Abstract
Previous studies have demonstrated that treatment with 17β-estradiol (E2) improves both spatial and nonspatial memory in young female mice. Still unclear, however, are the molecular mechanisms underlying the beneficial effects of E2 on memory. We have previously demonstrated that a single post-training intraperitoneal (i.p.) injection of 0.2 mg/kg E2 can enhance hippocampal-dependent spatial and object memory consolidation (e.g., Gresack and Frick, 2006b). Therefore, in the present study, we performed a microarray analysis on the dorsal hippocampi of 4 month-old female mice injected i.p. with vehicle or 0.2 mg/kg E2. Genes were considered differentially expressed following E2 treatment if they showed a greater than two-fold change in RNA expression levels compared to controls. Overall, out of a total of approximately 25,000 genes represented on the array, 204 genes showed altered mRNA expression levels upon E2 treatment, with 111 up-regulated and 93 down-regulated. Of these, 17 of the up-regulated and 6 of the down-regulated genes are known to be involved in learning and memory. mRNA expression changes in 5 of the genes were confirmed by real-time quantitative PCR analysis, and protein changes in these same genes were confirmed by Western blot analysis: Hsp70, a heat shock protein known to be estrogen responsive; Igfbp2, an IGF-I binding protein; Actn4, an actin binding protein involved in protein trafficking; Tubb2a, the major component of microtubules; and Snap25, a synaptosome-specific protein required for neurotransmitter release. The types of genes altered indicate that E2 may induce changes in the structural mechanics of cells within the dorsal hippocampus that could be conducive to promoting memory consolidation.
Keywords: Estrogen, microarray, memory, Igfbp2, Hsp70, Actn4, Tubb2a, Snap25
1. Introduction
The loss of estrogens at menopause has been associated with increases in dementia and age-related memory decline observed in aging women (Yaffe et al., 2000; Wolf and Kirschbaum, 2002). Despite the findings of the Women’s Health Initiative Memory Study (WHIMS), which demonstrated that giving estrogens alone or in combination with progesterone failed to prevent dementia in postmenopausal women (Shumaker et al., 2003; Shumaker et al., 2004), other evidence has shown that estrogen administration can have beneficial effects on memory. For example, giving estrogen to healthy postmenopausal women can improve spatial working memory (Duff and Hampson, 2000), object memory (Duka et al., 2000), and verbal memory (Kampen and Sherwin, 1994). Research has also demonstrated the beneficial effects of the potent estrogen, 17β-estradiol (E2), in rodent models. For example, E2 administered intraperitoneally (i.p.) immediately post-training enhances both spatial reference memory in the Morris water maze (Rissanen et al., 1999; Heikkinen et al., 2002; Gresack and Frick, 2006a) and novel object recognition memory (Luine et al., 2003; Gresack and Frick, 2006b) in mice and rats. Spatial and object memory are also enhanced by pre-training E2 treatments administered systemically by injection or silastic capsules (Daniel et al., 1997; Luine et al., 1998; Bimonte and Denenberg, 1999; Sandstrom and Williams, 2001; Vaucher et al., 2002; Sandstrom and Williams, 2004). However, systemic hormone administration in women can lead to a host of physiological problems, including an increased incidence of coronary artery disease, stroke, and invasive breast cancer (Mastorakos et al., 2006), calling into question whether the potential benefits of E2 on cognition outweigh the risks associated with hormone therapy.
An alternative approach to systemic hormone administration may be the specific targeting of proteins within the brain that are modulated by E2. For example, if the downstream effectors of E2 could be elucidated, then therapies that directly target these proteins could be developed that would enhance memory without the side effects of systemic hormone administration. E2 is known to increase dendritic spine density (Woolley and McEwen, 1993; Frick et al., 2004) and synaptic protein expression (Stone et al., 1998) in the CA1 region of the hippocampus and enhance neurogenesis in the hippocampal dentate gyrus (Tanapat et al., 1999; Galea et al., 2006). Additionally, acute E2 administration can activate several intracellular kinase cascades, including phosphatidylinositol 3-kinase (PI3K; Cardona-Gomez et al., 2002; Yokomaku et al., 2003; Mannella and Brinton, 2006) and extracellular signal-regulated kinase (ERK; Fernandez et al., 2008; Kuroki et al., 2000; Fitzpatrick et al., 2002; Wade and Dorsa, 2003), both of which can phosphorylate and activate CREB, an important protein involved in memory consolidation (Bozon et al., 2003). Previous microarray studies report that E2 alters expression of several genes in the hypothalamus (Malyala et al., 2004) and the hippocampus (Aenlle et al., 2007), but these studies have been conducted using chronic E2 administration. No microarray study has yet examined the effects of a single, acute dose of E2 on gene transcription in the hippocampus. Such information is important to the development of hormone-based drug treatments in order to more closely link E2-induced changes in signal transduction to alterations in gene transcription.Therefore, the present study endeavored to identify genes whose mRNA and protein expression levels were altered by an acute dose of water-soluble E2 known in young ovariectomized mice to enhance spatial and object memory (Gresack and Frick, 2006b) and activate the ERK cascade in the dorsal hippocampus (Fernandez et al., 2008; Lewis et al., 2008).
2. Methods
2.1. Subjects
Four month old female C57BL/6 mice were obtained from Taconic (Germantown, NY). Mice were bilaterally ovariectomized 1 week after arrival as per previously published methods (Fernandez and Frick, 2004), and housed up to 5/cage in a room with a 12:12 light/dark cycle (lights on at 07:00) for at least a week before treatments. Animals were handled 5 min per day over the course of 5 days, and had ad libitum access to food and water. All procedures were approved by the Institutional Animal Care and Usage Committee of Yale University, and conformed to the guidelines established by the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Mice were randomly assigned to groups receiving i.p. injections of either 0.2 mg/kg 17β-estradiol (E2) conjugated to the solubility enhancer 2-hydroxypropyl-β-cyclodextrin (HBC) and dissolved in physiological saline (E2 group, n=3) or HBC dissolved in an equal volume of physiological saline containing the same amount of cyclodextrin present in the HBC-E2 solution (vehicle group, n=3). HBC is a solubility-enhancer for steroid hormones that does not alter the bioefficacy of the hormones (Pitha and Pitha, 1985), but allows them to successfully cross the blood-brain barrier and rapidly dissociate into the tissue while the HBC remains in the circulation (Taylor et al., 1989). This hormone preparation is metabolized within 24 hours (Pitha et al., 1986; Taylor et al., 1989) and our laboratory has shown that that a single i.p. injection of the 0.2 mg/kg dose given to young female mice immediately after training specifically enhances memory consolidation in both spatial Morris water maze and novel object recognition tasks (Gresack and Frick, 2006a). Previous studies have shown that a 1 μg dose of estradiol dissolved in oil produces levels similar to those seen in the estrus phase of the estrous cycle, and a 10 μg dose produces levels similar to those seen during the proestrus phase of the cycle (Akinci and Johnston, 1997). Given mean body weight for young ovariectomized females of 22 g, the approximate estradiol levels for the 0.2 mg/kg dose is 4.4 μg, and thus near the middle of the physiological range.
In the present study, injections of vehicle or 0.2 mg/kg E2 were given 1 hour before sacrifice, as our previous work found an increase in dorsal hippocampal p42 ERK phosphorylation at this time point (Fernandez et al., 2008; Lewis et al., 2008). One hour (for RNA analyses) or 3 and 4 hours (for protein analyses) after injection, mice were cervically dislocated, and dorsal hippocampi were bilaterally dissected and stored at -80°C until use.
2.2. RNA Isolation
RNA was isolated from hippocampal tissue using the Trizol reagent isolation protocol (Invitrogen, Carlsbad, CA). Briefly, 800 μL Trizol reagent was added to each tube, and the tissue was homogenized by 10 passes of a Dounce homogenizer (Kontes Glass Co, Vineland, NJ). The homogenate was incubated for 5 min at room temperature and subsequently extracted with 0.2 mL of chloroform to remove proteins. The aqueous phase was transferred to a fresh tube, and the RNA was precipitated using 0.5 mL of isopropanol and centrifuged to pellet the RNA. The RNA pellet was washed twice by resuspension in 1 mL of 75% ethanol. After the last wash, the RNA pellet was allowed to air dry and was then resuspended in DEPC-treated water. The RNA was further purified using the RNAeasy Mini Kit RNA Cleanup protocol (Qiagen, Valencia, CA) per manufacturer’s instructions. The RNA concentration and purity were measured by reading the absorbance at 260 nm and 280 nm on a SmartSpec 3000 Spectrophotometer (BioRad, Hercules, CA). The quality of the RNA was further assessed using the Agilent Bioanalyser (Agilent, Santa Clara, CA).
