Abstract
Background
Immature human blood monocytoid dendritic cells (mDCs) express high affinity receptors for IgE (FcεRI); yet their exact function and regulation remain poorly understood.
Objective
To characterize FcεRI-dependent cytokine responses and their regulation in circulating human blood mDCs.
Methods
FcεRI-dependent cytokine responses of circulating mDCs were studied using anti-FcεRIα stimulation. Plasmacytoid dendritic cell (pDC) cross-regulation via TLR9 on these responses was investigated by examining the effects of exogenous IFN-α pretreatment and by co-culturing pDCs and mDCs stimulated with CpG. Culture supernatants were analyzed by ELISA to determine cytokine levels. Cell markers were determined by flow cytometry.
Results
MDCs express marked levels of FcεRI (nMFI=196±49, n=4). Following FcεRI-dependent activation in mDCs, TNF-α (2189±864 pg/106, n=3) was up-regulated within 4h, whereas, IL-10 (112±47 pg/106, n=3) was detectable only after 24h incubation. Adding IL-10 neutralizing Ab, TNF-α FcεRI-dependent responses were significantly augmented (3903±197 pg/106 mDCs, p< 0.01, n=3). Conversely, recombinant IL-10 dose-dependently inhibited FcεRI-mediated TNF-α responses up to 86±3%, (n=3, p< 0.001). Pretreatment of mDCs with IFN-α (100U/mL) enhanced FcεRI-dependent secretion of IL-10 by 3.2-fold (183±11 pg/106 mDCs, n=4) as compared with untreated cells (57±33 pg/106 mDCs, p<0.001, n=4). In pDC/mDC co-cultures pretreated with CpG, FcεRI-dependent IL-10 secretion by mDCs was similarly augmented by 3-fold.
Conclusions
Autocrine secretion of IL-10, a critical auto-regulator of FcεRI-dependent proinflammatory responses in mDCs, is cross-regulated by IFN-α, a major product of TLR9 responses in pDCs that normally promote Th1 immunity.
Keywords: Dendritic cells, FcεRI, IL-10, IFN-α, TNF-α
Introduction
Dendritic cells (DC’s) are potent professional antigen-presenting cells (APCs) that sample, capture, and process peripheral antigens for presentation to naive T cells. In mediating this activity, DCs play an important role in both the priming of adaptive immune responses and in the induction of peripheral tolerance.1 In human blood, there are two major types of immature DCs, namely, the CD123+, CD11c−, CD303+ (BDCA-2), CD304+ (BDCA-4+) plasmacytoid (pDCs) and CD123low, CD11chigh, CD1c (BDCA-1)+ monocytoid (mDCs).2 While both DC subtypes express Toll-like receptors (TLRs) that are pivotal in innate immunity, recent studies show that they also co-express the αγ2 variant of the high affinity IgE receptor (FcεRI) suggesting a role in allergy-associated adaptive immunity.3, 4 In addition, there is mounting evidence that innate immune stimuli are critical in determining the course of adaptive immune responses, including those associated with allergic diseases. It remains unclear, however, whether these two subsets of DCs work in concert or contribute differentially to the regulatory processes of allergic disease.
In this study, we characterize FcεRI-mediated cytokine responses in human blood mDCs. We have also investigated the potential for cytokine crosstalk between pDCs and mDCs by examining a role for type I IFN’s (e.g. IFN-α/β) in modulating these IgE receptor responses in mDCs. The results demonstrate that FcεRI-dependent proinflammatory cytokine responses in mDCs are suppressed by autocrine secretion of IL-10, with this regulation in of itself being augmented by pDC-derived IFN-α resulting from TLR9-dependent activation. Overall, these findings provide insight into potential mechanisms of how innate immunity can play a key role in regulating adaptive immune responses that are likely linked to the pathogenesis of allergic disease.
Materials and Methods
DC purification from human blood
Venipuncture was performed on consenting adults (age range, 21–55 years) using a protocol approved by the Western Institutional Review Board (Seattle, Washington). Donors were not selected based on allergic status. In fact, preparations also included cells procured from residual cell packs from anonymous subjects undergoing platelet pheresis within the Hemapheresis Unit at Johns Hopkins University. In all cases, mixed leukocyte suspensions were subjected to double-Percoll (1.075/1.081 g/ml) density centrifugation, which produced both basophil-depleted cell (BDC) and basophil-enriched cell suspensions, as previously described.5 PDCs were purified from the BDC suspensions using blood dendritic cell antigen (BDCA)-4 positive magnetic selection (Miltenyi Biotec, Auburn, CA). Cells not retained in the column during BDCA-4 selection (i.e. the BDCA-4 flow-through) were subsequently used for isolating mDCs using BDCA-1 selection (Miltenyi). This first involved depleting cell suspensions of CD19+ B cells and then a second pass through a different column to select for BDCA-1+ mDCs. Cells were counted using a Spiers-Levy chamber. Assessment of several mDC suspensions prepared in this manner indicated >95% purity, as determined by BDCA-1 staining. Several pDC suspensions indicated >95%, as determined by BDCA-2 staining.
