Abstract
Glucocorticoid receptor agonists are mainstays in the treatment of various malignancies of hematological origin. Glucocorticoids are included in therapeutic regimens for their ability to stimulate intracellular signal transduction cascades that culminate in alterations in the rate of transcription of genes involved in cell cycle progression and programmed cell death. Unfortunately, subpopulations of patients undergoing systemic glucocorticoid therapy for these diseases are or become insensitive to glucocorticoid-induced cell death, a phenomenon recognized as glucocorticoid resistance. Multiple factors contributing to glucocorticoid resistance have been identified. Here we summarize several of these mechanisms and describe the processes involved in generating a host of glucocorticoid receptor isoforms from one gene. The potential role of glucocorticoid receptor isoforms in determining cellular responsiveness to glucocorticoids is emphasized.
Keywords: glucocorticoid receptor, glucocorticoid resistance, hematological malignancy, alternative initiation of translation
Introduction
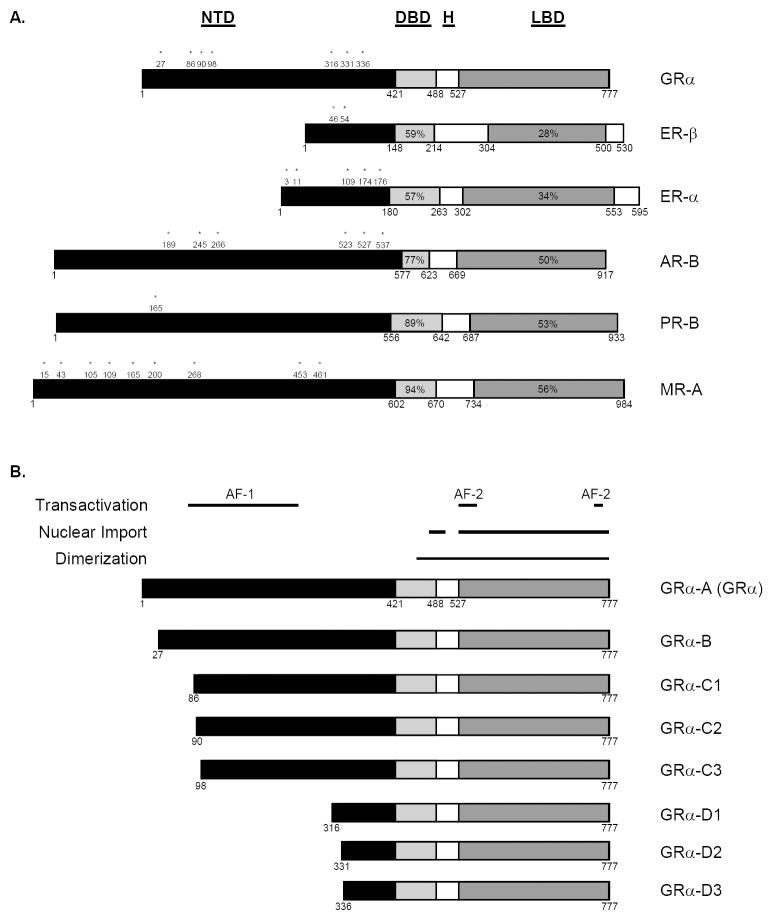
The nuclear receptor superfamily is comprised of a variety of ligand-dependent transcription factors. The steroid hormone receptors, such as the glucocorticoid, androgen, estrogen, mineralocorticoid, and progesterone receptors, are members of the nuclear receptor subfamily 3 (Nuclear Receptors Nomenclature Committee, 1999). These receptors are modular proteins that contain three distinct functional regions, the N-terminal transactivation domain (NTD), the central zinc-finger DNA-binding domain (DBD), and the C-terminal ligand-binding domain (LBD) (Figure 1A). The NTD is often referred to as the immunogenic region, as it is the most variable in length and primary sequence among steroid hormone receptors. Consistent with their evolution from a common ancestral precursor (Thornton, 2001), the DBD and LBD are highly conserved among members of the nuclear receptor subfamily 3 (Figure 1A). For example, the glucocorticoid (GR) and mineralocorticoid receptors (MR) share 94% and 56% amino acid identity in the DBD and LBD, respectively. Therefore, it is not surprising that these receptors regulate transcription from a common DNA element and exhibit overlapping hormone specificities. Given the degree of homology between functional domains within steroid hormone receptors and the promiscuous nature of steroid hormone receptors and ligands, it is remarkable that activated receptors specifically regulate a wide array of unrelated processes including, but not limited to, embryogenesis, reproduction, electrolyte homeostasis, glucose metabolism, inflammation, immunity, and cell proliferation and survival (Thompson, 1994; Lydon et al., 1995; Berger et al., 1998; Dupont et al., 2000; Cole et al., 2001; Chang et al., 2004; Schmidt et al., 2004; Rhen et al., 2005; Vihko et al., 2006). Multiple mechanisms, such as pre-receptor ligand metabolism, receptor isoform expression, and receptor-, tissue-, and cell type-specific factors, exist to generate diversity as well as specificity in the steroid hormone response (Vegeto et al., 1993; Wilson et al., 1994; Yeh et al., 1996; Nelson et al., 1999; Stewart et al., 1999; Flouriot et al., 2000; Quinkler et al., 2003; Rizner et al., 2003; Kobayashi et al., 2004; Pascual-Le Tallec et al., 2004; Lu et al., 2005; Pascual-Le Tallec et al., 2005; Chen et al., 2006; Grenier et al., 2006; Han et al., 2006; Heitzer et al., 2006; Blind et al., 2008).
Figure 1. Modular domain organization of steroid hormone receptors.
A. Steroid hormone receptors contain three major functional regions, the N-terminal transactivation domain (NTD), the central DNA-binding domain (DBD), and the C-terminal ligand-binding domain (LBD). The region located between the DBD and LBD is known as the hinge region (H). The DBD and LBD are highly conserved among receptors, and percentages indicate amino acid identity between these domains when compared to the respective domains within GRα. The numbers correspond to amino acid positions, asterisks represent internal N-terminal methionine residues conserved between the human, rat, and mouse. B. Domain organization of the GRα translational isoforms. Alternative initiation of translation gives rise to the GRα translational isoforms GRα-A, GRα-B, GRα-C1, GRα-C2, GRα-C3, GRα-D1, GRα-D2, and GRα-D3. The horizontal lines indicate functional regions embedded within the GRα protein. Due to deletions within the N-terminus, the translational isoforms lack portions of the NTD.
The pleiotropic effects of ligand-activated steroid hormone receptors under normal and pathological conditions provide an abundance of opportunities for pharmacological manipulation. Indeed, steroid hormone receptors have been exploited in the treatment of a variety of pathologies including proliferative disorders of the prostate, breast, and blood. For example, androgen receptor (AR) antagonists are used in combination with androgen ablation therapy in the treatment of androgen-dependent prostate cancer and offer a survival advantage when compared to androgen ablation therapy alone (Klotz, 2006). Similarly, selective estrogen receptor (ER) modulators that act as ER antagonists in the breast are used in the treatment of ER-positive breast cancer and, in conjunction with screening mammography, significantly reduce the rate of patient death (Berry et al., 2005). In contrast, GR agonists are the cornerstone in the treatment of a number of hematological malignancies, including leukemia, lymphoma, and myeloma (Frankfurt et al., 2004; Schmidt et al., 2004), and induce remission in 60% of children with newly-diagnosed acute lymphoblastic leukemia (ALL) (Gaynon et al., 1995). Unfortunately, a subpopulation of patients either fail to respond to steroid hormone analogue-based therapy initially or fail to respond at relapse despite response at presentation (Klumper et al., 1995; Gaynon et al., 1999; Renner et al., 2003). This phenomenon, known as steroid hormone resistance, exemplifies the variability in the steroid hormone response among individuals as well as within the same individual and presents a challenge to scientists and clinicians in the treatment of malignancies. A considerable amount of research has been devoted to understanding the complex molecular determinants of the steroid hormone response, as this knowledge may well improve the efficacy of existing treatment regimens. GR agonists (glucocorticoids) are among the most widely prescribed drugs worldwide; therefore, in this review, we will focus on the mechanisms of action and resistance to glucocorticoids, with an emphasis on the recently identified GR isoforms.
Mechanism of Glucocorticoid Action
Endogenous glucocorticoids are stress-induced hormones synthesized under the control of the hypothalamic-pituitary-adrenal axis. In response to a variety of stressors, the hypothalamic factor corticotropin-releasing hormone stimulates the release of adrenocorticotropic hormone (ACTH) from the pituitary, and ACTH induces glucocorticoid synthesis from cells within the zona fasciculata of the adrenal cortex. Glucocorticoids are highly lipophilic and are transported in the blood predominantly bound to corticosteroid-binding globulin (CBG). In many tissues, CBG-free glucocorticoids readily diffuse across the plasma membrane to exert their effects. Upon encountering a cytosolic, mature GR heterocomplex, the multi-step GR signal transduction pathway is initiated. GR heterocomplexes are formed through a series of dynamic but coordinated associations with molecular chaperones, such as heat shock proteins (hsp) 40, 70, and 90, and co-chaperones including hsp70-interacting protein (hip) and hsp70 / hsp90-organizing protein (hop) (Pratt et al., 1997; Cheung et al., 2000). The mature GR heterocomplex is made up of GR, an hsp90 dimer, a stabilizing protein called p23, and at least one of the following co-chaperones: FKBP52, FKBP51, cyclophilin 40, or protein phosphatase 5 (Pratt et al., 1997; Cheung et al., 2000). In the classic signal transduction pathway, ligand binding induces molecular rearrangement of the GR heterocomplex and promotes GR nuclear localization, homodimerization, and DNA-binding (Davies et al., 2002; Wochnik et al., 2005). The regions of the GR important for these activities are highlighted in Figure 1B. The activated GR homodimer exerts its genomic effect by interacting with specific DNA elements, termed glucocorticoid response elements (GRE), located within regulatory regions of glucocorticoid-responsive genes. After organized recruitment of coactivators or corepressors and the basal transcriptional machinery, the GR modulates the rate of gene transcription. The transcriptional response depends on the integrity of the activation function (AF) domains embedded within the NTD and LBD (Figure 1B). AF-1 is a strong transactivation domain and is able to modulate transcription in a hormone-independent fashion, whereas the relatively weak transcriptional activity of AF-2 requires hormone binding (Hollenberg et al., 1988). The direction of the transcriptional response depends in part on the nature of the GRE. Positive GREs mediate transcriptional up-regulation, whereas negative GREs (nGRE) mediate transcriptional down-regulation (Sakai et al., 1988; Drouin et al., 1989; Dostert et al., 2004). Activated GR can also influence transcription independent of a classical GRE. For example, the GR physically interacts with other transcription factors, such as nuclear factor κB (NFκB) (Ray et al., 1994), activator protein-1 (AP-1) (Yang-Yen et al., 1990), and signal transducers and activators of transcription (STAT) (Stocklin et al., 1996), and modulates their action at target genes. Deciphering the molecular mechanisms of glucocorticoid action is further complicated by the realization that glucocorticoids can induce rapid, non-genomic effects within the cytoplasm. Croxtall et al. showed that glucocorticoids stimulate the release of Src kinase from GR heterocomplexes, resulting in lipocortin 1 activation and inhibition of arachidonic acid release (Croxtall et al., 2000). Thus, the GR influences cellular processes through multiple genomic and non-genomic mechanisms.
