Abstract
Axon extension involves the coordinated regulation of the neuronal cytoskeleton. Actin filaments drive protrusion of filopodia and lamellipodia while microtubules invade the growth cone, thereby providing structural support for the nascent axon. Furthermore, in order for axons to extend the growth cone must attach to the substratum. Previous work indicates that myosin II activity inhibits the advance of microtubules into the periphery of growth cones, and myosin II has also been implicated in mediating integrin-dependent cell attachment. However, it is not clear how the functions of myosin II in regulating substratum attachment and microtubule advance are integrated during axon extension. We report that inhibition of myosin II function decreases the rate of axon extension on laminin, but surprisingly promotes extension rate on polylysine. The differential effects of myosin II inhibition on axon extension rate are attributable to myosin II having the primary function of mediating substratum attachment on laminin, but not on polylysine. Conversely, on polylysine the primary function of myosin II is to inhibit microtubule advance into growth cones. Thus, the substratum determines the role of myosin II in axon extension by controlling the functions of myosin II that contribute to extension.
Keywords: laminin, polylysine, blebbistatin, growth cone, vinculin, attachment, microtubule
Introduction
Axon extension occurs through a three-step process sequentially involving protrusion and substratum-attachment of filopodia and lamellipodia, followed by engorgement of the growth cone by microtubules and organelles, and finalized by the consolidation of a new stretch of axon shaft behind the advancing growth cone (Dent and Gertler, 2003; Loudon et al., 2006). Both protrusion and engorgement of the growth cone by microtubules contribute to the rate of axon extension (Letourneau et al., 1987; Dent and Gertler, 2003). We use the term engorgement, as defined by Goldberg and Burmeister (1986), to mean the advance of microtubule tips into lamellipodia and filopodia of growth cones. In addition, axons also require attachment to the substratum to extend and exert forces (Varum-Finney and Reichardt, 1994; Jay, 2001; Brown and Bridgman, 2003a; Woo and Gomez, 2006). Thus, axon extension is thought to be dependent on substratum attachment, protrusive activity and engorgement of the growth cone by microtubules. However, it is not clear how these different components of the mechanism of axon extension are integrated.
Myosin II is a mechanoenzyme that binds F-actin and generates cellular contractile forces. All known isoforms of myosin II are found in growth cones (Rochlin et al., 1995; Turney and Bridgman, 2005). Inhibition of myosin II function in neurons and neuronal cell lines has revealed multiple functions for myosin II in the extension and retraction of axons. When growing on laminin, inhibition of all myosin II activity or the activity of the IIB isoform alone decreases axon extension rate (reviewed in Brown and Bridgman, 2003a). Conversely, downregulation of myosin IIA, but not IIB, in Neuro-2A neuroblastoma cells decreases attachment through integrin receptors (Wylie and Chantler, 2001). Furthermore, myosin II activity is reported to inhibit microtubule engorgement of the peripheral domain of growth cones (Lin et al., 1996; Zhou and Cohan, 2001; Brown and Bridgman, 2003b). Myosin II activity drives the retrograde flow of actin in growth cones (Zhang et al., 2003), and actin undergoing retrograde flow may prevent microtubules from engorging the growth cone (Schaefer et al., 2002). Thus, myosin II is thought to regulate multiple aspects of the process of axon extension, including actin retrograde flow, engorgement of the growth cone by microtubules, and substratum attachment. However, it is not clear how the multiple functions of myosin II are integrated during axon extension.
We report that inhibition of myosin II activity on laminin coated substrata decreases axon extension rate. However, inhibition of myosin II increases axon extension rate on polyornithine-coated substrata (consistent with Turney and Bridgman, 2005). To investigate the mechanisms responsible for the substratum-dependent effects of myosin II inhibition on axon extension rate, we tested the hypothesis that different functions of myosin II in growth cones are engaged in a substratum-dependent manner (Fig. 1), thereby resulting in different effects of myosin II inhibition on axon extension as a function of the substratum. The hypothesis posits that (1) myosin II is required for attachment on substrata involving integrins (i.e. laminin) but not polycationic substrata (e.g., polylysine or polyornithine), and that (2) myosin II negatively regulates microtubule engorgement of the growth cone regardless of substratum. The data indicate that on laminin axon extension is attenuated following myosin II inhibition because of decreased substratum attachment, even though microtubule engorgement of the growth cone is promoted. However, on polylysine inhibition of myosin II does not alter attachment but increases engorgement of the growth cone by microtubules thereby promoting axon extension rate. We conclude that although myosin II has multiple functions during axon extension, the primary function of myosin II in regulating the rate of axon extension is determined by the substratum.
Figure 1.
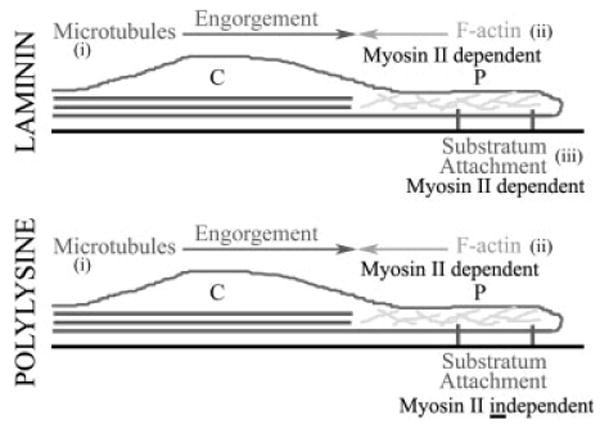
Main hypothesis. A schematic of a growth cone is shown in longitudinal cross-section. The central (C) and peripheral (P) domains are denoted. The peripheral domain contains actin filaments (F-actin) and the central domain contains microtubules. The hypothesis posits that on both laminin and polylysine coated substrata microtubule engorgement of the growth cone peripheral domain (i) is countered by myosin II-F-actin interactions (ii). The hypothesis also proposes that this function of myosin II is substratum-independent. Furthermore, the hypothesis posits that myosin II functions in a substratum dependent manner to regulate substratum attachment (iii). Specifically, the function of myosin II in mediating attachment is engaged by laminin but not polylysine.
Materials and Methods
Tissue Culture
Embryonic Day 7 chicken dorsal root ganglia were cultured as explants following published protocols (Lelkes et al., 2006). Explants were cultured in defined-medium (F12H with additives; Invitrogen, Carlsbad CA) and supplemented with 20 ng/mL nerve growth factor (R&D Systems, Minneapolis, MN). Explants were cultured in “video dishes” following published protocols (Gallo, 2004) using either 25 μg/mL laminin (Invitrogen) or 100 μg/mL of poly-d-lysine (70 KD MW; Sigma) as coated substrata. Briefly, “video dishes” were fabricated by drilling 1.4 cm holes in the center of Falcon 1006 dishes (Becton Dickinson, Franklin Lake, NJ) and affixing coverslips on the bottom of the dishes using aquarium sealant (Perfecto Manufacturing, Noblesville, IN).
Reagents
Taxol was purchased from Sigma (St. Louis, MO). Blebbistatin was obtained from Toronto Research Chemicals (North York, Ontario Canada). The W1B10 anti-β1 integrin antibody and the DM1A anti-tubulin antibody were purchased from Sigma. GFP-EB3 plasmids (kind gift of Dr. P.W. Baas, Drexel College of Medicine; Stepanova et al., 2003) were prepared and transfected into neurons using nucleofection as described in Jones et al. (2006).
Cell Attachment Assay
Cell attachment assays were performed as previously described (Silver and Gallo, 2005) and the number of attached neurons was determined by staining coverslips with neuron-specific βIII tubulin antibodies. Briefly, cells were plated in parallel on coverslips coated with laminin or polylysine for 3.5 h. The medium was then removed and the coverslips were washed three times to remove non-adherent cells by adding and removing 1 mL of fresh culture medium, allowing the medium to run over the coverslip. Following the final wash, the cultures were fixed and stained for neuron-specific βIII-tubulin.
Microscopy
Live imaging experiments were performed on a Zeiss 200 M microscope equipped with an AxioCam CCD camera (Zeiss, Gottingen Germany). Live cultures were observed using a 40× phase objective, or 100× for imaging of EB3. Time-lapse acquisition was driven by Zeiss AxioVision software. Imaging of EB3 comets was performed as described in Jones et al. (2006). The axons were observed for a total of 60 min, 30 min pretreatment and 30 min post-treatment with a 15 min period in between treatments. Images were acquired every 3 min of pretreatment and posttreatment. The stage was heated and held constant at 39°C using an ASI-400 air curtain incubator system (NevTek, Burnsville VA). Quantitative analysis of the axon extension rate was performed using the interactive measurement module of the AxioVision Software. The extension rates of axons were determined by distance in μm over elapsed time.
