Abstract
It is well-established that long-term stress leads to induction of tyrosine hydroxylase (TH) mRNA and TH protein in adrenal medulla and brain. This induction is usually associated with stimulation of TH gene transcription rate. However, a number of studies have reported major discrepancies between the stress-induced changes in TH gene transcription, TH mRNA and TH protein. These discrepancies suggest that post-transcriptional mechanisms also play an important role in regulating TH expression in response to stress and other stimuli. In this report we summarize some of our findings and literature reports that demonstrate these discrepancies in adrenal medulla, locus coeruleus and midbrain dopamine neurons. We then describe our recent work investigating the molecular mechanisms that mediate this post-transcriptional regulation in adrenal medulla and midbrain. Our results suggest that trans-acting factors binding to the polypyrimidine-rich region of the 3′UTR of TH mRNA play a role in these post-transcriptional mechanisms. A hypothetical cellular model describing this post-transcriptional regulation is proposed.
Keywords: tyrosine hydroxylase, stress, adrenal medulla, locus coeruleus, midbrain dopamine neurons, post-transcriptional regulation
Text
Most forms of repeated or chronic stress induce tyrosine hydroxylase (TH) in adrenal medulla, sympathetic ganglia and locus coeruleus, and some types of stress induce TH in midbrain dopamine neuron1-4. Current hypotheses describing the molecular and signaling mechanisms responsible for this response focus primarily on transcriptional regulation and are based primarily on results of studies performed in vivo using adrenal medulla or in cultured PC12 and neuroblastoma cells. According to these models, stress leads to increased firing of afferent nerve fibers, like the splanchnic nerve for the adrenal medulla, resulting in enhanced activation of multiple plasma membrane receptors on postsynaptic catecholaminergic cell bodies by neurotransmitters released from these afferents. This receptor activation causes stimulation of multiple signaling pathways, leading to activation and/or induction of transcription factors that stimulate the TH gene promoter. The resulting increase in TH gene transcription rate leads to induction of TH mRNA. The consequent increase in TH protein is presumably responsible for maintaining the appropriate levels of catecholamine neurotransmitters during periods of sustained catecholamine release, which occur during chronic or repeated stress.
This model is supported by a large amount of evidence. TH mRNA is induced by almost all stressors and this induction precedes the increases in TH protein 1-4. Using a number of technically-difficult assays, including nuclear run-on assays, measurement of changes in the levels of nuclear RNA primary transcripts, and measurement of changes in expression of reporter genes driven by the TH gene promoter in transgenic mice, a number of laboratories have shown that TH gene transcription rate increases in response to stress and other stimuli in both adrenal medulla and locus coeruleus. Finally, stress leads to expected changes in transcription factors that are known to regulate the TH gene promoter 5-11. Interestingly, in the adrenal medulla, the time course of this transcriptional response is dependent on the duration of the stress. Short-term stress leads to rapid, but transient transcriptional activation, whereas chronic or repeated stress is associated with sustained stimulation of the TH gene 5-14. This sustained stimulation is apparently mediated by different transcription factors than those that regulate the gene during short-term stress and results in long-term induction of TH mRNA and TH protein.
Even though this model is consistent with much of the available evidence, there are a number of important discrepancies that do not fit well. (1) The observed effect of a particular stressor on TH expression differs depending on the tissue being studied 2, 15, 16. For instance, the responses observed in the adrenal medulla are not always identical to those observed in brain regions. (2) Increases in TH mRNA levels do not always correlate closely with increases in TH gene transcription rate in response to some stressors 8, 9, 17-19, suggesting that TH mRNA stability is regulated. (3) Induction of TH mRNA does not always lead to induction of TH protein, suggesting that either TH protein synthesis or degradation is regulated by stress 14, 20-24. In this report, we will briefly discuss some of these discrepancies and relate them to the hypothesis that trans-factors binding to the 3′UTR of TH mRNA may be regulated by stress, resulting in regulation of TH mRNA stability and translation in different catecholaminergic cell types.
Adrenal Medulla
Since most of the research in this field has been performed using either the adrenal medulla or pheochromocytoma cells that are derived from the adrenal medulla, the evidence from this system fits most closely the transcriptional hypothesis of TH regulation. However, even in the adrenal, discrepancies exist. The best-studied example of post-transcriptional regulation of TH expression involves stabilization of TH mRNA by hypoxia. Early studies showed that TH mRNA in adrenal medulla is regulated in a complicated manner by oxygen deprivation 25-27. Subsequent studies using PC12 cells showed that hypoxia induces TH mRNA by both transcriptional and post-transcriptional mechanisms 28-30. The post-transcriptional mechanism involves stabilization of TH mRNA and is mediated by trans-acting proteins binding to a 27 nt polypyrimidine-rich sequence within the 3′UTR of rat TH mRNA. One candidate for this trans-acting factor is the poly-C binding protein (PCBP), which is discussed in more detail below.
A second example of post-transcriptional regulation in adrenal medulla occurs in response to treatment with the muscarinic agonist bethanechol. Both short-term and long-term treatment with bethanechol leads to 2-3 fold increases in adrenal TH mRNA 14, 23. However, TH protein and TH activity are not induced in response to this drug. This discrepant response suggests that stimulation of muscarinic acetylcholine receptors on adrenal chromaffin cells activates signaling pathways that induce TH mRNA, but either do not activate or inhibit post-transcriptional pathways that regulate TH protein synthesis or degradation.
