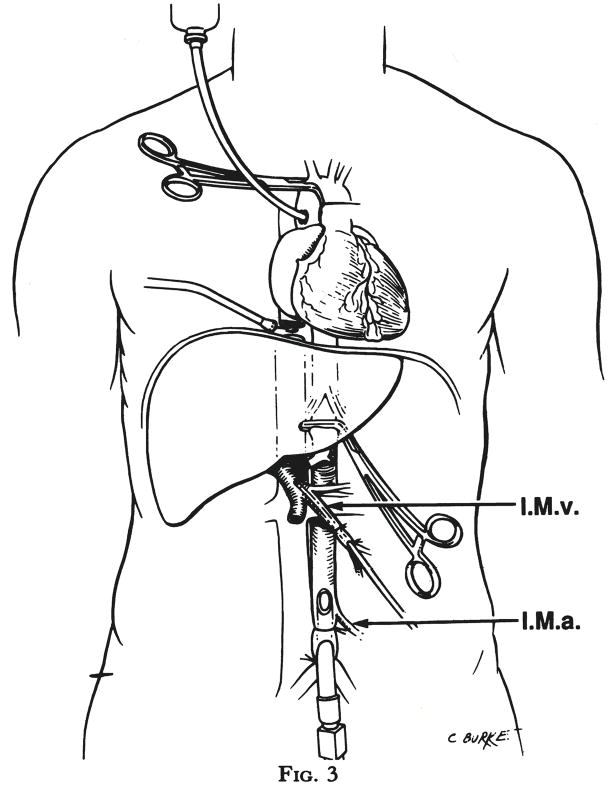
As soon as the cardiac team has discontinued effective circulation by occluding the vena caval inflow or by cross clamping the aorta, the previously encircled aorta is cross clamped at the diaphragmatic site. Cold infusion is begun through both the inferior mesenteric venous cannula and through the terminal aortic cannula (Fig. 3). Chilled lactated Ringer’s solution is our preference for this double infusion because of its low cost; however, some groups prefer to use one of the potassium rich Collin’s solutions from the outset. An outflow for the infusate is provided by transection of the vena cava at the level of the diaphragm (Fig. 3) or, if the cardiac team insists upon cross clamping or stapling the inferior vena cava within the pericardium, the vena cava near its abdominal bifurcation is cut to allow more distal escape of the fluid. The liver is observed as it blanches, during which time the cardiac team is completing the cardiectomy. The liver is not dissected further until it is palpably cold and free of blood. In adults, this requires 1 or 2 liters of portal infusion plus 2 to 4 liters of aortic infusion. The kidneys can be seen and felt to participate in the cooling process.
Fig. 3.
Cross clamping of aorta at the time of rapid infusion. I.M.v., Inferior mesenteric vein, and I.M.a., inferior mesenteric artery.
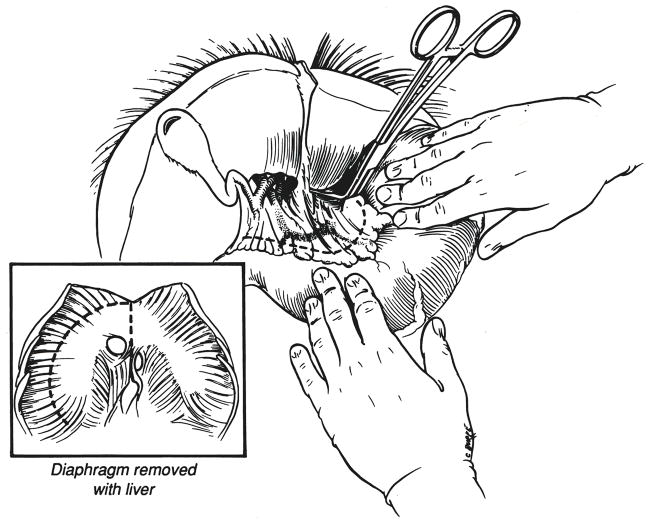
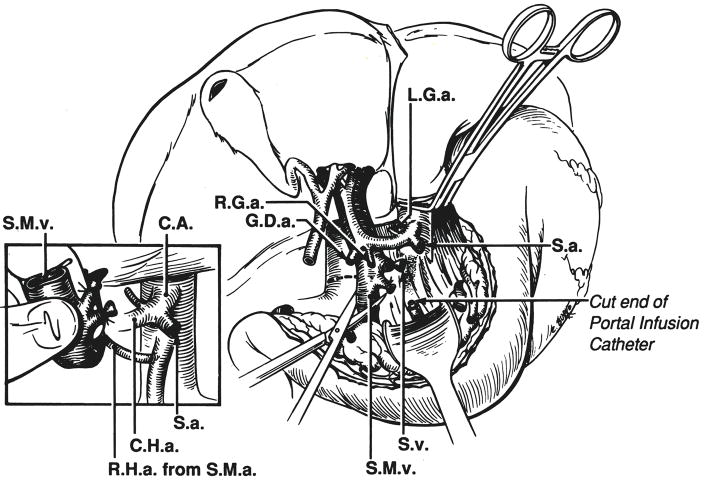
After the liver is cold and the heart has been removed, a patch of diaphragm is removed around the lumen of the suprahepatic inferior vena cava (Fig. 4, insert). Next, dissection of the hepatic hilum is begun in the bloodless field cutting or ligating the gastroduodenal, right gastric, splenic and left gastric arteries as far as possible from the celiac axis and common hepatic artery (Fig. 4). As this is accomplished, the portal vein is uncovered and followed inferiorly to the confluence of the splenic and superior mesenteric veins which are cut (Fig. 5).