Immunocytochemistry
For staining purposes, cultures were fixed with a final concentration of 0.25% gluteraldehyde for 15 min, followed by washing and treatment with 2 mg/mL sodium borohydride (Sigma) in PBS. Actin filaments were stained using rhodamine-phalloidin (Molecular Probes, Eugene OR; 8/100 μL of staining solution). For experiments involving vinculin staining or the determination of microtubule content, the cultures were simultaneously fixed and extracted (FEX) as described in Gallo et al. (2002). Staining was performed using monoclonal anti-vinculin antibody (1:200; Sigma) and a goat-anti-mouse TRITC conjugated secondary antibody (1:400; Jackson Immunochemicals, West Grove PA). For determination of microtubule distribution, cultures were fixed using FEX. Microtubules were stained using a FITC-conjugated anti-tubulin monoclonal antibody (DM1A clone, 1:100; Sigma) and actin filaments were stained using rhodamine phalloidin. For experiments determining the ratio of tyrosinated tubulin to total tubulin, cultures were treated with specific doses of taxol for 65 min, fixed using FEX, and stained with 1:400 anti-tyrosinated tubulin primary antibody (1:400, Sigma) and a goat-anti-mouse TRITC conjugated secondary antibody (1:400; Jackson Immunochemicals), blocked with 10% mouse serum in PBS, and followed by DM1A-FITC antibody. For cell counts in attachment assays coverslips were fixed with 0.25% glutaraldehyde, processed as afore mentioned, and stained with a neuron specific βIII-tubulin antibody (1:100; Sigma). All antibodies were applied in PBS supplemented with 10% goat serum and 0.1% triton X-100 (GST). Blocking was performed prior to each antibody application for 30 min using GST.
Quantification of Staining Intensity
Imaging of fluorescently labeled preparations was performed on a Zeiss 200 M microscope using a 100× objective. Images were acquired as dual channel stacks in identical register. Acquisition parameters were set for each channel so that no pixel was at saturation level. Measurements of F-actin total staining intensity were performed at the growth cone, defined as the structure comprised by lamellipodia and filopodia encompassing the distal most 10 μm of the axon. Vinculin staining intensity was similarly determined at the growth cone. To determine the extent that microtubules engorged individual filopodia, the distance that microtubules invaded filopodia from the base of the filopodium was determined and divided by the total length of the filopodium. The ratio of tyrosinated tubulin to total tubulin mean staining intensity was similarly determined in the distal 10 μm of the axon. The measurements of staining intensity were determined using the interactive measurement module of the AxioVision Software. Total staining intensity was determined by integrating the pixel intensities within the area of measurement. Background intensity values were subtracted from all images prior to quantification. For presentation purposes, we selected images at random from the set of all images that are within one standard deviation from the mean.
Quantification of EB3 Comet Invasion of Filopodia
To determine the maximal distance that individual EB3-labeled polymerizing microtubule tips invaded growth cone filopodia, we made time-lapse movies of the same growth cones for 5 min prior to and after experimental treatment. A 6-second inter-frame interval was selected because this acquisition protocol allowed us to detect the polymerizing microtubule tips in filopodia while minimizing photobleaching, in these experiments we did not attempt to determine polymerization rates or catastrophe events, which would require a shorter inter-frame interval. The filopodia were imaged in EB3-expressing cells by digitally increasing the brightness and contrast of the image, which reveals the filopodial shaft because of low diffuse background GFP fluorescence. The maximal distance EB3 comets invaded each filopodium, relative to the length of the filopodial shaft, was then determined using AxioVision software quantitative analysis tools. For this analysis neurons were cultured on laminin.
Results
Inhibition of Myosin II has Substratum-Dependent Effects on Axon Extension Rate
Blebbistatin is a pharmacological inhibitor of the ATPase activity of myosin II (Straight et al., 2003), and exhibits no activity against other myosin family members (Limouze et al., 2004). Blebbistatin blocks myosin II-dependent processes such as apoptotic blebbing, cytokinesis, and the maintenance of cell polarity (Straight et al., 2003; Rosenblatt et al., 2004; Guha et al., 2005; Loudon et al., 2006). Turney and Bridgman (2005) reported that both blebbistatin treatment and genetic knock out of myosin II function affect the extension rate of axons on laminin and polyornithine. For our studies we used blebbistatin at 50 μM, a concentration that maximally blocks spontaneous apoptotic membrane blebbing and disrupts stress fibers in primary embryonic kidney cells and fibroblasts (data not shown). Axon retraction is myosin II dependent (Gallo et al., 2002; Wylie and Chantler, 2003), and 50 μM blebbistatin also maximally inhibits axon retraction in our culturing system (Gallo, 2004). In our hands 50 μM blebbistatin results in changes in growth cone polarity and blocks apoptotic cell blebbing within 10–15 min following treatment (Loudon et al., 2006; data not shown), indicating that blebbistatin readily enters the cytoplasm and blocks myosin II activity. In addition, chronic treatment of fibroblasts (24 h) with blebbistatin causes persistent, but reversible upon washout, changes in morphology and loss of stress fibers, indicating that blebbistatin remains active in our culturing system over prolonged time periods (data not shown). Collectively, these considerations indicate that blebbistatin is a valuable and specific tool to inhibit myosin II function in living cells.
We tested the effects of blebbistatin treatment on embryonic chicken dorsal root ganglion neurons cultured on laminin or polylysine. Axons were observed for a total of 60 min, 30 min pretreatment and 30 min posttreatment with a 15 min period in between treatments. On laminin, blebbistatin treatment inhibited axon extension rate by ∼60% [Fig. 2(A)]. However, blebbistatin increased axon extension rate by ∼40% on polylysine [Fig. 2(A,B)]. Thus, inhibition of myosin II has opposite effects on axon extension rate depending on substratum, consistent with the report of Turney and Bridgman (2005).
Figure 2.
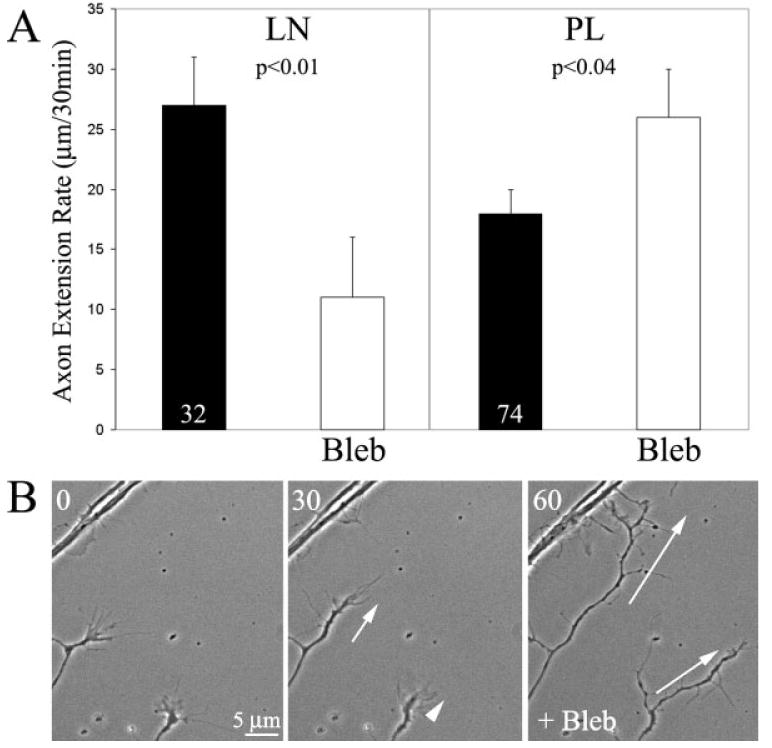
Inhibition of myosin II using blebbistatin (Bleb) differentially effects axon extension rate on laminin and polylysine. (A) Treatment with blebbistatin decreased axon extension on laminin (LN), but increased extension rate on polylysine (PL). In this and all other figures, numbers in bars represent sample size. n = axons. Axons were monitored before and after treatment with blebbistatin. Paired t-test used for comparisons. (B) Example of increased axon extension following blebbistatin treatment on polylysine. Numbers in top left corners reflect minutes elapsed from beginning of video sequence (t = 0). The white arrows at 30 min show the extension during the pre-treatment period. White arrows at 60 min denote the axon extension that occurred in the 30 min post blebbistatin treatment, for comparison to that occurring prior to blebbistatin treatment (30 min time point).
