Abstract
Elevated intraocular pressure (IOP) constitutes the best characterized risk for primary open-angle glaucoma (POAG). Elevated IOP is believed to result from an increase in aqueous humor outflow resistance at the level of the trabecular meshwork (TM)/ Schlemm's canal (SC). Malfunction of the TM in POAG is associated with the expression of markers for inflammation, cellular senescence, oxidative damage, and decreased cellularity. Current POAG treatments rely on lowering IOP, but there is no therapeutic approach available to delay the loss of function of the TM in POAG patients. We evaluated the effects of chronic administration of the dietary supplement resveratrol on the expression of markers for inflammation, oxidative damage, and cellular senescence in primary TM cells subjected to chronic oxidative stress (40% O2). Resveratrol treatment effectively prevented increased production of intracellular reactive oxygen species (iROS) and inflammatory markers (IL1α, IL6, IL8, and ELAM-1), and reduced expression of the senescence markers sa-β-gal, lipofuscin, and accumulation of carbonylated proteins. Furthermore, resveratrol exerted antiapoptotic effects that were not associated with a decrease in cell proliferation. These results suggest that resveratrol could potentially have a role in preventing the TM tissue abnormalities observed in POAG.
Keywords: Resveratrol, oxidative stress, glaucoma, trabecular meshwork
1. Introduction
Primary Open-Angle Glaucoma (POAG) is an age-related-disease affecting millions of people worldwide. This optic neuropathy is characterized by loss of retinal ganglion cell axons and remodeling of the optic nerve head that is accompanied by progressive visual field loss that can result in irreversible blindness. The main risk factor for POAG is elevated intraocular pressure (IOP) and lowering IOP is currently the only therapeutic approach available to delay the progression of the disease (Armaly, et al., 1980; Leske, et al., 2003). The trabecular meshwork (TM) is the tissue responsible for draining most of the aqueous humor from the anterior chamber of the eye, and the increase in IOP observed in POAG is believed to result from an increase in resistance to aqueous humor outflow at the level of the TM/ Schlemm's canal (SC) (Maepea and Bill, 1992; Moses, 1977).
The specific mechanisms leading to the failure of the TM to maintain normal levels of outflow resistance in POAG are not completely understood. However, it is known that the TM from glaucoma donors is characterized by the sustained activation of a stress response that results in expression of pro-inflammatory markers such as ELAM-1 (Wang, et al., 2001). In a comparative analysis between control and POAG tissues, several genes associated with inflammation and an acute-phase response, including ELAM-1, were up-regulated (Liton, et al., 2006). Glaucomatous TM cells produce constitutively IL1α that has been shown to lead to the up-regulation of ELAM-1 and other inflammatory mediators (Wang, et al., 2001; Zhang, et al., 2006). In addition, a polymorphism in IL1α that leads to increased IL1 expression has been reported to be a risk factor for POAG (Wang, et al., 2006). Together with the sustained activation of a pro-inflammatory response, the TM from glaucoma donors is also known to show some decrease in cellularity and an increase in the expression of the cellular senescence marker sa-beta-galactosidase (sa-β-gal) (Alvarado, et al., 1984; Liton, et al., 2005), which suggests that apoptosis and cellular senescence may contribute to the loss of function of the TM.
Oxidative stress may also be a contributing factor in the observed alterations of the TM in glaucoma. Acute treatment of TM cells with H2O2 has been shown to induce the expression of the glaucoma marker ELAM-1 (Zhou, et al., 2007) and chronic H2O2 treatment results in a sustained activation of a stress response similar to that observed in TM cells from glaucoma donors (Li, 2007). Furthermore, oxidative stress has been suggested to contribute to the loss in cellularity in the TM by inducing apoptosis (Alvarado, et al., 1981; Alvarado, et al., 1984) and is also known to contribute to an increased expression of sa-β-gal (Caballero, et al., 2003). The TM is subjected to relatively high oxygen concentration from the aqueous humor, between 5% and 6% (Helbig, et al., 1993), higher than in most tissues (around 3%). The pathogenic role of reactive oxygen species (ROS) in glaucoma is supported by additional experimental findings including the induction of TM degeneration and increase in resistance to aqueous humor outflow by hydrogen peroxide (Kahn, et al., 1983; Nguyen, et al., 1988); reduction in the antioxidant capacities of the TM with age (De La Paz and Epstein, 1996) and positive correlation between oxidative DNA damage in the TM with visual-field loss and increased IOP (Sacca, et al., 2005). Recently, the increased expression of the oxidative stress marker sPLA2-IIA has also been reported in association with glaucoma (Zhou, et al., 2007).