Fig. 4.
Dissection in bloodless field of hepatic hilar structures. A rim of diaphragm around the suprahepatic inferior vena cava is excised with the liver (inset) and later dissected off at the back table in the recipient hospital.
Fig. 5.
Completion of hilar transaction. After cutting the superior mesenteric vein and splenic vein, the portal vein is folded superiorly with the finger and an anomalous right hepatic artery is looked for posterior to the portal vein. L.G.a., Left gastric artery; S.a., splenic artery; S.v., splenic vein; S.M.v., superior mesenteric vein; R.H.a., right hepatic artery; S.M..a., superior mesenteric artery; C.H.a., common hepatic artery, and C.A., celiac axis.
The cut portal tributaries are folded superiorly with the finger (Fig. 5, insert) and an examination conducted for an anomalous right hepatic artery passing posterior to the portal vein. If an anomalous right hepatic artery is found, it usually originates from the superior mesenteric artery. In such an instance, a length of superior mesenteric artery is removed with a large Carrel patch from the aorta that also encompasses the origin of the celiac artery (3). Otherwise, the celiac-axis origin is removed with a simple Carrel patch (Fig. 6).
Fig. 6.
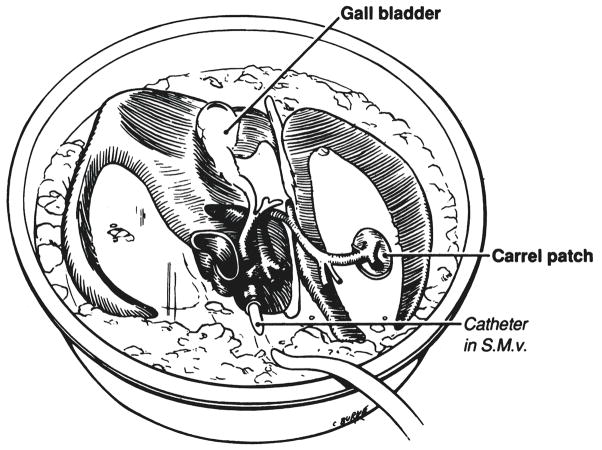
Excised liver in ice basin with a cannula secured in the portal vein, which is used to infuse chilled Collin’s solution. S.M.v., Superior mesenteric vein.
The chilled specimen is removed to an ice basin on the back table and a cannula is inserted into the portal vein (Fig. 6) through which 800 to 1,000 milliliters of cold Collin’s solution is infused for an adult liver and proportionately less for an organ of a child. The liver is packed in an ice chest and brought back to the recipient hospital where it is cleaned and prepared for transplantation at a formal back table procedure that requires about 30 minutes.
The foregoing removal of the cold and bloodless liver requires some 15 to 30 minutes, but during most of this time, effective cold perfusion of the kidneys in situ continues. Once the liver has been removed from the abdomen, both exposed kidneys can be removed en bloc within an additional five or ten minutes, as previously described (1). After their removal and immersion in an ice bath, the kidneys also are reperfused with Collin’s solution individually through the aorta if they have not been separated. Further preservation can then be achieved with cold storage or continuous perfusion. Removal of iliac vascular grafts for possible use in the recipient is then carried out as previously reported (1).
RESULTS
During the development and initial testing of the technique, the abdominal part of all the operations was performed by two of the authors. Because such uniformity of personnel was not a condition of procurements using our older method (1), meaningful comparisons could not be made with our retrospective experience. However, considerable information about the value of the procedure was obtained.
Organ function.—Renal grafts
The 48 donors provided a total of 87 renal grafts, including one pair of kidneys from a child which were transplanted en bloc to a single recipent. In three other very small donors who were children, the kidneys were not removed because a decision had been made not to use them. In two donors, one of the kidneys was congenitally absent, and in one other, a kidney was ruptured by trauma.