Effects of Myosin II Inhibition on Growth Cone F-Actin Content
F-actin is required for maintenance of normal axon extension rates (Letourneau et al., 1987). Previous studies using laminin as a substratum have reported decreased levels of F-actin in growth cones with decreased myosin II activity (Bridgman et al., 2001). Thus, myosin II inhibition could potentially differentially alter F-actin levels in growth cones in a substratum dependent manner resulting in different effects on axon extension rate. For example, myosin II inhibition may only decrease F-actin levels on laminin but not polylysine. Measurement of the total F-actin content of growth cones revealed that blebbistatin treatment decreased F-actin content on both laminin and polylysine [Fig. 3(A,B)]. This observation is consistent with the study by Bridgman et al. (2001) that found decreased levels of F-actin in growth cones of neurons cultured from myosin IIB knock out mice growing on a laminin substratum. Interestingly, blebbistatin decreased F-actin content by 73 and 41% on polylysine and laminin, respectively. Decreases in F-actin levels in sensory growth cones result in decreased axon extension rates on both polylysine and laminin substrata (Letourneau et al., 1987; Jones et al., 2006). Thus, because F-actin levels were decreased by blebbistatin treatment on both laminin and polylysine, but the effects of blebbistatin treatment on axon extension rate were dependent on the culturing substratum, these data indicate that the blebbistatin-induced decrease in growth cone F-actin content does not provide an explanation for the substratum-dependent effects of blebbistatin treatment on axon extension rate. Indeed, if decreases in growth cone F-actin levels were responsible for the observed changes in axon extension rates following myosin II inhibition, then extension rate should have been inhibited to a greater extent on polylysine than laminin
Figure 3.
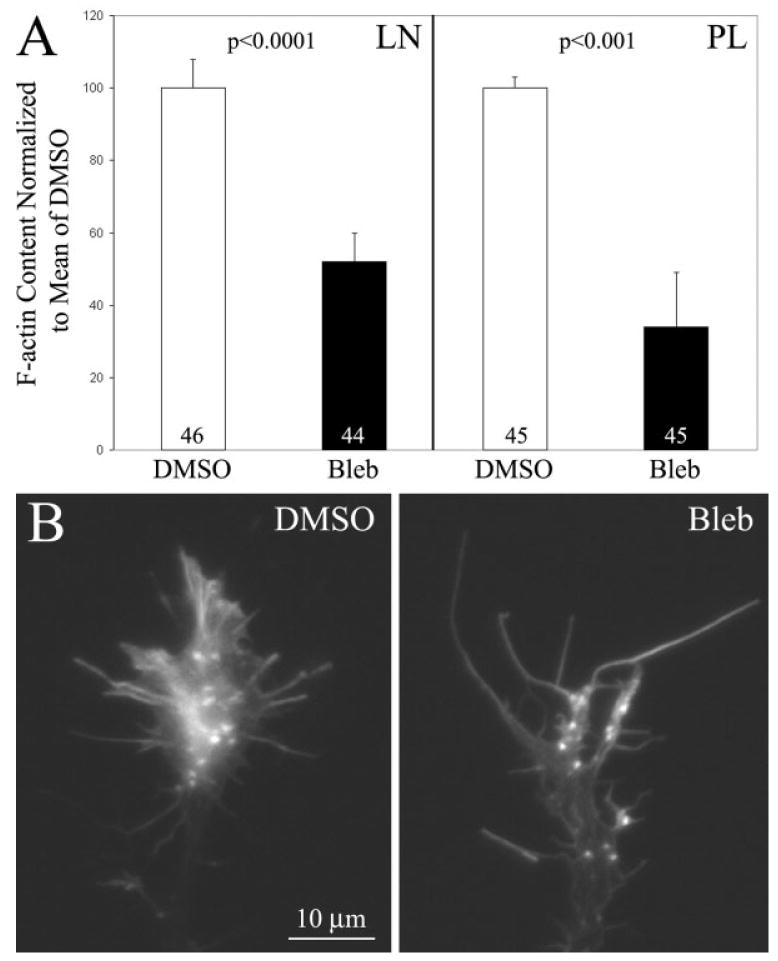
Growth cone F-actin content is decreased following inhibition of myosin II independent of substratum. (A) Inhibition of myosin II using blebbistatin (Bleb) decreased growth cone F-actin content on both laminin (LN) and polylysine (PL) relative to DMSO (vehicle control). Welch t-test used for comparison. n = growth cones. (B) Examples of growth cones on polylysine following DMSO or blebbistatin treatment. On both substrata F-actin was decreased mostly in lamellipodia, while filopodial F-actin appeared relatively unaffected, consistent with the report of Bridgman et al. (2001) for growth cones growing on laminin.
Myosin II Inhibition Decreases Substratum Attachment on Laminin but not Polylysine
Myosin II is involved in the regulation of integrin-mediated substratum attachment in neuro-2A neuroblastoma cells (Wylie and Chantler, 2001). However, it is not known whether myosin II alters attachment to substrata not dependent on integrins, or whether myosin II contributes to the attachment of primary neurons to integrin ligands (Brown and Bridgman, 2003a). Substratum attachment is required for neurons to extend axons. Therefore, differential inhibition of substratum attachment on laminin and polylysine following blockade of myosin II activity may underlie the decrease in axon extension rate on laminin. We compared the effects of inhibiting myosin II using blebbistatin on neuronal attachment to substrata coated with laminin or polylysine. The absolute number of neurons attached to PL and LN substrata in the absence of blebbistatin did not differ (19 ± 4 and 16 ± 5 cells/field on PL and LN respectively, p > 0.8), indicating that similar populations of neurons attach to LN and PL. Neuronal cell attachment assays revealed that inhibition of myosin II decreased cell attachment on laminin by ∼40% [Fig. 4(A)]. However, inhibition of myosin II did not alter attachment to polylysine [Fig. 4(A)]. Similarly, Wylie and Chantler (2001) also report partial inhibition of attachment to integrin-dependent substrata when myosin II levels were downregulated using antisense in a homogenous population of neuro-2A cells. Thus, in primary neurons myosin II partially contributes to attachment to laminin but not polylysine coated substrata.
Figure 4.
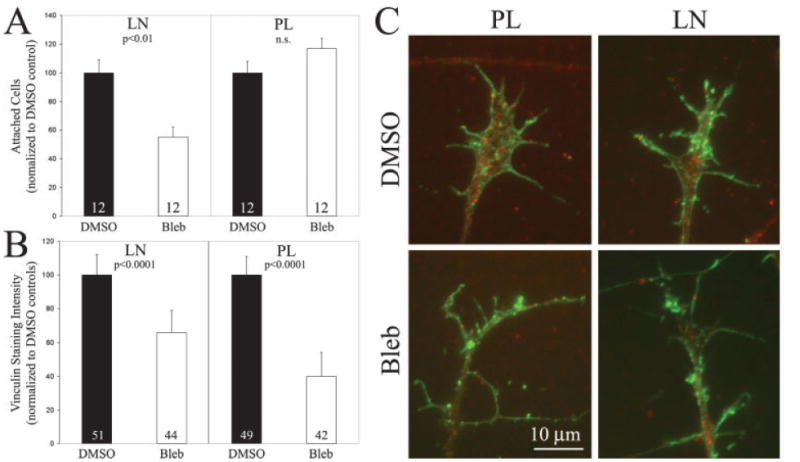
Myosin II activity is required for substratum attachment on laminin but not polylysine. (A) Cell attachment assays revealed that inhibition of myosin II using blebbistatin (Bleb) decreased cell attachment on laminin (LN) but not polylysine (PL), relative to vehicle control treatment (DMSO). n = cultures per group. Similar numbers of neurons attached to both laminin and polylysine in the absence of blebbistatin treatment (see text). Welch t-test used for comparisons. (B) Myosin II inhibition decreased the levels of cytoskeleton-associated vinculin, reflective of decreased substratum point contacts. n = growth cones. Welch t-test used for comparisons. (C) Examples of vinculin (red) and F-actin (green) staining in simultaneously fixed and extracted growth cones. Note decreased vinculin staining in growth cones and axons in blebbistatin treated cells. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Vinculin is a component of substratum adhesion point contacts in growth cones and nonneuronal cells, and its distribution and cytoskeletal association reports on substratum point contacts (Renaudin et al., 1999; Steketee and Tosney, 2002). Integrins engage vinculin and additional focal point proteins resulting in the formation of substratum contacts required for attachment. The importance of vinculin-based attachment in determining the rate of axon extension is emphasized by the demonstration that depletion of vinculin decreases neurite extension from PC12 cells (Varnum-Finney and Reichardt, 1994). To confirm the data obtained from cell attachment assays, and extend it to growth cones, we next investigated whether myosin II inhibition differentially altered the levels of cytoskeleton associated vinculin in growth cones using a simultaneous fixation and extraction protocol followed by quantitative immunocytochemistry (Steketee and Tosney, 2002). On laminin, myosin II inhibition decreased vinculin staining by 34% [Fig. 4(B,C)]. On polylysine, a substratum that does not require myosin II activity for attachment, myosin II inhibition decreased vinculin staining by 60% [Fig. 4(B,C)]. This indicates that although on polylysine myosin II contributes to vinculin association with the F-actin cytoskeleton, this interaction does not report on a role in cell attachment on polylysine. Moreover, in DMSO treated controls the total amount of vinculin staining in growth cones did not differ between laminin and polylysine substrata (integrated pixel intensities values 104× of 294 ± 26 and 329 ± 32, mean ± SEM on laminin and polylysine respectively, p > 0.3, n = 49–51 gowth cones/group), indicating that on both substrata similar amounts of vinculin associate with the cytoskeleton. Collectively, the attachment data and vinculin staining studies indicate that cell attachment on laminin, but not polylysine, is dependent on myosin II and the formation of focal contacts.