A third apparent post-transcriptional mechanism in adrenal medulla involves differences in expression of TH mRNA and TH protein in response to either a single or repeated stress. For instance, after a single immobilization stress, TH mRNA increases dramatically (8-fold at 3 hr after immobilization) and remains elevated ∼2-fold after 24 hr. Yet, this large effect produces very small or insignificant increases in TH protein and TH activity 22. In contrast, repeated immobilization stress over 2-7 days results in large increases in TH mRNA, TH protein and TH activity. We have confirmed this difference in response to single versus repeated immobilization stress in adrenal medulla 24. This discrepancy between changes in TH mRNA and TH protein also are observed in response to other short-term stressors 24. Recently, we tested whether this discrepancy is due to regulation of TH mRNA translation 24. Polysomal distribution assays were used to assess the percentage of TH mRNA associated with polyribosomes in adrenal medullae isolated from control rats or rats subjected to a single or repeated immobilization stress. TH mRNA was induced 2-fold 24 hr after a single immobilization, but the percentage of TH mRNA associated with polysomes was decreased significantly (from 33% ± 3 in controls to 21% ± 1.5 in singly-immobilized rat, N=3). In contrast, the percentage of TH mRNA associated with polysomes was essentially unchanged relative to controls in repeatedly-immobilized rats, even though TH mRNA was induced ∼2-fold. These results suggest that TH mRNA is induced to approximately the same extent in response to either single or repeated immobilization stress, but that the induced TH mRNA is not efficiently translated after a single immobilization. These findings support a model in which a single immobilization stress activates signaling pathways that stimulate TH gene transcription and induce TH mRNA, but do not activate mechanisms that are essential for translating the induced TH mRNA into TH protein. Repeated stress apparently activates both transcriptional and translational mechanisms, such that TH protein is induced. More work is needed to delineate the precise molecular mechanisms responsible for this translational response and to confirm that it occurs in response to other short-term stressors and that it plays a role in the lack of induction of TH protein observed in response to bethanechol treatment.
Locus Coeruleus
Stress, nicotine, antidepressants and many other stimuli regulate TH expression in the locus coeruleus 1-4. One of the most interesting findings over the past few years is that this regulation is apparently mediated by different mechanisms than that which occurs in the adrenal medulla. Our laboratory has shown that TH gene transcription increases in response to nicotine or stress in both LC and adrenal medulla, and that in the adrenal this activated transcriptional response is sustained for long periods of time when the animal is subjected to either drug treatment or stress repeatedly over many days 7, 8, 10, 11, 14. In contrast, the transcriptional response to each nicotine injection is short-lived or insignificant in the LC, even when the drug is administered repeatedly for 14 days 8, 10. Sabban and coworkers 31, 32 have recently shown that different transcription factors regulating TH gene promoter activity are induced in locus coeruleus during stress compared to those induced in adrenal medulla. These results suggest that the transcriptional response to stress or nicotine in these two tissues is mediated by different signals and molecular mechanisms.
Discrepant changes between TH gene transcription and TH mRNA induction have also been observed in response to stress or nicotine in the LC. Foot shock is associated with induction of TH mRNA in the LC that lasts for at least 24 hr 9. In contrast, TH gene transcription, assessed by measuring changes in TH gene primary RNA transcripts, increases for 15 min and 6 hr after foot shock, but not at 24 hr. Similar discordant changes occur in response to nicotine in LC. Sun et al 8 showed that chronic nicotine injections elicit a 2-3 fold induction of TH mRNA in LC and that this induction persists for at least 24 hr after the final nicotine injection. This induction is long-lasting, since TH mRNA remains elevated (∼1.5-fold) at 3 days after the final nicotine injection. In contrast, TH gene transcription increases 2-3 fold 2 hr after the final nicotine injection, but returns to control activity by 24 hr. Similarly, sustained increases in TH mRNA are observed after repeated immobilization stress in the LC (at least 48 hr after the final stress), but TH gene transcription is stimulated at 2 hr, not at 24 hr after the final stress 8. These discrepant time courses suggest that the long-term changes in TH mRNA that occur in response to stress or nicotine are not solely due to activation of transcriptional mechanisms, but may be mediated by post-transcriptional mechanisms that decrease TH mRNA degradation rate, as seen in response to hypoxia in PC12 cells.
Midbrain Dopamine Neurons
Very little is known about TH regulation in midbrain dopamine neurons. Severe stress, chronic nicotine treatment and reserpine treatment induce TH gene expression in these neurons, with ventral tegmental neurons being more responsive than those in the substantia nigra 33-36. However, this induction has not been observed in all studies and when observed, the effects are usually small or short-lived compared to those seen in adrenal medulla or locus coeruleus 37-39
Lesioning using neurotoxins like MPTP, 6-hydroxydopamine or high doses of amphetamines is another form of stress used commonly to study adaptive changes in dopamine neurons. Interestingly, only modest or insignificant increases and even decreases in TH mRNA levels are observed in surviving midbrain dopamine cell bodies after partial lesioning with these neurotoxins 40-44. In agreement, TH gene transcription rate does not increase in midbrain after treatment with 6-hydroxydopamine 45. In human Parkinsonian patients, decreases in midbrain TH mRNA levels have been observed 46, 47. This lack of compensatory induction of TH mRNA in the midbrain is puzzling, since one would expect the existence of homeostatic mechanisms to elevate TH gene expression in dopamine cell bodies to replenish neurotransmitter levels lost due to the lesion; compensatory induction of TH gene expression occurs in the peripheral nervous system in response to catecholamine-depleting neurotoxins or drugs 48-51. One explanation for this lack of response is that the signaling pathways mediating TH mRNA induction are not activated. However, to test this hypothesis is difficult, because so little is known about the regulation of TH in midbrain neurons. Hence, our laboratory initiated an extensive set of experiments to investigate the signaling mechanisms and receptors regulating midbrain TH mRNA.