Glucocorticoid Resistance
The glucocorticoids dexamethasone and prednisone are efficacious in the treatment of hematological malignancies presumably for their ability to regulate the expression of genes involved in cell cycle progression and programmed cell death (Renner et al., 2003; Frankfurt et al., 2004; Schmidt et al., 2004). The net effect of glucocorticoids in these disorders is induction of apoptosis, thereby inhibiting the expansion of cells that have escaped the normal constraints of the cell cycle. Glucocorticoid resistance or insensitivity is defined here as the inability of malignant cells to initiate the apoptotic program in response to a GR agonist. Remission failure or relapse in cancer patients undergoing systemic glucocorticoid therapy is characteristic of glucocorticoid resistance or acquired glucocorticoid resistance, respectively. As insensitivity to glucocorticoids limits the therapeutic benefit of GR agonists in disorders of proliferation and is positively correlated with poor prognosis (Dordelmann et al., 1999; Felice et al., 2001), it is of clinical importance to elucidate molecular mechanisms of glucocorticoid resistance.
Mutations in the GR Gene
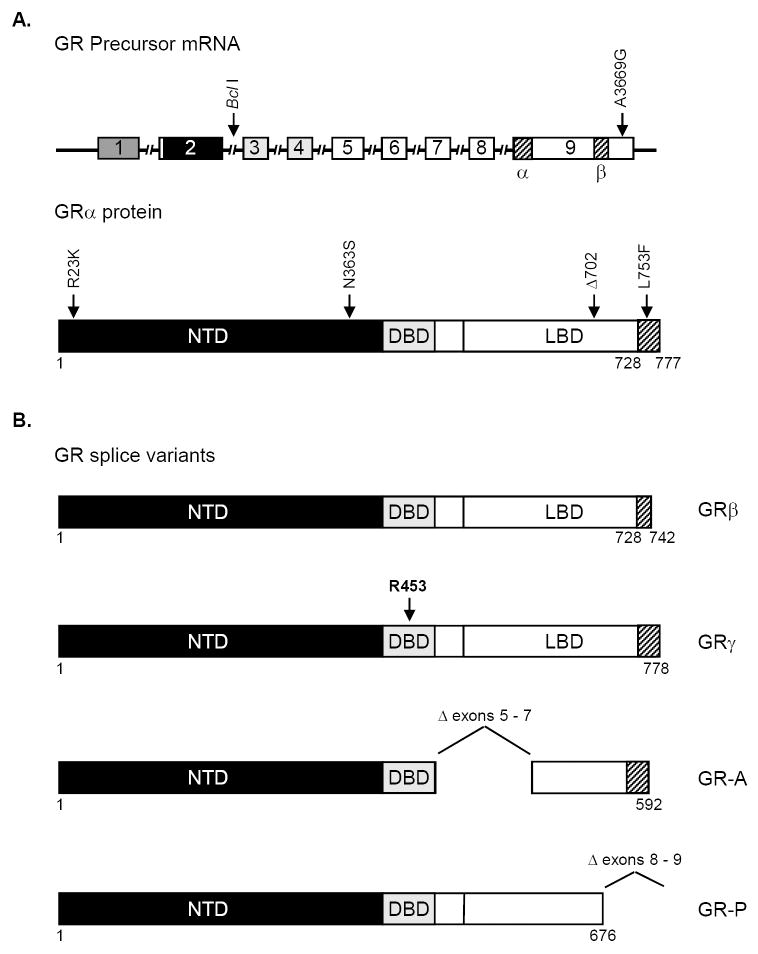
The GR is the protein product of one gene that has been mapped to chromosome 5 (5q31-32) (Francke et al., 1989; Theriault et al., 1989). Although GR gene mutations are the primary cause of an inherited form of generalized glucocorticoid resistance and in vitro acquisition of glucocorticoid resistance in various malignant cell lines (Danielsen et al., 1986; Ashraf et al., 1993; Powers et al., 1993; Lee et al., 1995; Strasser-Wozak et al., 1995; Hala et al., 1996; Riml et al., 2004; Schmidt et al., 2006), GR mutations in primary cells from glucocorticoid-resistant cancer patients had not been identified until recently (Soufi et al., 1995). Hillmann et al. were the first to discover a transactivation-deficient GR mutant (L753F) in cells from an individual with glucocorticoid-resistant ALL (Figure 2A) (Hillmann et al., 2000). Irving et al. subsequently identified a LBD-deficient GR mutant (Δ702) in cells from another individual with glucocorticoid-resistant ALL at relapse (Figure 2A) (Irving et al., 2005). The Δ702 mutation was not apparent at presentation but was evident in a day 28 remission sample, indicating that the subpopulation of cells harboring this mutation emerged as the dominant population at relapse (Irving et al., 2005). The acquisition of the GR gene mutation occurred without mention of generalized glucocorticoid resistance and has been attributed to either spontaneous mutation or mutation induced by DNA damaging agents that were used in conjunction with glucocorticoid therapy. Indeed, the chemotherapeutic agent bleomycin has been reported to induce deletions and/or chromosomal breaks in the GR gene resulting in the emergence of glucocorticoid-resistant cell lines (Palmer et al., 1992). The two cases described above are the only known examples of resistance to glucocorticoid therapy in malignancy attributable to acquired GR mutations in vivo. However, the sensitivity of conventional assays for identification of GR gene mutations may be inadequate for detection of aberrant GR in heterogeneous cell populations. Therefore, acquisition of GR mutations in vivo may be underrepresented as a mechanism of glucocorticoid resistance.
Figure 2. GR Variants.
A. GR polymorphisms and acquired mutations. Exons within the GR precursor mRNA are denoted with a rectangle and shading indicates that the NTD is encoded by exon 2, the DBD is encoded by exons 3 and 4, and the C-terminal region is encoded by exons 5-9. The proximal and distal regions of exon 9 are denoted α and β, respectively. Introns are marked with hashed bars. The arrows in the top panel indicate polymorphisms that do not result in amino acid changes, whereas the arrows in the bottom panel indicate polymorphisms that do result in amino acid changes. Furthermore, the locations of the L753F and Δ702 GR mutations that are associated with acquired glucocorticoid resistance to therapy in vivo are shown in the bottom panel. B. The GR splice variants. GRβ mRNA is produced from splicing of exon 8 to exon 9β, and the resulting protein contains 15 unique amino acids at the C-terminus (amino acids 728-742). GRγ mRNA is generated by alternative usage of a splice donor site at the exon 3 / exon 4 boundary and encodes for a protein containing an arginine insertion (R453) between the two zinc fingers of the DBD. The GR-A variant is produced from splicing of exon 4 to exon 8. Therefore, exons 5 – 7 are deleted. GR-P is formed by a failure to splice exon 7 to exon 8. Thus, GR-P contains the splice donor site of exon 7 followed by intact intronic sequences. Due to the deletion of 3’ exons, GR-A and GR-P lack significant portions of the LBD.
GR Expression Levels
The GR is a ubiquitously expressed protein, found in almost all human cell types and tissues at appreciable levels. It is well-established that the level of GR expression is closely correlated with the magnitude of the glucocorticoid response (Vanderbilt et al., 1987); therefore, it is not surprising that the sensitivity of malignant cells to glucocorticoids is partially dependent on the number of receptors found within the cell. Several groups have shown that reduced GR expression in primary ALL cells is associated with initial resistance to glucocorticoid therapy, relapse, and poor prognosis (Bloomfield et al., 1981; Wells et al., 1981; Ho et al., 1982; Pui et al., 1984; Federico et al., 1986; Sakurai et al., 1986). GR levels in cells are dynamic and are regulated in a cell-type specific manner by the surrounding concentration of ligand (Cidlowski et al., 1981; Hoeck et al., 1989; Silva et al., 1994; Alarid, 2006). In several cell lines and tissues, administration of GR agonists results in down-regulation of the GR (Cidlowski et al., 1981; Svec et al., 1981; Schlechte et al., 1982; Tornello et al., 1982; Lacroix et al., 1984; Sapolsky et al., 1984; Hoeck et al., 1989; Silva et al., 1994). The mechanism of glucocorticoid-induced GR down-regulation has been attributed to reduced transcription of the GR gene as well as decreased stability of the GR mRNA and protein (Dong et al., 1988; Rosewicz et al., 1988; Vedeckis et al., 1989). Promoter-dependent (Govindan et al., 1991) and -independent (Okret et al., 1986; Burnstein et al., 1990; Burnstein et al., 1994) DNA elements are likely involved in down-regulation of GR mRNA expression, whereas proteosome-mediated GR degradation contributes to increased turnover of the GR protein (Wallace et al., 2001). While hormone-induced down-regulation of GR represents a mechanism for maintaining glucocorticoid homeostasis in normal cells, it has the potential to limit therapeutic responses to glucocorticoids in malignant cells. For example, glucocorticoid-induced reductions in GR content in primary ALL cells between diagnosis and relapse have been observed and are associated with resistance to glucocorticoid therapy (Bloomfield et al., 1981; Pui et al., 1984; Pui et al., 1986). In contrast, hormone-induced up-regulation of GR (autoinduction) is associated with glucocorticoid sensitivity. For example, T-cell lines that exhibit GR autoinduction at the protein and/or mRNA level are sensitive to glucocorticoid-induced apoptosis (Eisen et al., 1988; Antakly et al., 1989; Gruol et al., 1989; Gomi et al., 1990; Ashraf et al., 1991; Denton et al., 1993), whereas T-cell lines that fail to autoinduce GR are resistant to this effect (Gomi et al., 1990; Ramdas et al., 1999; Pedersen et al., 2003; Riml et al., 2004; Schmidt et al., 2006). The mechanism of autoinduction is likely to be transcriptional as GREs have been identified within specific regions of the GR promoter (Breslin et al., 2001; Presul et al., 2007). Importantly, Tissing et al. showed no correlation between autoinduction of GR mRNA and glucocorticoid responsiveness in primary childhood T-cell ALL or precursor B-cell ALL (Tissing et al., 2006). It should be noted, however, that in this study GR protein level was not analyzed (Tissing et al., 2006). Thus, the precise role of GR autoinduction in determining glucocorticoid sensitivity in patients warrants further investigation. Taken together, these data suggest that GR expression level may be an important determinant of the glucocorticoid response in malignant cells. Moreover, glucocorticoid sensitivity may critically depend on the effect of GR agonists on the number of intracellular GR, i.e. whether glucocorticoid-induced down-regulation or autoinduction predominates.
Alternative Processing of the GR Gene
Experiments conducted in our laboratory and others have revealed multiple mechanisms for generating a host of GR proteins from the single GR gene (Hollenberg et al., 1985; Moalli et al., 1993; Rivers et al., 1999; Yudt et al., 2001; Geng et al., 2005; Lu et al., 2005; Turner et al., 2007). These isoforms exhibit tissue-specific patterns of expression as well as differences in subcellular localization and transcriptional activity (Oakley et al., 1997; Oakley et al., 1999; Rivers et al., 1999; Yudt et al., 2001; Geng et al., 2005; Lu et al., 2005; Turner et al., 2007). Therefore, the repertoire of GR subtypes expressed by a particular cell may contribute to the ability of the cell to respond to glucocorticoids. Below, we describe transcriptional, post-transcriptional, and translational mechanisms involved in controlling GR expression, and the potential roles for specific GR isoforms in glucocorticoid resistance are discussed.