Of the 87 renal grafts removed for transplantation, none were discarded. The requirement for dialysis in the first week post-transplantation was 18 per cent (16 of 87) (Table I). The requirement for dialysis in the first week often was in recipients with widely reacting cytotoxic antibodies whose grafts were undergoing an obvious accelerated rejection.
TABLE I.
FUNCTION OF GRAFTS FROM 48 CADAVERIC DONORS
| No. | Per cent | |
|---|---|---|
| Renal grafts | 87 | 100 |
| Immediate function | 71 | 82 |
| Need for dialysis in 1st week | 16 | 18 |
| Liver grafts | 47 | 100 |
| Immediate function | 46 | 98 |
| Primary nonfunction | 1 | 2 |
| Heart grafts | 18 | 100 |
| Primary function | 15 | 84 |
| Perioperative death | 3 | 16 |
Hepatic grafts
The 48 donors provided 47 livers that were engrafted. One liver was not used because the proposed recipient died before transplantation could be performed. Of the 47 hepatic grafts, 46 functioned promptly (Table I). There was only one example of primary nonfunction. The recipient was treated with retransplantation but died subsequently of infection.
Cardiac grafts
One heart was not used because of death of the recipient. Of the other 18 hearts, 15 functioned immediately and supported life for at least the first five postoperative days (Table I). Of the three perioperative deaths, one death was the result of primary cardiac nonfunction and two grafts failed to support life in desperately ill patients with severe pulmonary hypertension.
Procedure acceptance
The majority of operating room nurses, transplant co-ordinators and participating surgeons gave high marks to this so-called rapid approach (Table II). There were many remarks to the effect that what had previously been a tedious and grisly exercise consuming many hours had become almost a minor event. A number of surgeons already have adopted this new technique at their own centers. The most consistent accolades came from the cardiac surgeons who, with the new method, have not been asked to sit around for hours waiting for their time to come at the termination of a complex and lengthy procurement procedure.
TABLE II.
RAPID TECHNIQUE EVALUATION COMPARED WITH OLD METHOD
| Superior | No advantage | Poor | |
|---|---|---|---|
| Local co-ordinator | 40 (83) | 6 (12) | 2 (5) |
| O R. nurses | 38 (81) | 9 (19) | 0 (0) |
| Renal surgeons | 32 (70) | 11 (24) | 3 (6) |
| Cardiac surgeons | 16(84) | 3 (16) | 0 (0) |
O.R., Operating room.
Numbers in parentheses are percentages.
DISCUSSION
The collegiality between collaborating transplantation groups has been greatly improved with the rapid procurement operation discribed in this report. However, the advantages of speed and efficiency would be of little moment, if the quality of the organs were jeopardized by this approach.
There has been no such penalty. The ability to remove the liver and the kidney without any dissection has eliminated those unintentional and often unrecognized periods of ischemia that may occur if individual vessels are skeletonized with conventional meticulous isolation techniques. Immediate function of all kinds of grafts has been superior to that with the previously used technique. Consequently, the new procedure has completely supplanted the old at our center.
Nevertheless, it is self-evident that the rapid technique requires considerable surgical skill as well as anatomic knowledge. Those developing the rapid method had gone through a long apprenticeship with the other technique. However, we now are training our new fellows from the beginning with the rapid operation.
A specific advantage of the new method is the ease with which it can be used for the kind of unstable donors which usually have been discarded in the past. A number of the donors herein reported had unsatisfactory circulation at the time the donor operations were begun, and in two, cardiac arrest occurred just as the donors were being brought into the operating room. The ability to cool the organs within a few minutes and to have all of the grafts excised and cold stored within 30 to 60 minutes has greatly increased the practicality of procurement under these adverse circumstances. The method should be applicable in countries in which brain death laws are not in force.
Acknowledgments
This study was supported by Research Grants from the Veterans Administration and Project Grant No. AM-19961 from the National Institutes of Health, Bethesda, Maryland.
References
- 1.Starzl T, Hakala T, Shaw B, Jr, et al. Flexible procedure for multiple cadaveric organ procurement. Surg Gynecol Obstet. 1984;158:223. [PMC free article] [PubMed] [Google Scholar]
- 2.Starzl TE, Iwatsuki S, Shaw BW, Jr, Gordon RD. Orthotopic liver transplantation in 1984. Transplant Proc. 1985;17:250–258. [Google Scholar]
- 3.Gordon R, Shaw B, Jr, Iwatsuki S, et al. Simplified technique for revascularization of homografts of liver with a variant right hepatic artery from superior mesenteric artery. Surg Gynecol Obstet. 1985;160:474. [PMC free article] [PubMed] [Google Scholar]