In vivo studies
Our initial studies were performed in vivo, by microinjecting drugs either into the lateral ventricles or intranigrally at the same coordinates used by Leviel et al 52 in their studies on forskolin. The drugs that we chose were those that induce TH mRNA and stimulate TH gene transcription rate in other model systems, such as cAMP analogs, forskolin, phorbol esters or stimulatory agonists like glutamate, neurotensin, muscarine, nicotine or PACAP. To our surprise, none of these drugs reproducibly induced TH mRNA in the midbrain at any of the time points tested. Perhaps most surprising was the lack of response to forskolin, since Leviel et al 52 reported a dramatic induction (∼10-fold) of TH mRNA in substantia nigra 24 hr after a single injection of this drug. We performed the same experiment as in this previous report using the same dose and brain coordinates to inject forskolin intranigrally in both anesthetized (as in Leviel et al 52) and unanesthetized rats. We also injected 5 or 25 nmol of the membrane-permeable cAMP analog, 8-CPT-cAMP in the same manner and measured TH mRNA levels in the substantia nigra 4 or 24 hr after the injection. In all cases we observed no significant, reproducible effects on TH mRNA levels. We were concerned that the induction of TH mRNA might be short-lived and we were not measuring it at the appropriate time point (in Leviel et al 52 the induction of TH mRNA by forskolin was observed at 24 hr, but not at any time point before or after 24 hr). Hence, we used a semiquantitative RT-PCR assay to measure the levels of TH gene primary transcripts containing intron-2 sequences. An increase in the levels of TH gene primary transcripts is an indirect measurement of an increase in TH gene transcription rate. Using this assay, we repeated our in vivo experiments to test whether TH gene transcription rate increased rapidly in response to forskolin, cAMP analog or other stimulatory drugs. Again, our results were negative, in that significant and reproducible increases in TH intron-2-containing primary transcripts were not observed at either 15 min or 1-2 hr after intraventricular or intranigral injection of these drugs.
Midbrain slice explant studies
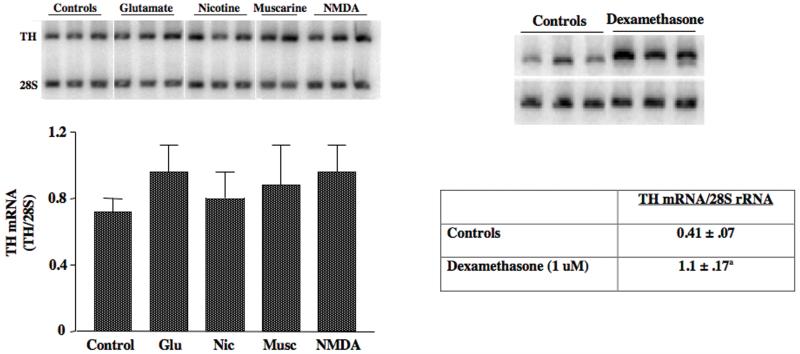
Since these in vivo experiments produced negative findings, we reasoned that the injection of these drugs might be producing effects on non-dopaminergic afferent neurons and hence, confounding our studies by potentially increasing inhibitory input to the the midbrain, which theoretically could inhibit TH gene activation. To avoid this potential confound, we developed an organotypic explant slice culture model system to test whether TH mRNA levels increased in response to treatment with different agonists for stimulatory receptors that are present on midbrain DA neurons. We did not observe an increase in TH mRNA levels in midbrain slices treated for 6 hr with any of these stimulatory drugs (Figure 1). In other experiments we treated slices with these drugs for 24 hr and still did not observe significant changes in TH mRNA levels. We also treated slices with GDNF, phorbol ester, and bicuculline (to block inhibitory GABA receptors); none of these drugs induced TH mRNA after 6 hr or 24 hr of treatment.
Figure 1. TH mRNA is induced by dexamethasone, but not by stimulatory receptor agonists in midbrain slices.
Organotypic midbrain slice cultures were incubated in the presence of 100 uM glutamate, 20 uM nicotine, 50 uM muscarine or 100 uM NMDA for 6 hr or by 1 uM dexamethasone for 24 hr. TH mRNA levels were measured using semiquantitative RT-PCR. The data represent the means ± SE from 3 cultures for each of the agonists in the bar graph and 5 cultures for the dexamethasone experiment.
