Abstract
Background: Despite advances in surgical treatment options, failure rates of rotator cuff repair have continued to range from 20% to 90%. Hence, there is a need for new repair strategies that provide effective mechanical reinforcement of rotator cuff repair as well as stimulate and enhance the intrinsic healing potential of the patient. The purpose of this study was to evaluate the extent to which augmentation of acute repair of rotator cuff tendons with a newly designed poly-L-lactide repair device would improve functional and biomechanical outcomes in a canine model.
Methods: Eight adult, male mongrel dogs (25 to 30 kg) underwent bilateral shoulder surgery. One shoulder underwent tendon release and repair only, and the other was subjected to release and repair followed by augmentation with the repair device. At twelve weeks, tendon retraction, cross-sectional area, stiffness, and ultimate load of the repair site were measured. Augmented repairs underwent histologic assessment of biocompatibility. In addition, eight pairs of canine cadaver shoulders underwent infraspinatus injury and repair with and without device augmentation with use of identical surgical procedures and served as time-zero biomechanical controls. Eight unpaired, canine cadaver shoulders were included as normal biomechanical controls.
Results: At time zero, repair augmentation significantly increased the ultimate load (23%) (p = 0.034) but not the stiffness of the canine infraspinatus tendon repair. At twelve weeks, the poly-L-lactide scaffold was observed to be histologically biocompatible, and augmented repairs demonstrated significantly less tendon retraction (p = 0.008) and significantly greater cross-sectional area (137%), stiffness (26%), and ultimate load (35%) than did repairs that had not been augmented (p < 0.001, p = 0.002, and p = 0.009, respectively).
Conclusions: While limiting but not eliminating tendon repair retraction, the augmentation device provided a tendon-bone bridge and scaffold for host tissue deposition and ingrowth, resulting in improved biomechanical function of the repair at twelve weeks.
Clinical Relevance: The augmentation device, applied in a similar manner as described in the present study, might offer a functional benefit to patients undergoing rotator cuff repair.
Rotator cuff tears are a common cause of debilitating pain, reduced shoulder function, and weakness, and 30,000 to 50,000 repairs are performed annually in the United States1. Despite improvements in the understanding of this disease process and advances in surgical treatment options, failure rates of rotator cuff repairs have ranged from 20% to 90%2-8, depending on patient age, tear size and chronicity, muscle atrophy and degeneration, tendon quality, repair technique, and the postoperative rehabilitation protocol9-17. Furthermore, recurrent and chronic rotator cuff tears may not be repairable. Treatment of symptomatic irreparable tears is extremely challenging and limited to nonsurgical management, débridement with partial repair18-21, or major reconstructive procedures such as muscle transfers22. Hence, there is a need for new repair strategies that provide effective mechanical reinforcement of the repair as well as stimulate and enhance the intrinsic healing potential of the patient2,23.
The mechanisms of rotator cuff repair failure are believed to be suture cutting through tendon secondary to excessive tension at the repair site and/or poor healing capacity of the involved tissues2,24,25. To address these challenges, natural and synthetic devices have been investigated for the reinforcement of rotator cuff repairs. Rotator cuff repair with devices derived from polylactic acid26-28, polytetrafluoroethylene29, extracellular matrix30-35, chitin36, and chitosan-hyaluronan37 have been studied in animal models over the past decade. Of these, only extracellular matrix derived from dermis, small intestine submucosa, fascia lata, and pericardium are commercially available for rotator cuff repair at the current time38. However, in their current configurations, commercially available extracellular matrices may possess some, but likely not all, of the mechanical and suture retention properties necessary for providing effective mechanical augmentation to rotator cuff repairs38-40. Despite the current clinical use of extracellular matrices for rotator cuff repair, the limited clinical data show mixed results with regard to surgical outcomes and complication rates41-49. In particular, several clinical studies investigating small intestine submucosa have found high retear rates, formation of noninfectious edema, swelling, pain, and increased skin temperature around the wound42-45, which have led to the conclusion that small intestine submucosa is not suitable for rotator cuff repair, at least in its current form45. Furthermore, the ability of any extracellular matrix device to improve healing rates of rotator cuff tendon repair has yet to be demonstrated in a prospective, controlled clinical study. Hence, there remains a need to establish an effective repair strategy for rotator cuff repair augmentation.
The objective of this study was to investigate the use of a poly-L-lactide repair device (X-Repair; Synthasome, San Diego, California) for rotator cuff repair augmentation. Poly-L-lactide, a slowly degrading and biocompatible polymer, has been approved for human use by the United States Food and Drug Administration (FDA) in the form of suture, screws, washers, and various other medical devices50-52. Poly-L-lactide scaffolds have been previously investigated for rotator cuff repair in animal models, both as an interpositional device for irreparable tears26 and to reinforce the suture repair line27,28. To date, however, poly-L-lactide scaffolds have not been used for augmenting or load-sharing with the tendon-bone repair.
The purpose of this study was to evaluate the extent to which augmentation of acute repair of rotator cuff tendons with the X-Repair device would improve functional and biomechanical outcomes in a canine model. Our primary hypotheses were that, compared with repairs without augmentation, augmented repairs would have (1) increased biomechanical properties at time zero and (2) less tendon retraction and increased biomechanical properties after twelve weeks of healing. We also hypothesized that the biomechanical properties of both unaugmented and augmented repairs at twelve weeks would be increased with respect to time zero but remain less than normal.
Materials and Methods
Experimental Design
Eight adult, male mongrel dogs (25 to 30 kg) underwent bilateral shoulder surgery30,31 as approved by the Institutional Animal Care and Use Committee at our institution. Both shoulders received the same tendon injury: partial release of the superior 8 to 9 mm of the infraspinatus tendon, which constitutes approximately two-thirds of the full tendon width. We chose a partial-width injury model on the basis of our previous work demonstrating a 100% rate of retear with a full-width injury in the canine model53. We reasoned that a partial-width injury might moderate the rate of repair failures and mimic the mechanical environment of many single-tendon tears in the human injury condition. The right shoulder underwent tendon release and repair only, and the left shoulder was subjected to release and repair followed by augmentation with the woven poly-L-lactide device. Tendon retraction, cross-sectional area, stiffness, ultimate load, and biocompatibility of the repair site were evaluated at twelve weeks after surgery. In addition, eight pairs of canine cadaver shoulders obtained from another study at our institution underwent infraspinatus injury and repair with and without device augmentation with use of identical surgical procedures and served as time-zero biomechanical controls. Eight unpaired, canine cadaver shoulders, also obtained from another study at our institution, were included as normal biomechanical controls.
Mechanical Properties of the X-Repair Device (Stiffness, Ultimate Load, and Suture Retention)
The X-Repair device is a 12-mm-wide by 34-mm-long by 0.8-mm-thick woven poly-L-lactide device, designed and fabricated by the manufacturer with the goal of load-sharing or so-called off-loading the rotator cuff tendon repair. To determine stiffness and ultimate load, three devices were clamped and tested in air in uniaxial tension at 30 mm/min. The stiffness of the device was defined by the slope of the load versus displacement data between 50 and 150 N. The ultimate load for retention of three simple, number-2 FiberWire sutures (Arthrex, Naples, Florida) in five devices was also determined in uniaxial tension at 30 mm/min.