Alternative promoter usage
The human GR cDNA was first cloned in 1985 (Hollenberg et al., 1985), and elucidation of genomic structure revealed the presence of 9 exons that span an 80 kB region (Encio et al., 1991; Oakley et al., 1996). Exon 1 contains the 5’ untranslated region, and recent studies have identified 9 alternative exon 1s (1A, 1B, 1C, 1D, 1E, 1F, 1H, 1I, and 1J) that are generated from unique promoters (Breslin et al., 2001; Turner et al., 2005; Presul et al., 2007). Additional variability in exon 1 is accomplished through alternative splicing of exon 1A into 1A1, 1A2, or 1A3 and exon 1C into 1C1, 1C2, or 1C3 (Breslin et al., 2001; Turner et al., 2005). Even though the heterogeneity in exon 1 does not affect the sequence of the GR protein per se, cell type-selective promoter usage exists and may serve to regulate GR protein levels (see section on GR expression levels) (Breslin et al., 2001; Presul et al., 2007), alternative splicing that occurs elsewhere in the primary GR transcript (see section on alternative splicing) (Russcher et al., 2007), and/or alternative initiation of translation of mature GR mRNA (see section on alternative translation initiation) (Pedersen et al., 2004), all of which are important factors in determining glucocorticoid sensitivity.
Alternative Splicing
The GR protein is encoded by exons 2 – 9 of the GR gene (Encio et al., 1991) (Figure 2A), and alternative splicing of GR precursor mRNA gives rise to 5 GR protein subtypes that have been termed GRα, GRβ, GRγ, GR-A, and GR-P (Figures 2A and 2B). The ubiquitously expressed GRα is the classic, functionally active receptor and is generated through splicing of exon 8 to the proximal end of exon 9 (9α), whereas GRβ is produced through splicing of exon 8 to the distal end of exon 9 (9β). GRα is made up of 777 amino acids, including 50 C-terminal residues derived from exon 9α. In contrast, GRβ is a shorter protein of 742 residues, the final 15 of which are encoded by exon 9β. Thus, GRα and GRβ proteins share identical N-termini encoded by exons 2-8 and are distinguished only by their C-termini (Hollenberg et al., 1985). The presence of the 15 unique C-terminal amino acids exerts a profound influence on GRβ function (Yudt et al., 2003). GRβ has been reported not to bind glucocorticoid agonists (Oakley et al., 1999) and was originally thought to control transcription only through a dominant-negative effect on GRα-induced gene expression (Bamberger et al., 1995; Oakley et al., 1999). Recently however, Lewis-Tuffin et al. demonstrated that GRβ regulates gene expression even in the absence of GRα and interacts with the GRα antagonist/partial agonist RU-486, which diminishes its transcriptional capacity (Lewis-Tuffin et al., 2007). Furthermore, in a manner similar to GRα, GRβ represses basal activity of the interleukin 5 (IL-5) and IL-13 cytokine promoters through recruitment of histone deacetylase 1 (Kelly et al., 2008). These recent studies indicate that GRβ may have a previously unappreciated role in cell signaling.
GRβ is detected in most tissues and cell lines, but it is generally expressed at low levels when compared to GRα (Bamberger et al., 1995; de Castro et al., 1996; Oakley et al., 1996; Oakley et al., 1997; Pujols et al., 2002). However, in support of a dominant-negative function for GRβ, resistance to glucocorticoid therapy in leukemia has been associated with high cellular levels of GRβ when compared to GRα (Shahidi et al., 1999; Longui et al., 2000; Koga et al., 2005). It is important to note that this relationship is controversial, as it has not been observed by other investigators (Haarman et al., 2004; Tissing et al., 2005a). Therefore, the contribution of relative GRβ levels in determining cellular sensitivity to glucocorticoids in individuals undergoing glucocorticoid therapy for malignancies remains unclear.
In addition to the GRα and GRβ isoforms, GRγ, GR-A, and GR-P splice variants have been described (Figure 2B). The GRγ isoform contains a three base insertion in the DBD between exons 3 and 4 that results in addition of arginine between the two zinc fingers of the DBD (Rivers et al., 1999). Analysis of various tissues revealed that GRγ is widely expressed, representing 3.8 to 8.7% of the total GR mRNA (Rivers et al., 1999). Since GRγ exhibits decreased transcriptional activity when compared to GRα (Kasai, 1990; Ray et al., 1996), it is not surprising that elevated levels of GRγ are associated with glucocortoid resistance in childhood leukemia (Beger et al., 2003; Haarman et al., 2004). The GR-A and GR-P splice variants lack portions of the LBD due to the absence of exons 5 through 7 and exons 8 through 9, respectively (Moalli et al., 1993). The GR-P isoform is preferentially derived from transcripts containing exon 1B and is widely expressed (de Lange et al., 2001; Russcher et al., 2007). Studies from a number of laboratories have revealed a correlation between elevated levels of GR-P or GR-A and glucocorticoid resistance in myeloma and leukemia (Moalli et al., 1993; Krett et al., 1995; de Lange et al., 2001). However, more recent studies directly contradict these earlier findings (Tissing et al., 2005a). Interestingly, even though the GR-P variant exhibits lower transactivation potential when compared to GRα, GR-P is capable of stimulating GRα-mediated transcription in certain cell lines (de Lange et al., 2001). Therefore, GR-P may act in a cell type-specific manner to enhance glucocorticoid responsiveness. Taken together, these data suggest that alternative splicing of a common precursor GR mRNA gives rise to multiple GR isoforms. Moreover, up-regulation of the GR-β, -γ, -A, or -P isoform may represent one of many mechanisms for modulating cellular responsiveness to glucocorticoids.
Alternative Translation Initiation
Recently, our laboratory has identified alternative translation initiation as yet another mechanism for generating diversity in GR protein expression. Yudt et al. first reported that GRα cDNA expressed either in vitro or transiently in COS-1 cells, which are known to be void of detectable endogenous GR, consistently appeared as a doublet of 94 kD and 91 kD in Western blots (Yudt et al., 2001; Lu et al., 2005). Although partial proteolysis or phosphorylation could potentially explain the appearance of the doublet, neither protease inhibitor nor phosphatase inhibitor treatment affected the GRα expression pattern (Yudt et al., 2001). Thus, another mechanism must account for generation of the two GRα forms. Since alternative translational start site usage is an important mechanism for producing isoforms of other steroid hormone receptors (see below), the potential for initiation of GR translation at internal AUG codons was investigated. The GRα cDNA was first examined for additional translational start sites downstream of the initial AUG codon corresponding to methionine 1. Indeed, an in-frame AUG codon (methionine 27) that is conserved among the human, rat and mouse was identified (Figures 1A and 1B) and is indispensable for expression of the rapidly migrating species within the GR doublet (Yudt et al., 2001). The 94 kD protein is the classic GRα that is now referred to as GRα-A, and the 91 kD protein has been termed GRα-B (Yudt et al., 2001). Subsequent analysis revealed that, in addition to GRα-A and GRα-B, proteins of 82-84 and 53-56 kD are also expressed from a single GRα mRNA species (Lu et al., 2005). AUG codons corresponding to methionines 86, 90, and 98 were required for formation of the 82-84 kD proteins, whereas methionines 316, 331, and 336 were necessary for expression of the 53-56 kD proteins (Lu et al., 2005). These AUG codons are also conserved among the human, rat, and mouse and initiate the expression of GRα isoforms designated GRα-C1, C2, C3, D1, D2, and D3, respectively (Figures 1A and 1B). The mechanism for generating the translational isoforms involves leaky 5’ ribosomal scanning and/or ribosomal shunting (Lu et al., 2005), which refers to discontinuous scanning of the mRNA by the translation initiation complex (Touriol et al., 2003). Both ribosomal scanning and shunting are likely controlled by mRNA-specific elements. For example, Pederson et al. reported that GRα-B is preferentially expressed from GRα transcripts containing exon 1A3 (Pedersen et al., 2004). Although all of the GRα translational variants were detected in rat and mouse tissues, the expression levels differed (Lu et al., 2005). These data indicate that alternative initiation of translation operates in vivo and gives rise to tissue-specific GRα isoform expression patterns. Additional complexity and diversity in GR isoform expression is implicated by the potential for alternative translation initiation of GR-β transcripts, as GRα and GRβ mRNA contain identical 5’ coding regions.
Since the translational isoforms are distinguished only by the length of their N-terminus, it is not surprising that they posses common as well as unique properties. All 8 isoforms exhibit a similar affinity for glucoccorticoids and most undergo hormone-induced nuclear localization followed by GRE binding and transcriptional regulation (Lu et al., 2005; Lu et al., 2007). The GRα-D3 variant is peculiar in that it localizes to the nucleus and can bind certain GREs even in the absence of hormone (Lu et al., 2005; Lu et al., 2007). The translational isoforms are further differentiated by their transcriptional activity in reporter assays. The GRα-C3 variant is the most active, whereas the GRα-D proteins were the least active (Lu et al., 2005). Furthermore, GRα-C3 enhanced the ligand-dependent transcriptional activity of GRα-A, but GRα-D3 did not (Lu et al., 2005). These data suggest that the glucocorticoid-induced transcriptional response reflects the composite actions of GRα isoforms and that the specific intracellular pool of GRα subtypes may determine cellular sensitivity to glucocorticoids. Microarray analysis revealed a similar hierarchy with respect to transcriptional capability (Lu et al., 2005; Lu et al., 2007). Moreover, in addition to regulating a common subset of endogenous genes, the translational isoforms also regulate unique, subtype-specific genes (Lu et al., 2005). The difference in gene regulatory profiles likely contributes to the distinct apoptotic phenotype of U2-OS osteosarcoma cells stably expressing the individual translational forms. Expression of the more active GRα-C3 correlated with increased sensitivity to glucocorticoid-induced apoptosis, and expression of the relatively inactive GRα-D3 was associated with resistance to glucocorticoid-induced apoptosis (Lu et al., 2007). Additional studies are needed to determine whether the relative expression levels of translational isoforms, particularly GRα-D3, correlate with resistance to glucocorticoid-induced apoptosis in malignancies.
Interestingly, other nuclear receptor subfamily 3 members have the potential for alternative translation initiation at internal AUG codons. Multiple methionines are found in the N-terminal regions of steroid hormone receptors, and these residues are conserved among the human, rat, and mouse (Figure 1A). In fact, some of these AUG codons have been confirmed as translational start sites. For example, the AUG codon corresponding to methionine 15 gives rise to MR-B (Pascual-Le Tallec et al., 2004), whereas alternative splicing followed by usage of a translation start site corresponding to methionine 174 gives rise to ERα-46 (Flouriot et al., 2000). In addition, it has been suggested that methionine 189 gives rise to the A isoform of the AR (Wilson et al., 1994; Wilson et al., 1996), although controversy exists (Gregory et al., 2001). The N-terminal variants of ER and AR have been shown to oppose the actions of the corresponding full length receptor (Flouriot et al., 2000; Liegibel et al., 2003). These data suggest that alternative translational initiation may represent a common mechanism for controlling cellular responses to steroid hormones. However, the relative expression levels of ER and AR isoforms in steroid hormone analogue-resistant prostate and breast cancer are unknown.
Polymorphisms in the GR Gene
Genetic polymorphisms are defined as variations in DNA that are observed in 1% or more of the population. Since polymorphisms in the GR gene have been associated with variations in GR function (Lamberts et al., 1996; Huizenga et al., 1998; van Rossum et al., 2002; van Rossum et al., 2003; Russcher et al., 2005a), it has been proposed that genetic alterations in the GR gene may account for the variability in the glucocorticoid response in individuals undergoing glucocorticoid therapy for hematological malignancies.