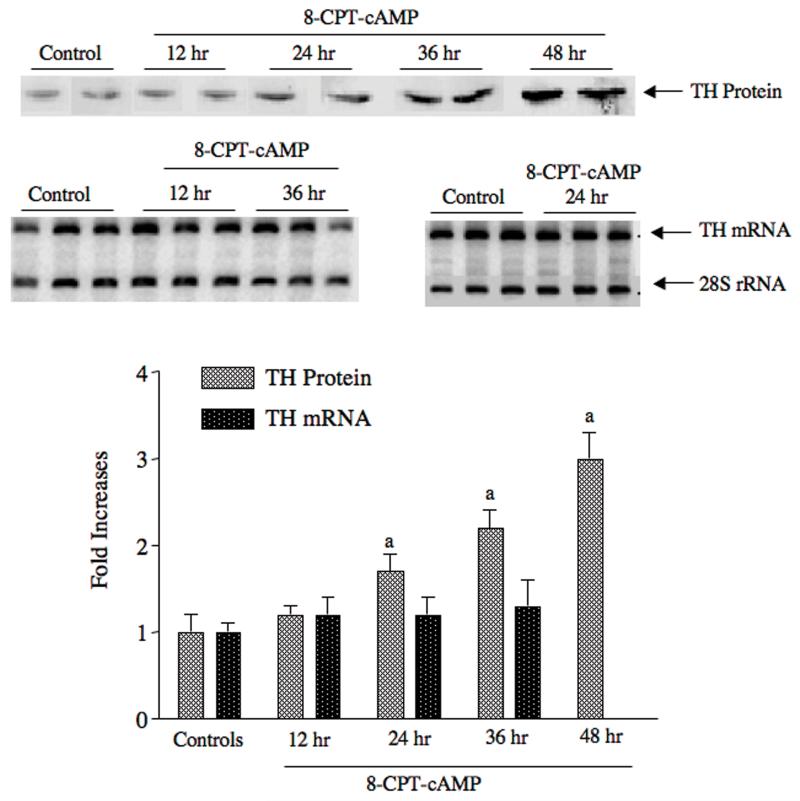
Finally, we tested whether cAMP induced TH gene expression in midbrain slices using forskolin or the cell-permeable cAMP analog, 8-CPT-cAMP. cAMP is a powerful inducer of both TH mRNA and TH protein in almost every other system tested 4, 53. In contrast, when midbrain slices were treated with 1 mM 8-CPT-cAMP or 10 uM forskolin, there was no induction of TH mRNA at any time point tested from 30 min to 36 hr (Figure 2). The only drug that induced TH mRNA in the midbrain slices was the synthetic glucocorticoid dexamethasone (P. Radcliffe, Ph.D. thesis, University of Rochester, 2006). This glucocorticoid induced TH mRNA 2-3 fold after 24 hr of treatment (Figure 1).
Figure 2. 8-CPT-cAMP elicits induction of TH protein, but not TH mRNA in midbrain slice cultures.
Organotypic midbrain slice cultures were incubated in the presence of 1 mM 8-CPT-cAMP for the periods of time designated in the figure. TH protein was measured using western analysis (see representative western blot above bar graph) and TH mRNA was measured using semiquantitative RT-PCR (see representative autoradiogram above bar graph). The bar graph presents the changes in TH mRNA and TH protein expressed as fold-increases over control values, which were 1.0 ± 0.1 for TH mRNA/28S and 12 ± 2 density units/ug protein for TH protein. The autoradiograms showing the RT-PCR results were overexposed for display purposes; quantification of the density values was made using a phosphorimager within the linear range. The data represent the means ± SE from 3-4 cultures.
We have described in detail these mostly “negative findings” for three major reasons: (1) These are extremely surprising results! It is generally accepted that TH mRNA is induced by drugs that depolarize catecholamine neurons or stimulate PKC or PKA. Clearly, this assumption is not applicable to midbrain DA neurons. (2) To demonstrate that TH gene expression is apparently regulated by different mechanisms in midbrain DA neurons than in peripheral systems or noradrenergic LC neurons. (3) These findings may partially explain why a compensatory induction of TH mRNA is not observed in neurotoxin-lesioned animal models.
cAMP-mediated induction of TH protein by a translational mechanism in midbrain
During the experiments with the midbrain slice explants, we also measured the effect of some of these drugs on TH protein levels. To our surprise, forskolin and cAMP analogs induced TH protein 2-3 fold in a dose-dependent manner, even though TH mRNA levels were not altered (Figure 2). This finding suggested that post-transcriptional mechanisms regulate TH protein expression in the midbrain. We have performed extensive studies characterizing this response in both midbrain slice cultures and MN9D cells, a cell line that is derived from the fusion of neuroblastoma cells with embryonic mouse midbrain neurons 54, Both of these cultured cell models respond to cAMP in similar manners (ie, induction of TH protein without induction of TH mRNA) (see Figure 2 for cAMP response in midbrain slice cultures). We show that TH protein synthesis rate increases in both models in response to cAMP, even though TH mRNA levels do not rise, and that cAMP elicits a greater percentage of TH mRNA associated with polysomes. These results suggest that cAMP produces an increase in TH mRNA translation in midbrain neurons.
To further investigate the mechanisms responsible for this translational regulation, we constructed TH-luciferase reporter genes, in which TH mRNA sequences were linked upstream and/or downstream of the luciferase coding region, introduced these reporters into MN9D cells and determined the effect of cAMP on luciferase activity and luciferase mRNA levels (unpublished data). TH mRNA sequences within the 280 nt 3′UTR conferred a cAMP response on luciferase activity, but not luciferase mRNA. Deletion and mutagenesis studies showed that the polypyrimidine-rich region within the TH mRNA 3′UTR was responsible for this response. Interestingly, this region is similar, if not identical to the one characterized by Czyzyk-Krzska and coworkers 29, 30, which mediated the stabilization of TH mRNA in response to hypoxia in PC12 cells. Since these workers present evidence that PCBPs bind to this polypyrimidine-rich region and may act as trans-factors mediating the hypoxic response, we tested whether PCBPs mediate the cAMP-mediated translational response. Our results indicate that the PCBP2 isoform is induced by cAMP in MN9D cells and that overexpression of this isoform leads to induction of TH protein, but not TH mRNA, even without an increase in cAMP (unpublished data).