RNA isolation
Total RNA was isolated from 0.25–1.0 × 106 cells using the RNA-Bee protocol (Tel-test Inc., Friendswood, TX). Following isopropanol precipitation, RNA was washed with ethanol and nearly dried under vacuum. The RNA was then resuspended in DNase-free water and stored below −70°C.
Quantitative RT-PCR for Detection of TNF-α mRNA
Real-time RT-PCR was performed using SYBR Green reagents and technology. Total RNA was first reverse transcribed in a Techne TC-thermocycler using the GeneAmp RNA PCR kit (Applied Biosystems, Branchburg, NJ) and oligo (dT)16 as primer, according to the manufacturer’s instructions. The cDNA product was then amplified in an Applied Biosystems 7300 Thermocycler using the SYBR Green PCR Master Mix kit (Applied Biosystems). Primer sets for TNF-α (F: CTTCTGCCTGCTGCACTTTG, R: CTGGGCCAGAGGGCTGAT) and the housekeeping gene ubiquitin (F: CACTTGGTCCTGCGCTTGA R: CAATTGGGAATGCAACAACTTTAT) have been described.6 Values were normalized to ubiquitin expression to correct for levels in total mRNA.
Flow Cytometry
Direct staining of cell surface marker proteins (i.e. FcεRIα, CD80, CD86) on unfixed and fixed (buffered 4% paraformaldehyde) mDCs was achieved with APC-, PE-, or FITC-conjugated antibodies and appropriate isotype controls using methods previously described.7 Flow cytometry was performed using a FACSCalibur machine. Histograms were prepared using the FlowJo graphics application (FlowJo LLC, Ashland, OR).
Culture Conditions
For the secretion of cytokines, DC subtypes (mDCs and pDCs) were cultured in C-IMDM containing human AB serum at 5%. Depending on the set of experiments, cells (2.5×104 to 1.0×105) were added to the wells of 96-well plates in volumes of either 0.100 ml or 0.125 ml. Those not requiring pre-incubation were done in 0.125 ml volumes. After incubating 15 min. to equilibrate to 37°C, 5% CO2, an equal volume of stimulus (2X) was added and the cells cultured for specific time points. In cultures requiring pre-incubation (such as with IFN-α) the cells were first added in 0.100 ml volumes, before adding an equal volume of IFN-α at 2x concentrations. Following 16 h pretreatment, IgE receptor cross-linking was then initiated by adding 50 µL of FcεRIα Ab (at 5x final concentration) to each designated well for up to 30 h incubation. FcεRI-mediated IL-10 neutralization experiments were performed by adding anti-IL-10 Ab (500 ng/mL, clone JESS-9D7, eBioscience) simultaneously with the AER-37 anti-FcεRIα Ab from eBioscience (1:167 of the stock or ~75 ng/mL) for 16 h.
Cytokine Measurements
ELISA protocols for the detection of TNF-α, IL-10 (eBioscience), and IFN-α (in-house) have all been described previously.8
Statistical analysis
Data are presented as mean±SEM unless otherwise indicated. Statistical analysis was performed with InStat3 software (Graphpad Software, San Diego, CA) and involved the use of the Student-Newman-Keuls Multiple Comparisons Test unless otherwise stated. P values ≤ 0.05 were considered significant.
Results
Time course of cytokine responses by immature blood mDCs following FcεRI cross-linking
Phenotypic analyses of PBMCs in previous studies have demonstrated that immature mDCs express high levels of FcεRI.3 We first sought to confirm these findings by staining for FcεRIα on mDCs freshly isolated from human blood. All freshly prepared, unfixed mDC suspensions examined with PE conjugated FcεRIα Ab show uniform and marked staining on flow histograms (nMFI 196±49, n=4). Based on initial dose-response studies, we found that maximal FcεRI-mediated cytokine responses (for TNF-α, and IL-10) in mDCs occurred at an FcεRIα Ab concentration of 75 ng/mL (or a 1:167 dilution of the Ab stock). In addition, we observed an intriguing relationship between the kinetics for TNF-α vs. IL-10. When left unstimulated using media alone or an isotype control, mDCs secreted only marginal TNF-α (34±28 pg/106 mDCs, n=3) with undetectable IL-10 levels. However, as shown in Figure 1A, a rapid and marked up-regulation in TNF-α was seen with FcεRI-dependent activation, with levels detected within 1h and peaking after 4h incubation (2189±864 pg/106 mDC). As indicated in Figure 1B, IL-10 secretion was also induced with FcεRI-cross-linking. Compared with TNF-α induction, IL-10 levels were substantially lower, and first appeared only after 4h and increased to 112±47 pg/106 mDC (n=3) after 30h incubation. Most importantly, TNF-α secretion was blunted at the onset of IL-10 secretion (4h) and progressively declined with increasing levels of IL-10. These findings therefore indicated IL-10 as a potential feedback regulator of FcεRI-mediated pro-inflammatory responses by human blood mDCs.