The ER22/23EK GR polymorphism that results in an arginine to lysine change (R23K) within the NTD (Figure 2A) is associated with decreased GR transcriptional activity in reporter assays and decreased expression of endogenous genes when compared to wild type GR (van Rossum et al., 2002; Russcher et al., 2005a). Upon further analysis, Russcher et al. discovered that the ER22/23EK GR polymorphism facilitated the expression of GRα-A, but had no effect on the expression of the GRα-B translational isoform (Russcher et al., 2005b). Since some studies have shown that GRα-A is transcriptionally less active when compared to GRα-B (Yudt et al., 2001), these molecular data suggest that the relative inactivity of ER22/23EK results from decreased relative levels of GRα-B and that ER22/23EK may correlate with glucocorticoid insensitivity (Russcher et al., 2005b). Although ER22/23EK was not associated with differences in responsiveness to exogenous glucocorticoids in ALL (Tissing et al., 2005c), adult carriers of the ER22/23EK polymorphism were shown to have decreased incidence of type 2 diabetes and decreased risk for cardiovascular disease (van Rossum et al., 2004a; van Rossum et al., 2004b). Similarly, the GRβ polymorphism A3669G located in the 3’ untranslated region (Figure 2A) results in enhanced expression of the dominant negative GRβ and is associated with favorable metabolic parameters (Syed et al., 2006). These data imply that ER22/23EK and A3669G carriers have a more favorable metabolic profile due to a relative insensitivity to endogenous glucocorticoids.
In contrast to the polymorphisms described above, two particular polymorphisms in the GR gene, Bcl I and N363S, are associated with generalized increases in glucocorticoid sensitivity (Huizenga et al., 1998; van Rossum et al., 2003; Russcher et al., 2005a) and metabolic disorders (Buemann et al., 1997; Dobson et al., 2001; Di Blasio et al., 2003; Lin et al., 2003). The Bcl I variant is a restriction fragment length polymorphism (RFLP) located in intron 2, whereas the N363S polymorphism occurs within the NTD and results in an asparagine to serine substitution (Figure 2A). Interestingly, microarray analysis revealed a unique, polymorphism-specific pattern of gene regulation for N363S when compared to GRα wild type (Jewell et al., 2007). Moreover, some reports suggest that N363S is not only associated with glucocorticoid hypersensitivity but also with decreased bone mineral density (van Rossum et al., 2004b). Although individuals harboring Bcl I or N363S have no identifiable therapeutic advantage when undergoing systemic glucocorticoid therapy for hematological malignancies (Tissing et al., 2005c), it is plausible to speculate that these variants may be associated with an increased sensitivity to dose-limiting side effects of glucocorticoid therapy, such as avascular necrosis of bone (Lamberts et al., 1996). Taken together, these data imply that polymorphisms in the GR may be an indicator of disease. Furthermore, the primary sequence of the GR may prove to be of prognostic value in glucocorticoid-managed proliferative disorders and a predictor of adverse reactions.
The GR Heterocomplex
Koper et al. discovered that approximately 9% of individuals failed to respond adequately to dexamethasone suppression tests by efficiently down-regulating endogenous glucocorticoid production (Koper et al., 1997). As these subjects exhibit an underlying glucocorticoid resistance that was not associated with mutations or polymorphisms in the GR gene, it has been suggested that mechanisms independent of the GR gene itself may contribute to the development of glucocorticoid resistance in a subpopulation of individuals challenged with systemic glucocorticoid therapy (Koper et al., 1997). Since the integrity of the mature GR heterocomplex is required for optimal ligand-binding and subsequent activation of the transcriptional response, abnormalities in the chaperones and co-chaperones that make up the heterocomplex may contribute to decreased glucocorticoid responsiveness. Kojika et al. showed that alterations in hsp90 and hsp70 were associated with decreased cellular sensitivity to glucocorticoids (Kojika et al., 1996). Furthermore, aberrant hsp90 protein and low hsp70 levels were identified in two out of nine glucocorticoid-resistant human leukemic cell lines (Kojika et al., 1996). In addition, altered levels of hsp90 were found in peripheral blood mononuclear cells from individuals with steroid-resistant forms of asthma (Qian et al., 2001), multiple sclerosis (Matysiak et al., 2008), and idiopathic nephrotic syndrome (Ouyang et al., 2006). However, Lauten et al. found no relationship between hsp90 mRNA or protein levels and resistance to glucocorticoids in primary cells from individuals with ALL (Lauten et al., 2003). In agreement with these data, Tissing et al. found no significant differences in hsp90 in addition to hsp70 mRNA expression in mononuclear cells from glucocorticoid-resistant individuals with ALL when compared to glucocorticoid-sensitive individuals (Tissing et al., 2005b). Therefore, the role of hsp90 and hsp70 proteins in glucocorticoid-resistant malignancies remains unclear.
The relative levels of FKBP51 and FKBP52 have been shown to be important determinants of cellular sensitivity to glucocorticoids in various systems (Reynolds et al., 1999; Denny et al., 2000; Scammell et al., 2001; Scammell et al., 2002; Riggs et al., 2003). For example, high levels of FKBP51 and low levels of FKBP52 were associated with glucocorticoid resistance in cell lines and tissues from several genera of New World primates (Reynolds et al., 1999; Denny et al., 2000; Scammell et al., 2001; Scammell et al., 2002). Furthermore, FKBP51 overexpression inhibits hormone-induced GR transactivation in mammalian cells (Denny et al., 2000; Scammell et al., 2001; Westberry et al., 2006), and co-expression of FKBP52 blocks the inhibitory effect of FKBP51 (Denny et al., 2005; Wochnik et al., 2005). These data suggest that the relative levels of FKBP51 and FKBP52 may impact cellular sensitivity to systemic glucocorticoid therapy in malignancies. However, no significant differences in FKBP51 or FKBP52 mRNA levels were found between glucocorticoid-sensitive and glucocorticoid-resistant individuals with ALL (Tissing et al., 2005b). Due to limited availability of patient material in the aforementioned studies, the hsp90, hsp70, FKBP51, and FKBP52 genes were not sequenced (Lauten et al., 2003; Tissing et al., 2005b). As mutated versions of chaperones and co-chaperones could alter signaling through the mature GR heterocomplex potentially leading to decreased cellular sensitivity to glucocorticoid-induced cell death, the role of these proteins in glucocorticoid-resistant malignancy cannot be ruled out (Denny et al., 2000; Denny et al., 2005).
Concluding Remarks
Glucocorticoids are used in the treatment of various malignant disorders of hematological origin for their ability to regulate the expression of genes involved in cell cycle progression and apoptosis, thereby inducing cell death. Glucocorticoids exert their effects through activation of the GR signal transduction pathway, and resistance to glucocorticoid therapy can occur through multiple mechanisms. Reduced GR expression, GR down-regulation, and acquired GR mutations are factors that may potentially limit the therapeutic response to glucocorticoids. Although the impact of GR polymorphisms and altered chaperone or co-chaperone expression on glucocorticoid responsiveness in hematological malignancies is not well established, GR polymorphisms are emerging as an important risk factor for diseases of metabolic origin. Data from our laboratory and others indicate that multiple GR isoforms are derived from the single GR gene. In addition to differences in subcellular localization, transcriptional activity, and sensitivity to glucocorticoid-induced apoptosis, these isoforms exhibit tissue-specific expression patterns. These data suggest that cellular glucocorticoid responsiveness may reflect the intracellular composition of GR isoforms. Whether or not glucocorticoid-resistant malignant cells exhibit an altered pattern of GR isoform expression remains to be determined.
Acknowledgments
We thank Robert H. Oakley and Amy J. Beckley for helpful comments and discussion concerning this manuscript. This research was supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alarid ET. Lives and times of nuclear receptors. Mol Endocrinol. 2006;20:1972–1981. doi: 10.1210/me.2005-0481. [DOI] [PubMed] [Google Scholar]
- Antakly T, Thompson EB, O’Donnell D. Demonstration of the intracellular localization and up-regulation of glucocorticoid receptor by in situ hybridization and immunocytochemistry. Cancer Res. 1989;49:2230s–2234s. [PubMed] [Google Scholar]
- Ashraf J, Kunapuli S, Chilton D, Thompson EB. Cortivazol mediated induction of glucocorticoid receptor messenger ribonucleic acid in wild-type and dexamethasone-resistant human leukemic (CEM) cells. J Steroid Biochem Mol Biol. 1991;38:561–568. doi: 10.1016/0960-0760(91)90313-t. [DOI] [PubMed] [Google Scholar]
- Ashraf J, Thompson EB. Identification of the activation-labile gene: a single point mutation in the human glucocorticoid receptor presents as two distinct receptor phenotypes. Mol Endocrinol. 1993;7:631–642. doi: 10.1210/mend.7.5.8316249. [DOI] [PubMed] [Google Scholar]
- Bamberger CM, Bamberger AM, de Castro M, Chrousos GP. Glucocorticoid receptor beta, a potential endogenous inhibitor of glucocorticoid action in humans. J Clin Invest. 1995;95:2435–2441. doi: 10.1172/JCI117943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beger C, Gerdes K, Lauten M, Tissing WJ, Fernandez-Munoz I, Schrappe M, Welte K. Expression and structural analysis of glucocorticoid receptor isoform gamma in human leukaemia cells using an isoform-specific real-time polymerase chain reaction approach. Br J Haematol. 2003;122:245–252. doi: 10.1046/j.1365-2141.2003.04426.x. [DOI] [PubMed] [Google Scholar]