2.3. Microarray Analysis
The microarrays used were OMM25K arrays developed by the Yale University WM Keck Foundation Biotechnology Resource Laboratory. The arrays were fabricated from a 70mer oligo set consisting of 16,463 oligos from the Operon Mouse Version 2.0 set and 8,097 oligos from the Operon Mouse Version 3.0 set, both designed from the publicly available Ensembl Mouse 14.30 database (http://www.ensembl.org/index.html) and the Mouse Genome Sequencing Project (http://www.hgsc.bcm.tmc.edu/projects/mouse). Isolated RNA was labeled by either Cy5 (RNA from vehicle-treated mice, n=3) or Cy3 (RNA from E2-treated mice, n=3) dyes and hybridized onto the arrays using the Genisphere Array900 Expression Array Detection kit (Genisphere, Hatfield, PA) according to the manufacturer’s protocol. Hybridizations were performed on an Advalytix SlideBooster hybridization station (Olympus America Inc, Concord, MA), and slides were subsequently scanned on an Axon GenPix 4100 scanner and imaged using GenePix 5.0 software (Axon, Sunnyvale, CA). The data were then analyzed according to the Genespring program (Agilent), in collaboration with the Biostatistics Department of the Yale University WM Keck Foundation Biotechnology Resource Laboratory. The intensity values for each spot were averaged across the three arrays to give an average intensity of the spot for each group, and these values were normalized to background intensity. Only those spots whose intensity was twice the background value were considered in the analysis. The data were first subjected to multiple t-tests, and then to a false discovery rate (FDR) correction. Genes were considered to have an altered expression with E2 treatment if they showed a greater than 2-fold change in expression level, and had an FDR-adjusted p-value of less than 0.05.
2.4. Quantitative real-time PCR
RNA was isolated from dorsal hippocampi according to the protocol described above. Total RNA (1.5 μg) was reverse transcribed in the presence of random hexamers using the SuperScript First-Strand Synthesis kit (Invitrogen) to synthesize cDNA. One ng of cDNA from each sample (vehicle-treated, n = 3; E2-treated, n = 3) was used in analysis. Quantitative real-time PCR (QPCR) was performed using the QuantiTect SYBR Green PCR Kit, with the QuantiTect Primer Assay PCR primers (Qiagen). Quantitation of PCR products was performed using the relative standard curve method. The cDNA standards were prepared from the liver of a 4 month-old mouse (for all genes except Snap25) or mouse brain reference RNA (Applied Biosystems, Foster City, CA, for Snap25) in the concentrations of 100, 50, 25, 10, and 1 ng/μL. Standards were loaded onto the reaction plate in each experimental run with each primer set. Standards were run in duplicate and samples in triplicate, with the housekeeping gene GAPDH run on each plate for internal normalization. The PCR reactions were performed on an ABI 7900 Sequence Detection System (Applied Biosystems) for 40 cycles (15 s at 95°C, 30 s at the annealing temperature, 30 s at 70°C), followed by a dissociation step at 60°C to visualize the melt curve and determine the purity of the product. The threshold cycle (Ct) value was calculated as the cycle number in which the SYBR green fluorescent signal crossed detection threshold limit set by the instrument, which is the midpoint of the log phase of the amplification reaction. A standard curve for each primer set was generated by plotting the concentration versus the Ct value for the reference samples. The concentrations of unknown samples were determined by substituting the Ct values for each sample into the best fit line where y = mx + b, and solving for the concentration x. The concentrations of each sample were reported as concentration equivalents of reference sample cDNA. Using SPSS 14.0 (SPSS Inc., Chicago, IL), separate independent samples t-tests were run for each gene comparing vehicle and E2 groups.
Western blot analysis
Dorsal hippocampi were homogenized and Western blot analysis performed as previously described (Fernandez et al., 2008; Lewis et al., 2008). Briefly, six μg of protein from each homogenate (vehicle-treated, n = 3; E2-treated, n = 3) were run on a 10% SDS-PAGE (BioRad, Hercules, CA), transferred to a PVDF membrane (Millipore, Temecula, CA), blocked in a 5% non-fat dry milk dissolved in Tris-buffered saline containing 0.1% Tween-20 (TTBS), and probed with Hsp70 (1:2000, #sc-33575, Santa Cruz, Santa Cruz, CA), IGFBP2 (1:1000, #06-107, Upstate, Lake Placid, NY), Actn 4 (1:2000, #05-384, Millipore), Tubb (1:4000, #3146, Cell Signaling, Danvers, MA), or SNAP-25 (1:1000, #610366, BD Biosciences, San Jose, CA) diluted in 5% bovine serum albumin (BSA) in TTBS. Secondary antibodies were either anti-rabbit horse radish peroxidase (HRP)-conjugated (1:2000, #7074, Cell Signaling, used for Hsp70, IGFBP2, and Tubb2a) or anti-mouse-HRP (1:2000, #7076, Cell Signaling, used for Actn 4 and SNAP-25). Blots were stripped and then re-probed with GAPDH (1:2500, #ab9484, Abcam, Cambridge, MA) for normalization. Band intensity was measured by densitometry using Kodak 1D 3.6 software on the Kodak Image Station 440 CF. Data were analyzed as described above.
3. Results
In order to identify the effects of E2 on gene expression in the hippocampus of young adult mice, the dorsal hippocampi of 4 month-old mice were subjected to microarray analyses using DNA oligo microarrays containing oligos from 25,000 genes. Overall, 73 genes were up-regulated and 53 genes were down-regulated by E2 treatment (p < 0.05 by FDR multiple t-test correction). Of the genes up-regulated by E2, 17 are known to be specifically involved in learning and memory. These genes were divided into functional categories by a PubMed search to elucidate their known activities in vivo. Two of the 17 genes encode for proteins that act as transcription factors, 3 encode proteins involved in metabolism, 6 encode structural proteins, 6 encode neuropeptides/growth factors/receptors, and 1 encodes a molecular chaperone (these 17 genes shown in bold and italics, Table 1). Functional categories for other up-regulated genes are also reported in Table 1. Of the genes down-regulated by E2, 6 are known to be specifically involved in learning and memory. Of the 6 genes known to be involved in learning and memory, 4 encode transcription factors, 1 encodes a transport protein, and 1 encodes an anti-apoptotic protein (these genes shown in bold and italics, Table 2). Functional categories for other down-regulated genes are also reported in Table 2.
Table 1. Genes whose expression was significantly up-regulated by E2 treatment.
The p-values listed in the table represent significance reported after the false discovery rate (FDR) analysis. Genes in bold and italics indicate those that are known to be involved in learning and memory based on a PubMed search. The genes marked with an asterisk indicate those that were chosen for further study by real-time PCR and Western blotting.