Inhibition of Laminin-Integrin Interactions Decreases Axon Extension Rate
Inhibition of myosin II, using blebbistatin, selectively decreased substratum attachment to laminin but not polylysine, suggesting that on laminin the decrease in axon extension rate observed following myosin II inhibition may be attributable to decreased integrin-laminin engagement. If decreases in laminin-integrin engagement following myosin II inhibition result in decreased axon extension rates, then similar decreases in axon extension rates should be detectable following direct experimental manipulation of laminin-integrin engagement. To determine the contribution of laminin-integrin binding in regulating the rate of axon extension, we treated axons growing on laminin coated substrata with a function blocking monoclonal antibody to β1 integrin that disrupts cell attachment to integrin-dependent substrata (W1B10 antibody; Hayashi et al., 1990). A dose response study revealed that treatment with 11 μg/mL of the β1 integrin antibody decreased axon extension rate by 57% [Fig. 5(A,B); p < 0.0001], similar to treatment with 50 μM blebbistatin [Fig. 2(A)]. Four out of 20 growth cones retracted in response to 11 μg/mL of the antibody, and these were not counted in the determination of axon extension rate. Interestingly, although the majority of antibody treated axons extended at slower rates, growth cones remained motile, actively extending filopodia and lamellipodia (not shown). Treatment with 22 μg/mL of the antibody resulted in axon retraction by 7 out of 10 axons, indicating complete detachment from the substratum. The remaining three axons stalled and did not extend following antibody treatment. Treatment with 2.2 μg/mL of the antibody did not affect axon extension rate [Fig. 5(A)]. As a control, treatment of cultures with a monoclonal antitubulin antibody (DM1A, Sigma) did not affect axon extension rates at a concentration of 22 μg/mL [Fig. 5(A)], the highest concentration of the β1 integrin antibody tested that results in pronounced axon retraction. We chose to use an antibody to an intracellular antigen as a control because antibodies to extracellular domains of receptors or cell adhesion molecules that have not been fully characterized can act as activators or inhibitors of their intended targets (e.g. the p75 neurotrophin receptor, Gehler et al., 2004). The tubulin antibody thus serves as a control for the addition of a monoclonal antibody to the cultures. As an additional control, treatment of cultures growing on polylysine with 11 μg/mL of the β1 integrin antibody, which decreases extension rate on laminin, did not affect axon extension rates [Fig. 5(A)], demonstrating the effects of the antibody are specific for substrata requiring integrin binding.
Figure 5.
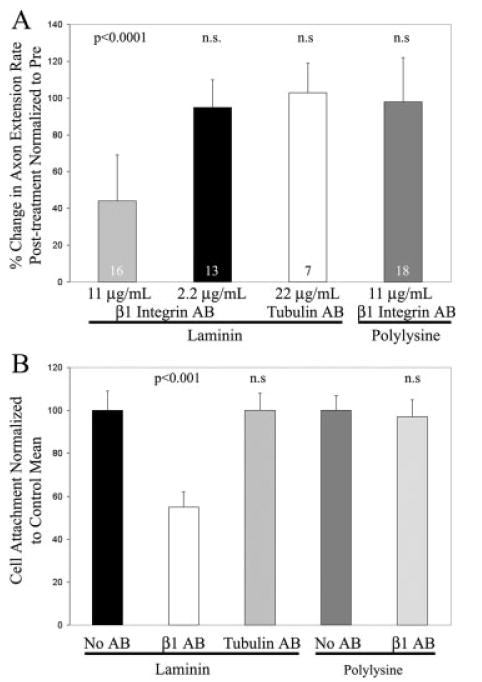
Inhibition of integrin-laminin interactions with a β1 integrin function-blocking antibody (AB) decreases axon extension rate and attachment. (A) On laminin coated substrata, 11 μg/mL of the β1 integrin antibody inhibited axon extension rate by 57%, while 2.2 μg/mL of the antibody did not affect axon extension rate. As a control, treatment with 22 μg/mL of an anti-tubulin antibody (DM1A) did not affect extension rate. Treatment of axons growing on polylysine with 11 μg/mL of the anti-β1 integrin antibody did not affect extension rate. Data collected from pre and post antibody treatment experiments. Paired t-test used for comparisons. n = axons. (B) Effects of the β1 integrin antibody on neuronal cell attachment to laminin or polylysine coated substrata. The concentration of β1 integrin antibody that decreased axon extension rate by 57% (11 μg/mL) inhibited cell attachment on laminin by 47%. Treatment with 11 μg/mL of the control anti-tubulin antibody did not affect attachment on laminin. Treatment with 11 μg/mL of the β1 integrin antibody did not affect attachment on polylysine. Welch t-test used for comparisons. n = 16 cultures per group.
To relate the effects of the β1 integrin antibody on axon extension to substratum attachment, we determined the effects of the antibody in cell attachment assays using substrata coated with laminin or polylysine. At a concentration of 11 μg/mL of antibody, which inhibits axon extension by 57% [Fig. 5(A)], cell attachment was inhibited by 47% relative to untreated controls [Fig. 5(B)]. Treatment with 11 μg/mL of the control anti-tubulin antibody did not affect cell attachment relative to untreated controls [Fig. 5(B)]. The β1 integrin antibody (11 μg/mL) did not affect attachment on polylysine [Fig. 5(B)]. These data indicate that the degree of integrin-laminin engagement regulates the rate of axon extension and substratum attachment, and are consistent with altered integrin-laminin interactions following myosin II inhibition mediating the decrease in axon extension rate.
Inhibition of Myosin II Promotes Engorgement of the Growth Cone by Microtubules on both Laminin and Polylysine
Axon extension requires microtubule advance into the growth cone, a process termed engorgement (Dent and Gertler, 2003). Previous work indicates that myosin II activity antagonizes the engorgement of microtubules into growth cone lamellipodia and filopodia (Lin et al., 1996; Zhou and Choan, 2001; Brown and Bridgman, 2003b). Therefore, promotion of microtubule-based engorgement of growth cone may contribute to the increased axon extension rate observed on polylysine following inhibition of myosin II. We determined the effects of myosin II inhibition on engorgement of growth cones by microtubules on polylysine and laminin. Filopodia represent the most distal portions of growth cones, and thus provide a good model system for determining the extent of microtubule engorgement. We determined the distance that microtubules invaded individual filopodia. Myosin II inhibition increased the distance that microtubules engorged into individual filopodia on both laminin and polylysine to a similar extent [Fig. 6(A,B)]. However, following myosin II inhibition, a greater percentage of all growth cone filopodia exhibited engorgement by one or more microtubules on laminin, but not on polylysine [Fig. 6(C)]. Blebbistatin treatment did not affect the number of growth cone filopodia on laminin (n = 43 gowth cones per group; p > 0.6). On Polylysine, blebbistatin treatment decreased the number of growth cone filopodia by 27% (n = 43 gowth cones per group; p < 0.0001). Untreated growth cones exhibited 50% more filopodia on polylysine than laminin (p < 0.0001). Comparison of the number of filopodia following blebbistatin treatment across the two substrata revealed no difference in the number of growth cone filopodia (means of 6.3 and 6.7 filopodia on laminin and polylysine, respectively; p > 0.2). Thus, myosin II inhibits the engorgement of growth cone filopodia by microtubules independent of substratum. Collectively, these data indicate that the difference in axon extension rates observed following myosin II inhibition on laminin and polylysine are not readily explained by substratum-dependent differences in the ability of microtubules to engorge growth cones.
Figure 6.