- Berger S, Bleich M, Schmid W, Cole TJ, Peters J, Watanabe H, Kriz W, Warth R, Greger R, Schutz G. Mineralocorticoid receptor knockout mice: pathophysiology of Na+ metabolism. Proc Natl Acad Sci U S A. 1998;95:9424–9429. doi: 10.1073/pnas.95.16.9424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry DA, Cronin KA, Plevritis SK, Fryback DG, Clarke L, Zelen M, Mandelblatt JS, Yakovlev AY, Habbema JD, Feuer EJ. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353:1784–1792. doi: 10.1056/NEJMoa050518. [DOI] [PubMed] [Google Scholar]
- Blind RD, Garabedian MJ. Differential recruitment of glucocorticoid receptor phospho-isoforms to glucocorticoid-induced genes. J Steroid Biochem Mol Biol. 2008;109:150–157. doi: 10.1016/j.jsbmb.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield CD, Smith KA, Peterson BA, Munck A. Glucocorticoid receptors in adult acute lymphoblastic leukemia. Cancer Res. 1981;41:4857–4860. [PubMed] [Google Scholar]
- Breslin MB, Geng CD, Vedeckis WV. Multiple promoters exist in the human GR gene, one of which is activated by glucocorticoids. Mol Endocrinol. 2001;15:1381–1395. doi: 10.1210/mend.15.8.0696. [DOI] [PubMed] [Google Scholar]
- Buemann B, Vohl MC, Chagnon M, Chagnon YC, Gagnon J, Perusse L, Dionne F, Despres JP, Tremblay A, Nadeau A, Bouchard C. Abdominal visceral fat is associated with a BclI restriction fragment length polymorphism at the glucocorticoid receptor gene locus. Obes Res. 1997;5:186–192. doi: 10.1002/j.1550-8528.1997.tb00292.x. [DOI] [PubMed] [Google Scholar]
- Burnstein KL, Jewell CM, Cidlowski JA. Human glucocorticoid receptor cDNA contains sequences sufficient for receptor down-regulation. J Biol Chem. 1990;265:7284–7291. [PubMed] [Google Scholar]
- Burnstein KL, Jewell CM, Sar M, Cidlowski JA. Intragenic sequences of the human glucocorticoid receptor complementary DNA mediate hormone-inducible receptor messenger RNA down-regulation through multiple mechanisms. Mol Endocrinol. 1994;8:1764–1773. doi: 10.1210/mend.8.12.7708063. [DOI] [PubMed] [Google Scholar]
- Chang C, Chen YT, Yeh SD, Xu Q, Wang RS, Guillou F, Lardy H, Yeh S. Infertility with defective spermatogenesis and hypotestosteronemia in male mice lacking the androgen receptor in Sertoli cells. Proc Natl Acad Sci U S A. 2004;101:6876–6881. doi: 10.1073/pnas.0307306101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Rogatsky I, Garabedian MJ. MED14 and MED1 differentially regulate target-specific gene activation by the glucocorticoid receptor. Mol Endocrinol. 2006;20:560–572. doi: 10.1210/me.2005-0318. [DOI] [PubMed] [Google Scholar]
- Cheung J, Smith DF. Molecular chaperone interactions with steroid receptors: an update. Mol Endocrinol. 2000;14:939–946. doi: 10.1210/mend.14.7.0489. [DOI] [PubMed] [Google Scholar]
- Cidlowski JA, Cidlowski NB. Regulation of glucocorticoid receptors by glucocorticoids in cultured HeLa S3 cells. Endocrinology. 1981;109:1975–1982. doi: 10.1210/endo-109-6-1975. [DOI] [PubMed] [Google Scholar]
- Cole TJ, Myles K, Purton JF, Brereton PS, Solomon NM, Godfrey DI, Funder JW. GRKO mice express an aberrant dexamethasone-binding glucocorticoid receptor, but are profoundly glucocorticoid resistant. Mol Cell Endocrinol. 2001;173:193–202. doi: 10.1016/s0303-7207(00)00407-x. [DOI] [PubMed] [Google Scholar]
- Croxtall JD, Choudhury Q, Flower RJ. Glucocorticoids act within minutes to inhibit recruitment of signalling factors to activated EGF receptors through a receptor-dependent, transcription-independent mechanism. Br J Pharmacol. 2000;130:289–298. doi: 10.1038/sj.bjp.0703272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsen M, Northrop JP, Ringold GM. The mouse glucocorticoid receptor: mapping of functional domains by cloning, sequencing and expression of wild-type and mutant receptor proteins. Embo J. 1986;5:2513–2522. doi: 10.1002/j.1460-2075.1986.tb04529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies TH, Ning YM, Sanchez ER. A new first step in activation of steroid receptors: hormone-induced switching of FKBP51 and FKBP52 immunophilins. J Biol Chem. 2002;277:4597–4600. doi: 10.1074/jbc.C100531200. [DOI] [PubMed] [Google Scholar]
- de Castro M, Elliot S, Kino T, Bamberger C, Karl M, Webster E, Chrousos GP. The non-ligand binding beta-isoform of the human glucocorticoid receptor (hGR beta): tissue levels, mechanism of action, and potential physiologic role. Mol Med. 1996;2:597–607. [PMC free article] [PubMed] [Google Scholar]
- de Lange P, Segeren CM, Koper JW, Wiemer E, Sonneveld P, Brinkmann AO, White A, Brogan IJ, de Jong FH, Lamberts SW. Expression in hematological malignancies of a glucocorticoid receptor splice variant that augments glucocorticoid receptor-mediated effects in transfected cells. Cancer Res. 2001;61:3937–3941. [PubMed] [Google Scholar]
- Denny WB, Prapapanich V, Smith DF, Scammell JG. Structure-function analysis of squirrel monkey FK506-binding protein 51, a potent inhibitor of glucocorticoid receptor activity. Endocrinology. 2005;146:3194–3201. doi: 10.1210/en.2005-0027. [DOI] [PubMed] [Google Scholar]
- Denny WB, Valentine DL, Reynolds PD, Smith DF, Scammell JG. Squirrel monkey immunophilin FKBP51 is a potent inhibitor of glucocorticoid receptor binding. Endocrinology. 2000;141:4107–4113. doi: 10.1210/endo.141.11.7785. [DOI] [PubMed] [Google Scholar]
- Denton RR, Eisen LP, Elsasser MS, Harmon JM. Differential autoregulation of glucocorticoid receptor expression in human T- and B-cell lines. Endocrinology. 1993;133:248–256. doi: 10.1210/endo.133.1.8319574. [DOI] [PubMed] [Google Scholar]
- Di Blasio AM, van Rossum EF, Maestrini S, Berselli ME, Tagliaferri M, Podesta F, Koper JW, Liuzzi A, Lamberts SW. The relation between two polymorphisms in the glucocorticoid receptor gene and body mass index, blood pressure and cholesterol in obese patients. Clin Endocrinol (Oxf) 2003;59:68–74. doi: 10.1046/j.1365-2265.2003.01798.x. [DOI] [PubMed] [Google Scholar]
- Dobson MG, Redfern CP, Unwin N, Weaver JU. The N363S polymorphism of the glucocorticoid receptor: potential contribution to central obesity in men and lack of association with other risk factors for coronary heart disease and diabetes mellitus. J Clin Endocrinol Metab. 2001;86:2270–2274. doi: 10.1210/jcem.86.5.7465. [DOI] [PubMed] [Google Scholar]
- Dong Y, Poellinger L, Gustafsson JA, Okret S. Regulation of glucocorticoid receptor expression: evidence for transcriptional and posttranslational mechanisms. Mol Endocrinol. 1988;2:1256–1264. doi: 10.1210/mend-2-12-1256. [DOI] [PubMed] [Google Scholar]
- Dordelmann M, Reiter A, Borkhardt A, Ludwig WD, Gotz N, Viehmann S, Gadner H, Riehm H, Schrappe M. Prednisone response is the strongest predictor of treatment outcome in infant acute lymphoblastic leukemia. Blood. 1999;94:1209–1217. [PubMed] [Google Scholar]
- Dostert A, Heinzel T. Negative glucocorticoid receptor response elements and their role in glucocorticoid action. Curr Pharm Des. 2004;10:2807–2816. doi: 10.2174/1381612043383601. [DOI] [PubMed] [Google Scholar]
- Drouin J, Trifiro MA, Plante RK, Nemer M, Eriksson P, Wrange O. Glucocorticoid receptor binding to a specific DNA sequence is required for hormone-dependent repression of pro-opiomelanocortin gene transcription. Mol Cell Biol. 1989;9:5305–5314. doi: 10.1128/mcb.9.12.5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M. Effect of single and compound knockouts of estrogen receptors alpha (ERalpha) and beta (ERbeta) on mouse reproductive phenotypes. Development. 2000;127:4277–4291. doi: 10.1242/dev.127.19.4277. [DOI] [PubMed] [Google Scholar]
- Eisen LP, Elsasser MS, Harmon JM. Positive regulation of the glucocorticoid receptor in human T-cells sensitive to the cytolytic effects of glucocorticoids. J Biol Chem. 1988;263:12044–12048. [PubMed] [Google Scholar]
- Encio IJ, Detera-Wadleigh SD. The genomic structure of the human glucocorticoid receptor. J Biol Chem. 1991;266:7182–7188. [PubMed] [Google Scholar]
- Federico MH, Martins VR, Pozzi DH, Llacer PE, Brentani MM. Glucocorticoid receptors in acute leukemia. Braz J Med Biol Res. 1986;19:167–172. [PubMed] [Google Scholar]
- Felice MS, Zubizarreta PA, Alfaro EM, Sackmann-Muriel F. Childhood acute lymphoblastic leukemia: prognostic value of initial peripheral blast count in good responders to prednisone. J Pediatr Hematol Oncol. 2001;23:411–415. doi: 10.1097/00043426-200110000-00004. [DOI] [PubMed] [Google Scholar]
- Flouriot G, Brand H, Denger S, Metivier R, Kos M, Reid G, Sonntag-Buck V, Gannon F. Identification of a new isoform of the human estrogen receptor-alpha (hER-alpha) that is encoded by distinct transcripts and that is able to repress hER-alpha activation function 1. Embo J. 2000;19:4688–4700. doi: 10.1093/emboj/19.17.4688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francke U, Foellmer BE. The glucocorticoid receptor gene is in 5q31-q32 [corrected] Genomics. 1989;4:610–612. doi: 10.1016/0888-7543(89)90287-5. [DOI] [PubMed] [Google Scholar]
- Frankfurt O, Rosen ST. Mechanisms of glucocorticoid-induced apoptosis in hematologic malignancies: updates. Curr Opin Oncol. 2004;16:553–563. doi: 10.1097/01.cco.0000142072.22226.09. [DOI] [PubMed] [Google Scholar]
- Gaynon PS, Carrel AL. Glucocorticosteroid therapy in childhood acute lymphoblastic leukemia. Adv Exp Med Biol. 1999;457:593–605. doi: 10.1007/978-1-4615-4811-9_66. [DOI] [PubMed] [Google Scholar]
- Gaynon PS, Lustig RH. The use of glucocorticoids in acute lymphoblastic leukemia of childhood. Molecular, cellular, and clinical considerations. J Pediatr Hematol Oncol. 1995;17:1–12. doi: 10.1097/00043426-199502000-00001. [DOI] [PubMed] [Google Scholar]
- Geng CD, Pedersen KB, Nunez BS, Vedeckis WV. Human glucocorticoid receptor alpha transcript splice variants with exon 2 deletions: evidence for tissue- and cell type-specific functions. Biochemistry. 2005;44:7395–7405. doi: 10.1021/bi047485e. [DOI] [PubMed] [Google Scholar]
- Gomi M, Moriwaki K, Katagiri S, Kurata Y, Thompson EB. Glucocorticoid effects on myeloma cells in culture: correlation of growth inhibition with induction of glucocorticoid receptor messenger RNA. Cancer Res. 1990;50:1873–1878. [PubMed] [Google Scholar]
- Govindan MV, Pothier F, Leclerc S, Palaniswami R, Xie B. Human glucocorticoid receptor gene promotor-homologous down regulation. J Steroid Biochem Mol Biol. 1991;40:317–323. doi: 10.1016/0960-0760(91)90197-d. [DOI] [PubMed] [Google Scholar]