| Common Name | Fold change | p-value | PubMed Number | |
|---|---|---|---|---|
| Transcription | Junb | 2.35 | 0.0465 | NM_008416 |
| Pik3cb | 2.13 | 0.0368 | AK003230 | |
| Tcf4 | 2.24 | 0.0368 | NM_013685 | |
| Eef1d | 2.07 | 0.0491 | NM_023240 | |
| Metabolism | Odc | 2.42 | 0.0487 | NM_013614 |
| Got2 | 2.35 | 0.0368 | NM_010325 | |
| Atp1b3 | 2.28 | 0.0463 | NM_007502 | |
| Sult4a1 | 2.16 | 0.0368 | NM_013873 | |
| ATP F1 | 3.38 | 0.0203 | BC013607 | |
| Dlat | 2.03 | 0.0203 | BC003202 | |
| Nec1 | 2.01 | 0.0368 | AK018159 | |
| Atp6b2 | 2.07 | 0.0435 | BC012497 | |
| Pmca1b | 2.63 | 0.0398 | AK013291 | |
| E2RG | 10.42 | 0.0368 | AK019895 | |
| Agk | 2.05 | 0.0368 | BC019145 | |
| Hatpaseb1 | 2.06 | 0.0464 | BC017127 | |
| Structure | Myo6 | 2.13 | 0.0435 | NM_008662 |
| Tubb2a* | 2.67 | 0.042 | M28739 | |
| Actn4* | 2.63 | 0.0368 | NM_021895 | |
| Tmsb4x | 2.29 | 0.0368 | NM_021278 | |
| Dctn4 | 2.4 | 0.0491 | BC006677 | |
| Snap25* | 2.84 | 0.0487 | BC018249 | |
| Myh2 | 9.73 | 0.0368 | BC008538 | |
| Esm1 | 12.28 | 0.0435 | BC020038 | |
| Arfl10C | 2.66 | 0.0465 | BC013719 | |
| Tpk2 | 2.45 | 0.0464 | BC019149 | |
| Structure | Mtrp6 | 2.3 | 0.0368 | BC020019 |
| Cct6a | 2.26 | 0.0203 | NM_009838 | |
| Ccol | 2.28 | 0.0368 | NM_177177 | |
| NP/GF/Rec | Rfrp | 2.03 | 0.0465 | NM_021892 |
| Hgfl | 3.69 | 0.0471 | NM_008243 | |
| Erbb4 | 2.32 | 0.0402 | AF059177 | |
| Neurod2 | 2.54 | 0.0368 | NM_010895 | |
| Pafah1b1 | 2.4 | 0.0368 | NM_013625 | |
| Kcna1 | 2.04 | 0.0471 | NM_010595 | |
| Olfr, put | 2.01 | 0.0368 | X89678 | |
| Olfr67 | 2.78 | 0.0491 | NM_013619 | |
| Gpr124 | 2.05 | 0.0368 | NM_054044 | |
| lgsf1 | 2.35 | 0.0464 | AY227771 | |
| Scube1 | 2.34 | 0.046 | NM_022723 | |
| Eva | 5 09 | 0.0368 | BC015076 | |
| Cd209e | 2.39 | 0.0368 | AF373412 | |
| Neph | 2.36 | 0.0471 | NM_025684 | |
| Chaperone | HSP70-1* | 2.11 | 0.0368 | M12573 |
| Differentiation | Morf | 2.04 | 0.0464 | NM_017479 |
| Cd81 | 2.21 | 0.049 | BC011433 | |
| Gcm2 | 2.5 | 0.0368 | NM_008104 | |
| Ctf1 | 2.84 | 0.0368 | NM_007795 | |
| Alcam | 2.07 | 0.0435 | U95030 | |
| Mmd | 2.37 | 0.0368 | NM_026178 | |
| Nuf2r | 8.42 | 0.0368 | NM_023284 | |
| Ash1l | 2.11 | 0.0389 | AF247132 | |
| Oxidative Stress | Pxf | 2.56 | 0.0368 | AK012785 |
| Inflammation | Tcra | 2.49 | 0.0368 | X72904 |
| Aldh1a7 | 2 | 0.0465 | NM_011921 | |
| Egln1 | 2.07 | 0.0386 | BC006903 | |
| CS1 | 2.23 | 0.0368 | AK014517 | |
| Protein deg | Rbx1 | 2.05 | 0.0435 | AK005127 |
| Rnf5 | 2.1 | 0.0398 | NM_019403 | |
| Bean | 21.34 | 0.0368 | AF240460 | |
| Pep4 | 6.54 | 0.0368 | NM_008820 | |
| Serp23 | 2.18 | 0.0311 | BC018517 | |
| Psmd1 | 2.1 | 0.0368 | AK010596 | |
| Psmb2 | 2.06 | 0.0498 | NM_011970 | |
| Transport | SV2 | 2.13 | 0.0399 | AK013742 |
| Rab1b | 2.54 | 0.0368 | BC016408 | |
| MLC1 | 2.11 | 0.0466 | AF449425 | |
| Snta1 | 2.17 | 0.0465 | NM_009228 | |
| Slc14a1 | 2.08 | 0.0389 | NM_028122 | |
| Rcc1 | 2.36 | 0.0465 | BC019807 | |
| Itgb4bp | 2.22 | 0.0368 | BC015274 | |
| Ergic1 | 2.33 | 0.0464 | NM_026170 | |
| Ssra | 2.07 | 0.0386 | NM_025965 |
Abbreviations: Transcription, transcription factor; NP/GF/Rec, neuropeptides, growth factors, or receptors; Protein deg, involved in protein degradation.
Table 2. Genes whose expression was significantly down-regulated by E2 treatment.
The p-values listed in the table represent significance reported after the false discovery rate (FDR) analysis. Genes in bold and italics indicate those that are known to be involved in learning and memory based on a PubMed search. The genes marked with an asterisk indicate those that were chosen for further study by real-time PCR and Western blotting.
| Common Name | Fold change | p-value | PubMed ID | |
|---|---|---|---|---|
| Transcription | Plc | 16.67 | 0.0466 | AK011892 |
| lnpp5d | 3.57 | 0.0468 | NM_010566 | |
| Pla2g1b | 3.85 | 0.0465 | NM_011107 | |
| lgfbp2* | 2.38 | 0.0389 | NM_008342 | |
| AF-9 | 20 | 0.0368 | AK008707 | |
| Brd7 | 25 | 0.0465 | NM_012047 | |
| Ikbkb | 10 | 0.0363 | AF088910 | |
| Rbm8 | 3.23 | 0.0368 | NM_025875 | |
| Smarca3 | 6.67 | 0.0368 | AF165911 | |
| Lztfl1 | 2.13 | 0.0466 | NM_033322 | |
| Transport | Konj8 | 2.13 | 0.0389 | NM_008428 |
| Apoptosis | Birc4 | 7.14 | 0.0368 | NM_009688 |
| Ca2+-assoc | Calb2 | 2.5 | 0.0368 | BC017646 |
| Cacng3 | 2.56 | 0.0491 | NM_019430 | |
| Calmbp1 | 5 | 0.0402 | NM_009791 | |
| Cacybp | 11.11 | 0.0368 | U97327 | |
| Trpv6 | 2.17 | 0.0368 | NM_022413 | |
| Pln | 3.85 | 0.0466 | AK002622 | |
| Metabolism | Anpep | 2.38 | 0.0368 | NM_008486 |
| Lrp6 | 2.17 | 0.0368 | NM_008514 | |
| Atic | 2.56 | 0.0465 | AK010611 | |
| Fabp4 | 11.11 | 0.0465 | BC002148 | |
| Cyp40 | 50 | 0.0368 | AB006034 | |
| Nit1 | 3.03 | 0.0465 | AK004988 | |
| Ethi6 | 20 | 0.0435 | AK010551 | |
| Pit1 | 50 | 0.0389 | AF196476 | |
| Structure | Lox | 11.11 | 0.0368 | NM_010728 |
| Siat8d | 2.17 | 0.0368 | NM_009183 | |
| Dpt | 100 | 0.0368 | NM_019759 | |
| Mtap4 | 50 | 0.0435 | AK019611 | |
| Pxn10 | 2.17 | 0.0203 | - | |
| Cypt6 | 2.22 | 0.0368 | NM_025738 | |
| Adam18 | 2.63 | 0.049 | NM_010084 | |
| Adam rep | 2.17 | 0.0491 | AB112362 | |
| Differentiation | Rho | 7.14 | 0.0368 | BC013125 |
| Wnt4 | 3.85 | 0.0368 | NM_009523 | |
| Gli5 | 3.33 | 0.0368 | BC021517 | |
| Pcdh15 | 2.86 | 0.0435 | NM_023115 | |
| Pax5 | 8.33 | 0.0464 | NM_008782 | |
| Pax9 | 2.78 | 0.0468 | NM_011041 | |
| Ccna1 | 4.35 | 0.0203 | NM_007628 | |
| Pol theta | 2.7 | 0.0368 | AK020790 | |
| Cdc2l2 | 2.22 | 0.0368 | NM_007661 | |
| GF/Rec | Pglp | 50 | 0.0368 | AJ293619 |
| Cpr2 | 6.67 | 0.0401 | BC016483 | |
| Gadd45a | 20 | 0.0464 | NM_007836 | |
| Protein deg | Arih2 | 2.86 | 0.0495 | NM_011790 |
| Psg4-1 | 2.08 | 0.0464 | AK017550 | |
| Immune sys | Cd2 | 2.38 | 0.0384 | NM_013486 |
| Itgae | 4.17 | 0.0435 | NM_008399 | |
| Tslp | 2.22 | 0.0471 | NM_021367 | |
| Fcnb | 3.7 | 0.0368 | AK010913 | |
| BCA3 | 2.13 | 0.0465 | NM_020616 |
Abbreviations: Transcription, transcription factor; Ca2+-assoc, involved in calcium signaling or processing; GF/Rec, growth factors or receptors; Protein deg, involved in protein degradation; Immune sys, genes related to the immune system.