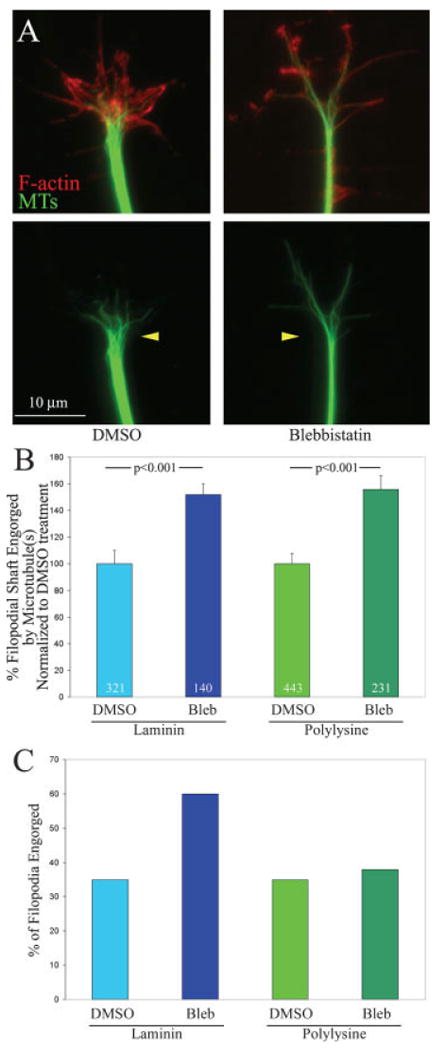
Myosin II negatively regulates engorgement of the growth cone by microtubules independent of substratum. (A) Examples of growth cones on laminin double stained for microtubules (green) and F-actin (red). In DMSO treated controls microtubules do not extend deep into the growth cone peripheral domain. However, following blebbistatin treatment microtubules engorge the peripheral domain and the tips of microtubules are found very close to the leading edge of the peripheral domain. The bottom panels show the microtubule staining alone, and the yellow arrowheads denote the location of the base of the growth cone, the point where microtubules splay out from the bundled axonal array. In blebbistatin treated growth cones, microtubules extend further into the growth cone from the base. (B) Quantification of microtubule engorgement into individual filopodia, the most distal portions of the growth cone peripheral domain. The distance microtubules engorged individual filopodial shafts was determined and expressed relative to the total length of the filopodium. Only filopodia exhibiting engorgement by microtubules were used in this analysis. Blebbistatin treatment promotes the distance microtubules engorge filopodia by ∼50% regardless of substratum. Data are shown normalized to the DMSO control values. Welch t-test used for comparison. n = filopodia. (C) The percentage of all filopodia at growth cones engorged by one or more microtubules is increased by blebbistatin on laminin but not polylysine. The same data set used to obtain the data in (B) was used for determining the data in (C). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Live Imaging of the Engorgement of Filopodia by Microtubule Tips
To confirm the immunocytochemical observation of increased engorgement of growth cone filopodia by microtubules following inhibition of myosin II, we directly imaged microtubule tips in living neurons expressing fluorescently labeled EB3 (as previously reported in Jones et al., 2006). EB3 is a microtubule tip tracking protein that reports on the dynamics of microtubule tips that are actively polymerizing (Stepanova et al., 2003). Blebbistatin is not a suitable reagent for imaging GFP/CFP/YFP constructs due to phototoxicity (Kolega, 2004; Loudon et al., 2006). We therefore attempted expressing mRFP-EB3 in chicken DRG neurons. In chicken neurons mRFP-EB3 expression was not reliable and we did not observe a characteristic EB3-expression pattern, although the same construct we used works well in mammalian neurons (Dr. P.W. Baas, Drexel College of Medicine, personal communication). Therefore, we expressed GFP-EB3 and inhibited myosin II function indirectly by treating the neurons with 10 μM y-27632, an inhibitor of RhoA-kinase. We have previously determined that this treatment decreases phosphorylation of regulatory myosin light chains by greater than 80% in growth cones in our culturing system (Loudon et al., 2006).
GFP-EB3 expression in cells results in the presence of “comets” [Fig. 7(A)], each comet reporting on the polymerization of one microtubule tip (Stepanova et al., 2003). We monitored the localization of EB3-comets in growth cone filopodia before and after treatment with y-27632 in neurons cultured on laminin. y-27632 treatment increased the distance that individual microtubule tips advanced into filopodia by 50% relative to treatment with DMSO [Fig. 7(B)], consistent with the promotion of engorgement following inhibition of myosin II. Measurements of filopodial length revealed that treatment with y-27632 does not result in a decrease in filopodial shaft length (p > 0.27, comparing pretreatment and posttreatment filopodial lengths), which could indirectly account for increased microtubule tip engorgement of filopodia independent of inhibition of myosin II activity. Similarly, DMSO treatment had no effect on filopodial length (p > 0.63). In addition, we found no differences between the number of EB3-comets that engorged filopodia (p > 0.49 for both DMSO and y-27632, respectively), or the total number of EB3 comets in the growth cone (p > 0.57 and p > 0.89 for DMSO and y-27632, respectively), following treatment with DMSO or y-27632. These results are consistent with the immunocytochemical analysis of increased engorgement of growth cones and filopodia by microtubules following direct inhibition of myosin II using blebbistatin. We however cannot rule out that an additional target of RhoA-kinase, other than myosin II activity, contributed to the increase in EB3-comet advance into filopodia.
Figure 7.
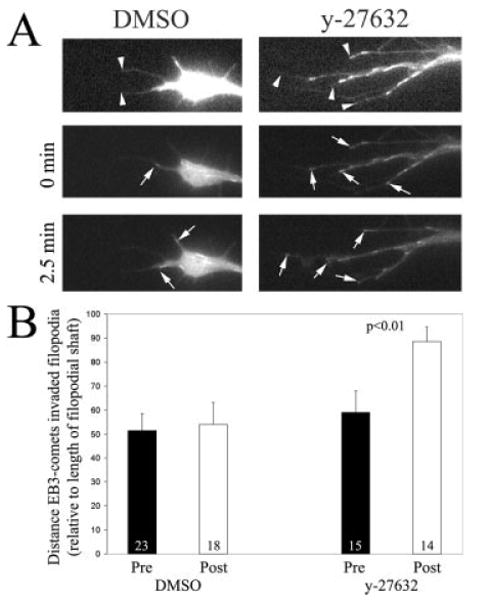
Inhibition of RhoA-kinase promotes the distance microtubule tips invade filopodia in living growth cones. (A) Example of GFP-EB3 comets in neuronal filopodia in growth cones treated with DMSO or with 10 μM y-27632. The top panel shows the growth cone expressing GFP-EB3 that has been digitally enhanced to reveal the growth cone because of low background fluorescence. The tips of filopodia are denoted by arrowheads. The two panels below show the extent of microtubule tip invasion into the filopodia (arrows point to individual EB3-comets). Note that the EB3-comets in the y-27632 treated growth cone extend further into the filopodial shaft than in the DMSO treated growth cone. The difference in total comet number is reflective of differences between individual growth cones. The quantitative analysis was carried out within growth cone before and after treatment. (B) Results of the quantitative analysis of EB3-comet advance into individual growth cone filopodia. Five growth cones were monitored for 5 min prior to and 5 min after treatment with y-27632 or DMSO starting at 15 min post treatment. The maximum distance, relative to the length of the filopodial shaft, that EB3 comets were noted to invade each filopodium was used as a metric. The number of filopodia analyzed is shown in the bars. Paired t-test was used for the comparison.
Engorgement of the Growth Cone by Microtubules is Required for the Increase in Axon Extension Rate Following Inhibition of Myosin II on Polylysine
Inhibition of myosin II promoted engorgement of growth cones by microtubules on both laminin and polylysine. Microtubules engorge growth cones through the dynamic polymerization of their tips (Tanaka and Kirschner, 1991, 1995; Gallo, 1998; Dent and Gertler, 2003), a process termed dynamic instability. To determine whether microtubule engorgement is required for the increase in axon extension rate observed on polylysine following myosin II inhibition, we treated cultures with low nano molar doses of taxol to inhibit microtubule tip dynamic instability. Taxol is a pharmacological compound that at low concentrations specifically inhibits microtubule dynamic instability (Jordan et al., 1993; Derry et al., 1995), and thereby inhibits microtubule advance into growth cones (Gallo, 1998). Cultures were pretreated with varying concentrations of taxol prior to treatment with 50 μM blebbistatin. Taxol dose dependently inhibited the blebbistatin-induced increase in extension rate [Fig. 8(A)]. At the concentration that blocked increases in axon extension rates (0.25 nM), taxol treatment alone did not decrease axon extension rate [Fig. 8(B)]. This indicates that 0.25 nM taxol is buffering increases in axon extension rate, without directly affecting the extension rate of axons.
Figure 8.