- Gregory CW, He B, Wilson EM. The putative androgen receptor-A form results from in vitro proteolysis. J Mol Endocrinol. 2001;27:309–319. doi: 10.1677/jme.0.0270309. [DOI] [PubMed] [Google Scholar]
- Grenier J, Trousson A, Chauchereau A, Cartaud J, Schumacher M, Massaad C. Differential recruitment of p160 coactivators by glucocorticoid receptor between Schwann cells and astrocytes. Mol Endocrinol. 2006;20:254–267. doi: 10.1210/me.2005-0061. [DOI] [PubMed] [Google Scholar]
- Gruol DJ, Rajah FM, Bourgeois S. Cyclic AMP-dependent protein kinase modulation of the glucocorticoid-induced cytolytic response in murine T-lymphoma cells. Mol Endocrinol. 1989;3:2119–2127. doi: 10.1210/mend-3-12-2119. [DOI] [PubMed] [Google Scholar]
- Haarman EG, Kaspers GJ, Pieters R, Rottier MM, Veerman AJ. Glucocorticoid receptor alpha, beta and gamma expression vs in vitro glucocorticod resistance in childhood leukemia. Leukemia. 2004;18:530–537. doi: 10.1038/sj.leu.2403225. [DOI] [PubMed] [Google Scholar]
- Hala M, Hartmann BL, Bock G, Geley S, Kofler R. Glucocorticoid-receptor-gene defects and resistance to glucocorticoid-induced apoptosis in human leukemic cell lines. Int J Cancer. 1996;68:663–668. doi: 10.1002/(SICI)1097-0215(19961127)68:5<663::AID-IJC17>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Han SJ, DeMayo FJ, Xu J, Tsai SY, Tsai MJ, O’Malley BW. Steroid receptor coactivator (SRC)-1 and SRC-3 differentially modulate tissue-specific activation functions of the progesterone receptor. Mol Endocrinol. 2006;20:45–55. doi: 10.1210/me.2005-0310. [DOI] [PubMed] [Google Scholar]
- Heitzer MD, DeFranco DB. Hic-5/ARA55, a LIM domain-containing nuclear receptor coactivator expressed in prostate stromal cells. Cancer Res. 2006;66:7326–7333. doi: 10.1158/0008-5472.CAN-05-2379. [DOI] [PubMed] [Google Scholar]
- Hillmann AG, Ramdas J, Multanen K, Norman MR, Harmon JM. Glucocorticoid receptor gene mutations in leukemic cells acquired in vitro and in vivo. Cancer Res. 2000;60:2056–2062. [PubMed] [Google Scholar]
- Ho AD, Hunstein W, Ganeshaguru K, Hoffbrand AV, Brandeis WE, Denk B. Therapeutic and prognostic implications of glucocorticoid receptors and terminal deoxynucleotidyl transferase in acute leukemia. Leuk Res. 1982;6:1–8. doi: 10.1016/0145-2126(82)90037-6. [DOI] [PubMed] [Google Scholar]
- Hoeck W, Rusconi S, Groner B. Down-regulation and phosphorylation of glucocorticoid receptors in cultured cells. Investigations with a monospecific antiserum against a bacterially expressed receptor fragment. J Biol Chem. 1989;264:14396–14402. [PubMed] [Google Scholar]
- Hollenberg SM, Evans RM. Multiple and cooperative trans-activation domains of the human glucocorticoid receptor. Cell. 1988;55:899–906. doi: 10.1016/0092-8674(88)90145-6. [DOI] [PubMed] [Google Scholar]
- Hollenberg SM, Weinberger C, Ong ES, Cerelli G, Oro A, Lebo R, Thompson EB, Rosenfeld MG, Evans RM. Primary structure and expression of a functional human glucocorticoid receptor cDNA. Nature. 1985;318:635–641. doi: 10.1038/318635a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizenga NA, Koper JW, De Lange P, Pols HA, Stolk RP, Burger H, Grobbee DE, Brinkmann AO, De Jong FH, Lamberts SW. A polymorphism in the glucocorticoid receptor gene may be associated with and increased sensitivity to glucocorticoids in vivo. J Clin Endocrinol Metab. 1998;83:144–151. doi: 10.1210/jcem.83.1.4490. [DOI] [PubMed] [Google Scholar]
- Irving JA, Minto L, Bailey S, Hall AG. Loss of heterozygosity and somatic mutations of the glucocorticoid receptor gene are rarely found at relapse in pediatric acute lymphoblastic leukemia but may occur in a subpopulation early in the disease course. Cancer Res. 2005;65:9712–9718. doi: 10.1158/0008-5472.CAN-05-1227. [DOI] [PubMed] [Google Scholar]
- Jewell CM, Cidlowski JA. Molecular evidence for a link between the N363S glucocorticoid receptor polymorphism and altered gene expression. J Clin Endocrinol Metab. 2007;92:3268–3277. doi: 10.1210/jc.2007-0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai Y. Two naturally-occurring isoforms and their expression of a glucocorticoid receptor gene from an androgen-dependent mouse tumor. FEBS Lett. 1990;274:99–102. doi: 10.1016/0014-5793(90)81339-p. [DOI] [PubMed] [Google Scholar]
- Kelly A, Bowen H, Jee YK, Mahfiche N, Soh C, Lee T, Hawrylowicz C, Lavender P. The glucocorticoid receptor beta isoform can mediate transcriptional repression by recruiting histone deacetylases. J Allergy Clin Immunol. 2008;121:203–208. e201. doi: 10.1016/j.jaci.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Klotz L. Combined androgen blockade: an update. Urol Clin North Am. 2006;33:161–166. v–vi. doi: 10.1016/j.ucl.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Klumper E, Pieters R, Veerman AJ, Huismans DR, Loonen AH, Hahlen K, Kaspers GJ, van Wering ER, Hartmann R, Henze G. In vitro cellular drug resistance in children with relapsed/refractory acute lymphoblastic leukemia. Blood. 1995;86:3861–3868. [PubMed] [Google Scholar]
- Kobayashi S, Shibata H, Yokota K, Suda N, Murai A, Kurihara I, Saito I, Saruta T. FHL2, UBC9, and PIAS1 are novel estrogen receptor alpha-interacting proteins. Endocr Res. 2004;30:617–621. doi: 10.1081/erc-200043789. [DOI] [PubMed] [Google Scholar]
- Koga Y, Matsuzaki A, Suminoe A, Hattori H, Kanemitsu S, Hara T. Differential mRNA expression of glucocorticoid receptor alpha and beta is associated with glucocorticoid sensitivity of acute lymphoblastic leukemia in children. Pediatr Blood Cancer. 2005;45:121–127. doi: 10.1002/pbc.20308. [DOI] [PubMed] [Google Scholar]
- Kojika S, Sugita K, Inukai T, Saito M, Iijima K, Tezuka T, Goi K, Shiraishi K, Mori T, Okazaki T, Kagami K, Ohyama K, Nakazawa S. Mechanisms of glucocorticoid resistance in human leukemic cells: implication of abnormal 90 and 70 kDa heat shock proteins. Leukemia. 1996;10:994–999. [PubMed] [Google Scholar]
- Koper JW, Stolk RP, de Lange P, Huizenga NA, Molijn GJ, Pols HA, Grobbee DE, Karl M, de Jong FH, Brinkmann AO, Lamberts SW. Lack of association between five polymorphisms in the human glucocorticoid receptor gene and glucocorticoid resistance. Hum Genet. 1997;99:663–668. doi: 10.1007/s004390050425. [DOI] [PubMed] [Google Scholar]
- Krett NL, Pillay S, Moalli PA, Greipp PR, Rosen ST. A variant glucocorticoid receptor messenger RNA is expressed in multiple myeloma patients. Cancer Res. 1995;55:2727–2729. [PubMed] [Google Scholar]
- Lacroix A, Bonnard GD, Lippman ME. Modulation of glucocorticoid receptors by mitogenic stimuli, glucocorticoids and retinoids in normal human cultured T cells. J Steroid Biochem. 1984;21:73–80. doi: 10.1016/0022-4731(84)90062-1. [DOI] [PubMed] [Google Scholar]
- Lamberts SW, Huizenga AT, de Lange P, de Jong FH, Koper JW. Clinical aspects of glucocorticoid sensitivity. Steroids. 1996;61:157–160. doi: 10.1016/0039-128x(96)00005-0. [DOI] [PubMed] [Google Scholar]
- Lauten M, Beger C, Gerdes K, Asgedom G, Kardinal C, Welte K, Schrappe M. Expression of heat-shock protein 90 in glucocorticoid-sensitive and -resistant childhood acute lymphoblastic leukaemia. Leukemia. 2003;17:1551–1556. doi: 10.1038/sj.leu.2403027. [DOI] [PubMed] [Google Scholar]
- Lee S, Duncan KA, Chou H, Chen D, Kohli K, Huang CF, Stallcup MR. A somatic cell genetic method for identification of untargeted mutations in the glucocorticoid receptor that cause hormone binding deficiencies. Mol Endocrinol. 1995;9:826–837. doi: 10.1210/mend.9.7.7476966. [DOI] [PubMed] [Google Scholar]
- Lewis-Tuffin LJ, Jewell CM, Bienstock RJ, Collins JB, Cidlowski JA. Human glucocorticoid receptor beta binds RU-486 and is transcriptionally active. Mol Cell Biol. 2007;27:2266–2282. doi: 10.1128/MCB.01439-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liegibel UM, Sommer U, Boercsoek I, Hilscher U, Bierhaus A, Schweikert HU, Nawroth P, Kasperk C. Androgen receptor isoforms AR-A and AR-B display functional differences in cultured human bone cells and genital skin fibroblasts. Steroids. 2003;68:1179–1187. doi: 10.1016/j.steroids.2003.08.016. [DOI] [PubMed] [Google Scholar]
- Lin RC, Wang XL, Dalziel B, Caterson ID, Morris BJ. Association of obesity, but not diabetes or hypertension, with glucocorticoid receptor N363S variant. Obes Res. 2003;11:802–808. doi: 10.1038/oby.2003.111. [DOI] [PubMed] [Google Scholar]
- Longui CA, Vottero A, Adamson PC, Cole DE, Kino T, Monte O, Chrousos GP. Low glucocorticoid receptor alpha/beta ratio in T-cell lymphoblastic leukemia. Horm Metab Res. 2000;32:401–406. doi: 10.1055/s-2007-978661. [DOI] [PubMed] [Google Scholar]
- Lu NZ, Cidlowski JA. Translational regulatory mechanisms generate N-terminal glucocorticoid receptor isoforms with unique transcriptional target genes. Mol Cell. 2005;18:331–342. doi: 10.1016/j.molcel.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Lu NZ, Collins JB, Grissom SF, Cidlowski JA. Selective regulation of bone cell apoptosis by translational isoforms of the glucocorticoid receptor. Mol Cell Biol. 2007;27:7143–7160. doi: 10.1128/MCB.00253-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA, Jr, Shyamala G, Conneely OM, O’Malley BW. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9:2266–2278. doi: 10.1101/gad.9.18.2266. [DOI] [PubMed] [Google Scholar]
- Matysiak M, Makosa B, Walczak A, Selmaj K. Patients with multiple sclerosis resisted to glucocorticoid therapy: abnormal expression of heat-shock protein 90 in glucocorticoid receptor complex. Mult Scler. 2008 doi: 10.1177/1352458508090666. [DOI] [PubMed] [Google Scholar]
- Moalli PA, Pillay S, Krett NL, Rosen ST. Alternatively spliced glucocorticoid receptor messenger RNAs in glucocorticoid-resistant human multiple myeloma cells. Cancer Res. 1993;53:3877–3879. [PubMed] [Google Scholar]
- Nelson CC, Hendy SC, Shukin RJ, Cheng H, Bruchovsky N, Koop BF, Rennie PS. Determinants of DNA sequence specificity of the androgen, progesterone, and glucocorticoid receptors: evidence for differential steroid receptor response elements. Mol Endocrinol. 1999;13:2090–2107. doi: 10.1210/mend.13.12.0396. [DOI] [PubMed] [Google Scholar]
- Nuclear Receptors Nomenclature Committee. A unified nomenclature system for the nuclear receptor superfamily. Cell. 1999;97:161–163. doi: 10.1016/s0092-8674(00)80726-6. [DOI] [PubMed] [Google Scholar]