Figure 1. Kinetics for FcεRI-dependent TNF-α and IL-10 secretion by blood mDCs.
mDCs were isolated from blood as described in the Materials & Methods and stimulated with FcεRIα Ab, isotype control, or medium alone. Cell-free supernatants were then harvested at the indicated time points and analyzed for TNF-α (A) and IL-10 (B) protein by ELISA. Values represent the mean±SEM, n=3.
IL-10 inhibits de novo synthesis of TNF-α by blood mDCs stimulated with FcεRIα Ab
Several different approaches were taken to address whether IL-10 accounts for the down-regulation of FcεRI-dependent proinflammatory cytokine secretion by mDCs. We first evaluated the consequences of neutralizing IL-10 activity by using an Ab that prevents IL-10 binding to the IL-10 receptor. As shown in Figure 2A, unstimulated mDCs secreted low levels of TNF-α. (220±78 pg/106 mDC, n=3). When mDCs were stimulated with FcεRIα Ab for 16h, a pronounced increase of TNF-α secretion was observed (3050±241 pg/106 mDC, n=3). This response was significantly augmented by 28% when mDCs were simultaneously treated with both the FcεRIα Ab and the IL-10 neutralizing Ab (3903±197 pg/106 mDCs, p < 0.01, n=3). In contrast, as shown in Figure 2B, the FcεRI-dependent TNF-α responses by mDCs concomitantly treated with IL-10 at 0.1, 1, or 10 ng/mL for 4h, were rapidly reduced to 1857±249, 716±70, and 349±43 pg/106 cells, which corresponded to percent inhibitions of 36±10%, 75±5%, and 86±3%, respectively (n=3, p < 0.001).In addition, as indicated in Figure 2C, the magnitude of TNF-α transcription, as measured by real time RT-PCR, was reduced within just 2h of FcεRI cross-linking (~45-fold) and reduced by 74% (p<0.001, n=3) in cultures simultaneously treated with IL-10 (10 ng/ml).
Figure 2. IL-10 inhibits FcεRI-dependent TNF-α expression by blood mDCs.
A, Neutralizing IL-10 during FcεRIα-dependent activation enhances TNF-α secretion by mDCs. B, Recombinant IL-10, when simultaneously added to mDC cultures stimulated with anti-FcεRI Ab, markedly inhibits TNF-α secretion (at 4h) and C, mRNA expression (2h). Values in each panel represent the mean±SEM., n=3.
Importantly, the inhibitory effect of IL-10 on FcεRI-dependent pro-inflammatory cytokine responses did not appear to be secondary to toxic effects. Trypan Blue analyses indicated no differences in viabilities for mDCs incubated 24h in: 1) medium control (80%), 2) FcεRIα Ab (82%), 3) IL-10 at 10 ng/ml (86%), or 4) FcεRIα Ab + IL-10 (85%).
IL-10 does not affect the expression of co-stimulatory molecules or FcεRI on blood mDCs
IL-10 is known for its inhibition of co-stimulatory molecule expression on antigen-presenting cells 9, but its effect on FcεRI expression is not yet established. The inhibitory effect of IL-10 on FcεRI-dependent inflammatory cytokine responses may be a result of its potential inhibition of mDC maturation and/or FcεRI expression. Hence, the effect of IL-10 on CD80, CD86, and FcεRI expression by blood mDCs was investigated. As indicated by a representative panel of flow cytometry histograms displayed in Figure 3A, CD80 and CD86 staining of mDCs cultured with IL-10, FcεRIα Ab, or a combination of FcεRIα Ab and IL-10 appeared unaffected when compared to that of un-stimulated mDCs. Of note, blood mDCs expressed 10 to 20 fold higher levels of CD86 than what was observed for CD80. However, these levels were not statistically altered with IL-10 treatment. Similarly, as shown in Figure 3B, when blood mDCs were cultured with FcεRIα Ab or a combination of FcεRIα Ab and IL-10 for 24 h, FcεRI expression was unaltered with nMFI’s of 82±49 and 73±44, respectively (n=3). These data indicated that IL-10 unlikely affected mDC cytokine responses by suppressing FcεRI (or co-stimulatory molecule) expression.
Figure 3. Inhibitory effects of IL-10 on FcεRI-dependent responses in mDCs are not from a down-regulation in FcεRIα and co-stimulatory molecule expression.
CD80/CD86 (A) and FcεRIα (B) expression on mDCs following incubation (16h) in medium alone, IL-10 (10 ng/ml), anti-FcεRIα (75 ng/ml) or the combination of anti-FcεRIα and IL-10. Representative flow histograms are shown along with nMFI values (mean±SEM, n=3).