Of the genes whose expression was altered by E2, 5 were selected for further analysis by quantitative real-time PCR (QPCR), followed by Western blot analysis based on reports in the literature strongly linking them with learning and memory. The first chosen was heat shock protein 70 (Hsp70-1), as it is not only associated with increased memory retention in the Morris water maze (Pizarro et al., 2003), but is also known to be up-regulated by E2 (Olazabal et al., 1992). Hsp70-1 mRNA levels increased 3-fold by QPCR (t(4) = 3.731, p = 0.02; Figure 2A), matching the 2.1-fold increase seen on the microarray (Table 1). Additionally, when protein levels of Hsp70-1 were measured by Western blot analysis, there was a 1.4-fold increase at 4 hours after treatment with E2 (t(5) = 4.418, p = 0.007; Figure 2A).
Figure 2.
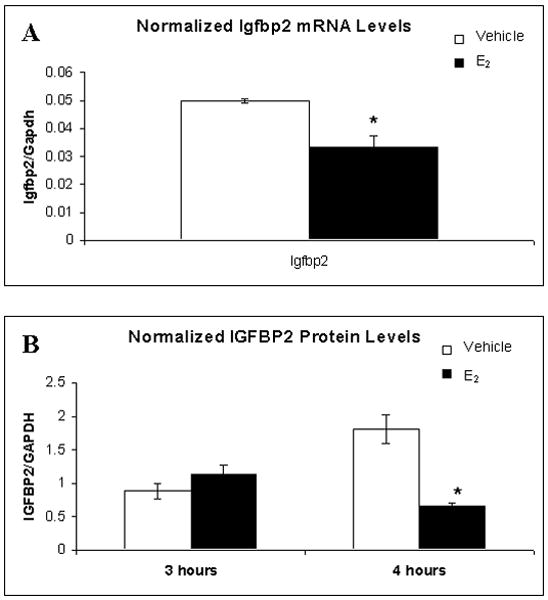
IGFBP2 gene and protein expression levels following E2 treatment. A. Igfbp2 mRNA expression levels significantly decreased 1 hour after E2 treatment. B. IGFBP2 protein levels were also significantly lower than vehicle 4 hours after E2 treatment. *p < 0.05 relative to vehicle.
The next gene chosen was the transcription factor-associated gene insulin-like growth factor binding protein 2 (Igfbp2). IGFBP2 is a serum protein secreted by a variety of cells, and is a part of the insulin-like growth factor (IGF-I) signaling cascade. IGF-I is known to be involved in memory, as long-term IGF-I treatment in aged rats improves learning and memory (Sonntag et al., 2005), and is thought to exert its memory-enhancing affects by promoting adult neurogenesis (Aberg et al., 2000; Perez-Martin et al., 2003) or neuronal survival (Subramaniam et al., 2005). IGFBP2 binds IGF-I in the serum and prevents it from binding its receptor and activating the cellular signaling cascade (Chesik et al., 2007); additionally, the serum levels of IGFBP2 are down- regulated in humans after conjugated equine estrogen treatment (Heald et al., 2005). The mRNA levels of Igfbp2 were down-regulated 2.4-fold on the microarrays (Table 2), a finding which was verified by QPCR, with a significant decrease of 1.5-fold relative to vehicle (t(4) = 3.90, p = 0.18; Figure 2B). Additionally, protein levels of IGFBP2 showed a 2-fold decrease 4 hours after E2 treatment (t(2.145) = 5.402, p = 0.028; Figure 2B), further confirming the mRNA data and indicating that IGFBP2 levels were decreased by E2 treatment.
The last 3 genes analyzed by QPCR were all structural proteins: α-actinin 4 (Actn 4), tubulin β2 (Tubb), and synaptosomal-associated protein 25 (Snap-25). Because estradiol is known to increase dendritic spine density (Woolley and McEwen, 1993) and enhance neurogenesis (Tanapat et al., 1999; Galea et al., 2006), these genes were predicted to be up-regulated by E2 treatment. Actn 4, an actin binding protein that regulates hippocampus spine morphology and density (Nakagawa et al., 2004), showed a 2.6-fold increase in mRNA levels upon microarray analysis (Table 1), a smaller but significant 0.5-fold increase relative to vehicle with QPCR (t(4) = 3.562, p = 0.024; Figure 2C), and 1.3-fold increase in protein levels 3 hours after treatment (t(5) = 3.603, p = 0.015; Figure 2C). Additionally, Tubb, an isoform of the microtubule-forming protein tubulin that is used as a marker for neuronal differentiation and whose expression increases with isoflavone treatment (Bu and Lephart, 2005), showed a 2.7-fold increase in mRNA levels on the microarrays (Table 1), a significant 2-fold increase relative to vehicle upon QPCR analysis (t(4) = 3.069, p = 0.037; Figure 2C), and a 1.7–fold increase in protein levels 4 hours after E2 treatment (t(5) = 2.568, p = 0.049; Figure 2C). Lastly, SNAP-25, a protein that localizes to synapses and may be required for long-term memory formation (Hou et al., 2006), showed a 2.8-fold increase in expression on the microarrays (Table 1), a significant 1.5-fold increase relative to vehicle upon QPCR analysis (t(4) = 4.623, p = 0.01; Figure 2C), and a 1.9–fold increase in protein levels 4 hours after E2 treatment (t(5) = 2.887, p = 0.034; Figure 2C).
4. Discussion
E2 administration led to increased RNA levels of 73 genes and decreased RNA levels of 53 genes. Of these, 17 of the up-regulated and 6 of the down-regulated genes have been previously associated with learning and memory as described above. Five of these genes were chosen for verification of RNA expression level changes by QPCR based on the availability of information in the literature relating to their involvement in learning and memory. The changes in mRNA and protein expression levels of all 5 of the genes were confirmed by QPCR and Western blot analysis: Hsp70-1, Actn 4, Tubb, and Snap-25 were up-regulated with E2 treatment, and Igfbp2 was down-regulated with E2 treatment. The increase in Hsp70 mRNA and protein levels was consistent with previous work, as estradiol has been shown to enhance Hsp70 expression (Olazabal et al., 1992). The decrease in IGFBP2 mRNA and protein levels may suggest a role for the IGF-I signaling pathway in mediating the mnemonic response to E2, whereas the increases in mRNA and protein levels of Actn 4, Tubb, and Snap-25 suggest that E2-induced alterations in the expression of these structural genes may play a role in the beneficial effects of E2 on memory consolidation in the dorsal hippocampus. Interestingly, of the 5 genes examined, only one, Hsp70, contains an estrogen response element in its promoter (Hamilton et al., 2004), which suggests that most of the gene expression changes observed were likely mediated primarily by mechanisms other than the classical estrogen receptor binding. Although the present analyses cannot directly link E2-induced alterations in gene and protein expression to those in learning and memory, the fact that a behaviorally effective dose of E2 was used in this study lends strength to the correlative evidence linking E2-induced changes in the hippocampus to memory. More study will be needed to determine the extent to which the molecular alterations observed in the present study are necessary for E2 to modulate hippocampal memory function.
Hsp70-1 belongs to the extensive heat shock protein family, and is responsible for assisting in protein folding (Burston and Clarke, 1995). Hsp70 is also an integral part of the cytosolic estrogen receptor (ER) protein complex that keeps ER in an inactive state until it binds its estrogens and translocates to the nucleus (Whitesell and Lindquist, 2005). Hsp70 expression has been well-documented to increase with E2 treatment in a wide variety of cell types, such as breast cancer cells (Takahashi et al., 1994), heart (Hamilton et al., 2004), and brain, where it may show differential expression based on sex (Olazabal et al., 1992). Hsp70 isoforms have also been implicated in learning. Hsp70-1 mRNA and protein levels are increased in the hippocampus of rats trained in the spatial Morris water maze (Pizarro et al., 2003), and the inducible form of Hsp70, Hsp72, is increased in the cerebellum during acquisition of a two-way avoidance task in rats (Ambrosini et al., 1999). Further, when Hsp72 is knocked out in mice, this deletion prevents spatial memory acquisition in the radial arm maze (Ambrosini et al., 2005), suggesting an active and potentially critical role for Hsp70 isoforms in learning and memory beyond their traditional function of simply complexing with ER in the cytoplasm and maintaining it in the inactive state.