- Oakley RH, Jewell CM, Yudt MR, Bofetiado DM, Cidlowski JA. The dominant negative activity of the human glucocorticoid receptor beta isoform. Specificity and mechanisms of action. J Biol Chem. 1999;274:27857–27866. doi: 10.1074/jbc.274.39.27857. [DOI] [PubMed] [Google Scholar]
- Oakley RH, Sar M, Cidlowski JA. The human glucocorticoid receptor beta isoform. Expression, biochemical properties, and putative function. J Biol Chem. 1996;271:9550–9559. doi: 10.1074/jbc.271.16.9550. [DOI] [PubMed] [Google Scholar]
- Oakley RH, Webster JC, Sar M, Parker CR, Jr, Cidlowski JA. Expression and subcellular distribution of the beta-isoform of the human glucocorticoid receptor. Endocrinology. 1997;138:5028–5038. doi: 10.1210/endo.138.11.5501. [DOI] [PubMed] [Google Scholar]
- Okret S, Poellinger L, Dong Y, Gustafsson JA. Down-regulation of glucocorticoid receptor mRNA by glucocorticoid hormones and recognition by the receptor of a specific binding sequence within a receptor cDNA clone. Proc Natl Acad Sci U S A. 1986;83:5899–5903. doi: 10.1073/pnas.83.16.5899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang J, Jiang T, Tan M, Cui Y, Li X. Abnormal expression and distribution of heat shock protein 90: potential etiologic immunoendocrine mechanism of glucocorticoid resistance in idiopathic nephrotic syndrome. Clin Vaccine Immunol. 2006;13:496–500. doi: 10.1128/CVI.13.4.496-500.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer LA, Hukku B, Harmon JM. Human glucocorticoid receptor gene deletion following exposure to cancer chemotherapeutic drugs and chemical mutagens. Cancer Res. 1992;52:6612–6618. [PubMed] [Google Scholar]
- Pascual-Le Tallec L, Demange C, Lombes M. Human mineralocorticoid receptor A and B protein forms produced by alternative translation sites display different transcriptional activities. Eur J Endocrinol. 2004;150:585–590. doi: 10.1530/eje.0.1500585. [DOI] [PubMed] [Google Scholar]
- Pascual-Le Tallec L, Simone F, Viengchareun S, Meduri G, Thirman MJ, Lombes M. The elongation factor ELL (eleven-nineteen lysine-rich leukemia) is a selective coregulator for steroid receptor functions. Mol Endocrinol. 2005;19:1158–1169. doi: 10.1210/me.2004-0331. [DOI] [PubMed] [Google Scholar]
- Pedersen KB, Geng CD, Vedeckis WV. Three mechanisms are involved in glucocorticoid receptor autoregulation in a human T-lymphoblast cell line. Biochemistry. 2004;43:10851–10858. doi: 10.1021/bi049458u. [DOI] [PubMed] [Google Scholar]
- Pedersen KB, Vedeckis WV. Quantification and glucocorticoid regulation of glucocorticoid receptor transcripts in two human leukemic cell lines. Biochemistry. 2003;42:10978–10990. doi: 10.1021/bi034651u. [DOI] [PubMed] [Google Scholar]
- Powers JH, Hillmann AG, Tang DC, Harmon JM. Cloning and expression of mutant glucocorticoid receptors from glucocorticoid-sensitive and -resistant human leukemic cells. Cancer Res. 1993;53:4059–4065. [PubMed] [Google Scholar]
- Pratt WB, Toft DO. Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocr Rev. 1997;18:306–360. doi: 10.1210/edrv.18.3.0303. [DOI] [PubMed] [Google Scholar]
- Presul E, Schmidt S, Kofler R, Helmberg A. Identification, tissue expression, and glucocorticoid responsiveness of alternative first exons of the human glucocorticoid receptor. J Mol Endocrinol. 2007;38:79–90. doi: 10.1677/jme.1.02183. [DOI] [PubMed] [Google Scholar]
- Pui CH, Costlow ME. Sequential studies of lymphoblast glucocorticoid receptor levels at diagnosis and relapse in childhood leukemia: an update. Leuk Res. 1986;10:227–229. doi: 10.1016/0145-2126(86)90046-9. [DOI] [PubMed] [Google Scholar]
- Pui CH, Dahl GV, Rivera G, Murphy SB, Costlow ME. The relationship of blast cell glucocorticoid receptor levels to response to single-agent steroid trial and remission response in children with acute lymphoblastic leukemia. Leuk Res. 1984;8:579–585. doi: 10.1016/0145-2126(84)90006-7. [DOI] [PubMed] [Google Scholar]
- Pujols L, Mullol J, Roca-Ferrer J, Torrego A, Xaubet A, Cidlowski JA, Picado C. Expression of glucocorticoid receptor alpha- and beta-isoforms in human cells and tissues. Am J Physiol Cell Physiol. 2002;283:C1324–1331. doi: 10.1152/ajpcell.00363.2001. [DOI] [PubMed] [Google Scholar]
- Qian X, Zhu Y, Xu W, Lin Y. Glucocorticoid receptor and heat shock protein 90 in peripheral blood mononuclear cells from asthmatics. Chin Med J (Engl) 2001;114:1051–1054. [PubMed] [Google Scholar]
- Quinkler M, Meyer B, Oelkers W, Diederich S. Renal inactivation, mineralocorticoid generation, and 11beta-hydroxysteroid dehydrogenase inhibition ameliorate the antimineralocorticoid effect of progesterone in vivo. J Clin Endocrinol Metab. 2003;88:3767–3772. doi: 10.1210/jc.2003-030092. [DOI] [PubMed] [Google Scholar]
- Ramdas J, Liu W, Harmon JM. Glucocorticoid-induced cell death requires autoinduction of glucocorticoid receptor expression in human leukemic T cells. Cancer Res. 1999;59:1378–1385. [PubMed] [Google Scholar]
- Ray A, Prefontaine KE. Physical association and functional antagonism between the p65 subunit of transcription factor NF-kappa B and the glucocorticoid receptor. Proc Natl Acad Sci U S A. 1994;91:752–756. doi: 10.1073/pnas.91.2.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray DW, Davis JR, White A, Clark AJ. Glucocorticoid receptor structure and function in glucocorticoid-resistant small cell lung carcinoma cells. Cancer Res. 1996;56:3276–3280. [PubMed] [Google Scholar]
- Renner K, Ausserlechner MJ, Kofler R. A conceptual view on glucocorticoid-lnduced apoptosis, cell cycle arrest and glucocorticoid resistance in lymphoblastic leukemia. Curr Mol Med. 2003;3:707–717. doi: 10.2174/1566524033479357. [DOI] [PubMed] [Google Scholar]
- Reynolds PD, Ruan Y, Smith DF, Scammell JG. Glucocorticoid resistance in the squirrel monkey is associated with overexpression of the immunophilin FKBP51. J Clin Endocrinol Metab. 1999;84:663–669. doi: 10.1210/jcem.84.2.5429. [DOI] [PubMed] [Google Scholar]
- Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids--new mechanisms for old drugs. N Engl J Med. 2005;353:1711–1723. doi: 10.1056/NEJMra050541. [DOI] [PubMed] [Google Scholar]
- Riggs DL, Roberts PJ, Chirillo SC, Cheung-Flynn J, Prapapanich V, Ratajczak T, Gaber R, Picard D, Smith DF. The Hsp90-binding peptidylprolyl isomerase FKBP52 potentiates glucocorticoid signaling in vivo. Embo J. 2003;22:1158–1167. doi: 10.1093/emboj/cdg108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riml S, Schmidt S, Ausserlechner MJ, Geley S, Kofler R. Glucocorticoid receptor heterozygosity combined with lack of receptor auto-induction causes glucocorticoid resistance in Jurkat acute lymphoblastic leukemia cells. Cell Death Differ. 2004;11(Suppl 1):S65–72. doi: 10.1038/sj.cdd.4401413. [DOI] [PubMed] [Google Scholar]
- Rivers C, Levy A, Hancock J, Lightman S, Norman M. Insertion of an amino acid in the DNA-binding domain of the glucocorticoid receptor as a result of alternative splicing. J Clin Endocrinol Metab. 1999;84:4283–4286. doi: 10.1210/jcem.84.11.6235. [DOI] [PubMed] [Google Scholar]
- Rizner TL, Lin HK, Peehl DM, Steckelbroeck S, Bauman DR, Penning TM. Human type 3 3alpha-hydroxysteroid dehydrogenase (aldo-keto reductase 1C2) and androgen metabolism in prostate cells. Endocrinology. 2003;144:2922–2932. doi: 10.1210/en.2002-0032. [DOI] [PubMed] [Google Scholar]
- Rosewicz S, McDonald AR, Maddux BA, Goldfine ID, Miesfeld RL, Logsdon CD. Mechanism of glucocorticoid receptor down-regulation by glucocorticoids. J Biol Chem. 1988;263:2581–2584. [PubMed] [Google Scholar]
- Russcher H, Dalm VA, de Jong FH, Brinkmann AO, Hofland LJ, Lamberts SW, Koper JW. Associations between promoter usage and alternative splicing of the glucocorticoid receptor gene. J Mol Endocrinol. 2007;38:91–98. doi: 10.1677/jme.1.02117. [DOI] [PubMed] [Google Scholar]
- Russcher H, Smit P, van den Akker EL, van Rossum EF, Brinkmann AO, de Jong FH, Lamberts SW, Koper JW. Two polymorphisms in the glucocorticoid receptor gene directly affect glucocorticoid-regulated gene expression. J Clin Endocrinol Metab. 2005a;90:5804–5810. doi: 10.1210/jc.2005-0646. [DOI] [PubMed] [Google Scholar]
- Russcher H, van Rossum EF, de Jong FH, Brinkmann AO, Lamberts SW, Koper JW. Increased expression of the glucocorticoid receptor-A translational isoform as a result of the ER22/23EK polymorphism. Mol Endocrinol. 2005b;19:1687–1696. doi: 10.1210/me.2004-0467. [DOI] [PubMed] [Google Scholar]
- Sakai DD, Helms S, Carlstedt-Duke J, Gustafsson JA, Rottman FM, Yamamoto KR. Hormone-mediated repression: a negative glucocorticoid response element from the bovine prolactin gene. Genes Dev. 1988;2:1144–1154. doi: 10.1101/gad.2.9.1144. [DOI] [PubMed] [Google Scholar]
- Sakurai M, Kamiya H, Kawai K, Kawasaki H, Komada Y, Ido M, Ochiai H, Akane H, Sato K. Predictable risk factors in children with acute lymphoblastic leukemia. Med Pediatr Oncol. 1986;14:140–143. doi: 10.1002/mpo.2950140307. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Krey LC, McEwen BS. Stress down-regulates corticosterone receptors in a site-specific manner in the brain. Endocrinology. 1984;114:287–292. doi: 10.1210/endo-114-1-287. [DOI] [PubMed] [Google Scholar]
- Scammell JG, Denny WB, Valentine DL, Smith DF. Overexpression of the FK506-binding immunophilin FKBP51 is the common cause of glucocorticoid resistance in three New World primates. Gen Comp Endocrinol. 2001;124:152–165. doi: 10.1006/gcen.2001.7696. [DOI] [PubMed] [Google Scholar]
- Scammell JG, Tucker JA, King JA, Moore CM, Wright JL, Tuck-Muller CM. A kidney epithelial cell line from a Bolivian squirrel monkey. In Vitro Cell Dev Biol Anim. 2002;38:258–261. doi: 10.1290/1071-2690(2002)038<0258:AKECLF>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Schlechte JA, Ginsberg BH, Sherman BM. Regulation of the glucocorticoid receptor in human lymphocytes. J Steroid Biochem. 1982;16:69–74. doi: 10.1016/0022-4731(82)90145-5. [DOI] [PubMed] [Google Scholar]
- Schmidt S, Irving JA, Minto L, Matheson E, Nicholson L, Ploner A, Parson W, Kofler A, Amort M, Erdel M, Hall A, Kofler R. Glucocorticoid resistance in two key models of acute lymphoblastic leukemia occurs at the level of the glucocorticoid receptor. Faseb J. 2006;20:2600–2602. doi: 10.1096/fj.06-6214fje. [DOI] [PubMed] [Google Scholar]