Interferons modulate FcεRI-dependent IL-10 secretion by blood mDCs
Interferons (IFNs) are key regulators of innate and adaptive immunity.10 Our previous studies suggested that IFN-α, a major Type I interferon from pDCs, plays a role in suppressing FcεRI expression and function in pDCs.8 IFN-α is also reported to influence the differentiation of human monocyte-derived dendritic cells.11, 12 Therefore, we investigated the potential for cross-regulation between pDCs and mDCs by examining whether Type I IFNs (IFN-α and IFN-β) modulate FcεRI-dependent responses of human blood mDCs. IFNs alone did not induce IL-10 secretion by human blood mDCs. However, as shown in Figure 4A, when mDCs were pretreated 16h with IFN-α at 100 U/mL with subsequent IgE receptor cross-linking for 24h, IL-10 production was enhanced 3.2-fold (183±11 pg/106 mDC, n=4) compared to those cells not pretreated with IFN-α (57±33 pg/106 mDC, p<0.001, n=4). If FcεRIα Ab and IFN-α were added simultaneously, there was no enhancement of IL-10, suggesting that the altering effect of IFN-α was not immediate (data not shown). In addition, as illustrated in Figure 4B derived from another 4 experiments, the indices of stimulation per 106 mDCs for IFN-α and IFN-β were 2.4±0.4 (p < 0.05, n=4) and 1.5±0.3 (p < 0.05, n=4), respectively. In sharp contrast, IFN-γ (a Type II IFN) markedly inhibited FcεRI-dependent IL-10 secretion by blood mDCs as demonstrated by it producing only a fraction of the control response (0.14±0.3; p < 0.05, n=4).
Figure 4. Interferons modulate FcεRI-dependent IL-10 secretion by mDCs.
A, mDCs pretreated 16h with doses of IFN-α before stimulating 24h with anti-FcεRIα for IL-10 secretion (mean±SEM, n=4). B, comparison of IFN-α, IFN-β and IFN-γ on this response using mDCs from 4 additional subjects. Values are normalized (mean±SEM, n=4) to IL-10 in control cultures receiving anti-FcεRIα alone (88±33 pg/106).
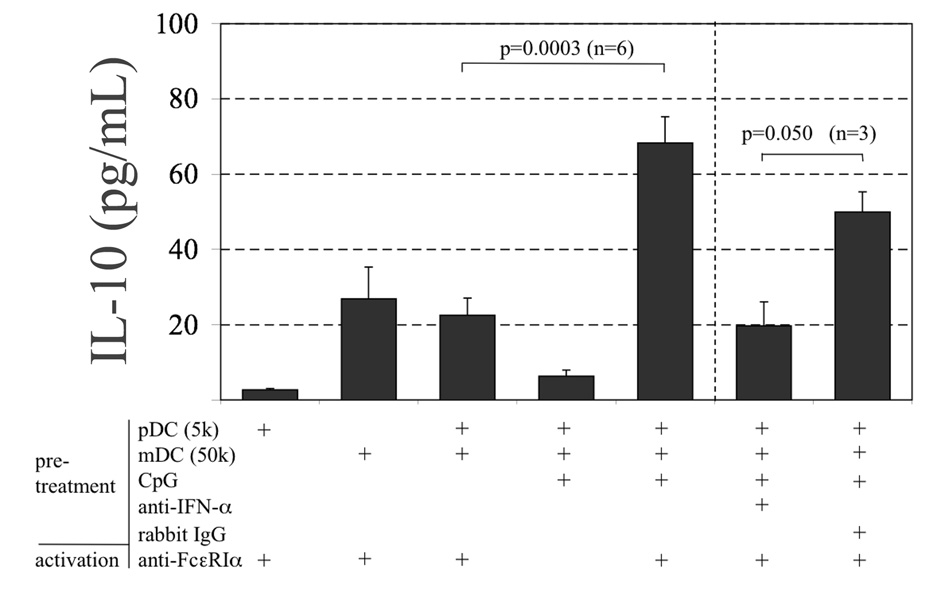
The results showing recombinant IFN-α as an important immunomodulator of the FcεRI-dependent IL-10 response by blood mDCs prompted an investigation of whether pDCs can directly mediate this effect on mDCs. As shown in Figure 5, when pDCs/mDCs co-cultures (1:10 ratio) were pretreated 16h with Type A CpG (ODN-2216) with subsequent FcεRI-dependent activation for 24h, IL-10 production was enhanced 3-fold (68.3±6.9 pg, n=6) compared to those co-cultures not pretreated with CpG (22.5±4.6 pg, p<0.003, n=6). Three of these six experiments were also treated with an Ab that neutralizes IFN-α activity. All 3 showed IL-10 responses that were reduced when compared with an isotype control (50±5 vs. 20±6 pg, p = 0.05). As expected, IFN-α protein was detected but only in cultures receiving CpG (1807±470 pg/ml, n=6), which were the same as those that showed greater IL-10 upon FcεRI-dependent activation. It is important to note that mDC culture supernatants (without pDCs) were negative for IFN-α following incubation with CpG (data not shown), which was expected since this DC subtype is not known to express TLR9.