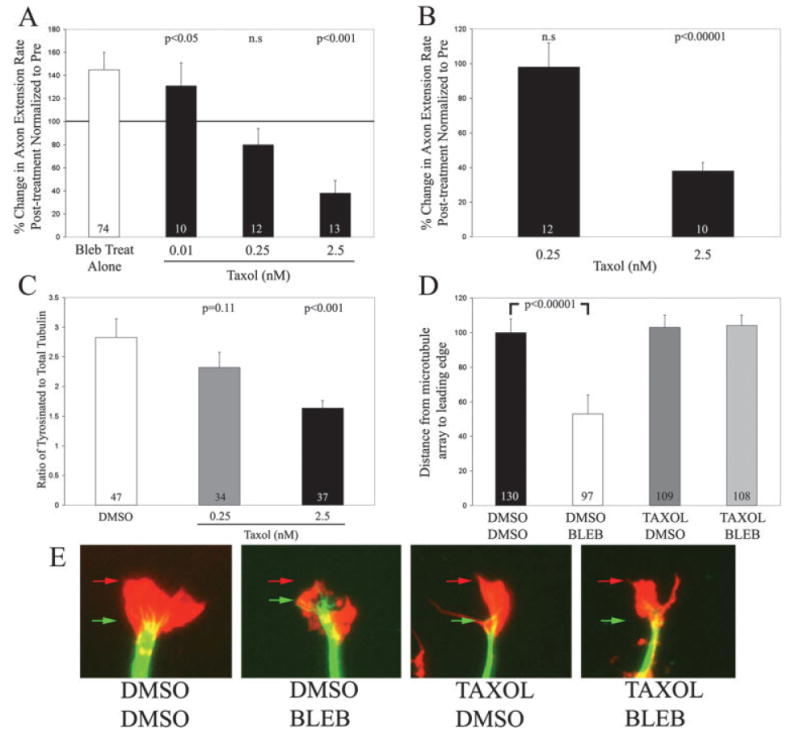
Inhibition of microtubule dynamic instability prevents the increase in axon extension rates observed on polylysine following inhibition of myosin II. (A) Dose dependent effects of taxol on the blebbistatin-induced increase in axon extension rate. Treatment with blebbistatin alone (Bleb Treat Alone) increased axon extension rate. Pretreatment with 0.01 nM taxol, prior to treatment with blebbistatin, resulted in a 25% decrease in axon extension rate relative to blebbistatin treatment alone, but blebbistatin treatment significantly elevated axon extension rate relative to pre-treatment (p < 0.05). Pretreatment with 0.25 nM taxol prevented the blebbistatin-induced increase in axon extension rate. No statistically significant difference was observed between pre and post blebbistatin treatment imaging periods. Pretreatment with 2.5 nM taxol resulted in a decrease in axon extension rate following blebbistatin treatment (p < 0.001). Matched t-test used for prepost treatment comparisons. n = axons. (B) Treatment with taxol inhibits axon extension rate in a dose dependent manner. The concentration of taxol that blocked the blebbistatin-induced increase in axon extension rate (0.25 nM) did not by itself affect axon extension rate. However, 2.5 nM taxol decreased axon extension rate. Matched t-test used for pre-post treatment comparisons. n = axons. (C) Effects of taxol on net microtubule dynamics. Quantification of the ratio of tyrosinated tubulin staining to total tubulin staining in distal axons revealed that 0.25 nM taxol decreased net microtubule dynamics by 19%, although this difference did not reach significance (p = 0.11). Treatment with 2.5 nM taxol significantly decreased net microtubule dynamics. Welch t-test used for comparison. n = axons. (D) Taxol treatment prevented the engorgement of the growth cone by microtubules induced by blebbistatin treatment. Cultures were treated for 30 min with either DMSO or 0.25 nM taxol prior to treatment with 50 μM blebbistatin or DMSO for an additional 30 min prior to simultaneous fixation and extraction. Blebbistatin treatment (DMSO BLEB) decreased the distance between the front of the microtubule array and the leading edge of growth cones relative to the control (DMSO DMSO), confirming the promotion of microtubule engorgement into the growth cone (Fig. 6). Treatment with TAXOL alone (TAXOL DMSO) did not alter engorgement relative to controls (DMSO DMSO, p > 0.7). Treatment with blebbistatin following pretreatment with taxol (TAXOL BLEB) prevented the effects of blebbistatin on engorgement of the growth cone (p > 0.7 compared to DMSO DMSO and p < 0.0.00001 compared to DMSO BLEB). Data is expressed normalized to the DMSO DMSO control. Welch t-test used for comparison. n = growth cones. (E) Examples of the distributions of microtubules and F-actin in growth cones exposed to various treatment paradigms as detailed in panel (D). Microtubules are shown in green and F-actin is shown in red. The red arrows denote the distal most F-actin staining and the green arrows denote the front of the microtubule array. Note that in the DMSO BLEB example the microtubules extend closer to the leading edge of the growth cone than in the other examples. For presentation purposes, the F-actin signal in blebbistatin treated growth cones was digitally enhanced to reveal the whole extent of growth cone morphology. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
We were surprised to observe that at a higher taxol concentration (2.5 nM) blebbistatin treatment decreased axon extension rate on polylysine [Fig. 8(A)]. Treatment with 2.5 nM taxol alone decreased axon extension rate by 60% [Fig. 8(B)]. Following treatment with blebbistatin, axon extension rate was further decreased by ∼60%, relative to taxol treatment alone, resulting in only minimal axon extension. This observation suggests an additional level of interaction between the dynamics of the microtubule cytoskeleton and the actomyosin system during axon extension. When microtubule dynamics are largely dampened, resulting in decreased axon extension rates, the activity of myosin II-dependent processes becomes a large determinant of axon extension.
We next determined the effects of taxol treatment on net microtubule dynamics and organization in growth cones. Cultures were treated with 0.25 and 2.5 nM taxol for 45 min, simultaneously fixed and extracted, and double labeled for total-tubulin and tyrosinated tubulin. A 45-min time point was chosen because this represents the state of microtubule dynamics in taxol treated cultures at the midway time point of blebbistatin treatment. Taxol treatment resulted in a dose dependent decrease in the levels of tyrosinated tubulin relative to total tubulin [Fig. 8(C)]. Treatment with 0.25 nM taxol resulted in a 19% decrease in the ratio of tyrosinated to total tubulin, although the decrease did not reach statistical significance (p = 0.11). Treatment with 2.5 nM taxol resulted in a 42% decrease in the ratio of tyrosinated to total tubulin (p < 0.001). Thus, consistent with the lack of overt effects of 0.25 nM taxol on axon extension rate, this concentration of taxol also did not have large effects on net microtubule dynamics, although it blocked the increase in growth rate resulting from blebbistatin treatment. Collectively, these data indicate that 0.25 nM taxol buffers the ability of microtubules to engorge the growth cone following inhibition of myosin II, and does not by itself affect normal levels of microtubule dynamics and engorgement.
Finally, we determined the effects of 0.25 nM taxol on the distribution of microtubules in growth cones with and without blebbistatin treatment. Cultures were pretreated with taxol or DMSO prior to treatment with blebbistatin or DMSO. The distance microtubules engorged the growth cone was then determined by measuring the distance between the front of main microtubule array and the leading edge of the growth cone directly in front of the microtubule array. This measurement method was chosen because we observed increases in filopodial lengths in subsets of growth cones following blebbistatin treatment in cultures pretreated with taxol (data not shown), thereby confounding the measurement method used in Figure 6 that relies on measurements of microtubule invasion of filopodia. These measurements confirmed that blebbistatin treatment alone promotes the engorgement of the growth cone by microtubules [Fig. 8(D,E), compare DMSO DMSO to DMSO BLEB]. Following blebbistatin treatment microtubules were found in closer proximity to the leading edge of growth cones than in controls [Fig. 8(E)]. Consistent with the block of increases in axon extension rate following blebbistatin treatment by 0.25 nM taxol, pretreatment with 0.25 nM taxol prevented the promotion of microtubule engorgement of growth cones induced by blebbistatin [Fig. 8(D)]. Similarly, consistent with the lack of an effect of 0.25 nM taxol on axon extension rate [Fig. 8(B)], treatment with 0.25 nM taxol alone did not affect engorgement of the growth cone by microtubules [Fig. 8(D,E)]. These data confirm that treatment with 0.25 nM taxol prevents the promotion of microtubule engorgement of the growth cone induced by blebbistatin treatment.
Discussion
Axon extension proceeds through a three step process sequentially involving leading edge protrusion, followed by the engorgement of the growth cone by microtubules, and the consolidation of a new segment of axon shaft behind the advancing growth cone (reviewed in Dent and Gertler, 2003). The engorgement of the growth cone by microtubules is a necessary step in axon extension. Indeed, when axons are depleted of actin filaments, axon extension becomes dependent on microtubule polymerization (Jones et al., 2006), and pharmacological promotion of microtubule polymerization increases axon extension rates (Letourneau et al., 1987). The attachment of the growth cone to the substratum is also of fundamental importance (McKerracher et al., 1996; Woo and Gomez, 2006). Substratum attachment in axon extension is required for growth cones to physically connect forces generated by the actomyosin system to the substratum through links between membrane receptors and the intracellular F-actin cytoskeleton (Bridgman et al., 2001; Jay, 2001). Although both substratum attachment and microtubule-based engorgement have been shown to be important for determining the rate of axon extension, it is not clear how these two aspects of the mechanism of axon extension are integrated. We suggest that myosin II is a key player in the integration of substratum attachment and microtubule-based engorgement on laminin. However, on polylysine myosin II has the primary function of controlling microtubule-based engorgement and not substratum attachment.