Conclusions
There is considerable evidence that post-transcriptional mechanisms play a major role in regulating TH mRNA and TH protein expression. Regulation of TH mRNA stability and translation has been observed in adrenal medulla, locus coeruleus and midbrain in response to stress or chronic drug treatments. The best-characterized examples of post-transcriptional regulation are stabilization of TH mRNA by hypoxia in PC12 cells, changes in TH mRNA translational efficiency in response to short-term or long-term stress in adrenal medulla and regulation of TH mRNA translation by cAMP in midbrain dopamine neurons. However, it is likely that other examples will be identified as techniques and model systems necessary to study post-transcriptional regulation improve.
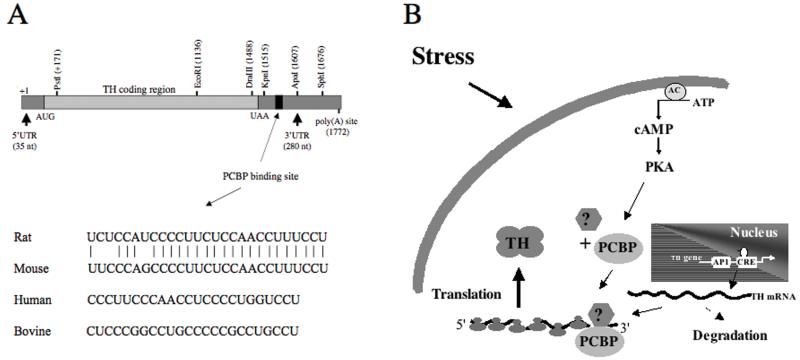
The mechanisms and signaling pathways responsible for these post-transcriptional responses remain obscure. The studies on hypoxia-mediated regulation of TH mRNA stability and cAMP-mediated reguation of TH mRNA translation indicate that a polypyrimidine-rich region within the 3′UTR of rat TH mRNA participates in this response (Figure 3A). This polypyrimidine-rich region is conserved very closely between rat and mouse (only a three nucleotide mismatch). Polypyrimidine-rich regions are also found in the 3′ UTRs of human and bovine TH mRNAs at approximately the same position as that seen in the rodent TH mRNAs (20-40 nt downstream from the translational stop codon), suggesting that this region may play an important role in TH mRNA expression in numerous species. However, the trans-acting factors that bind to this region and elicit the post-transcriptional responses remain unclear. The best candidates for these trans-acting factors are the PCBPs 55. These are a family of proteins derived from four genes that bind to single-stranded RNAs rich in polypyrimidines. At least two of these PCBP isoforms are expressed in PC12 and MN9D cells; the other two isoforms, PCBP3 and PCBP4, remain to be investigated. Hypoxia induces PCBP1 in PC12 cells, whereas cAMP induces PCBP2 in MN9D cells. Since these inductions correlate with the effects on TH mRNA stability and translation, respectively, in these two cell lines, it is reasonable to postulate that these factors play a role in these responses. A hypothetical model based on the available evidence is presented in Figure 3B. We postulate that stress, hypoxia, cAMP and probably many other stimuli induce PCBP isoforms that bind to the polypyrimidine-rich region of TH mRNA. This binding leads to increased interaction of the PCBP-TH mRNA complexes with other factors, stabiliizing the mRNA and/or increasing its association with polysomes leading to increased translation. Numerous questions remain, including: Do these PCBPs actually bind to the polypyrimidine-rich region of TH mRNA? Is there redundancy between the different PCBP isoforms mediating these responses? Do other trans-factors participate in the formation of this binding complex, such as micro RNAs? Why do these trans-factors elicit TH mRNA stabilization in PC12 cells, but activate TH mRNA translation in MN9D cells? What proteins or factors interact with the PCBP-mRNA complexes to mediate their effects on stability or translation? What signaling pathways and mechanisms regulate PCBP levels and activities? Which of these isoforms exist in adrenal medulla and central catecholamine neurons under in vivo conditions, and how are they regulated in response to stress or drug treatments? Answers to these (and other) questions are necessary to fully understand the mechanisms that regulate TH gene expression under different physiological, pathological and pharmacological states.
Figure 3.
(A) Diagram of TH mRNA, showing the sequences comprising the putative polypyrimidine-rich region of TH mRNA in different species. (B) Hypothetical mechanism by which cAMP, stress and other stimuli regulate TH mRNA translation and degradation via the induction and consequent increased binding of PCBP and possibly other unknown factors to the polypyrimidine-rich region of the 3′UTR.