Figure 5. TLR9-induced IFN-α from pDCs enhances FcεRI-dependent IL-10 secretion by mDCs.
pDC/mDC co-cultures (1:10 ratio; “K” denotes 1000) were pretreated 16h with and without CpG (100 nM). Cells were then stimulated with anti-FcεRIα for an additional 24h for IL-10 secretion. Three co-cultures additionally received anti-IFN-α (2µg/ml) or rabbit isotype control during pretreatment with CpG. Values are the mean±SEM.
Discussion
DCs are implicated in playing a significant role in both the sensitization and effector phases of allergic inflammation.13 In support of the latter, increased numbers of DCs (mDCs and pDCs) are reportedly recovered in BAL from the airways of allergic asthmatics following experimental allergen challenge 14, 15, 16, 17. It therefore follows that DC involvement in either phase is partially dependent on the nature of the co-stimulatory molecules expressed by these cells, with IgE receptor (FcεRI) being particularly important during the effector phase of disease. Indeed, it has long been suggested that the function of the αγ2 variant of FcεRI expressed on DCs and other APCs is to facilitate allergen presentation.18 Serum IgE levels, which are typically elevated in allergic disease, also play an important role in determining the level of FcεRI expression by DCs,3, 19, 20 just as they do for basophils and mast cells. Despite its prominence among the markers expressed by DCs, the exact role(s) of FcεRI on DC subsets remains poorly understood.
Our previous work and that of others have highlighted a potentially unique functional dichotomy among specific innate and adaptive immune receptors inherently expressed by pDCs.8, 21 Foremost, we showed that autocrine TNF-α secretion resulting from IgE-dependent activation plays a critical role in suppressing TLR9-dependent responses in pDCs that normally promote TH1 activity.22 In the present study, we pursued an observation of a potential regulatory mechanism, which was detected during our experiments looking at the kinetics of FcεRI-dependent cytokine secretion by mDCs. In particular, the production of TNF-α, which rapidly peaked between 4 to 8 h after activation, declined at about the same time when IL-10 secretion became evident (Figure 1). This prompted the notion that IL-10 was acting in an autocrine fashion to suppress FcεRI-dependent cytokine secretion. It is important to note that both TNF-α and IL-10 were also produced by mDCs under activation conditions that used anti-IgE Ab, as observed with pDCs.8, 22 However, we opted for the anti-FcεRI Ab in the present study, since this reagent contained relatively low endotoxin levels that might otherwise additionally activate mDCs through TLR4. We therefore cannot rule-out qualitative differences between these stimuli. Nonetheless, responses in cytokine patterns have been identical (data not shown).
Experiments using neutralizing Ab and exogenous recombinant cytokine confirmed that IL-10 is, indeed, a key regulator of the acute FcεRI-dependent proinflammatory responses in mDCs (Figure 2). The degree of inhibition by exogenous IL-10, at levels as low as 0.1 ng/mL, correlated with the observed inhibition by low endogenous IL-10 levels (i.e. < 35 to 183 pg/mL) detected in supernatants of FcεRI-activated blood mDCs. The fact that exogenous IL-10 rapidly mediated this inhibitory effect on TNF-α, both at the transcript and protein levels conspicuously suggested that IL-10 interrupts the signal transduction associated with IgE receptor cross-linking. Such a notion was further supported by our flow cytometry data, which indicated the lack of a significant IL-10 effect on mDC maturation markers and lack of a reduction in FcεRI expression (Figure 3). The latter observation was particularly important, since IL-10 has been reported to reduce FcεRI expression on mast cells exposed to this cytokine for up to 3 days.23 However, the inhibition mediated by IL-10 in the experiments here required no pre-incubation, making down regulation of FcεRI an unlikely mechanism to explain the inhibitory effects of this cytokine on mDC proinflammatory cytokine secretion. It is also unlikely that apoptotic activity (another function attributed to IL-10) played a role in inhibiting the TNF-α induced with FcεRI-dependent activation. Duramad, et al. reported that pDCs required up to 48h incubation with IL-10 before apoptotic effects were detected in pDCs.24 Taken together, our findings indicate that inhibition of FcεRI-dependent inflammatory responses in mDCs is accomplished through the autocrine effects of IL-10, which suppresses de novo synthesis of TNF-α by acting on signals induced through the IgE receptor. Naturally, additional studies are required to formally address this hypothesis.
While these observations bear a number of similarities to those previously reported by Novak et al, there are a few distinctions worth noting. First, our results are the first to comprehensively characterize FcεRI-dependent responses in freshly isolated immature blood mDCs as opposed to using monocytes and/or culture-derived DCs. The latter were reported as being prepared from atopic individual and generally required passive sensitization with IgE before responses were induced.9 We did not observe the same requirements using mDCs freshly prepared from blood, nor did we report similar needs when investigating FcεRI-dependent responses in pDCs.8, 22 However, preliminary work has indicated that FcεRI-dependent cytokine responses are significantly greater using cells from atopic individuals, who typically have elevated serum IgE and have greater receptor expression.20 Thus, we suspect that the same might also be true for the mDCs, but this is not yet determined. A second distinction regards the inhibitory activity of IL-10. Whereas, this cytokine is reported to prevent the in vitro maturation of monocytes into mDCs,9 such studies only allude to IL-10 being an inhibitor of FcεRI dependent responses. Our findings definitively show that IL-10 rapidly inhibits FcεRI-dependent responses through an autocrine mechanism.