- Schmidt S, Rainer J, Ploner C, Presul E, Riml S, Kofler R. Glucocorticoid-induced apoptosis and glucocorticoid resistance: molecular mechanisms and clinical relevance. Cell Death Differ. 2004;11(Suppl 1):S45–55. doi: 10.1038/sj.cdd.4401456. [DOI] [PubMed] [Google Scholar]
- Shahidi H, Vottero A, Stratakis CA, Taymans SE, Karl M, Longui CA, Chrousos GP, Daughaday WH, Gregory SA, Plate JM. Imbalanced expression of the glucocorticoid receptor isoforms in cultured lymphocytes from a patient with systemic glucocorticoid resistance and chronic lymphocytic leukemia. Biochem Biophys Res Commun. 1999;254:559–565. doi: 10.1006/bbrc.1998.9980. [DOI] [PubMed] [Google Scholar]
- Silva CM, Powell-Oliver FE, Jewell CM, Sar M, Allgood VE, Cidlowski JA. Regulation of the human glucocorticoid receptor by long-term and chronic treatment with glucocorticoid. Steroids. 1994;59:436–442. doi: 10.1016/0039-128x(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Soufi M, Kaiser U, Schneider A, Beato M, Westphal HM. The DNA and steroid binding domains of the glucocorticoid receptor are not altered in mononuclear cells of treated CLL patients. Exp Clin Endocrinol Diabetes. 1995;103:175–183. doi: 10.1055/s-0029-1211347. [DOI] [PubMed] [Google Scholar]
- Stewart PM, Krozowski ZS. 11 beta-Hydroxysteroid dehydrogenase. Vitam Horm. 1999;57:249–324. [PubMed] [Google Scholar]
- Stocklin E, Wissler M, Gouilleux F, Groner B. Functional interactions between Stat5 and the glucocorticoid receptor. Nature. 1996;383:726–728. doi: 10.1038/383726a0. [DOI] [PubMed] [Google Scholar]
- Strasser-Wozak EM, Hattmannstorfer R, Hala M, Hartmann BL, Fiegl M, Geley S, Kofler R. Splice site mutation in the glucocorticoid receptor gene causes resistance to glucocorticoid-induced apoptosis in a human acute leukemic cell line. Cancer Res. 1995;55:348–353. [PubMed] [Google Scholar]
- Svec F, Rudis M. Glucocorticoids regulate the glucocorticoid receptor in the AtT-20 cell. J Biol Chem. 1981;256:5984–5987. [PubMed] [Google Scholar]
- Syed AA, Irving JA, Redfern CP, Hall AG, Unwin NC, White M, Bhopal RS, Weaver JU. Association of glucocorticoid receptor polymorphism A3669G in exon 9beta with reduced central adiposity in women. Obesity (Silver Spring) 2006;14:759–764. doi: 10.1038/oby.2006.86. [DOI] [PubMed] [Google Scholar]
- Theriault A, Boyd E, Harrap SB, Hollenberg SM, Connor JM. Regional chromosomal assignment of the human glucocorticoid receptor gene to 5q31. Hum Genet. 1989;83:289–291. doi: 10.1007/BF00285175. [DOI] [PubMed] [Google Scholar]
- Thompson EB. Apoptosis and steroid hormones. Mol Endocrinol. 1994;8:665–673. doi: 10.1210/mend.8.6.7935482. [DOI] [PubMed] [Google Scholar]
- Thornton JW. Evolution of vertebrate steroid receptors from an ancestral estrogen receptor by ligand exploitation and serial genome expansions. Proc Natl Acad Sci U S A. 2001;98:5671–5676. doi: 10.1073/pnas.091553298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissing WJ, Lauten M, Meijerink JP, den Boer ML, Koper JW, Sonneveld P, Pieters R. Expression of the glucocorticoid receptor and its isoforms in relation to glucocorticoid resistance in childhood acute lymphocytic leukemia. Haematologica. 2005a;90:1279–1281. [PubMed] [Google Scholar]
- Tissing WJ, Meijerink JP, Brinkhof B, Broekhuis MJ, Menezes RX, den Boer ML, Pieters R. Glucocorticoid-induced glucocorticoid-receptor expression and promoter usage is not linked to glucocorticoid resistance in childhood ALL. Blood. 2006;108:1045–1049. doi: 10.1182/blood-2006-01-0261. [DOI] [PubMed] [Google Scholar]
- Tissing WJ, Meijerink JP, den Boer ML, Brinkhof B, Pieters R. mRNA expression levels of (co)chaperone molecules of the glucocorticoid receptor are not involved in glucocorticoid resistance in pediatric ALL. Leukemia. 2005b;19:727–733. doi: 10.1038/sj.leu.2403681. [DOI] [PubMed] [Google Scholar]
- Tissing WJ, Meijerink JP, den Boer ML, Brinkhof B, van Rossum EF, van Wering ER, Koper JW, Sonneveld P, Pieters R. Genetic variations in the glucocorticoid receptor gene are not related to glucocorticoid resistance in childhood acute lymphoblastic leukemia. Clin Cancer Res. 2005c;11:6050–6056. doi: 10.1158/1078-0432.CCR-04-2097. [DOI] [PubMed] [Google Scholar]
- Tornello S, Orti E, De Nicola AF, Rainbow TC, McEwen BS. Regulation of glucocorticoid receptors in brain by corticosterone treatment of adrenalectomized rats. Neuroendocrinology. 1982;35:411–417. doi: 10.1159/000123429. [DOI] [PubMed] [Google Scholar]
- Touriol C, Bornes S, Bonnal S, Audigier S, Prats H, Prats AC, Vagner S. Generation of protein isoform diversity by alternative initiation of translation at non-AUG codons. Biol Cell. 2003;95:169–178. doi: 10.1016/s0248-4900(03)00033-9. [DOI] [PubMed] [Google Scholar]
- Turner JD, Muller CP. Structure of the glucocorticoid receptor (NR3C1) gene 5’ untranslated region: identification, and tissue distribution of multiple new human exon 1. J Mol Endocrinol. 2005;35:283–292. doi: 10.1677/jme.1.01822. [DOI] [PubMed] [Google Scholar]
- Turner JD, Schote AB, Keipes M, Muller CP. A new transcript splice variant of the human glucocorticoid receptor: identification and tissue distribution of hGR Delta 313-338, an alternative exon 2 transactivation domain isoform. Ann N Y Acad Sci. 2007;1095:334–341. doi: 10.1196/annals.1397.037. [DOI] [PubMed] [Google Scholar]
- van Rossum EF, Feelders RA, van den Beld AW, Uitterlinden AG, Janssen JA, Ester W, Brinkmann AO, Grobbee DE, de Jong FH, Pols HA, Koper JW, Lamberts SW. Association of the ER22/23EK polymorphism in the glucocorticoid receptor gene with survival and C-reactive protein levels in elderly men. Am J Med. 2004a;117:158–162. doi: 10.1016/j.amjmed.2004.01.027. [DOI] [PubMed] [Google Scholar]
- van Rossum EF, Koper JW, Huizenga NA, Uitterlinden AG, Janssen JA, Brinkmann AO, Grobbee DE, de Jong FH, van Duyn CM, Pols HA, Lamberts SW. A polymorphism in the glucocorticoid receptor gene, which decreases sensitivity to glucocorticoids in vivo, is associated with low insulin and cholesterol levels. Diabetes. 2002;51:3128–3134. doi: 10.2337/diabetes.51.10.3128. [DOI] [PubMed] [Google Scholar]
- van Rossum EF, Koper JW, van den Beld AW, Uitterlinden AG, Arp P, Ester W, Janssen JA, Brinkmann AO, de Jong FH, Grobbee DE, Pols HA, Lamberts SW. Identification of the BclI polymorphism in the glucocorticoid receptor gene: association with sensitivity to glucocorticoids in vivo and body mass index. Clin Endocrinol (Oxf) 2003;59:585–592. doi: 10.1046/j.1365-2265.2003.01888.x. [DOI] [PubMed] [Google Scholar]
- van Rossum EF, Lamberts SW. Polymorphisms in the glucocorticoid receptor gene and their associations with metabolic parameters and body composition. Recent Prog Horm Res. 2004b;59:333–357. doi: 10.1210/rp.59.1.333. [DOI] [PubMed] [Google Scholar]
- Vanderbilt JN, Miesfeld R, Maler BA, Yamamoto KR. Intracellular receptor concentration limits glucocorticoid-dependent enhancer activity. Mol Endocrinol. 1987;1:68–74. doi: 10.1210/mend-1-1-68. [DOI] [PubMed] [Google Scholar]
- Vedeckis WV, Ali M, Allen HR. Regulation of glucocorticoid receptor protein and mRNA levels. Cancer Res. 1989;49:2295s–2302s. [PubMed] [Google Scholar]
- Vegeto E, Shahbaz MM, Wen DX, Goldman ME, O’Malley BW, McDonnell DP. Human progesterone receptor A form is a cell- and promoter-specific repressor of human progesterone receptor B function. Mol Endocrinol. 1993;7:1244–1255. doi: 10.1210/mend.7.10.8264658. [DOI] [PubMed] [Google Scholar]
- Vihko P, Herrala A, Harkonen P, Isomaa V, Kaija H, Kurkela R, Pulkka A. Control of cell proliferation by steroids: the role of 17HSDs. Mol Cell Endocrinol. 2006;248:141–148. doi: 10.1016/j.mce.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Wallace AD, Cidlowski JA. Proteasome-mediated glucocorticoid receptor degradation restricts transcriptional signaling by glucocorticoids. J Biol Chem. 2001;276:42714–42721. doi: 10.1074/jbc.M106033200. [DOI] [PubMed] [Google Scholar]
- Wells RJ, Mascaro K, Young PC, Cleary RE, Bachner RL. Glucocorticoid receptors in the lymphoblasts of patients with glucocorticoid-resistant childhood acute lymphocytic leukemia. Am J Pediatr Hematol Oncol. 1981;3:259–264. [PubMed] [Google Scholar]
- Westberry JM, Sadosky PW, Hubler TR, Gross KL, Scammell JG. Glucocorticoid resistance in squirrel monkeys results from a combination of a transcriptionally incompetent glucocorticoid receptor and overexpression of the glucocorticoid receptor co-chaperone FKBP51. J Steroid Biochem Mol Biol. 2006;100:34–41. doi: 10.1016/j.jsbmb.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Wilson CM, McPhaul MJ. A and B forms of the androgen receptor are present in human genital skin fibroblasts. Proc Natl Acad Sci U S A. 1994;91:1234–1238. doi: 10.1073/pnas.91.4.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CM, McPhaul MJ. A and B forms of the androgen receptor are expressed in a variety of human tissues. Mol Cell Endocrinol. 1996;120:51–57. doi: 10.1016/0303-7207(96)03819-1. [DOI] [PubMed] [Google Scholar]
- Wochnik GM, Ruegg J, Abel GA, Schmidt U, Holsboer F, Rein T. FK506-binding proteins 51 and 52 differentially regulate dynein interaction and nuclear translocation of the glucocorticoid receptor in mammalian cells. J Biol Chem. 2005;280:4609–4616. doi: 10.1074/jbc.M407498200. [DOI] [PubMed] [Google Scholar]
- Yang-Yen HF, Chambard JC, Sun YL, Smeal T, Schmidt TJ, Drouin J, Karin M. Transcriptional interference between c-Jun and the glucocorticoid receptor: mutual inhibition of DNA binding due to direct protein-protein interaction. Cell. 1990;62:1205–1215. doi: 10.1016/0092-8674(90)90396-v. [DOI] [PubMed] [Google Scholar]
- Yeh S, Chang C. Cloning and characterization of a specific coactivator, ARA70, for the androgen receptor in human prostate cells. Proc Natl Acad Sci U S A. 1996;93:5517–5521. doi: 10.1073/pnas.93.11.5517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudt MR, Cidlowski JA. Molecular identification and characterization of a and b forms of the glucocorticoid receptor. Mol Endocrinol. 2001;15:1093–1103. doi: 10.1210/mend.15.7.0667. [DOI] [PubMed] [Google Scholar]
- Yudt MR, Jewell CM, Bienstock RJ, Cidlowski JA. Molecular origins for the dominant negative function of human glucocorticoid receptor beta. Mol Cell Biol. 2003;23:4319–4330. doi: 10.1128/MCB.23.12.4319-4330.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]