Surgical Methods
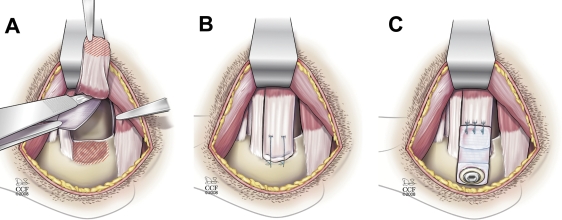
The dogs were initially anesthetized with an intravenous dose of sodium pentothal (20 mg/kg) to effect and then were intubated orotracheally and maintained on isoflurane in oxygen (3%). The infraspinatus tendon was approached with use of a 5-cm transverse incision. The superior two-thirds of the infraspinatus tendon was sharply detached from its insertion at the greater tuberosity, and a 1.5 by 2-cm portion of the underlying joint capsule was excised to model an intra-articular injury (Fig. 1, A). The infraspinatus tendon was immediately repaired back to its insertion on the humerus with use of two number-0 FiberWire sutures brought through separate transosseous tunnels in the humerus and passed through the tendon in a Mason-Allen configuration (Fig. 1, B). In the left shoulder, a sterile X-Repair device was affixed over the tendon repair (Fig. 1, C). The device was attached first to the tendon medially with use of three number-0 FiberWire Mason-Allen sutures. The device was then laid down over the repair and was tensioned by advancing the lateral edge approximately 2 mm laterally for the osseous attachment. Fixation to the humerus was achieved with use of a stainless-steel cortical screw (2.7 mm in diameter) with a polyetheretherketone spiked washer (8.0-mm outer diameter) (Synthes, West Chester, Pennsylvania). There was no fixation on the anterior or posterior edges. The wounds were irrigated and closed in layers. The dogs were exempted from normal exercise but were allowed free cage activity in runs with lowered ceilings to limit jumping or excessive weight-bearing on their front limbs.
Fig. 1.
Bilateral rotator cuff injury and repair in a canine model. A: The superior two-thirds of the infraspinatus tendon was sharply detached from its insertion at the greater tuberosity, and a 1.5 x 2-cm portion of the underlying joint capsule was excised. B: The infraspinatus tendon was immediately repaired back to its insertion on the humerus with use of two transosseous sutures. C: In one shoulder from each dog, a 12-mm-wide x 34-mm-long poly-L-lactide scaffold was affixed over the tendon repair. The scaffold was attached first to the tendon medially with use of three number-0 FiberWire modified Mason-Allen sutures. The device was then laid down over the repair and was tensioned by advancing the lateral edge approximately 2 mm laterally for the osseous attachment. Fixation to the humerus was achieved with use of a low-carbon stainless-steel cortical screw with a polyetheretherketone spiked washer (Synthes, West Chester, Pennsylvania). (Illustration by David Schumick, BS, CMI. Reprinted with the permission of the Cleveland Clinic Center for Medical Art and Photography; copyright 2009. All rights reserved.)
Killing and Dissection
At twelve weeks, the dogs were killed with use of a lethal injection of approximately 1 mL/10 lb (4.5 kg) of barbiturate (Beuthanasia-D; W.A. Butler, Dublin, Ohio). The infraspinatus muscle, the tendon repair construct, and 20 cm of the proximal part of the humerus were harvested for analysis. The joint capsule was released along its insertion with both the glenoid and the humeral head, and loose regions of capsule were dissected from the deep surface of the samples. It was not possible to reproducibly separate the intact portion of the tendon from the repaired portion, so the entire tendon (and the X-Repair device in samples that contained a device) was included as the so-called repair construct. The muscle-repair construct-bone samples were stored at −20°C until biomechanical analysis.
Tendon Retraction During Healing
To assess tendon retraction distance, visual inspection and palpation were used to identify the approximate position of the tendon stump within the fibrous tissue at the repair site. Calipers were then used to measure the distance between the retracted stump and the osseous repair site. The subjective nature of identifying the position of the retracted tendon stump led us to report the tendon retraction data categorically in three groups: (1) ≤1 cm, (2) between 1 and 2 cm, or (3) ≥2 cm.
Mechanical Properties of the Repair Construct (Cross-Sectional Area, Stiffness, and Ultimate Load)
At mechanical testing, samples were thawed at 4°C and equilibrated in phosphate-buffered saline solution at 37°C for one hour. The humeri were secured in aluminum pots with Cerrobend (Cerro bismuth alloy; McMaster-Carr, Robinsonville, New Jersey). The cross-sectional area of the repair construct was estimated from caliper measurements31 of the width and thickness of the tissue at the bone repair site, assuming an elliptical cross-section. A custom cryo-clamp was used to grip the muscle belly32. Mechanical testing of the repair construct was then performed. Optical markers were affixed to the tissue at the osseous insertion site and on the repair construct at 30 mm medial to the bone marker. This position was medial to the X-Repair attachment site on the tendon. Samples were positioned and tested in tension along the anatomic direction of pull. Testing was conducted in air at room temperature, and samples were kept moist by spraying with saline solution. Samples underwent 100 prefailure loading cycles from 5 to 100 N at 0.25 Hz54 and then were immediately tested to failure at 30 mm/min (MTS Systems, Eden Prairie, Minnesota). Load was recorded with a 5000-N load-cell (Honeywell Sensotec, Columbus, Ohio). A custom optical system, synchronized with the load data and sampling at 20 Hz, was used to track the optical markers and calculate the local displacements across the tendon-bone repair site55 with use of custom texture correlation software56 (Matlab; The MathWorks, Natick, Massachusetts). Repair stiffness was defined from failure testing as the slope of the load-displacement curve between 50 and 400 N. Ultimate load was defined as the maximum load that the sample reached during failure testing.
Host Cell Response to the X-Repair Device
After mechanical testing, the tendon-bone repair sites of the augmented repairs, including the polymer scaffolds, were retrieved for histologic evaluation of biocompatibility. Samples were fixed in 10% neutral buffered formalin (Sigma Diagnostics, St. Louis, Missouri) for three to seven days, decalcified in Surgipath Decalcifier I (Surgipath Medical Industries, Richmond, Illinois) for one to two weeks, processed routinely, and embedded in paraffin. Six-micrometer-thick sections were cut and stained with hematoxylin and eosin and were reviewed for the presence of inflammatory cells, such as neutrophils, macrophages, giant cells, and lymphocytes.