Insulin-like growth factor binding protein 2 (Igfbp2) is the predominant IGF-I binding protein secreted by neuronal and glial cells in the brain (Bezchlibnyk et al., 2006). Igfbp2 binds IGF-I in the serum and prevents it from activating the IGF-I receptor (IGF-IR) and initiating intracellular signaling cascades such as PI3K and ERK (Aberg et al., 2006; Chesik et al., 2007). Thus, an E2-induced down-regulation of Igfbp2 may lead to greater IGF-I availability, and subsequently to increased activation of the PI3K and ERK cascades. Links between the IGF-I signaling cascade and E2 are well-established. For example, treatment with E2 can activate IGF-IR signaling, and IGF-I has been shown to regulate the transcriptional activation of the classical estrogen receptors (ER) alpha and beta (Garcia-Segura et al., 2006). Administration of the ER antagonist ICI 182,780 blocks IGF-I-induced neurogenesis in female rats (Perez-Martin et al., 2003), suggesting that interactions with classical ERs are important in mediating the effects of IGF-I on hippocampal plasticity. Previous microarray analyses have shown a 1.7-fold increase in mRNA levels of another IGF-I binding protein, Igfbp6, in the hippocampus of male intact middle-aged rats relative to young rats (Blalock et al., 2003), and a decrease in the expression of Igfbp2 mRNA in the dentate gyrus of rats that have undergone passive avoidance training relative to untrained controls (O’Sullivan et al., 2007). An age-related increase in Igfbp6 mRNA could suggest that less IGF-I is available for binding to its receptor, which would block downstream signaling cascades. In contrast, the decrease in Igfbp2 mRNA observed after passive avoidance and in the present study could suggest more IGF-I available for binding, and may indicate an activity- or E2-induced increase in the activity of the downstream signaling cascades associated with IGF-I, as we have previously observed with ERK after 0.2 mg/kg E2 treatment (Fernandez et al., 2008).
Cytoskeletal structural genes are involved in several aspects of cellular function in the brain, including transport of proteins to various cellular locations (Antar et al., 2005), endocytosis and exocytosis of neurotransmitters (Chin et al., 2000), cell division during neurogenesis (German and Eisch, 2004), neuronal plasticity (Bianchi et al., 2005), and dendritic spine morphogenesis (Sekino et al., 2007). Several microarray studies have found alterations in the expression patterns of structural genes with learning. For example, synaptojanin II, a protein required for synaptic vesicle recycling, is up-regulated in aged rats who were classified as superior learners in the spatial Morris water maze task as compared to age-matched control learners (Burger et al., 2007). In addition, the mRNA levels of procollagen-type I, collagen type III and coronin, an actin binding protein, were down-regulated with age in the rat hippocampus, although vimentin and α-tubulin were both up-regulated with age (Blalock et al., 2003). Fewer structural genes appear to be influenced by E2 alone in array studies. The vesicle-associated membrane protein 2 and synaptogyrin were up-regulated in the basal hypothalamus of female guinea pigs following E2 treatment (Malyala et al., 2004). In non-array based studies, however, α-actinin 4 (Actn 4), a structural protein that binds to the actin cytoskeleton and is involved in protein trafficking within the cell, was reportedly up-regulated by the synthetic estrogen clomiphene citrate in rat uteri (Hosie et al., 2008) and was up-regulated by E2 treatment in the present study as well. Actn 4 mRNA levels are also increased in the amygdala during fear conditioning (Ressler et al., 2002), suggesting a role for this structural protein both in response to E2 and in learning and memory.
Tubulin-β (Tubb) is the major structural component of the microtubule network inside of cells. Column retention studies using hippocampal cell lysate have shown that tubulin-β binds directly to E2 (Ramirez et al., 2001). Additionally, yeast two-hybrid screens using MCF-7 cells indicate that microtubules may mediate the response of E2 by binding to and helping to shuttle the ERs to various locations within the cell (Manavathi et al., 2006). On the other hand, studies in breast cancer cells show that E2 actually disrupts microtubule formation (Aizu-Yokota et al., 1994), casting some doubt on whether there is a positive interaction between Tubb and E2. Tubulin-β is often used as a marker for neurogenesis (Rai et al., 2007), indicating that the increase in Tubb mRNA levels seen in this study may be due to cells preparing for future cell division and neurogenesis. Given that E2 has been shown to enhance neurogenesis in the hippocampal dentate gyrus (Tanapat et al., 1999; Galea et al., 2006), the increase in Tubb may not reflect a direct interaction between E2 and tubulin-β, but rather be an indirect result of E2-induced neurogenesis.
The final gene examined that was up-regulated by the microarray and QPCR analysis, as well as the Western blot analysis, was the synaptosomal-associated protein of 25 kD (Snap-25). SNAP-25 is a part of the synaptic vesicle docking and fusion process and is essential for neurotransmitter release (Wang and Tang, 2006). Interestingly, previous in situ hybridization data indicated that E2 treatment of ovariectomized rats decreased Snap-25 mRNA levels in the pituitary (Jacobsson et al., 1998), and another microarray study indicated that Snap-25 mRNA levels decrease after passive avoidance learning in the dentate gyrus of male rats (O’Sullivan et al., 2007). Although both of these findings conflict with the present data, other studies have shown that SNAP-25 is required for memory formation. For example, granule cells of the dentate gyrus show an increase in Snap-25 mRNA expression 2 hours after LTP stimulation (Roberts et al., 1998), and a Snap-25 antisense oligonucleotide added to either rat cortical neurons or PC12 cells impairs axon growth (Osen-Sand et al., 1993). Male intact rats infused with a SNAP-25 antisense oligonucleotide directly into either CA1 (Hou et al., 2004) or CA3 (Hou et al., 2006) exhibited impaired consolidation of contextual fear memory and spatial memory, suggesting that SNAP-25 is involved in memory consolidation in the hippocampus. As such, the increase in Snap-25 mRNA levels observed in our study indicate that treatment with a dose of E2 that enhances hippocampal memory consolidation (Gresack and Frick, 2006b) has a beneficial effect on the expression of this important gene, perhaps by influencing an increase in neurotransmitter release.
In conclusion, this study demonstrates that a single i.p. injection of a dose of E2 that enhances spatial and object memory consolidation (Gresack and Frick, 2006b) can influence the mRNA and protein expression levels of genes involved in learning and memory within 60 minutes of injection. We have found that E2 can enhance the expression of genes that act as chaperone proteins, such as Hsp70; transcription factor-related genes such as Igfbp2; genes involved in intracellular trafficking pathways, such as α-actinin 4 and tubulin-β; and synaptosome-associated proteins, such as Snap-25. These expression changes demonstrate that E2 may exert its effects on a wide variety of intracellular mechanisms and render cells within the hippocampus more amenable to the physical changes necessary to consolidate memories. More work is needed to understand if these gene expression changes specifically affect memory consolidation within the hippocampus. Nevertheless, the present data provide information that will be critical to understanding how E2 and similar hormones modulate memory.
Figure 1.
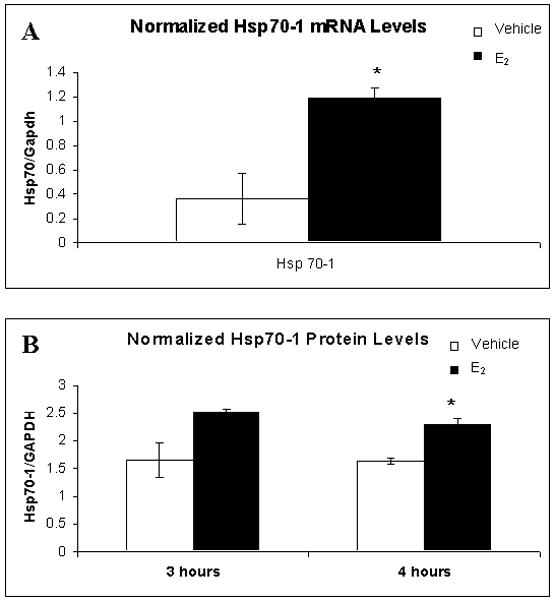
Heat-shock protein 70-1 (Hsp70-1) gene and protein expression levels following E2 treatment. A. Hsp70-1 mRNA expression levels significantly increased 1 hour after E2 treatment. B. Hsp70-1 protein levels were also significantly higher than vehicle 4 hours after E2 treatment. *p < 0.05 relative to vehicle.