References
- 1.Wong DL, Tank AW. Stress-induced catecholaminergic function: transcriptional and post-transcriptional control. Stress. 2007;10:121–30. doi: 10.1080/10253890701393529. [DOI] [PubMed] [Google Scholar]
- 2.Sabban EL, Kvetnansky R. Stress-triggered activation of gene expression in catecholaminergic systems: dynamics of transcriptional events. Trends in Neurosciences. 2001;24:91–8. doi: 10.1016/s0166-2236(00)01687-8. [DOI] [PubMed] [Google Scholar]
- 3.Sabban EL, et al. Regulation of gene expression of catecholamine biosynthetic enzymes by stress. Adv Pharmacol. 1998;42:564–7. doi: 10.1016/s1054-3589(08)60813-3. [DOI] [PubMed] [Google Scholar]
- 4.Kumer SC, Vrana KE. Intricate regulation of tyrosine hydroxylase activity and gene expression. J. Neurochem. 1996;67:443–462. doi: 10.1046/j.1471-4159.1996.67020443.x. [DOI] [PubMed] [Google Scholar]
- 5.Fossom LH, Carlson CD, Tank AW. Stimulation of tyrosine hydroxylase gene transcription rate by nicotine in rat adrenal medulla. Mol Pharmacol. 1991;40:193–202. [PubMed] [Google Scholar]
- 6.Serova LI, et al. Heightened transcription for enzymes involved in norepinephrine biosynthesis in the rat locus coeruleus by immobilization stress. Biological Psychiatry. 1999;45:853–862. doi: 10.1016/s0006-3223(98)90360-2. [DOI] [PubMed] [Google Scholar]
- 7.Sun B, Sterling CR, Tank AW. Chronic nicotine treatment leads to sustained stimulation of tyrosine hydroxylase gene transcription rate in rat adrenal medulla. Journal of Pharmacology & Experimental Therapeutics. 2003;304:575–588. doi: 10.1124/jpet.102.043596. [DOI] [PubMed] [Google Scholar]
- 8.Sun B, et al. Chronic nicotine treatment leads to induction of tyrosine hydroxylase in locus coeruleus neurons: The role of transcriptional activation. Molecular Pharmacology. 2004;66:1011–1021. doi: 10.1124/mol.104.001974. [DOI] [PubMed] [Google Scholar]
- 9.Chang MS, et al. Increased transcription of the tyrosine hydroxylase gene in individual locus coeruleus neurons following footshock stress. Neuroscience. 2000;101:131–9. doi: 10.1016/s0306-4522(00)00352-3. [DOI] [PubMed] [Google Scholar]
- 10.Osterhout C, et al. Induction of tyrosine hydroxylase in the locus coeruleus of transgenic mice in response to stress or nicotine treatment: lack of activation of tyrosine hydroxylase promoter activity. J Neurochem. 2005;94:731–741. doi: 10.1111/j.1471-4159.2005.03222.x. [DOI] [PubMed] [Google Scholar]
- 11.Osterhout CA, Chikaraishi DM, Tank AW. Induction of tyrosine hydroxylase protein and a transgene containing tyrosine hydroxylase 5′ flanking sequences by stress in mouse adrenal gland. Journal of Neurochemistry. 1997;68:1071–7. doi: 10.1046/j.1471-4159.1997.68031071.x. [DOI] [PubMed] [Google Scholar]
- 12.Nankova BB, Tank AW, Sabban EL. Transient or sustained transcriptional activation of the genes encoding rat adrenomedullary catecholamine biosynthetic enzymes by different durations of immobilization stress. Neuroscience. 1999;94:803–8. doi: 10.1016/s0306-4522(99)00290-0. [DOI] [PubMed] [Google Scholar]
- 13.Nankova BB, et al. Fos-related antigen 2: potential mediator of the transcriptional activation in rat adrenal medulla evoked by repeated immobilization stress. Journal of Neuroscience. 2000;20:5647–53. doi: 10.1523/JNEUROSCI.20-15-05647.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshimura R, et al. Nicotinic and muscarinic acetylcholine receptors are essential for the long-term response of tyrosine hydroxylase gene expression to chronic nicotine treatment in rat adrenal medulla. Molecular Brain Research. 2004;126:188–197. doi: 10.1016/j.molbrainres.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 15.Rusnak M, et al. Different effects of insulin and 2-deoxy-D-glucose administration on tyrosine hydroxylase gene expression in the locus coeruleus and the adrenal medulla in rats. Brain Research Bulletin. 1998;46:447–452. doi: 10.1016/s0361-9230(98)00033-1. [DOI] [PubMed] [Google Scholar]
- 16.Rusnak M, et al. Effect of novel stressors on gene expression of tyrosine hydroxylase and monoamine transporters in brainstem noradrenergic neurons of long-term repeatedly immobilized rats. Brain Research. 2001;899:20–35. doi: 10.1016/s0006-8993(01)02126-6. [DOI] [PubMed] [Google Scholar]
- 17.Czyzyk-Krzeska MF, et al. Post-transcriptional regulation of tyrosine hydroxylase gene expression by oxygen in PC12 cells. Kidney Int. 1997;51:585–90. doi: 10.1038/ki.1997.84. [DOI] [PubMed] [Google Scholar]
- 18.Czyzyk-Krzeska MF, et al. Regulation of tyrosine hydroxylase mRNA stability by oxygen in PC12 cells. Adv Exp Med Biol. 1996;410:143–50. doi: 10.1007/978-1-4615-5891-0_21. [DOI] [PubMed] [Google Scholar]