Power Analysis and Sample Size Justification
This study was powered primarily to detect significant differences between unaugmented and augmented repairs at time zero and after twelve weeks of healing. We chose a mean difference of 40 N/mm in stiffness and 200 N in ultimate load between unaugmented and augmented repairs to be clinically important, on the basis of the rationale that these values would correspond to a difference on the order of 25% of the properties of unaugmented tendon repairs at time zero. We were unable to estimate the expected variance of the mean paired difference in stiffness or ultimate load from previous work because data from similar studies have not been reported in this manner. Hence, for sample size estimation, we chose a standard deviation for the mean difference in stiffness of 33 N/mm and the difference in ultimate load of 167 N (an effect size of 1.2). It was estimated that a sample size of eight would allow us to detect an effect size of 1.2 with alpha = 0.05 and power = 0.8. On the basis of a sample size of eight for the paired analyses, we also used eight shoulders for the control groups.
Statistical Analysis
Stiffness and ultimate load were compared between paired unaugmented and augmented cadaver shoulders at time zero with use of a paired t test. Cross-sectional area, stiffness, and ultimate load were compared between paired unaugmented and augmented shoulders at twelve weeks with use of a paired t test, and tendon retraction distance was compared with use of a sign test. The nonparametric Jonckheere-Terpstra test was used to test for significant ordered differences in tendon retraction distance associated with each biomechanical property (stiffness and ultimate load). Regression analysis was used to test for correlations between each biomechanical property(stiffness and ultimate load) and cross-sectional area. For both unaugmented and augmented repair groups, the stiffness and ultimate load at twelve weeks were compared with the respective time-zero and normal controls with use of analysis of variance with a Tukey post hoc test. For both unaugmented and augmented repair groups, the cross-sectional area at twelve weeks was compared with normal with use of a Mann-Whitney rank-sum test. For all comparisons, a p value of <0.05 was considered significant.
Source of Funding
Synthasome, Inc. (San Diego, California) provided the funding and X-Repair devices for this study through a grant from the National Institutes of Health (NIH; R44 AR051260). The NIH provided salary support (T32 AR 050959-01) for two authors (M.J.C. and J.A.M.) and funding for our histology core facility (P30 AR-050953).
Results
Time Zero
Mechanical Properties of the X-Repair Device
The stiffness and ultimate load of the three X-Repair devices were a mean (and standard deviation) of 195 ± 2 N/mm and 796 ± 34 N, respectively. The ultimate load for the retention of three simple, number-2 FiberWire sutures in the five X-Repair devices was a mean of 397 ± 18 N.
Mechanical Properties of the Repair Construct
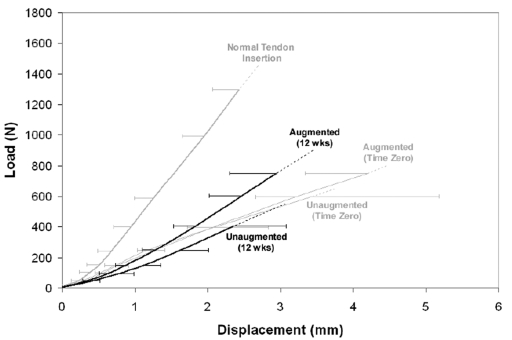
At time zero, there was no difference in stiffness between unaugmented and augmented repairs (p = 0.76) (Figs. 2 and 3-A). However, the ultimate load of augmented repairs averaged 133 ± 143 N (23% ± 25%) more than that of unaugmented repairs (p = 0.034) (Figs. 2 and 3-B). The failure mode of unaugmented repairs was predominantly suture pulling through tendon (seven of eight repairs), whereas four of eight augmented repairs failed alternatively by osseous avulsion of the intact portion of the tendon (Table I). Normal control tendon failed consistently by osseous avulsion of the tendon from the humeral head (Table I).
Fig. 2.
Average load-displacement curves for each repair group up to where the first sample in each group failed. These curves show the average shape and variance of the biomechanical data prior to failure. The average curves are only well-behaved until the data from individual samples begin to expire and, hence, cannot be plotted to the point of so-called average failure load. The error bars represent standard deviations of six to eight curves per group.
Fig. 3-A Fig. 3-B.
Figs. 3-A and 3-B Biomechanical properties of the paired unaugmented and augmented repairs at time zero and twelve weeks. Fig. 3-A At time zero, there was no difference in stiffness between unaugmented and augmented repairs (p = 0.76). At twelve weeks, the stiffness of augmented repairs was an average (and standard deviation) of 44 ± 26 N/mm (26%) more than that of unaugmented repairs (p = 0.002). Fig. 3-B At time zero, the ultimate load of augmented repairs averaged 133 ± 143 N (23%) more than that of unaugmented repairs (p = 0.034). At twelve weeks, the ultimate load of augmented repairs averaged 246 ± 143 N (35%) more than that of six unaugmented repairs (p = 0.009).
TABLE I.
Failure Modes for Mechanical Test Samples
| Time Zero
|
12 Weeks
|
||||
|---|---|---|---|---|---|
| Failure Modes | Normal Controls (N = 8) | Unaugmented (N = 8) | Augmented (N = 8) | Unaugmented (N = 8) | Augmented (N = 8) |
| Soft tissue* | 0 | 7 | 4 | 7 | 6 |
| Bone avulsion of intact tendon strut | 0 | 1 | 4 | 0 | 0 |
| Bone avulsion of humeral head | 8 | 0 | 0 | 0 | 0 |
| Grip artifact† | 0 | 0 | 0 | 1 | 2 |
Failure by suture pulling through tendon for time-zero samples; failure by rupture of the repair or intact portion of the tendon—at or away from bone—for twelve-week samples.
Excluded from analysis.
Twelve Weeks of Healing
Adverse Events
All of the dogs were walking by one to three days postoperatively, and no adverse events were noted throughout the twelve-week healing period.
Tendon Retraction During Healing
All of the repairs at twelve weeks were considered to show tendon retraction to some extent (Table II). Tendon retraction was significantly greater in unaugmented control repairs than in augmented repairs (p = 0.008). Five of eight unaugmented repairs retracted by ≥2 cm, whereas five of eight augmented repairs retracted ≤1 cm and none of the augmented repairs retracted >2 cm.
TABLE II.
In Vivo Tendon Retraction at Twelve Weeks
| Tendon Retraction Distance | Unaugmented Repairs (N = 8) | Augmented Repairs (N = 8) |
|---|---|---|
| ≤1 cm | 1 | 5 |
| 1-2 cm | 2 | 3 |
| ≥2 cm | 5 | 0 |
Mechanical Properties of the Repair Construct
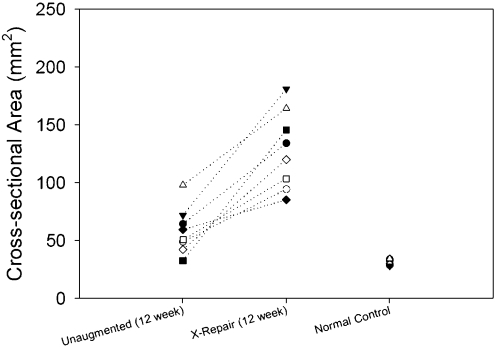
After twelve weeks of healing, the cross-sectional area of the augmented repairs averaged 70 ± 30 mm2 (137% ± 95%) more than that of the paired, unaugmented control repairs (p < 0.001) (Fig. 4).