In this report we address the paradoxical observation that inhibition of myosin II can either inhibit or promote axon extension rate as a function of the culturing substratum. Our data show that on substrata requiring myosin II function for attachment to the substratum, such as laminin, inhibition of myosin II results in decreased axon extension rate, even though the engorgement of the growth cone by microtubules is promoted. Thus, on substrata that require myosin II for attachment the primary function of myosin II in determining the rate of axon extension is to regulate substratum attachment. Consistent with this idea, inhibition of attachment to laminin using a function blocking integrin antibody slows axon extension and decreases attachment in a manner similar to inhibition of myosin II. However, on substrata that do not require myosin II activity for attachment, such as polylysine, the primary role of myosin II is to inhibit microtubules from engorging the growth cone, thereby preventing axons from extending at greater extension rates.
Myosin II is required for substratum attachment on laminin but not polylysine. Wylie and Chantler (2001) demonstrated a role for myosin II in mediating attachment to integrin ligands in Neuro-2A cells. We now report that myosin II has a similar function in primary embryonic sensory neurons. In future work it will be of interest to address the question of what aspects of substratum attachment point dynamics are altered when myosin II is inhibited. Woo and Gomez (2006) recently reported that inhibition of a myosin II upstream regulator, RhoA-kinase, results in more dynamic attachment points at the expense of more stable attachment points. Our data showing decreased vinculin staining in neurons treated with blebbistatin are consistent with the results of Woo and Gomez (2006). Although the effects of directly inhibiting myosin II on attachment point dynamics remain to be resolved, our data demonstrate that myosin II contributes to attachment on laminin.
Following inhibition of myosin II using blebbistatin, on laminin both cell attachment and the levels of cytoskeleton associated vinculin decreased to a similar extent (40 and 34%, respectively). However, on polylysine, inhibition of myosin II had no effect on cell attachment but decreased the levels of cytoskeleton associated vinculin to a greater extent than on laminin (60%). These data indicate that myosin II is required for integrin-mediated attachment on laminin but not on polylysine, and that although on polylysine vinculin associates with the F-actin cytoskeleton in a myosin-II dependent manner, this association is not of functional significance with regard to the mechanism of substratum attachment on polylysine. Cytoskeleton-associated vinculin on polylysine may represent a pool of potential substratum attachment mechanisms ready for use when axons encounter a substratum requiring focal point adhesions for axon extension, such as laminin. Indeed, growth cones turn to remain on laminin at borders between laminin and polyornithine, and inhibition of myosin II prevents axons from making guidance decisions at such borders (Turney and Bridgman, 2005). The above considerations suggest that the inability to make a full complement of substratum adhesion points on laminin during guidance at a border between laminin and polycationic substrata, under conditions of myosin II inhibition, may contribute to the failure of axons to turn and preferentially extend on laminin.
Inhibition of laminin-integrin engagement decreases axon extension rate. Experiments using a function blocking β1 integrin antibody support the notion that the degree of laminin-based substratum attachment is a determinant of axon extension rate. Lemmon et al. (1992) did not find that the degree of substratum attachment is a good predictor of axon extension rate across different substrata. However, consistent with the report of Condic and Letourneau (1997), in the case of laminin we find that the rate of axon extension and the degree of substratum attachment are related. Thus, a minimal degree of attachment to laminin must be maintained to exhibit ‘normal’ axon extension rates. Alternatively, decreased integrin-laminin interactions may decrease integrin-mediated signaling pathways that contribute to axon extension in a substratum-dependent but attachment-independent manner. For example, Kuhn et al. (1998) demonstrated that the Rac1 GTPase is involved in axon extension on integrin-dependent substrata but not polylysine. Thus, blocking integrin-laminin interactions with a blocking antibody could affect axon extension rate by inhibiting the physical interaction between receptor and ligand required for substratum attachment, or by the resultant loss of signaling through the receptor. Regardless, myosin II is required for the attachment of primary neurons to laminin substrata, and the integrin antibody affects both axon extension rates and substratum attachment.
The increase in axon extension rate on polylysine following inhibition of myosin II is attributable to increased engorgement of the growth cone by microtubules. The engorgement of growth cones by microtubules is required for axon extension (reviewed in Dent and Gertler, 2003), and promotion of engorgement of growth cones by microtubules is expected to positively contribute to the rate of axon extension (Letourneau et al., 1987). Prior evidence indicates that myosin II-based contractility restricts microtubules from engorging the growth cone (Lin et al., 1996; Zhou and Cohan, 2001; Schaefer et al., 2002; Brown and Bridgman, 2003b). Our results confirm that myosin II regulates engorgement of the growth cone by microtubules on both laminin and polylysine. Thus, although increased engorgement of growth cones by microtubules is expected to positively contribute to axon extension rate (Letourneau et al., 1987), on laminin the promotion of engorgement following inhibition of myosin II fails to increase axon extension rate, indicating that diminished substratum attachment is the primary cause for the observed decrease in axon extension rate. On laminin, relative to polylysine, we observed a greater percentage of filopodia becoming engorged by microtubules following inhibition of myosin II [Fig. 6(C)]. It is not currently clear how this increase in the number of engorged filopodia may affect axon extension rate, and we cannot rule out that increased numbers of filopodia engorged by microtubules may also partially contribute to the effects of myosin II inhibition on axon extension rate on laminin. Importantly, experiments using taxol to inhibit microtubule dynamics demonstrate that on polylysine the promotion of engorgement is required for the increase in axon extension rate induced by myosin II inhibition. Collectively these data demonstrate that the promotion of microtubule engorgement by myosin II inhibition is responsible for the increased axon extension rate exhibited following myosin II inhibition on polylysine-coated substrata. In future work it will be of interest to determine whether different isoforms of myosin II have substratum specific functions during axon extension in primary neurons as it has been suggested for neuron-like cell lines (Wylie and Chantler, 2001). However, Turney and Bridgman (2005) report that myosin II activity inhibits peripheral nervous system axon extension rate on polyornithine independent of isoform, suggesting that in primary neurons isoforms of myosin II act in concert and are interchangeable.
In conclusion, we propose that on laminin myosin II integrates substratum attachment and microtubule-based engorgement. On laminin, the role of myosin II in mediating attachment to laminin substrata is the major determinant of the rate of axon extension, and supercedes the role of myosin II in regulating microtubule engorgement of the growth cone. Indeed, in order to maintain a constant extension rate, neurons regulate the expression levels of cell surface integrins to match the extracellular density of laminin (Condic and Letourneau, 1997). Conversely, when myosin II is not required to mediate substratum attachment (i.e. on polylysine), its primary function in regulating axon extension rate is that of attenuating the engorgement of microtubules into the growth cone, resulting in suboptimal axon extension rates.
Acknowledgments
This research was supported by a NIH grant to GG (NS048090). AK and SJ contributed equally to this project.
Contract grant sponsor: NIH; contract grant number: NS048090.