- 19.Alterio J, Mallet J, Biguet NF. Multiple complexes involved in tyrosine hydroxylase mRNA stability in rat adrenal medulla, after reserpine stimulation. Molecular & Cellular Neurosciences. 2001;17:179–89. doi: 10.1006/mcne.2000.0930. [DOI] [PubMed] [Google Scholar]
- 20.Baruchin A, et al. Effects of cold exposure on rat adrenal tyrosine hydroxylase: an analysis of RNA, protein, enzyme activity, and cofactor levels. Journal of Neurochemistry. 1990;54:1769–75. doi: 10.1111/j.1471-4159.1990.tb01232.x. [DOI] [PubMed] [Google Scholar]
- 21.Fernandez E, Craviso GL. Protein synthesis blockade differentially affects the degradation of constitutive and nicotinic receptor-induced tyrosine hydroxylase protein level in isolated bovine chromaffin cells. Journal of Neurochemistry. 1999;73:169–78. doi: 10.1046/j.1471-4159.1999.0730169.x. [DOI] [PubMed] [Google Scholar]
- 22.Nankova B, et al. Induction of tyrosine hydroxylase gene expression by a nonneuronal nonpituitary-mediated mechanism in immobilization stress. Proc.Natl.Acad.Sci.USA. 1994;91:5937–5941. doi: 10.1073/pnas.91.13.5937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piech-Dumas KM, Sterling CR, Tank AW. Regulation of tyrosine hydroxylase gene expression by muscarinic agonists in rat adrenal medulla. Journal of Neurochemistry. 1999;73:153–61. doi: 10.1046/j.1471-4159.1999.0730153.x. [DOI] [PubMed] [Google Scholar]
- 24.Xu L, et al. Evidence for regulation of tyrosine hydroxylase mRNA translation by stress in rat adrenal medulla. Brain Res. 2007;1158:1–10. doi: 10.1016/j.brainres.2007.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Czyzyk-Krzeska MF, et al. Regulation of tyrosine hydroxylase gene expression in the rat carotid body by hypoxia. J Neurochem. 1992;58:1538–46. doi: 10.1111/j.1471-4159.1992.tb11376.x. [DOI] [PubMed] [Google Scholar]
- 26.DeCristofaro JD, LaGamma EF. Neonatal stress: effects of hypoglycemia and hypoxia on adrenal tyrosine hydroxylase gene expression. Pediatr Res. 1994;36:719–23. doi: 10.1203/00006450-199412000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Holgert H, et al. Birth-related up-regulation of mRNA encoding tyrosine hydroxylase, dopamine beta-hydroxylase, neuropeptide tyrosine, and prepro-enkephalin in rat adrenal medulla is dependent on postnatal oxygenation. Pediatr Res. 1995;37:701–6. doi: 10.1203/00006450-199506000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Czyzyk-Krzeska MF, et al. Hypoxia stimulates binding of a cytoplasmic protein to a pyrimidine-rich sequence in the 3′-untranslated region of rat tyrosine hydroxylase mRNA. Journal of Biological Chemistry. 1994;269:9940–5. [PubMed] [Google Scholar]
- 29.Czyzyk-Krzeska MF, Beresh JE. Characterization of the hypoxia-inducible protein binding site within the pyrimidine-rich tract in the 3′-untranslated region of the tyrosine hydroxylase mRNA. Journal of Biological Chemistry. 1996;271:3293–9. doi: 10.1074/jbc.271.6.3293. [DOI] [PubMed] [Google Scholar]
- 30.Paulding WR, Czyzyk-Krzeska MF. Regulation of tyrosine hydroxylase mRNA stability by protein-binding, pyrimidine-rich sequence in the 3′-untranslated region. Journal of Biological Chemistry. 1999;274:2532–8. doi: 10.1074/jbc.274.4.2532. [DOI] [PubMed] [Google Scholar]
- 31.Sabban EL, et al. Differential effects of stress on gene transcription factors in catecholaminergic systems. Ann N Y Acad Sci. 2004;1032:130–40. doi: 10.1196/annals.1314.010. [DOI] [PubMed] [Google Scholar]
- 32.Hebert MA, Serova LI, Sabban EL. Single and repeated immobilization stress differentially trigger induction and phosphorylation of several transcription factors and mitogen-activated protein kinases in the rat locus coeruleus. J Neurochem. 2005;95:484–98. doi: 10.1111/j.1471-4159.2005.03386.x. [DOI] [PubMed] [Google Scholar]
- 33.Ortiz J, et al. Biochemical adaptations in the mesolimbic dopamine system in response to repeated stress. Neuropsychopharmacology. 1996;14:443–52. doi: 10.1016/0893-133X(95)00152-4. [DOI] [PubMed] [Google Scholar]
- 34.Angulo JA, et al. Isolation stress increases tyrosine hydroxylase mRNA in the locus coeruleus and midbrain and decreases proenkephalin mRNA in the striatum and nucleus accumbens. Brain Research. Molecular Brain Research. 1991;11:301–8. doi: 10.1016/0169-328x(91)90039-z. [DOI] [PubMed] [Google Scholar]
- 35.Pasinetti GM, et al. Tyrosine hydroxylase mRNA concentration in midbrain dopaminergic neurons is differentially regulated by reserpine. J Neurochem. 1990;55:1793–9. doi: 10.1111/j.1471-4159.1990.tb04970.x. [DOI] [PubMed] [Google Scholar]
- 36.Serova L, Sabban EL. Involvement of alpha 7 nicotinic acetylcholine receptors in gene expression of dopamine biosynthetic enzymes in rat brain. J Pharmacol Exp Ther. 2002;303:896–903. doi: 10.1124/jpet.102.039198. [DOI] [PubMed] [Google Scholar]