Fig. 4.
The cross-sectional area of the paired unaugmented and augmented repairs at twelve weeks. The cross-sectional area of augmented repairs averaged 70 ± 30 mm2 more than that of unaugmented repairs (p < 0.001).
Biomechanical failure started at the soft-tissue freezing front (i.e., “grip artifact”) for two samples in the augmented group and one sample in the unaugmented group at twelve weeks; hence, the ultimate loads for these samples were excluded from the analysis.
After twelve weeks of healing, the stiffness of the augmented repairs averaged 44 ± 26 N/mm (26% ± 21%) more than paired, unaugmented repairs (p = 0.002) (Fig. 3-A). Further, at twelve weeks, the ultimate load of augmented repairs averaged 246 ± 143 N (35% ± 29%) more than the six paired, unaugmented repairs (p = 0.009) (Fig. 3-B). The failure mode of all twelve-week samples in both groups started in soft tissue, either by failure of the repair or failure of the intact portion of the tendon (Table I). Because soft tissues covered the device and/or tendon in all twelve-week samples, it was not possible to determine more precisely the location where failure started. In no instance was osseous avulsion observed nor did the X-Repair device fail during mechanical testing.
Correlations Between Biomechanical Outcomes and Tendon Retraction or Cross-Sectional Area
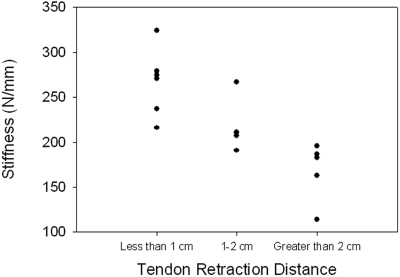
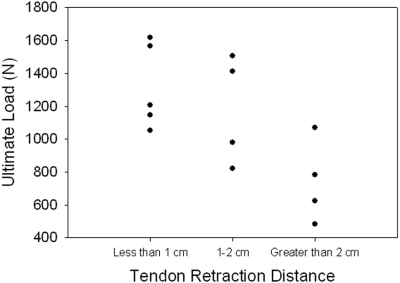
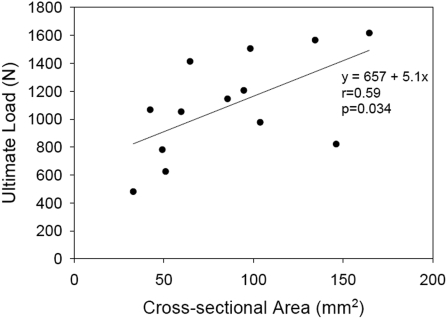
A significant ordered difference was found between tendon retraction distance and both stiffness (p = 0.004) (Fig. 5-A) and ultimate load (p = 0.006) (Fig. 5-B). Stiffness was not found to be significantly correlated to the sample cross-sectional area (comparison not shown). Ultimate load was moderately correlated to sample cross-sectional area (r = 0.59, p = 0.034) (Fig. 6).
Fig. 5-A Fig. 5-B.
Correlations between biomechanical outcomes and tendon retraction distance. A significant ordered difference was found between tendon retraction distance and both stiffness (p = 0.004) (Fig. 5-A) and ultimate load (p = 0.006) (Fig. 5-B).
Fig. 6.
Correlations between biomechanical outcomes and cross-sectional area. Ultimate load was moderately correlated to sample cross-sectional area (r = 0.59, p = 0.034).
Host Cell Response to the X-Repair Device
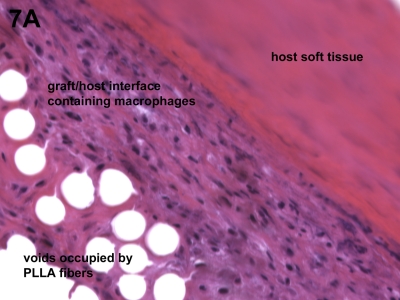
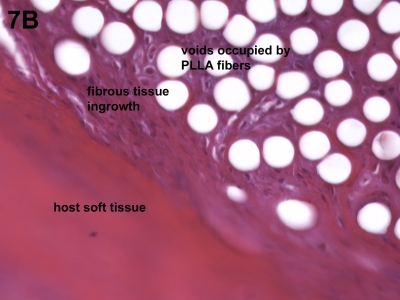
Histologic analysis revealed only the expected host cell response to a biocompatible biomaterial such as poly-L-lactide. Specifically, macrophages and giant cells were identified in sporadic regions along the surface of the X-Repair device, but no neutrophils or lymphocytes were observed (Fig. 7-A). Regions of fibrous tissue ingrowth were observed as well as occasional areas that appeared fibrocartilage-like (Fig. 7-B).
Fig. 7-A Fig. 7-B.
Figs. 7-A and 7-B Host cell response to the augmentation device. Histologic analysis revealed only the expected host cell response to a biocompatible biomaterial such as poly-L-lactide (PLLA). Fig. 7-A Macrophages and giant cells were identified in sporadic regions along the surface of the device, but no neutrophils or lymphocytes were observed (hematoxylin and eosin, ×40). Fig. 7-B Regions of fibrous tissue ingrowth were observed as well as occasional areas that appeared fibrocartilage-like (hematoxylin and eosin, ×40).
Comparison of Twelve-Week Repairs with Time-Zero Controls and Normal Controls
The cross-sectional area of both the unaugmented and augmented repairs at twelve weeks was two to fourfold greater than that of the normal controls (p < 0.001; Table III).
TABLE III.
Comparison of Twelve-Week Repairs with Normal Control Group and Respective Time-Zero Groups
| Unaugmented Repairs*
|
Augmented Repairs*
|
||||
|---|---|---|---|---|---|
| Normal Controls* | Time Zero | 12 Weeks | Time Zero | 12 Weeks | |
| Cross-sectional area (mm2) | 32 ± 2 | NA | 59 ± 20† | NA | 129 ± 34† |
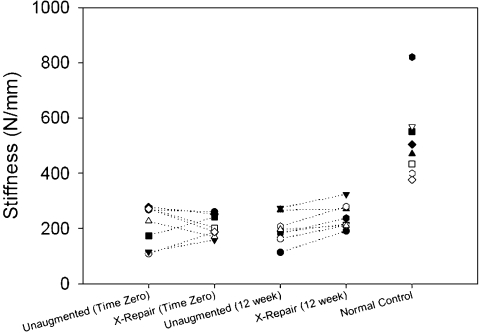
| Stiffness (N/mm) | 515 ± 141 | 214 ± 72† | 199 ± 53† | 207 ± 39† | 243 ± 45† |
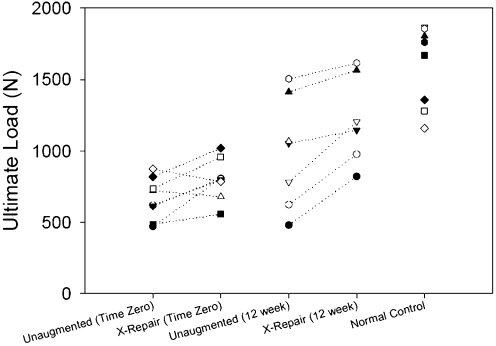
| Ultimate load (N) | 1595 ± 285 | 668 ± 146† | 990 ± 386† | 801 ± 145† | 1223 ± 316†‡ |
The values are given as the mean and the standard deviation. Each group had eight samples except for ultimate load testing in both the unaugmented group at twelve weeks (seven samples) and the augmented group at twelve weeks (six samples). NA = not available.