Although there is great deal of research aimed at preventing the loss of ganglion cells using neuroprotective agents (Cheung, et al., 2008; Weber, et al., 2008), current treatments for POAG rely on lowering the IOP and, at the present, there is no therapy that can help to prevent or delay the loss of function of the TM in POAG patients. Resveratrol, a naturally occurring polyphenol found in berries, nuts, and red wine, can enhance stress resistance and extend the life span of various organisms from yeast to vertebrates (Bauer, et al., 2004; Baur, et al., 2006; Howitz, et al., 2003; Valenzano, et al., 2006). It has been reported to exert anti-inflammatory, anti-oxidant, and anti-apoptotic effects, and there is experimental evidence supporting the beneficial effects of resveratrol in preventing or slowing down a wide variety of age related diseases (Baur and Sinclair, 2006; Cucciolla, et al., 2007; Holme and Pervaiz, 2007; Kahn, et al., 1983). Therefore, we hypothesized that resveratrol may help to prevent the alterations induced by chronic oxidative stress in TM cells. To evaluate the potential of resveratrol to protect TM function we analyzed the effects of chronic resveratrol treatment on the expression of markers for inflammation, oxidative damage, and cellular senescence in primary TM cells subjected to chronic oxidative stress, as well as on the levels of apoptosis induced by acute oxidative injury.
2. Material and Methods
2.1. Cell culture
Primary porcine TM cells were obtained from pig eyes, and cultured as previously described (Stamer, et al., 1995). Cell cultures were maintained at 37°C in 5% CO2 in media (low glucose Dulbecco's Modified Eagle Medium with L-glutamine, 110mg/mL sodium pyruvate, 10% fetal bovine serum, 100μM non-essential aminoacids, 100 units/mL penicillin, 100μg/mL streptomicyn sulfate and 0.25μg/mL amphotericin B). All the reagents were obtained from Invitrogen Corporation (Carlsbad, CA).
2.2. Chronic Oxidative stress and resveratrol treatment
For most of the experiments, confluent primary porcine TM cells were submitted to chronic treatment with resveratrol (25μM in DMSO, from Sigma, Saint Louis, MO) or vehicle (1μl DMSO/mL cell culture media) every three days for 15 days. Cells under resveratrol or vehicle treatment were incubated under oxidative stress conditions (40% oxygen). Additional control cultures were treated with vehicle and incubated at physiological oxygen concentration (5%). After 15 days cells were subjected to different assays.
2.3 Cytotoxicity Assay
Cytotoxicity was measured using the Cyto Tox 96® Non-Radioactive Cytotoxicity assay (Promega, Madison,WI) following the manufacturer's instructions. Briefly the release of lactate dehydrogenase (LDH) is converted into a red formazan product proportional to the number of lysed cells.
2.4. Senescence associated beta-galactosidase (sa-β-gal) activity
Activity of sa-β-gal was measured by flow cytometry using the fluorogenic substrate C12FDG (Molecular Probes, Eugene, OR) as previously described (Fiering, et al., 1991). Briefly, cells were incubated with 300μM of chloroquine for 30 minutes to modulate the intracellular pH. Cell cultures were then washed with phosphate buffered saline (PBS), trypsinized, resuspended in PBS, and incubated for 5 minutes at 37°C in a water bath, mixed gently with 2mM of FDG, and incubated for 1 minute. The cell suspension was placed on ice, diluted 10 times with cold PBS, and incubated on ice until flow cytometry analysis (FACScaliber, Becton Dickinson, CA, USA). The average number of cells analyzed for each experiment was 10,000.
2.5. Cell proliferation
Cell proliferation was assayed using 10μM BrdU (5-bromo-2′-deoxyuridine, from Sigma, Sant Louis, MO). Briefly, after two weeks of treatment, cells in 6 well plates were trypsinized and seeded in 10cm plates, incubated 48 hours and labeled with BrDu for 5 hours. After labeling, cells were trypsinized and fixed in cold methanol overnight. Cells were washed in PBS/BSA, denaturated with 2M HCl, neutralized with 0.1M sodium borate, and hybridized with anti BrdU-FITC antibody (Santa Cruz Biothecnology, Santa Cruz, CA), and resuspended in propidium iodine (10μg/mL) containing 300μg/mL RNase (Sigma, MO, USA). BrdU incorporation was calculated from 10,000 cells by flow cytometry.
2.6. Analysis of autofluorescence
Cell autofluorescence (lipofuscin-like or ceroid-like material) was determined by flow cytometry at 563-607nm. The average number of cells analyzed for each experiment was 10,000.