- 37.Smith KM, Mitchell SN, Joseph MH. Effects of chronic and subchronic nicotine on tyrosine hydroxylase activity in noradrenergic and dopaminergic neurones in the rat brain. J Neurochem. 1991;57:1750–6. doi: 10.1111/j.1471-4159.1991.tb06377.x. [DOI] [PubMed] [Google Scholar]
- 38.Biguet NF, et al. Time course of the changes of TH mRNA in rat brain and adrenal medulla after a single injection of reserpine. Embo J. 1986;5:287–91. doi: 10.1002/j.1460-2075.1986.tb04211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Melia KR, et al. Coordinate regulation of the cyclic AMP system with firing rate and expression of tyrosine hydroxylase in the rat locus coeruleus: effects of chronic stress and drug treatments. J Neurochem. 1992;58:494–502. doi: 10.1111/j.1471-4159.1992.tb09748.x. [DOI] [PubMed] [Google Scholar]
- 40.Bowyer JF, et al. Long-term effects of amphetamine neurotoxicity on tyrosine hydroxylase mRNA and protein in aged rats. J Pharmacol Exp Ther. 1998;286:1074–85. [PubMed] [Google Scholar]
- 41.Blanchard V, et al. Long-term induction of tyrosine hydroxylase expression: compensatory response to partial degeneration of the dopaminergic nigrostriatal system in the rat brain. J Neurochem. 1995;64:1669–79. doi: 10.1046/j.1471-4159.1995.64041669.x. [DOI] [PubMed] [Google Scholar]
- 42.Pasinetti GM, et al. Chronic lesions differentially decrease tyrosine hydroxylase messenger RNA in dopaminergic neurons of the substantia nigra. Brain Res Mol Brain Res. 1989;5:203–9. doi: 10.1016/0169-328x(89)90036-3. [DOI] [PubMed] [Google Scholar]
- 43.Pasinetti GM, et al. Slow changes of tyrosine hydroxylase gene expression in dopaminergic brain neurons after neurotoxin lesioning: a model for neuron aging. Brain Res Mol Brain Res. 1992;13:63–73. doi: 10.1016/0169-328x(92)90045-d. [DOI] [PubMed] [Google Scholar]
- 44.Kastner A, et al. Decreased tyrosine hydroxylase content in the dopaminergic neurons of MPTP-intoxicated monkeys: effect of levodopa and GM1 ganglioside therapy. Ann Neurol. 1994;36:206–14. doi: 10.1002/ana.410360213. [DOI] [PubMed] [Google Scholar]
- 45.Sherman TG, Moody CA. Alterations in tyrosine hydroxylase expression following partial lesions of the nigrostriatal bundle. Brain Res Mol Brain Res. 1995;29:285–96. doi: 10.1016/0169-328x(94)00259-h. [DOI] [PubMed] [Google Scholar]
- 46.Javoy-Agid F, et al. Decreased tyrosine hydroxylase messenger RNA in the surviving dopamine neurons of the substantia nigra in Parkinson’s disease: an in situ hybridization study. Neuroscience. 1990;38:245–53. doi: 10.1016/0306-4522(90)90389-l. [DOI] [PubMed] [Google Scholar]
- 47.Kastner A, et al. Immunocytochemical quantification of tyrosine hydroxylase at a cellular level in the mesencephalon of control subjects and patients with Parkinson’s and Alzheimer’s disease. J Neurochem. 1993;61:1024–34. doi: 10.1111/j.1471-4159.1993.tb03616.x. [DOI] [PubMed] [Google Scholar]
- 48.Trocme C, et al. CREM and ICER are differentially implicated in trans-synaptic induction of tyrosine hydroxylase gene expression in adrenal medulla and sympathetic ganglia of rat. J Neurosci Res. 2001;65:91–9. doi: 10.1002/jnr.1132. [DOI] [PubMed] [Google Scholar]
- 49.Thoenen H. Trans-synaptic enzyme induction. Life Sci. 1974;14:223–35. doi: 10.1016/0024-3205(74)90052-6. [DOI] [PubMed] [Google Scholar]
- 50.Mueller RA, Thoenen H, Axelrod J. Increase in tyrosine hydroxylase activity after reserpine administration. J Pharmacol Exp Ther. 1969;169:74–9. [PubMed] [Google Scholar]
- 51.Black IB, Chikaraishi DM, Lewis EJ. Trans-synaptic increase in RNA coding for tyrosine hydroxylase in a rat sympathetic ganglion. Brain Res. 1985;339:151–3. doi: 10.1016/0006-8993(85)90635-3. [DOI] [PubMed] [Google Scholar]
- 52.Leviel V, et al. Induction of tyrosine hydroxylase in the rat substantia nigra by local injection of forskolin. J Neurosci Res. 1991;30:427–32. doi: 10.1002/jnr.490300219. [DOI] [PubMed] [Google Scholar]
- 53.Lewis-Tuffin LJ, Quinn PG, Chikaraishi DM. Tyrosine hydroxylase transcription depends primarily on cAMP response element activity, regardless of the type of inducing stimulus. Molecular & Cellular Neurosciences. 2004;25:536–47. doi: 10.1016/j.mcn.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 54.Choi HK, et al. Specific modulation of dopamine expression in neuronal hybrid cells by primary cells from different brain regions. Proc Natl Acad Sci U S A. 1992;89:8943–7. doi: 10.1073/pnas.89.19.8943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Makeyev AV, Liebhaber SA. The poly(C)-binding proteins: a multiplicity of functions and a search for mechanisms. RNA. 2002;8:265–78. doi: 10.1017/s1355838202024627. [DOI] [PMC free article] [PubMed] [Google Scholar]