Compared with normal controls, the difference was significant (p < 0.05).
Compared with augmented time-zero group, the difference was significant (p = 0.016).
The stiffness of unaugmented repairs did not increase significantly between time zero and twelve weeks of healing (p = 0.944; Table III) and remained significantly less (an average of 39%) compared with that of normal controls at twelve weeks (p < 0.001; Table III). The ultimate load of unaugmented repairs was not significantly increased between time zero and twelve weeks of healing (p = 0.097, post hoc power = 0.585; Table III), and remained significantly less (an average of 62%) compared with that of normal controls at twelve weeks (p = 0.002; Table III).
The stiffness of augmented repairs was not significantly increased between time zero and twelve weeks of healing (p = 0.709; Table III), and it remained significantly less (an average of 47%) than that of normal controls at twelve weeks (p < 0.001; Table III). In contrast to the results in unaugmented repairs, the ultimate load of augmented repairs at twelve weeks was significantly increased compared with time zero (p = 0.016; Table III), yet it remained significantly less (an average of 77%) than that of normal controls (p = 0.034; Table III).
Discussion
We evaluated the extent to which augmentation of acute rotator cuff tendon repairs with a newly designed poly-L-lactide repair device would affect stiffness, ultimate load, and failure mode of the repair in a canine model. The device has high suture retention (approximately 400 N), and its stiffness (approximately 200 N/mm) and ultimate load (approximately 800 N) are similar to those of human rotator cuff tendon strips of similar width57-59.
At time zero, device augmentation did not significantly increase the stiffness of the repair construct in this animal model compared with repairs without augmentation, despite the use of a device with mechanical properties similar to the tendon and deliberately pretensioning the device so as to off-load the repair. (The lack of difference should not be interpreted as meaning the groups were equivalent, as this study was underpowered to detect differences of <40 N/mm.) The potential for device augmentation to increase construct stiffness may have been abrogated by the prefailure loading cycles. The cyclic protocol (100 loading cycles from 5 to 100 N) was intended to represent a realistic early loading paradigm; hence, it should be appreciated that the potential clinical benefit of device augmentation (even with pretensioning) may be mitigated by so-called suture setting in the device and tendon fibers during the early loading period. However, the parallel organization of the canine infraspinatus tendon likely makes it more sensitive to suture setting and/or slippage than the more interwoven organization of the human rotator cuff. The 23% increased ultimate load achieved with device augmentation at time zero may simply be the result of having five points of tendon fixation (two sutures between tendon and bone as well as three sutures between device and tendon) rather than three points. Note that the ultimate load with device augmentation sometimes occurred at a point after the tendon had completely separated from the bone. In these cases, the device would act as a bridge between the bone and tendon beyond what would have been the failure point for an unaugmented repair.
At twelve weeks after surgery, all of the repairs were considered to show evidence of tendon retraction, revealing that the loads and/or displacements experienced by all repair constructs were in excess of those required to pull sutures through tendon to some extent. So-called gap formation (or tendon retraction) following rotator cuff tendon repair has been reported previously in animal models23,33, and is a common mode of failure in patients following rotator cuff repair24,25. Augmented repairs demonstrated significantly lessretraction than repairs that had not been augmented, preventing massive (>2 cm) tendon retraction and maintaining a connected tendon-bone bridge. The relevance of this outcome is made manifest by the significant relationship found between tendon retraction distance and both stiffness and ultimate load—that is, the repairs that retracted less had higher stiffness and higher ultimate load. A similar, inverse correlation between retraction distance and repair strength and stiffness has been shown in healing flexor tendon repair60,61. Together, these data emphasize that achieving less retraction by way of device augmentation translates into a stiffer and stronger tendon repair.
At twelve weeks, augmented repairs demonstrated significantly greater stiffness (an average increase of 26%) than did repairs that had not been augmented. Our data showed that increased stiffness is not explained by increased cross-sectional area of the repair. It is possible that repair stiffness actually first decreases from time zero during the early weeks following repair as the suture attachments that primarily govern stiffness at time zero soften62,63. By twelve weeks, the stiffness of the repairs may be increasing by way of device integration to host tissues, tendon healing, and/or new tissue deposition. At twelve weeks, we would expect minimal degradation of the poly-L-lactide device; therefore, if the device has become integrated to some degree with the host tissue, it could contribute to the functional properties of the repair.
Similarly, at twelve weeks, augmented repairs demonstrated significantly greater ultimate load (an average increase of 35%) than did repairs that had not been augmented. Ultimate load was moderately correlated with cross-sectional area, so the increased tissue mass associated with augmented repairs may explain at least in part the increase in ultimate load. As with stiffness, device integration with host tissues and/or new tissue deposition may also play a role. The ultimate load of augmented repairs at twelve weeks was significantly increased compared with time-zero controls and was 77% of normal. One caveat must be raised in interpreting this result: the failure mode of normal tendon was exclusively osseous avulsion of the humeral head, indicating that normal control tendon strength is even greater than the ultimate loads measured. Notwithstanding this consideration, the results suggest that the ultimate load of augmented repairs is improving toward a normal functional outcome in this animal model.
The ability of scaffold devices to provide rotator cuff repair augmentation and support tendon regeneration has been investigated in animal models. Studies in which devices were investigated for repair augmentation (i.e., the device was applied over the primary tendon-bone repair)28,33,64 are more appropriate for comparison with our work than are studies in which devices were investigated as interpositional scaffolds to replace a resected tendon26,29-31,34,36,37. Schlegel et al. performed full-width infraspinatus injury and repair in sheep33. They placed a 10 × 20-mm patch of small intestine submucosa over the superficial aspect of the repaired tendon. The control was tendon repair without a graft. They reported that “both constructs showed evidence of gap formation as the tendon healed medial to the original repair site,” suggesting that graft augmentation was insufficient to prevent tendon retear in this animal model. At twelve weeks, repairs augmented with small intestine submucosa were significantly stiffer (39%) than unaugmented repairs, and stiffness was 40% of normal. The ultimate load of augmented repairs averaged 27% more than that of unaugmented repairs; however, this result was not significant. Nicholson et al. performed a partial-width infraspinatus injury and repair in sheep, investigating the effect of repair augmentation with small intestine submucosa or cross-linked porcine dermis grafts64. They reported little or no difference in ultimate load between graft-augmented and unaugmented repairs at nine or twenty-four weeks of healing. MacGillivray et al. performed the only study with use of a woven poly-L-lactide scaffold for rotator cuff repair augmentation28. Using the goat model, they created a full-width infraspinatus tendon injury with a 6 × 6-mm tendon defect prior to repair with or without augmentation. The poly-L-lactide device was fixed to the superficial aspect of the repaired tissue in a manner that may have offered some resistance to suture pull-through27. Ultimate loads of augmented repairs were not significantly different from unaugmented controls at twelve weeks.