Based on our previous results indicating functional counter-regulation between FcεRI and TLR9 in pDCs,8 along with current understanding indicating crosstalk between DC subsets, 13 we suspected that type I IFNs might also act on FcεRI-dependent responses in mDCs. In the present study, Type I IFNs preferentially up-regulated (IFN-α > IFN-β) the FcεRI-dependent IL-10 responses by mDCs (Figure 4). This effect was not immediate, but required mDCs to be pretreated (~16h) with Type I IFNs for enhanced production of IL-10. IFN-γ (i.e. a Type II IFN) did not evoke this same effect, but actually inhibited mDCs from producing IL-10 in response to IgE receptor-dependent activation (Figure 5).
Interestingly, these findings concerning Type I IFNs bear remarkable similarity to observations recently reported for culture-derived mast cells.25 However, our additional discovery that IL-10 is an extremely potent negative regulator of FcεRI-dependent activation in DCs points to the possibility that Type I IFNs play an important role in suppressing mDC responses during allergic inflammation. For example, it is conceivable that allergen, by binding to specific IgE on immature DCs, stimulates pro-inflammatory cytokines (i.e. TNF-α) that potentially augment allergic disease. However, if first exposed to an environment rich in Type I IFNs, mDC function may become altered to be better IL-10 producers upon encountering allergen. The autocrine and paracrine effects of enhanced IL-10 secretion could then simultaneously act on other DCs to make them less effective in producing TNF-α (and IL-6), thus potentially altering all down-stream responses (e.g. T cell responses) normally attributed to the allergic inflammation.
In agreement with the pro-Th1 nature of IFN-α, it is also important to note that recombinant forms of this Type I interferon have been used therapeutically to treat steroid resistant asthma among other Th2-like inflammatory disease conditions.26 In particular, IFN-α therapy was found to decrease leukocyte numbers, increase the relative numbers of CD4+ T cells with a predominate Th1 phenotype, and to increase IL-10 expression in PBMC.27 It therefore seems possible that the efficacy observed with this form of therapy is somehow linked to IFN-α’s effects on mDCs, although this belief remains to be determined.
Finally, it has been suggested that pDCs play an important role in promoting tolerance in rodent models of allergic inflammation.13, 28 It seems appropriate to speculate that the in vitro data presented here are consistent with this belief and further suggest than Type I IFNs are critical modulators of this activity. Hence, pDCs are the predominant source of Type I IFN’s among immune cells, particularly in response to TLR9 ligation.29 Previous findings indicate that pDCs exposed to TLR9 agonists, such as CpG-DNA, down-regulate IgE receptor expression (and function).8 Our current findings show that IFN-α, exogenous (figure 4) as well as that naturally derived from pDCs following TLR9 activation (figure 5), opposes the FcεRI-dependent proinflammatory cytokine secretion in mDCs by augmenting the FcεRI-dependent IL-10 response. Assuming the possibility that pDCs and mDCs co-localize in tissue, then we predict that TLR9/FcεRI crosstalk at the DC level plays a critical role in the TH2/TH1 balance. Whether such activity helps to explain the efficacy recently seen using CpG-based immunotherapy 30 remains to be seen but seems probable at this time.
In conclusion, we have shown that FcεRI-dependent pro-inflammatory cytokine responses in human blood mDCs are regulated by IL-10 in an autocrine fashion. The mechanism for IL-10 inhibition is mediated by reducing de novo synthesis of FcεRI-dependent proinflammatory cytokines. Priming with Type I IFNs enhances FcεRI-dependent IL-10 production by mDCs. We therefore predict that FcεRI-dependent signaling is modulated by crosstalk with innate immune responses (i.e. TLR9-dependent) that are known to stimulate Type I IFNs from pDCs. Taken together, the understanding of cytokine responses by blood DCs and their modulation via a TLR9-FcεRI axis may provide an immunologic basis for both the development of allergic sensitization and the potential tolerance mediated by TLR9 agonists.
Acknowledgments
Funding: This work was supported (in part) by research funding from NIH/NIAID grants: The Asthma and Allergic Diseases Cooperative Research Centers grant AI070345-01 (project 3: JTS), R01AI42221 (JTS), AI052468 (SKH). TL was supported by NIH/NIAID training grant AI07007 and by the Eudowood Foundation (RAW). Funding for SKH was also provided by The Philip Morris Company, USA. The authors declare no competing financial interests.