2.7. Intracellular Reactive Oxygen Species (iROS) measurement
Cells were loaded with 10μM of 2′7′-dichlorofluorescein diacetate (DCFDA) (Calbiochem, La Jolla, CA) incubated for 30 minutes in PBS and washed with PBS. This was followed by incubation for 20 minutes in media and trypsinization. Finally, the cells were collected in PBS and kept on ice until analyzed by flow cytometry. The average number of cells analyzed for each experiment was 10,000.
2.8. RNA extraction
For RNA extraction, porcine primary TM cells were washed with PBS and immediately submerged in RNA-later (Ambion Inc, Austin, TX). Total RNA was then isolated using RNeasy kit (Qiagen Inc. Valencia, CA) according to the manufacturer's instructions and treated with DNase. RNA yields were determined using RiboGreen fluorescent dye (Molecular Probes Inc. Eugene, OR).
2.9. Quantitative real-time PCR (RT-PCR)
First strand cDNA was synthesized from total RNA (1μg) by reverse transcription using oligo-dT and Superscript II reverse transcriptase (Invitrogen, Carlsbad, CA) according to manufacturer's instructions. RT-PCR reactions were performed in 20μL mixture containing 1μL of the cDNA preparation, 1X iQ SYBR Green Supermix (Bio-Rad, Hercules, CA), using the following PCR parameters: 95°C for 5 minutes followed by 50 cycles of 95°C for 15 seconds, 65°C for 15 seconds, and 72°C for 15 seconds. The fluorescence threshold value (Ct) was calculated using the iCycle system software. The absence of nonspecific products was confirmed by both analysis of the melting curves and electrophoresis in 3% Super acrylAgarose gels. β-actin was used as an internal standard of mRNA expression. Primers for RT-PCR were designed using Primer3 (v. 0.4.0) software (Rozen, 2000) and are showed in Table 1.
Table 1.
| Name | Genebank | Start position | Forward 5′-3′ | Reverse 5′-3′ |
|---|---|---|---|---|
| Acc # | ||||
| IL-1α | NM_14029 | 842 | AAGTGTTGACAGGCCGTATG | TACCAGACTTCGCTCCCTCT |
| ELAM-1 | NM_214268 | 697 | CCCATGGAACACAACCTGTGCATT | AGCTTTACACGTTGGCTTCTTGCC |
| IL-8 | NM_213867 | 92 | AAACTGGCTGTTGCCTTCTT | ATTTATGCACTGGCATCGAA |
| IL-6 | NM_214399 | 365 | GCTTCCAATCTGGGTTCAAT | CTAATCTGCACAGCCTCGAC |
| β-actin | AY550069 | 1063 | AAGATCAAGATCATCGCGCCTCCA | TGGAATGCAACTAACAGTCCGCCT |
2.10. Protein extraction and protein carbonylation assay
For protein extraction cells were washed in PBS and lysated in 1X RIPA buffer, and protein concentration was determined using Micro BCA Protein Assay Kit (Pierce, Rockford, IL). The OxyBlot™ Protein Oxidation Detection Kit (Chemicon, Temecula, CA) was employed for the comparative analysis of total protein carbonylation according to the manufacturer's instructions. Briefly, 2,4-dinitrophenylhy-drazine (DNPH) derivatization was carried out followed by SDS-PAGE under reducing conditions and blots were incubated with anti-DNP antibody. Blots were developed using a chemiluminescence detection system (ECL-Plus from Amersham, Buckinghamshire, UK). The comparison between the samples was calculated using the ratio between densitometric values of the total oxyblot bands and the total proteins stained with Commassie Blue on the same blot.
2.11. DNA damage assay
DNA damage quantification kit (Dojindo, Gaithersburg, MD) detects DNA modifications that result in the formation of aldehyde groups, which are the open ring form of the abasic sites (AP sites). Briefly, an excess of Aldehyde Reactive Probe (ARP) conjugated to avidin was incubated with DNA samples. After labeling, the AP sites were quantified by a colorimetric reaction using anti avidin antibody conjugated to peroxidase, following the manufacturer's instructions.
2.12. Proteasome activity assay
Chymotrypsin-like (CT-L), trypsin-like (T-L), and caspase-like (PGPH) activities of the proteasome were assayed using the fluorogenic peptides (from Sigma, Sant Louis, MO) N- Succinyl-Leu-Leu-Val-Tyr-AMC (Suc-LLVY-AMC at 70μM), Z-Leu-Leu-Glu-AMC (ZLLG-AMC at 70μM) and Boc-Leu-Arg-Arg-AMC (BOLAA at 200μM). Briefly, cells were lysated (20mM Tris–HCl, pH 7.5; 10% glycerol; 5mM ATP; 0.2% Np40) and 20μg of protein extract diluted in assay buffer (50mM Hepes/ KOH pH 7.5). Assays were carried out at 37°C for 30 minutes. The fluorescence of the samples was evaluated using a BIO-TEK Synergy HT spectrofluorimeter at excitation/emission wavelengths of 360/460. Activity was determined as the difference between total activity of crude extracts and the remaining activity with 20μM MG132 (Sigma, St Louis, MO), a proteasome inhibitor.