As we continue to assess device augmentation strategies in animal models, it is clear that our interpretation and comparison of various approaches would be greatly aided by the adoption of some commonalities in experimental design with respect to species, surgical injury, study design, surgical technique, outcome measures, and spectrum of controls. In this study, we reasoned that a partial-width-injury model might moderate the rate of repair failures and mimic the mechanical environment of many single tendon tears in the human injury condition. However, we observed a 100% rate of retear with the partial-width model, which, in hindsight, we postulate to be a consequence of the parallel aligned fascicles in the canine tendon that are able to retract independently when the muscle contracts.
Our study was not without limitations. First, the twelve-week time point, while commonly used33, does not allow us to investigate the long-term effects of device augmentation. Second, while all repairs had four holes (two transosseous tunnels) drilled in the bone, repairs with the device had a fifth hole in the bone. This fifth hole was subsequently filled with the cortical screw; however, it is possible that the extra bone hole may have slightly biased healing in the augmented group. Third, we performed histologic analysis after mechanical testing and only on the augmented repair samples to assess biocompatibility of the X-Repair device. This study did not seek to evaluate differences in histologic outcomes between the unaugmented and augmented groups. Finally, the study was powered primarily to evaluate the clinically important differences of 40 N/mm in stiffness and 200 N in ultimate load between paired unaugmented and augmented samples at time zero and at twelve weeks. To detect the same-sized differences between unpaired groups, the study was underpowered. To more rigorously address comparisons among time zero, twelve weeks, and normal control groups, a greater sample size would be required in order to accommodate the increased between-dog variance that is introduced by the unpaired analysis.
In conclusion, rotator cuff repair augmentation with a poly-L-lactide device reduces tendon retraction distance and improves the stiffness and ultimate load of the repair at twelve weeks in the canine model. The mechanically robust poly-L-lactide scaffold was biocompatible and would be expected to be resorbed by the host over a period of months or years. While limiting but not eliminating tendon repair retraction, the augmentation device provided a tendon-bone bridge for host tissue deposition and ingrowth, resulting in improved biomechanical function of the repair at twelve weeks. Such a device, applied in a similar manner, might offer a functional benefit to human patients undergoing rotator cuff repair. However, if the fourfold increase in cross-sectional area associated with device augmentation in this animal model were to occur in human repairs, impingement against the acromial arch could occur. 
Supplementary Material
Disclosure: In support of their research for or preparation of this work, one or more of the authors received, in any one year, outside funding or grants in excess of $10,000 from the National Institutes of Health (T32 AR 050959-01, P30 AR-050953, and R44 AR051260). One or more of the authors or a member of his or her immediate family received, in any one year, payments or other benefits of less than $10,000 or a commitment or agreement to provide such benefits from commercial entities (Wyeth and United HealthCare), and one or more of the authors or a member of his or her immediate family received, in any one year, payments or other benefits in excess of $10,000 or a commitment or agreement to provide such benefits from commercial entities (DePuy and Tornier). Also, a commercial entity (Synthasome, Inc.) paid or directed in any one year, or agreed to pay or direct, benefits in excess of $10,000 to a research fund, foundation, division, center, clinical practice, or other charitable or nonprofit organization with which one or more of the authors, or a member of his or her immediate family, is affiliated or associated.
Investigation performed at the Departments of Orthopaedic Surgery and Biomedical Engineering, Cleveland Clinic, Cleveland, Ohio
References
- 1.American Academy of Orthopaedic Surgeons. Number of patients, number of procedures, average patient age, average length of stay—National Hospital Discharge Survey 1998-2005. http://www.aaos.org/Research/stats/Rotator_Cuff_Repair.pdf.
- 2.Accousti KJ, Flatow EL. Technical pearls on how to maximize healing of the rotator cuff. Instr Course Lect. 2007;56:3-12. [PubMed] [Google Scholar]
- 3.Bishop J, Klepps S, Lo IK, Bird J, Gladstone JN, Flatow EL. Cuff integrity after arthroscopic versus open rotator cuff repair: a prospective study. J Shoulder Elbow Surg. 2006;15:290-9. [DOI] [PubMed] [Google Scholar]
- 4.Boileau P, Brassart N, Watkinson DJ, Carles M, Hatzidakis AM, Krishnan SG. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87:1229-40. [DOI] [PubMed] [Google Scholar]
- 5.Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86:219-24. [DOI] [PubMed] [Google Scholar]
- 6.Gazielly DF, Gleyze P, Montagnon C. Functional and anatomical results after rotator cuff repair. Clin Orthop Relat Res. 1994;304:43-53. [PubMed] [Google Scholar]
- 7.Gerber C, Fuchs B, Hodler J. The results of repair of massive tears of the rotator cuff. J Bone Joint Surg Am. 2000;82:505-15. [DOI] [PubMed] [Google Scholar]
- 8.Harryman DT 2nd, Mack LA, Wang KY, Jackins SE, Richardson ML, Matsen FA 3rd. Repairs of the rotator cuff. Correlation of functional results with integrity of the cuff. J Bone Joint Surg Am. 1991;73:982-9. [PubMed] [Google Scholar]
- 9.Romeo AA, Hang DW, Bach BR Jr, Shott S. Repair of full thickness rotator cuff tears. Gender, age, and other factors affecting outcome. Clin Orthop Relat Res. 1999;367:243-55. [PubMed] [Google Scholar]
- 10.Cofield RH, Parvizi J, Hoffmeyer PJ, Lanzer WL, Ilstrup DM, Rowland CM. Surgical repair of chronic rotator cuff tears. A prospective long-term study. J Bone Joint Surg Am. 2001;83:71-7. [DOI] [PubMed] [Google Scholar]