Figure 3.
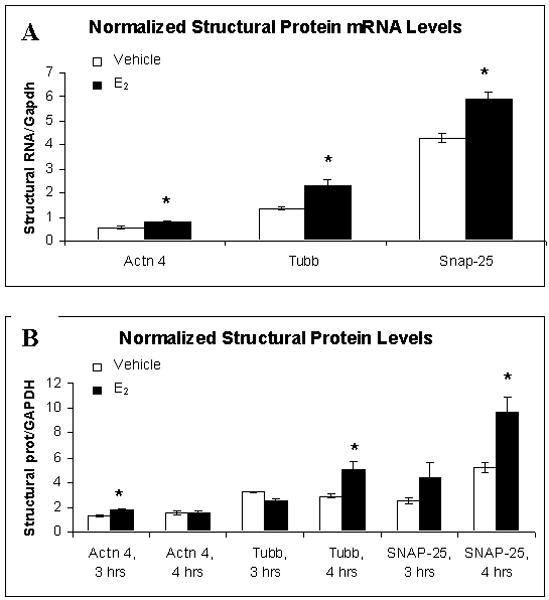
Structural protein gene and protein expression levels following E2 treatment. A. Actn4, Tubb, and Snap-25 mRNA expression levels were all significantly increased 1 hour after E2 treatment. B. Actn4 protein levels were increased 3 hours after E2 treatment, whereas, SNAP-25 and Tubb protein levels were significantly higher than vehicle 4 hours after treatment. *p < 0.05 relative to vehicle.
Acknowledgments
This work was supported by NIH grant RO1 AG022525 to KMF and Yale University. The authors gratefully acknowledge Irina Tikhovna, Ainpin Lin, Sheila Westman, and the Yale University W.M. Keck Foundation Biotechnology Resource Laboratory for their assistance with the microarray studies and for use of their real-time PCR machine. The authors would also like to thank Drs. Jonathan Ploski and Michael Lewis for their helpful comments on this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aberg MA, Aberg ND, Hedbacker H, Oscarsson J, Eriksson PS. Peripheral infusion of IGF-I selectively induces neurogenesis in the adult rat hippocampus. Journal of Neuroscience. 2000;20:2896–2903. doi: 10.1523/JNEUROSCI.20-08-02896.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aberg ND, Brywe KG, Isgaard J. Aspects of growth hormone and insulin-like growth factor-I related to neuroprotection, regeneration, and functional plasticity in the adult brain. ScientificWorldJournal. 2006;18:53–80. doi: 10.1100/tsw.2006.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aenlle KK, Kumar A, Cui L, Jackson TC, Foster TC. Estrogen effects on cognition and hippocampal transcription in middle-aged mice. Neurobiology of Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.09.004. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizu-Yokota E, Ichinoseki K, Sato Y. Microtubule disruption induced by estradiol in estrogen receptor-positive and -negative human breast cancer cell lines. Carcinogenesis. 1994;15:1875–1879. doi: 10.1093/carcin/15.9.1875. [DOI] [PubMed] [Google Scholar]
- Akinci MK, Johnston GA. Sex differences in the effects of gonadectomy and acute swim stress on GABAA receptor binding in mouse forebrain membranes. Neurochemistry International. 1997;31:1–10. doi: 10.1016/s0197-0186(96)00143-x. [DOI] [PubMed] [Google Scholar]
- Ambrosini MV, Mariucci G, Tantucci M, Brushchelli G, Giuditta A. Induction of cerebellar hsp72 in rats learning a two-way active avoidance task. Molecular Brain Research. 1999;70:164–166. doi: 10.1016/s0169-328x(99)00144-8. [DOI] [PubMed] [Google Scholar]
- Ambrosini MV, Mariucci G, Tantucci M, Van Hooijdonk L, Ammassari-Teule M. Hippocampal 72-kD heat shock protein expression varies according to mice learning performance independently from chronic exposure to stress. Hippocampus. 2005;15:413–417. doi: 10.1002/hipo.20069. [DOI] [PubMed] [Google Scholar]
- Antar LN, Dictenburg JB, Plociniak M, Afroz R, Bassell GJ. Localization of FMRP-associated mRNA granules and requirement of microtubules for activity-dependent trafficking in hippocampal neurons. Genes, Brain and Behavior. 2005;4:350–359. doi: 10.1111/j.1601-183X.2005.00128.x. [DOI] [PubMed] [Google Scholar]
- Bezchlibnyk YB, Wang J-F, Shao L, Young LT. Insulin-like growth factor binding protein-2 expression is decreased by lithium. Molecular Neuroscience. 2006;17:897–901. doi: 10.1097/01.wnr.0000220143.37036.32. [DOI] [PubMed] [Google Scholar]
- Bianchi M, Hagan JJ, Heidbreder CA. Neuronal plasticity, stress and depression: involvement of the cytoskeletal microtubular system? Current Drug Targets CNS Neurologic Disorders. 2005;4:597–611. doi: 10.2174/156800705774322012. [DOI] [PubMed] [Google Scholar]
- Bimonte HA, Denenberg VH. Estradiol facilitates performance as working memory load increases. Psychoneuroendocrinology. 1999;24:161–173. doi: 10.1016/s0306-4530(98)00068-7. [DOI] [PubMed] [Google Scholar]
- Blalock EM, Chen K-C, Sharrow K, Herman JP, Porter NM, Foster TC, Landfield PW. Gene microarrays in hippocampal aging: Statistical profiling identifies novel processes correlated with cognitive impairment. Journal of Neuroscience. 2003;23:3807–3819. doi: 10.1523/JNEUROSCI.23-09-03807.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozon B, Kelly A, Josselyn SA, Silva AJ, Davis S, Laroche S. MAPK, CREB, and zif268 are all required for the consolidation of recognition memory. Phil Trans R Soc Lond. 2003;358:805–814. doi: 10.1098/rstb.2002.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu L, Lephart ED. Soy isoflavones modulate the expression of BAD and neuron-specific beta III tubulin in male rat brain. Neuroscience Letters. 2005;385:153–157. doi: 10.1016/j.neulet.2005.05.040. [DOI] [PubMed] [Google Scholar]
- Burger C, Lopez MC, Feller JA, Baker HV, Muzyczka N, Mandel RJ. Changes in transcription within the CA1 field of the hippocampus are associated with age-related spatial learning impairments. Neurobiology of Learning and Memory. 2007;87:21–41. doi: 10.1016/j.nlm.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Burston SG, Clarke AR. Molecular chaperones: physical and mechanistic properties. Essays Biochemistry. 1995;29:125–136. [PubMed] [Google Scholar]
- Cardona-Gomez GP, Mendez P, Garcia-Segura LM. Synergistic interaction of estradiol and insulin-like growth factor-I in the activation of PI3K/Akt signaling in the adult rat hypothalamus. Molecular Brain Research. 2002;107:80–88. doi: 10.1016/s0169-328x(02)00449-7. [DOI] [PubMed] [Google Scholar]
- Chesik D, De Keyser J, Wilczak N. Insulin-like growth factor binding protein-2 as a regulator of IGF actions in CNS: Implications in multiple sclerosis. Cytokine and Growth Factor Reviews. 2007;18:267–278. doi: 10.1016/j.cytogfr.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Chin L-S, Nugent RD, Raynor MC, Vavalle JP, Li L. SNIP, a novel SNAP-25-interacting protein implicated in regulated exocytosis. Journal of Biological Chemistry. 2000;275:1191–1200. doi: 10.1074/jbc.275.2.1191. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Fader AJ, Spencer AL, Dohanich GP. Estrogen enhances performance of female rats during acquisition of a radial arm maze. Hormones and Behavior. 1997;32:217–225. doi: 10.1006/hbeh.1997.1433. [DOI] [PubMed] [Google Scholar]
- Duff SJ, Hampson E. A beneficial effect of estrogen on working memory in postmenopausal women taking hormone replacement therapy. Hormones and Behavior. 2000;38:262–276. doi: 10.1006/hbeh.2000.1625. [DOI] [PubMed] [Google Scholar]
- Duka T, Tasker R, McGowan JF. The effect of 3-week estrogen hormone replacement on cognition in elderly healthy females. Psychopharmacology (Berl) 2000;149:129–139. doi: 10.1007/s002139900324. [DOI] [PubMed] [Google Scholar]
- Fernandez SM, Frick KM. Chronic oral estrogen affects memory and neurochemistry in middle-aged female mice. Behavioral Neuroscience. 2004;118:1340–1351. doi: 10.1037/0735-7044.118.6.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez SM, Lewis MC, Pechenino AS, Orr PT, Gresack JE, Harburger LL, Schafe GE, Frick KM. Estradiol-induced enhancement of object memory consolidation involves hippocampal ERK and membrane-bound estrogen receptors. Journal of Neuroscience. 2008 doi: 10.1523/JNEUROSCI.1968-08.2008. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick JL, Mize AL, Wade CB, Harris JA, Shapiro RA, Dorsa DM. Estrogen-mediated neuroprotection against beta-amyloid toxicity requires expression of estrogen receptor alpha or beta and activation of the MAPK pathway. Journal of Neurochemistry. 2002;82:674–682. doi: 10.1046/j.1471-4159.2002.01000.x. [DOI] [PubMed] [Google Scholar]