Non-standard Abbreviations
- DC
dendritic cell
- pDC
plasmacytoid dendritic cell
- mDC
monocytoid dendritic cell
- TLR
Toll-like receptor
- FcεRI
high affinity IgE receptor
- BDCA
blood dendritic cell antigen
- BDC
basophil-depleted cell
- BEC
basophil-enriched cell
- CpG-DNA
DNA containing unmethylated CpG motifs
- APC
antigen presenting cell
Footnotes
Clinical implications: The understanding of adaptive FcεRI-dependent cytokine responses and TLR9-FcεRI cross-regulation between DC subtypes may provide insight into an immunologic basis for the development of allergic sensitization and induction of tolerance.
Capsule Summary: Immature myeloid dendritic cells secrete both pro-inflammatory (TNF-α) and auto-regulatory cytokines (IL-10) in response to FcεRIα-dependent activation. The latter acts in an autocrine fashion to suppress DC activation and is augmented by Type I IFNs.
References
- 1.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 2.Liu YJ. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol. 2005;23:275–306. doi: 10.1146/annurev.immunol.23.021704.115633. [DOI] [PubMed] [Google Scholar]
- 3.Foster B, Metcalfe DD, Prussin C. Human dendritic cell 1 and dendritic cell 2 subsets express FcepsilonRI: correlation with serum IgE and allergic asthma. J Allergy Clin Immunol. 2003;112:1132–1138. doi: 10.1016/j.jaci.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 4.Novak N, Kraft S, Bieber T. IgE receptors. Curr Opin Immunol. 2001;13:721–726. doi: 10.1016/s0952-7915(01)00285-0. [DOI] [PubMed] [Google Scholar]
- 5.Schroeder JT, MacGlashan DW, Jr, Kagey-Sobotka A, White JM, Lichtenstein LM. IgE-dependent IL-4 secretion by human basophils. The relationship between cytokine production and histamine release in mixed leukocyte cultures. J Immunol. 1994;153:1808–1817. [PubMed] [Google Scholar]
- 6.Marshall JD, Fearon K, Abbate C, Subramanian S, Yee P, Gregorio J, et al. Identification of a novel CpG DNA class and motif that optimally stimulate B cell and plasmacytoid dendritic cell functions. J Leukoc Biol. 2003;73:781–792. doi: 10.1189/jlb.1202630. [DOI] [PubMed] [Google Scholar]
- 7.Schroeder JT, Miura K, Kim HH, Sin A, Cianferoni A, Casolaro V. Selective expression of nuclear factor of activated T cells 2/c1 in human basophils: evidence for involvement in IgE-mediated IL-4 generation. J Allergy Clin Immunol. 2002;109:507–513. doi: 10.1067/mai.2002.122460. [DOI] [PubMed] [Google Scholar]
- 8.Schroeder JT, Bieneman AP, Xiao H, Chichester KL, Vasagar K, Saini S, et al. TLR9-and FcepsilonRI-mediated responses oppose one another in plasmacytoid dendritic cells by down-regulating receptor expression. J Immunol. 2005;175:5724–5731. doi: 10.4049/jimmunol.175.9.5724. [DOI] [PubMed] [Google Scholar]
- 9.Novak N, Bieber T, Katoh N. Engagement of Fc epsilon RI on human monocytes induces the production of IL-10 and prevents their differentiation in dendritic cells. J Immunol. 2001;167:797–804. doi: 10.4049/jimmunol.167.2.797. [DOI] [PubMed] [Google Scholar]
- 10.Taniguchi T, Takaoka A. A weak signal for strong responses: interferon-alpha/beta revisited. Nat Rev Mol Cell Biol. 2001;2:378–386. doi: 10.1038/35073080. [DOI] [PubMed] [Google Scholar]
- 11.Blanco P, Palucka AK, Gill M, Pascual V, Banchereau J. Induction of dendritic cell differentiation by IFN-alpha in systemic lupus erythematosus. Science. 2001;294:1540–1543. doi: 10.1126/science.1064890. [DOI] [PubMed] [Google Scholar]
- 12.Boruchov AM, Heller G, Veri MC, Bonvini E, Ravetch JV, Young JW. Activating and inhibitory IgG Fc receptors on human DCs mediate opposing functions. J Clin Invest. 2005;115:2914–2923. doi: 10.1172/JCI24772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hammad H, Lambrecht BN. Recent progress in the biology of airway dendritic cells and implications for understanding the regulation of asthmatic inflammation. J Allergy Clin Immunol. 2006;118:331–336. doi: 10.1016/j.jaci.2006.03.041. [DOI] [PubMed] [Google Scholar]
- 14.Lommatzsch M, Bratke K, Bier A, Julius P, Kuepper M, Luttmann W, et al. Airway dendritic cell phenotypes in inflammatory diseases of the human lung. Eur Respir J. 2007;30:878–886. doi: 10.1183/09031936.00036307. [DOI] [PubMed] [Google Scholar]