- 11.Bartolozzi A, Andreychik D, Ahmad S. Determinants of outcome in the treatment of rotator cuff disease. Clin Orthop Relat Res. 1994;308:90-7. [PubMed] [Google Scholar]
- 12.Riley GP, Harrall RL, Constant CR, Chard MD, Cawston TE, Hazleman BL. Tendon degeneration and chronic shoulder pain: changes in the collagen composition of the human rotator cuff tendons in rotator cuff tendinitis. Ann Rheum Dis. 1994;53:359-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goutallier D, Postel JM, Gleyze P, Leguilloux P, Van Driessche S. Influence of cuff muscle fatty degeneration on anatomic and functional outcomes after simple suture of full-thickness tears. J Shoulder Elbow Surg. 2003;12:550-4. [DOI] [PubMed] [Google Scholar]
- 14.Hamada K, Tomonaga A, Gotoh M, Yamakawa H, Fukuda H. Intrinsic healing capacity and tearing process of torn supraspinatus tendons: in situ hybridization study of alpha 1 (I) procollagen mRNA. J Orthop Res. 1997;15:24-32. [DOI] [PubMed] [Google Scholar]
- 15.Thomopoulos S, Soslowsky LJ, Flanagan CL, Tun S, Keefer CC, Mastaw J, Carpenter JE. The effect of fibrin clot on healing rat supraspinatus tendon defects. J Shoulder Elbow Surg. 2002;11:239-47. [DOI] [PubMed] [Google Scholar]
- 16.Iannotti JP. Full-thickness rotator cuff tears: factors affecting surgical outcome. J Am Acad Orthop Surg. 1994;2:87-95. [DOI] [PubMed] [Google Scholar]
- 17.Uhthoff HK, Trudel G, Himori K. Relevance of pathology and basic research to the surgeon treating rotator cuff disease. J Orthop Sci. 2003;8:449-56. [DOI] [PubMed] [Google Scholar]
- 18.Melillo AS, Savoie FH 3rd, Field LD. Massive rotator cuff tears: debridement versus repair. Orthop Clin North Am. 1997;28:117-24. [DOI] [PubMed] [Google Scholar]
- 19.Rockwood CA Jr, Williams GR Jr, Burkhead WZ Jr. Débridement of degenerative, irreparable lesions of the rotator cuff. J Bone Joint Surg Am. 1995;77:857-66. [DOI] [PubMed] [Google Scholar]
- 20.Hawkins RH, Dunlop R. Nonoperative treatment of rotator cuff tears. Clin Orthop Relat Res. 1995;321:178-88. [PubMed] [Google Scholar]
- 21.Burkhart SS. Partial repair of massive rotator cuff tears: the evolution of a concept. Orthop Clin North Am. 1997;28:125-32. [DOI] [PubMed] [Google Scholar]
- 22.Dines DM, Moynihan DP, Dines JS, McCann P. Irreparable rotator cuff tears: what to do and when to do it; the surgeon's dilemma. Instr Course Lect. 2007;56:13-22. [PubMed] [Google Scholar]
- 23.Rodeo SA, Potter HG, Kawamura S, Turner AS, Kim HJ, Atkinson BL. Biologic augmentation of rotator cuff tendon-healing with use of a mixture of osteoinductive growth factors. J Bone Joint Surg Am. 2007;89:2485-97. [DOI] [PubMed] [Google Scholar]
- 24.Burkhart SS, Diaz Pagàn JL, Wirth MA, Athanasiou KA. Cyclic loading of anchor-based rotator cuff repairs: confirmation of the tension overload phenomenon and comparison of suture anchor fixation with transosseous fixation. Arthroscopy. 1997;13:720-4. [DOI] [PubMed] [Google Scholar]
- 25.Cummins CA, Murrell GA. Mode of failure for rotator cuff repair with suture anchors identified at revision surgery. J Shoulder Elbow Surg. 2003;12:128-33. [DOI] [PubMed] [Google Scholar]
- 26.Aoki M, Miyamoto S, Okamura K, Yamashita T, Ikada Y, Matsuda S. Tensile properties and biological response of poly(L-lactic acid) felt graft: an experimental trial for rotator-cuff reconstruction. J Biomed Mater Res B Appl Biomater. 2004;71:252-9. [DOI] [PubMed] [Google Scholar]
- 27.Koh JL, Szomor Z, Murrell GA, Warren RF. Supplementation of rotator cuff repair with a bioresorbable scaffold. Am J Sports Med. 2002;30:410-3. [DOI] [PubMed] [Google Scholar]
- 28.MacGillivray JD, Fealy S, Terry MA, Koh JL, Nixon AJ, Warren RF. Biomechanical evaluation of a rotator cuff defect model augmented with a bioresorbable scaffold in goats. J Shoulder Elbow Surg. 2006;15:639-44. [DOI] [PubMed] [Google Scholar]
- 29.Kimura A, Aoki M, Fukushima S, Ishii S, Yamakoshi K. Reconstruction of a defect of the rotator cuff with polytetrafluoroethylene felt graft. Recovery of tensile strength and histocompatibility in an animal model. J Bone Joint Surg Br. 2003;85:282-7. [DOI] [PubMed] [Google Scholar]
- 30.Adams JE, Zobitz ME, Reach JS Jr, An KN, Steinmann SP. Rotator cuff repair using an acellular dermal matrix graft: an in vivo study in a canine model. Arthroscopy. 2006;22:700-9. [DOI] [PubMed] [Google Scholar]
- 31.Dejardin LM, Arnoczky SP, Ewers BJ, Haut RC, Clarke RB. Tissue-engineered rotator cuff tendon using porcine small intestine submucosa. Histologic and mechanical evaluation in dogs. Am J Sports Med. 2001;29:175-84. [DOI] [PubMed] [Google Scholar]
- 32.Sano H, Kumagai J, Sawai T. Experimental fascial autografting for the supraspinatus tendon defect: remodeling process of the grafted fascia and the insertion into bone. J Shoulder Elbow Surg. 2002;11:166-73. [DOI] [PubMed] [Google Scholar]
- 33.Schlegel TF, Hawkins RJ, Lewis CW, Motta T, Turner AS. The effects of augmentation with Swine small intestine submucosa on tendon healing under tension: histologic and mechanical evaluations in sheep. Am J Sports Med. 2006;34:275-80. [DOI] [PubMed] [Google Scholar]
- 34.Zalavras CG, Gardocki R, Huang E, Stevanovic M, Hedman T, Tibone J. Reconstruction of large rotator cuff tendon defects with porcine small intestinal submucosa in an animal model. J Shoulder Elbow Surg. 2006;15:224-31. [DOI] [PubMed] [Google Scholar]
- 35.Zheng MH, Chen J, Kirilak Y, Willers C, Xu J, Wood D. Porcine small intestine submucosa (SIS) is not an acellular collagenous matrix and contains porcine DNA: possible implications in human implantation. J Biomed Mater Res B Appl Biomater. 2005;73:61-7. [DOI] [PubMed] [Google Scholar]
- 36.Funakoshi T, Majima T, Suenaga N, Iwasaki N, Yamane S, Minami A. Rotator cuff regeneration using chitin fabric as an acellular matrix. J Shoulder Elbow Surg. 2006;15:112-8. [DOI] [PubMed] [Google Scholar]
- 37.Funakoshi T, Majima T, Iwasaki N, Suenaga N, Sawaguchi N, Shimode K, Minami A, Harada K, Nishimura S. Application of tissue engineering techniques for rotator cuff regeneration using a chitosan-based hyaluronan hybrid fiber scaffold. Am J Sports Med. 2005;33:1193-201. [DOI] [PubMed] [Google Scholar]