Footnotes
This article contains supplementary material available via the Internet at http://www.interscience.wiley.com/jpages/1932-8451/suppmat
References
- Bridgman PC, Dave S, Asnes CF, Tullio AN, Adelstein RS. Myosin IIB is required for growth cone motility. J Neurosci. 2001;21:6159–6169. doi: 10.1523/JNEUROSCI.21-16-06159.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J, Bridgman PC. Role of myosin II in axon outgrowth. J Histochem Cytochem. 2003a;51:421–428. doi: 10.1177/002215540305100403. [DOI] [PubMed] [Google Scholar]
- Brown J, Bridgman PC. Retrograde flow rate is increased in growth cones from myosin IIB knockout mice. J Cell Sci. 2003b;116:1087–1094. doi: 10.1242/jcs.00335. [DOI] [PubMed] [Google Scholar]
- Condic ML, Letourneau PC. Ligand-induced changes in integrin expression regulate neuronal adhesion and neurite outgrowth. Nature. 1997;389:852–856. doi: 10.1038/39878. [DOI] [PubMed] [Google Scholar]
- Dent EW, Gertler FB. Cytoskeletal dynamics and transport in growth cone motility and axon guidance. Neuron. 2003;40:209–227. doi: 10.1016/s0896-6273(03)00633-0. [DOI] [PubMed] [Google Scholar]
- Derry WB, Wilson L, Jordan MA. Substoichiometric binding of taxol suppresses microtubule dynamics. Biochemistry. 1995;34:2203–2211. doi: 10.1021/bi00007a014. [DOI] [PubMed] [Google Scholar]
- Gallo G. Involvement of microtubules in the regulation of neuronal growth cone morphologic remodeling. J Neurobiol. 1998;35:121–140. [PubMed] [Google Scholar]
- Gallo G, Yee HF, Jr, Letourneau PC. Actin turnover is required to prevent axon retraction driven by endogenous actomyosin contractility. J Cell Biol. 2002;158:1219–1228. doi: 10.1083/jcb.200204140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo G. Myosin II activity is required for severing-induced axon retraction in vitro. Exp Neurol. 2004;189:112–121. doi: 10.1016/j.expneurol.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Gehler S, Gallo G, Veien E, Letourneau PC. p75 neurotrophin receptor signaling regulates growth cone filopodial dynamics through modulating RhoA activity. J Neurosci. 2004;24:4363–4372. doi: 10.1523/JNEUROSCI.0404-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg DJ, Burmeister DW. Stages in axon formation: Observations of growth of Aplysia axons in culture using video-enhanced contrast-differential interference contrast microscopy. J Cell Biol. 1986;103:1921–1931. doi: 10.1083/jcb.103.5.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha M, Zhou M, Wang YL. Cortical actin turnover during cytokinesis requires myosin II. Curr Biol. 2005;15:732–736. doi: 10.1016/j.cub.2005.03.042. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Haimovich B, Reszka A, Boettiger D, Horwitz A. Expression and function of chicken integrin β 1 subunit and its cytoplasmic domain mutants in mouse NIH 3T3 cells. J Cell Biol. 1990;110:175–184. doi: 10.1083/jcb.110.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay D. A Src-astic response to mounting tension. J Cell Biol. 2001;155:327–330. doi: 10.1083/jcb.200110019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S, Selzer M, Gallo G. Developmental regulation of sensory axon regeneration in the absence of growth cones. J Neurobiol. 2006;66:1630–1645. doi: 10.1002/neu.20309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan MA, Toso RJ, Thrower D, Wilson L. Mechanism of mitotic block and inhibition of cell proliferation by taxol at low concentrations. Proc Natl Acad Sci USA. 1993;90:9552–9556. doi: 10.1073/pnas.90.20.9552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolega J. Phototoxicity and photoinactivation of blebbistatin in UV and visible light. Biochem Biophys Res Commun. 2004;320:1020–1025. doi: 10.1016/j.bbrc.2004.06.045. [DOI] [PubMed] [Google Scholar]
- Kuhn TB, Brown MD, Bamburg JR. Rac1-dependent actin filament organization in growth cones is necessary for β1-integrin-mediated advance but not for growth on poly-d-lysine. J Neurobiol. 1998;37:524–540. doi: 10.1002/(sici)1097-4695(199812)37:4<524::aid-neu3>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Lelkes PI, Unsworth BR, Saporta S, Cameron DF, Gallo G. Culture of neuroendocrine and neuronal cells for tissue engineering. In: Vunjak-Novakovic G, Freshney RI, editors. Culture of Cells for Tissue Engineering. chapter 14 Wiley; 2006. [Google Scholar]
- Lemmon V, Burden SM, Payne HR, Elmslie GJ, Hlavin ML. Neurite growth on different substrates: Permissive versus instructive influences and the role of adhesive strength. J Neurosci. 1992;12:818–826. doi: 10.1523/JNEUROSCI.12-03-00818.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letourneau PC, Shattuck TA, Ressler AH. “Pull” and “Push” in neurite elongation: Observations on the effects of different concentrations of Cytochalasin B and taxol. Cell Motil Cytoskeleton. 1987;8:193–209. doi: 10.1002/cm.970080302. [DOI] [PubMed] [Google Scholar]
- Limouze J, Straight AF, Mitchison T, Sellers JR. Specificity of blebbistatin, an inhibitor of myosin II. J Muscle Res Cell Motil. 2004;25:337–341. doi: 10.1007/s10974-004-6060-7. [DOI] [PubMed] [Google Scholar]
- Lin CH, Espreafico EM, Mooseker MS, Forscher P. Myosin drives retrograde F-actin flow in neuronal growth cones. Neuron. 1996;16:769–782. doi: 10.1016/s0896-6273(00)80097-5. [DOI] [PubMed] [Google Scholar]
- Loudon RP, Silver LD, Yee HF, Jr, Gallo G. RhoA-kinase and myosin II are required for the maintenance of growth cone polarity and guidance by nerve growth factor. J Neurobiol. 2006;66:847–867. doi: 10.1002/neu.20258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKerracher L, Chamoux M, Arregui CO. Role of laminin and integrin interactions in growth cone guidance. Mol Neurobiol. 1996;12:95–116. doi: 10.1007/BF02740648. [DOI] [PubMed] [Google Scholar]
- Renaudin A, Lehmann M, Girault J, McKerracher L. Organization of point contacts in neuronal growth cones. J Neurosci Res. 1999;55:458–471. doi: 10.1002/(SICI)1097-4547(19990215)55:4<458::AID-JNR6>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Rochlin MW, Itoh K, Adelstein RS, Bridgman PC. Localization of myosin II A and B isoforms in cultured neurons. J Cell Sci. 1995;108:3661–3670. doi: 10.1242/jcs.108.12.3661. [DOI] [PubMed] [Google Scholar]
- Rosenblatt J, Cramer LP, Baum B, McGee KM. Myosin II-dependent cortical movement is required for centrosome separation and positioning during mitotic spindle assembly. Cell. 2004;117:361–372. doi: 10.1016/s0092-8674(04)00341-1. [DOI] [PubMed] [Google Scholar]
- Schaefer AW, Kabir N, Forscher P. Filopodia and actin arcs guide the assembly and transport of two populations of microtubules with unique dynamic parameters in neuronal growth cones. J Cell Biol. 2002;158:139–152. doi: 10.1083/jcb.200203038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver L, Gallo G. Extracellular muscle myosin ii promotes sensory axon formation. DNA Cell Biol. 2005;24:438–445. doi: 10.1089/dna.2005.24.438. [DOI] [PubMed] [Google Scholar]
- Steketee MB, Tosney KW. Three functionally distinct adhesions in filopodia: Shaft adhesions control lamellar extension. J Neurosci. 2002;22:8071–8083. doi: 10.1523/JNEUROSCI.22-18-08071.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanova T, Slemmer J, Hoogenraad CC, Lansbergen G, Dortland B, De Zeeuw CI, Grosveld F, van Cappellen G, Akhmanova A, Galjart N. Visualization of microtubule growth in cultured neurons via the use of EB3-GFP (end-binding protein 3-green fluorescent protein) J Neurosci. 2003;23:2655–2664. doi: 10.1523/JNEUROSCI.23-07-02655.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straight AF, Cheung A, Limouze J, Chen I, Westwood NJ, Sellers JR, Mitchison TJ. Dissecting temporal and spatial control of cytokinesis with a myosin II Inhibitor. Science. 2003;299:1743–1747. doi: 10.1126/science.1081412. [DOI] [PubMed] [Google Scholar]
- Tanaka EM, Kirschner MW. Microtubule behavior in the growth cones of living neurons during axon elongation. J Cell Biol. 1991;115:345–363. doi: 10.1083/jcb.115.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka E, Ho T, Kirschner MW. The role of microtubule dynamics in growth cone motility and axonal growth. J Cell Biol. 1995;128:139–155. doi: 10.1083/jcb.128.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turney SG, Bridgman PC. Laminin stimulates and guides axonal outgrowth via growth cone myosin II activity. Nat Neurosci. 2005;8:717–719. doi: 10.1038/nn1466. [DOI] [PubMed] [Google Scholar]
- Varnum-Finney B, Reichardt LF. Vinculin-deficient PC12 cell lines extend unstable lamellipodia and filopodia and have a reduced rate of neurite outgrowth. J Cell Biol. 1994;127:1071–1084. doi: 10.1083/jcb.127.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo S, Gomez TM. Rac1 and RhoA promote neurite outgrowth through formation and stabilization of growth cone point contacts. J Neurosci. 2006;26:1418–1428. doi: 10.1523/JNEUROSCI.4209-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie SR, Chantler PD. Separate but linked functions of conventional myosins modulate adhesion and neurite outgrowth. Nat Cell Biol. 2001;3:88–92. doi: 10.1038/35050613. [DOI] [PubMed] [Google Scholar]
- Wylie SR, Chantler PD. Myosin IIA drives neurite retraction. Mol Biol Cell. 2003;14:465–466. doi: 10.1091/mbc.E03-03-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XF, Schaefer AW, Burnette DT, Schoonderwoert VT, Forscher P. Rho-dependent contractile responses in the neuronal growth cone are independent of classical peripheral retrograde actin flow. Neuron. 2003;40:931–944. doi: 10.1016/s0896-6273(03)00754-2. [DOI] [PubMed] [Google Scholar]
- Zhou FQ, Cohan CS. Growth cone collapse through coincident loss of actin bundles and leading edge actin without actin depolymerization. J Cell Biol. 2001;153:1071–1084. doi: 10.1083/jcb.153.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]