- 15.Jahnsen FL, Lund-Johansen F, Dunne JF, Farkas L, Haye R, Brandtzaeg P. Experimentally induced recruitment of plasmacytoid (CD123high) dendritic cells in human nasal allergy. J Immunol. 2000;165:4062–4068. doi: 10.4049/jimmunol.165.7.4062. [DOI] [PubMed] [Google Scholar]
- 16.Masten BJ, Olson GK, Tarleton CA, Rund C, Schuyler M, Mehran R, et al. Characterization of myeloid and plasmacytoid dendritic cells in the human lung. J Immunol. 2006;177:7784–7793. doi: 10.4049/jimmunol.177.11.7784. [DOI] [PubMed] [Google Scholar]
- 17.Bratke K, Lommatzsch M, Julius P, Kuepper M, Kleine HD, Luttmann W, et al. Dendritic cell subsets in human bronchoalveolar lavage fluid after segmental allergen challenge. Thorax. 2007;62:168–175. doi: 10.1136/thx.2006.067793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Novak N, Kraft S, Bieber T. Unraveling the mission of FcepsilonRI on antigen-presenting cells. J Allergy Clin Immunol. 2003;111:38–44. doi: 10.1067/mai.2003.2. [DOI] [PubMed] [Google Scholar]
- 19.Prussin C, Griffith DT, Boesel KM, Lin H, Foster B, Casale TB. Omalizumab treatment downregulates dendritic cell FcepsilonRI expression. J Allergy Clin Immunol. 2003;112:1147–1154. doi: 10.1016/j.jaci.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Tversky JR, Le TV, Bieneman AP, Chichester KL, Hamilton RG, Schroeder JT. Human blood dendritic cells from allergic subjects have impaired capacity to produce interferon-alpha via toll-like receptor 9. Clin Exp Allergy. 2008 doi: 10.1111/j.1365-2222.2008.02954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cao W, Rosen DB, Ito T, Bover L, Bao M, Watanabe G, et al. Plasmacytoid dendritic cell-specific receptor ILT7-Fc epsilonRI gamma inhibits Toll-like receptor-induced interferon production. J Exp Med. 2006;203:1399–1405. doi: 10.1084/jem.20052454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schroeder JT, Chichester KL, Bieneman AP. Toll-like receptor 9 suppression in plasmacytoid dendritic cells after IgE-dependent activation is mediated by autocrine TNF-alpha. J Allergy Clin Immunol. 2008;121:486–491. doi: 10.1016/j.jaci.2007.09.049. [DOI] [PubMed] [Google Scholar]
- 23.Gillespie SR, DeMartino RR, Zhu J, Chong HJ, Ramirez C, Shelburne CP, et al. IL-10 inhibits Fc epsilon RI expression in mouse mast cells. J Immunol. 2004;172:3181–3188. doi: 10.4049/jimmunol.172.5.3181. [DOI] [PubMed] [Google Scholar]
- 24.Duramad O, Fearon KL, Chan JH, Kanzler H, Marshall JD, Coffman RL, et al. IL-10 regulates plasmacytoid dendritic cell response to CpG-containing immunostimulatory sequences. Blood. 2003;102:4487–4492. doi: 10.1182/blood-2003-07-2465. [DOI] [PubMed] [Google Scholar]
- 25.Fujita T, Kambe N, Uchiyama T, Hori T. Type I interferons attenuate T cell activating functions of human mast cells by decreasing TNF-alpha production and OX40 ligand expression while increasing IL-10 production. J Clin Immunol. 2006;26:512–518. doi: 10.1007/s10875-006-9043-1. [DOI] [PubMed] [Google Scholar]
- 26.Tilg H, Kaser A. Type I interferons and their therapeutic role in Th2-regulated inflammatory disorders. Expert Opin Biol Ther. 2004;4:468–481. doi: 10.1517/14712598.4.4.469. [DOI] [PubMed] [Google Scholar]
- 27.Simon HU, Seelbach H, Ehmann R, Schmitz M. Clinical and immunological effects of low-dose IFN-alpha-2b treatment in patients with corticosteroid-resistant asthma. Allergy. 2003;58:1250–1255. doi: 10.1046/j.1398-9995.2003.00424.x. [DOI] [PubMed] [Google Scholar]
- 28.van Rijt LS, Jung S, Kleinjan A, Vos N, Willart M, Duez C, et al. In vivo depletion of lung CD11c+ dendritic cells during allergen challenge abrogates the characteristic features of asthma. J Exp Med. 2005;201:981–991. doi: 10.1084/jem.20042311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siegal FP, Kadowaki N, Shodell M, Fitzgerald-Bocarsly PA, Shah K, Ho S, et al. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–1837. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- 30.Creticos PS, Schroeder JT, Hamilton RG, Balcer-Whaley SL, Khattignavong AP, Lindblad R, et al. Immunotherapy with a ragweed-toll-like receptor 9 agonist vaccine for allergic rhinitis. N Engl J Med. 2006;355:1445–1455. doi: 10.1056/NEJMoa052916. [DOI] [PubMed] [Google Scholar]