- 38.Aurora A, McCarron J, Iannotti JP, Derwin K. Commercially available extracellular matrix materials for rotator cuff repairs: state of the art and future trends. J Shoulder Elbow Surg. 2007;16(5 Suppl):S171-8. [DOI] [PubMed] [Google Scholar]
- 39.Derwin KA, Baker AR, Spragg RK, Leigh DR, Iannotti JP. Commercial extracellular matrix scaffolds for rotator cuff tendon repair. Biomechanical, biochemical, and cellular properties. J Bone Joint Surg Am. 2006;88:2665-72. [DOI] [PubMed] [Google Scholar]
- 40.Barber FA, Herbert MA, Coons DA. Tendon augmentation grafts: biomechanical failure loads and failure patterns. Arthroscopy. 2006;22:534-8. [DOI] [PubMed] [Google Scholar]
- 41.Metcalf MH, Savoie FH 3rd, Kellum B. Surgical technique for xenograft (SIS) augmentation of rotator-cuff repairs. Oper Tech Orthop. 2002;12:204-8. [Google Scholar]
- 42.Iannotti JP, Codsi MJ, Kwon YW, Derwin K, Ciccone J, Brems JJ. Porcine small intestine submucosa augmentation of surgical repair of chronic two-tendon rotator cuff tears. A randomized, controlled trial. J Bone Joint Surg Am. 2006;88:1238-44. [DOI] [PubMed] [Google Scholar]
- 43.Malcarney HL, Bonar F, Murrell GA. Early inflammatory reaction after rotator cuff repair with a porcine small intestine submucosal implant: a report of 4 cases. Am J Sports Med. 2005;33:907-11. [DOI] [PubMed] [Google Scholar]
- 44.Sclamberg SG, Tibone JE, Itamura JM, Kasraeian S. Six-month magnetic resonance imaging follow-up of large and massive rotator cuff repairs reinforced with porcine small intestinal submucosa. J Shoulder Elbow Surg. 2004;13:538-41. [DOI] [PubMed] [Google Scholar]
- 45.Walton JR, Bowman NK, Khatib Y, Linklater J, Murrell GA. Restore orthobiologic implant: not recommended for augmentation of rotator cuff repairs. J Bone Joint Surg Am. 2007;89:786-91. [DOI] [PubMed] [Google Scholar]
- 46.Burkhead WZ Jr, Schiffern SC, Krishnan SG. Use of Graft Jacket as an augmentation for massive rotator cuff tears. Semin Arthrop. 2007;18:11-8. [Google Scholar]
- 47.Dopirak R, Bond JL, Snyder SJ. Arthroscopic total rotator cuff replacement with an acellular human dermal allograft matrix. Intl J Shoulder Surg. 2007;1:7-15. [Google Scholar]
- 48.Badhe SP, Lawrence TM, Smith FD, Lunn PG. An assessment of porcine dermal xenograft as an augmentation graft in the treatment of extensive rotator cuff tears. J Shoulder Elbow Surg. 2008;17(1 Suppl):35S-9S. [DOI] [PubMed] [Google Scholar]
- 49.Soler JA, Gidwani S, Curtis MJ. Early complications from the use of porcine dermal collagen implants (Permacol) as bridging constructs in the repair of massive rotator cuff tears. A report of 4 cases. Acta Orthop Belg. 2007;73:432-6. [PubMed] [Google Scholar]
- 50.Lee S, Mahar A, Bynum K, Pedowitz R. Biomechanical comparison of bioabsorbable sutureless screw anchor versus suture anchor fixation for rotator cuff repair. Arthroscopy. 2005;21:43-7. [DOI] [PubMed] [Google Scholar]
- 51.Tominaga K, Habu M, Khanal A, Mimori Y, Yoshioka I, Fukuda J. Biomechanical evaluation of different types of rigid internal fixation techniques for subcondylar fractures. J Oral Maxillofac Surg. 2006;64:1510-6. [DOI] [PubMed] [Google Scholar]
- 52.Barber FA, Boothby MH. Bilok interference screws for anterior cruciate ligament reconstruction: clinical and radiographic outcomes. Arthroscopy. 2007;23:476-81. [DOI] [PubMed] [Google Scholar]
- 53.Derwin KA, Baker AR, Codsi MJ, Iannotti JP. Assessment of the canine model of rotator cuff injury and repair. J Shoulder Elbow Surg. 2007;16(5 Suppl):S140-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ma CB, Comerford L, Wilson J, Puttlitz CM. Biomechanical evaluation of arthroscopic rotator cuff repairs: double-row compared with single-row fixation. J Bone Joint Surg Am. 2006;88:403-10. [DOI] [PubMed] [Google Scholar]
- 55.Baker AR, Abreu EL, Mascha E, Derwin KA. Homotypic variation of canine flexor tendons: implications for the design of experimental studies in animal models. J Biomech. 2004;37:959-68. Erratum in: J Biomech. 2004;37:1955. [DOI] [PubMed] [Google Scholar]
- 56.Bey MJ, Song HK, Wehrli FW, Soslowsky LJ. A noncontact, nondestructive method for quantifying intratissue deformations and strains. J Biomech Eng. 2002;124:253-8. [DOI] [PubMed] [Google Scholar]
- 57.Itoi E, Berglund LJ, Grabowski JJ, Schultz FM, Growney ES, Morrey BF, An KN. Tensile properties of the supraspinatus tendon. J Orthop Res. 1995;13:578-84. [DOI] [PubMed] [Google Scholar]
- 58.Halder A, Zobitz ME, Schultz E, An KN. Structural properties of the subscapularis tendon. J Orthop Res. 2000;18:829-34. [DOI] [PubMed] [Google Scholar]
- 59.Halder A, Zobitz ME, Schultz F, An KN. Mechanical properties of the posterior rotator cuff. Clin Biomech (Bristol, Avon). 2000;15:456-62. [DOI] [PubMed] [Google Scholar]
- 60.Gelberman RH, Boyer MI, Brodt MD, Winters SC, Silva MJ. The effect of gap formation at the repair site on the strength and excursion of intrasynovial flexor tendons. An experimental study on the early stages of tendon-healing in dogs. J Bone Joint Surg Am. 1999;81:975-82. [DOI] [PubMed] [Google Scholar]
- 61.Thomopoulos S, Zampiakis E, Das R, Silva MJ, Gelberman RH. The effect of muscle loading on flexor tendon-to-bone healing in a canine model. J Orthop Res. 2008;26:1611-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yildirim Y, Kara H, Cabukoglu C, Esemenli T. Suture holding capacity of the Achilles tendon during the healing period: an in vivo experimental study in rabbits. Foot Ankle Int. 2006;27:121-4. [DOI] [PubMed] [Google Scholar]
- 63.McDowell CL, Marqueen TJ, Yager D, Owen J, Wayne JS. Characterization of the tensile properties and histologic/biochemical changes in normal chicken tendon at the site of suture insertion. J Hand Surg [Am]. 2002;27:605-14. [DOI] [PubMed] [Google Scholar]
- 64.Nicholson GP, Breur GJ, Van Sickle D, Yao JQ, Kim J, Blanchard CR. Evaluation of a cross-linked acellular porcine dermal patch for rotator cuff repair augmentation in an ovine model. J Shoulder Elbow Surg. 2007;16(5 Suppl):S184-90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.