- Frick KM, Fernandez SM, Bennett JC, Prange-Kiel J, MacLusky NJ, Leranth C. Behavioral training interferes with the ability of gonadal hormones to increase CA1 spine density in ovariectomized female rats. Behavior and Brain Research. 2004;19:3026–3032. doi: 10.1111/j.1460-9568.2004.03427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea LAM, Spritzer MD, Barker JM, Pawluski JL. Gonadal hormone modulation of hippocampal neurogenesis in the adult. Hippocampus. 2006;16:225–232. doi: 10.1002/hipo.20154. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM, Sanz A, Mendez P. Cross-talk between IGF-1 and estradiol in the brain: focus on neuroprotection. Neurosteroids and Neuroprotection. 2006;84:275–279. doi: 10.1159/000097485. [DOI] [PubMed] [Google Scholar]
- German DC, Eisch AJ. Mouse models of Alzheimer’s disease: insight into treatment. Reviews in Neuroscience. 2004;15:353–369. doi: 10.1515/revneuro.2004.15.5.353. [DOI] [PubMed] [Google Scholar]
- Gresack JE, Frick KM. Effects of continuous and intermittent estrogen treatments on memory in aging female mice. Brain Research. 2006a;1115:135–147. doi: 10.1016/j.brainres.2006.07.067. [DOI] [PubMed] [Google Scholar]
- Gresack JE, Frick KM. Post-training estrogen enhances spatial and object memory consolidation in female mice. Pharmacology, Biochemistry and Behavior. 2006b;84:112–119. doi: 10.1016/j.pbb.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Hamilton KL, Gupta L, Knowlton A. Estrogen and regulation of heat shock protein expression in female cardiomyocytes: cross-talk with NFkappaB signaling. Journal of Molecular and Cellular Cardiology. 2004;36:577–584. doi: 10.1016/j.yjmcc.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Heald A, Kaushal K, Anderson S, Redpath M, Durrington PN, Selby PL, Gibson MJ. Effects of hormone replacement therapy on insulin-like growth factor (IGF)-1, IGF-II and IGF binding protein (IGFBP)-1 to IGFBP-4: Implications for cardiovascular risk. Gynecological Endocrinology. 2005;20:176–182. doi: 10.1080/09513590400027406. [DOI] [PubMed] [Google Scholar]
- Heikkinen T, Puolivali J, Liu L, Rissanen A, Tanila H. Effects of ovariectomy and estrogen treatment on learning and hippocampal neurotransmitters in mice. Hormones and Behavior. 2002;41:22–32. doi: 10.1006/hbeh.2001.1738. [DOI] [PubMed] [Google Scholar]
- Hosie M, Adamson M, Penny C. Actin binding protein expression is altered in uterine luminal epithelium by clomiphene citrate, a synthetic estrogen receptor modulator. Theriogenology. 2008;69:700–713. doi: 10.1016/j.theriogenology.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Hou Q-L, Gao X, Zhang X-H, Kong LW, Wang X, Bian W, Tu YY, Jin ML, Zhao G-P, Li B-M, Jing N-H, Yu L. SNAP-25 in hippocampal CA1 region is involved in memory consolidation. European Journal of Neuroscience. 2004;20:1593–1603. doi: 10.1111/j.1460-9568.2004.03600.x. [DOI] [PubMed] [Google Scholar]
- Hou Q-L, Gao X, Lu Q, Zhang X-H, Tu Y-Y, Jin M-L, Zhao G-P, Yu L, Jing N-H, Li B-M. SNAP-25 in hippocampal CA3 region is required for long-term memory formation. Biochemical and Biophysical Research Communications. 2006;347:955–962. doi: 10.1016/j.bbrc.2006.06.184. [DOI] [PubMed] [Google Scholar]
- Jacobsson G, Razani H, Ogren SO, Meister B. Estrogen down-regulates mRNA encoding the exocytotic protein SNAP-25 in the rat pituitary gland. Journal of Neuroendocrinology. 1998;10:157–163. doi: 10.1046/j.1365-2826.1998.00178.x. [DOI] [PubMed] [Google Scholar]
- Kampen DL, Sherwin BB. Estrogen and verbal memory in healthy postmenopausal women. Obstetrics and Gynocology. 1994;83:979–983. doi: 10.1097/00006250-199406000-00017. [DOI] [PubMed] [Google Scholar]
- Kuroki Y, Fukushima K, Kanda Y, Mizuno K, Watanabe Y. Putative membrane-bound estrogen receptors possibly stimulate mitogen-activated protein kinase in the rat hippocampus. European Journal of Pharmacology. 2000;400:205–209. doi: 10.1016/s0014-2999(00)00425-8. [DOI] [PubMed] [Google Scholar]
- Lewis MC, Kerr KM, Orr PT, Frick KM. Estradiol-induced enhancement of object memory consolidation involves NMDA receptors and protein kinase A in the dorsal hippocampus of female C57BL/6 mice. Behavioral Neuroscience. 2008;122:716–721. doi: 10.1037/0735-7044.122.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine VN, Richards ST, Wu VY, Beck KD. Estradiol enhances learning and memory in a spatial memory task and effects levels of monoaminergic neurotransmitters. Hormones and Behavior. 1998;34:149–162. doi: 10.1006/hbeh.1998.1473. [DOI] [PubMed] [Google Scholar]
- Luine VN, Jacome LF, MacClusky NJ. Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology. 2003;144:2836–44. doi: 10.1210/en.2003-0004. [DOI] [PubMed] [Google Scholar]
- Malyala A, Pattee P, Nagalla SR, Kelly MJ, Ronnekleiv OK. Suppression subtractive hybridization and microarray identification of estrogen-regulated hypothalamic genes. Neurochemical Research. 2004;29:1189–1200. doi: 10.1023/b:nere.0000023606.13670.1d. [DOI] [PubMed] [Google Scholar]
- Manavathi B, Acconcia F, Rayala SK, Kumar R. An inherent role of microtubule network in the action of nuclear receptor. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:15981–15986. doi: 10.1073/pnas.0607445103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannella P, Brinton RK. Estrogen receptor protein interaction with phosphatidylinositol 3-kinase leads to activation of phosphorylated Akt and extracellular signal-regulated kinase 1/2 in the same population of cortical neurons: a unified mechanism of estrogen action. Journal of Neuroscience. 2006;26:9439–9447. doi: 10.1523/JNEUROSCI.1443-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastorakos G, Sakkas EGR, Xydakis AM, Creatsas G. Pitfalls of the WHIs Women’s Health Initiative. Annals of the New York Acadamy of Sciences. 2006;1092:331–340. doi: 10.1196/annals.1365.030. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Engler JA, Sheng M. The dynamic turnover and functional roles of alpha-actinin in dendritic spines. Neuropharmacology. 2004;47:734–745. doi: 10.1016/j.neuropharm.2004.07.022. [DOI] [PubMed] [Google Scholar]
- O’Sullivan NC, McGettigan PA, Sheridan GK, Pickering M, Conboy L, O’Connor JJ, Moynagh PN, Higgins DG, Regan CM, Murphy KJ. Temporal changes in gene expression in the rat dentate gyrus following passive avoidance learning. Journal of Neurochemistry. 2007;101:1085–1098. doi: 10.1111/j.1471-4159.2006.04418.x. [DOI] [PubMed] [Google Scholar]
- Olazabal UE, Pfaff DW, Mobbs CV. Sex differences in the regulation of heat shock protein 70 kDa and 90 kDa in the rat ventromedial hypothalamus by estrogen. Brain Research. 1992;596:311–314. doi: 10.1016/0006-8993(92)91563-t. [DOI] [PubMed] [Google Scholar]
- Osen-Sand A, Catsicas M, Staple JK, Jones KA, Ayala G, Knowles J, Grenningloh G, Stephan C. Inhibition of axonal growth by SNAP-25 antisense oligonucleotides in vitro and in vivo. Nature. 1993;364:445–448. doi: 10.1038/364445a0. [DOI] [PubMed] [Google Scholar]
- Perez-Martin M, Azcoitia I, Trejo JL, Sierra A, Garcia-Segura LM. An antagonist of estrogen receptors blocks the induction of adult neurogenesis by insulin-like growth factor-1 in the dentate gyrus of adult female rat. European Journal of Neuroscience. 2003;18:923–930. doi: 10.1046/j.1460-9568.2003.02830.x. [DOI] [PubMed] [Google Scholar]
- Pitha J, Pitha J. Amorphous water soluble derivatives of cyclodextrins: Nontoxic dissolution enhancing excipients. Journal of Pharmaceutical Sciences. 1985;74:987–990. doi: 10.1002/jps.2600740916. [DOI] [PubMed] [Google Scholar]
- Pitha J, Harman SM, Michel ME. Hydrophilic cyclodextrin derivatives enable effective oral administration of steroidal hormones. Journal of Pharmaceutical Sciences. 1986;75:165–167. doi: 10.1002/jps.2600750213. [DOI] [PubMed] [Google Scholar]
- Pizarro JM, Haro LS, Barea-Rodriguez EJ. Learning associated increase in heat shock cognate 70 mRNA and protein expression. Neurobiology of Learning and Memory. 2003;79:142–151. doi: 10.1016/s1074-7427(02)00008-4. [DOI] [PubMed] [Google Scholar]
- Rai KS, Hattiangady B, Shetty AK. Enhanced production and dendritic growth of new dentate granule cells in the middle-aged hippocampus following intracerebroventricular FGF-2 infusions. European Journal of Neuroscience. 2007;26:1765–1779. doi: 10.1111/j.1460-9568.2007.05820.x. [DOI] [PubMed] [Google Scholar]
- Ramirez VD, Kipp JL, Joe I. Estradiol, in the CNS, targets several physiologically relevant membrane-associated proteins. Brain Research Reviews. 2001;37:141–152. doi: 10.1016/s0165-0173(01)00114-x. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Paschall G, Zhou X-l, Davis M. Regulation of synaptic plasticity genes during consolidation of fear conditioning. Journal of Neuroscience. 2002;22:7892–7902. doi: 10.1523/JNEUROSCI.22-18-07892.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissanen A, Puolivali J, van Groen T, Riekkinen PJ. In mice tonic estrogen replacement therapy improves non-spatial and spatial memory in a water maze task. NeuroReport. 1999;10:1369–1372. doi: 10.1097/00001756-199904260-00039. [DOI] [PubMed] [Google Scholar]
- Roberts LA, Morris BJ, O’Shaughnessy CT. Involvement of two isoforms of SNAP-25 in the expression of long-term potentiation in the rat hippocampus. NeuroReport. 1998;9:33–36. doi: 10.1097/00001756-199801050-00007. [DOI] [PubMed] [Google Scholar]
- Sandstrom NJ, Williams CL. Memory retention is modulated by acute estradiol and progesterone replacement. Behavioral Neuroscience. 2001;115:383–393. [PubMed] [Google Scholar]
- Sandstrom NJ, Williams CL. Spatial memory retention is enhanced by acute and continuous estradiol replacement. Hormones and Behavior. 2004;45:128–135. doi: 10.1016/j.yhbeh.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Sekino Y, Kojima N, Shirao T. Role of actin cytoskeleton in dendritic spine morphogenesis. Neurochemistry International. 2007;51:92–104. doi: 10.1016/j.neuint.2007.04.029. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, Hendrix SL, Jones BN, III, Assaf AR, Jackson RD, Kotchen JM, Wassertheil-Smoller S, Wactawski-Wende J. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women. Journal of the American Medical Association. 2003;289:2651–2662. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Lane DS, Fillit H, Stefanick ML, Hendrix SL, Lewis CE, Masaki K, Coker LH. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women. Journal of the American Medical Association. 2004;291:2947–2958. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- Sonntag WE, Ramsey M, Carter CS. Growth hormone and insulin-like growth factor-1 (IGF-1) and their influence on cognitive aging. Ageing Research Reviews. 2005;4:195–212. doi: 10.1016/j.arr.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Stone DJ, Rozovsky I, Morgan TE, Anderson CP, Finch CE. Increased synaptic sprouting in response to estrogen via an apolipoprotein E-dependent mechanism: implications for Alzheimer’s disease. Journal of Neuroscience. 1998;18:3180–3185. doi: 10.1523/JNEUROSCI.18-09-03180.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam S, Shahani N, Strelau J, Laliberte C, Brandt R, Kaplan D, Unsicker K. Insulin-like growth factor 1 inhibits extracellular signal-regulated kinase to promote neuronal survival via the phosphatidylinositol 3-kinase/protein kinase A/c-Raf pathway. Journal of Neuroscience. 2005;25:2838–2852. doi: 10.1523/JNEUROSCI.5060-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S, Mikami T, Watanabe Y, Okazaki M, Okazaki Y, Okazaki A, Sato T, Asaishi K, Hirata K, Narimatsu E. Correlation of heat shock protein 70 expression with estrogen receptor levels in invasive breast cancer. American Journal of Clinical Pathology. 1994;101:519–525. doi: 10.1093/ajcp/101.4.519. [DOI] [PubMed] [Google Scholar]
- Tanapat P, Hastings NB, Reeves AJ, Gould E. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. Journal of Neuroscience. 1999;19:5792–5801. doi: 10.1523/JNEUROSCI.19-14-05792.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor GT, Weiss J, Pitha J. Testosterone in a cyclodextrin-containing formulation: Behavioral and physiological effects of episode-like pulses in rats. Pharmaceutical Research. 1989;6:641–646. doi: 10.1023/a:1015922019038. [DOI] [PubMed] [Google Scholar]
- Vaucher E, Reymond I, Najaffe R, Kar S, Quirion R, Miller M, Franklin K. Estrogen effects on object memory and cholinergic receptors in young and old female mice. Neurobiology of Aging. 2002;23:87–95. doi: 10.1016/s0197-4580(01)00250-0. [DOI] [PubMed] [Google Scholar]
- Wade CB, Dorsa DM. Estrogen activation of cyclic adenosine 5’-monophosphate response element-mediated transcription requires the extracellularly regulated kinase/mitogen-activated protein kinase pathway. Endocrinology. 2003;144:832–838. doi: 10.1210/en.2002-220899. [DOI] [PubMed] [Google Scholar]
- Wang Y, Tang BL. SNAREs in neurons-beyond vesicle exocytosis (review) Molecular Membrane Biology. 2006;23:377–384. doi: 10.1080/09687860600776734. [DOI] [PubMed] [Google Scholar]
- Whitesell L, Lindquist SL. Hsp90 and the chaperoning of cancer. Nature Reviews. 2005;5:761–772. doi: 10.1038/nrc1716. [DOI] [PubMed] [Google Scholar]
- Wolf OT, Kirschbaum C. Endogenous estradiol and testosterone levels are associated with cognitive performance in older women and men. Hormones and Behavior. 2002;41:259–266. doi: 10.1006/hbeh.2002.1770. [DOI] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. Journal of Comparative Neurology. 1993;8:293–306. doi: 10.1002/cne.903360210. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Lui LY, Grady D, Cauley J, Kramer J, Cummings SR. Cognitive decline in women in relation to non-protein-bound oestradiol concentrations. Lancet. 2000;356:708–712. doi: 10.1016/S0140-6736(00)02628-3. [DOI] [PubMed] [Google Scholar]
- Yokomaku D, Numakawa T, Numakawa Y, Suzuki S, Matsumoto T, Adachi N, Nishio C, Taguchi T, Hatanaka H. Estrogen enhances depolarization-induced glutamate release through activation of phosphatidylinositol 3-kinase and moitogen-activated protein kinase in cultured hippocampal neurons. Molecular Endocrinology. 2003;17:831–844. doi: 10.1210/me.2002-0314. [DOI] [PubMed] [Google Scholar]