2.13. Apoptotic analysis
Apoptosis was measured using the Vybrant Apoptosis Assay kit (Invitrogen, Eugene, OR). Briefly, apoptosis was induced using several concentrations of H2O2 (0, 200, 400 and 800μM). Cells were treated with 25μM of resveratrol or vehicle 24 hours before H2O2 treatment and during the treatment. Cells were trypsinized four hours after H2O2 treatment, washed with cold PBS, resuspended in PBS with YO-PRO and propidium iodine dyes, incubated on ice for 30 minutes, and analyzed by flow cytometry. The average number of cells analyzed for each experiment was 10,000.
2.14. Statistical analysis
Results are expressed as mean value ± SD in at least three independent experiments. The SAS Software package (SAS/STAT, version 8, Cary, NC: SAS Institute Inc., 1999) was used for the statistical analysis. P-values were calculated by a non paired two sided t-test and by the non parametric test Kruskal-Wallis (that is the same as the Wilcoxon test when there are only two groups). In the figures “*” denoted p-values based on t-test and “#” p-values based on the Kruskal-Wallis test.
3. Results
3.1. Cytotoxicity of chronic treatment with resveratrol in TM cells
In order to evaluate potential cytotoxic effects of resveratrol, TM cell cultures incubated at physiologic oxygen concentration (5%) were subjected to chronic treatment with different concentrations of resveratrol or vehicle for 15 days. A single treatment with high concentrations of resveratrol (200 and 400μM) resulted in extensive cell death in less than 48 hours. However, chronic treatment with resveratrol at concentrations of 100μM or lower did not show any increase in cytotoxity compared to the vehicle treated controls. Treatment with resveratrol at concentrations of 50 and 100μM resulted in a small but significant decrease in cytotoxicity compared to control cultures treated with vehicle (Figure 1).
Figure 1.
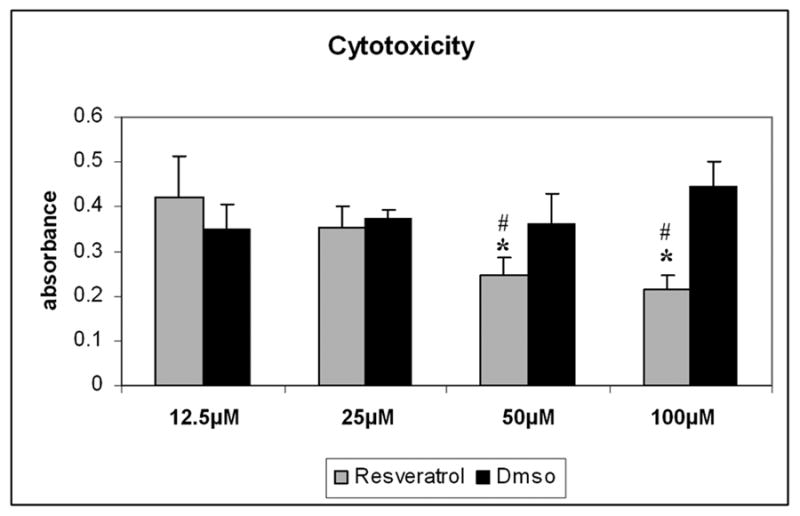
Cytotoxic effects of chronic treatment with resveratrol in TM cells incubated at physiological oxygen concentration (5%). Cytotoxicity was evaluated using Cyto Tox 96 assay after 15 days of chronic treatment with different concentrations of resveratrol (12.5 to 400μM) or vehicle. A single treatment with high concentrations of resveratrol (200 and 400μM) resulted in extensive cell death in less than 48 hours. Since these cultures did not survive a chronic treatment for 15 days, cytotoxicity data for these concentrations is not included in the figure (N=3, t-test *P ≤ 0.05, # Kruskal-Wallis test P ≤ 0.049).
3.2. Resveratrol inhibits intracellular ROS production
To evaluate the effects of resveratrol on the induction of intracellular ROS production mediated by chronic oxidative stress, confluent primary cultures of porcine TM cells were subjected to chronic oxidative stress (15 days at 40% oxygen) and chronic treatment with resveratrol or vehicle (every three days during 15 days). Non-stressed control cultures were incubated in parallel at 5% oxygen concentration. The induction of endogenous ROS production in this model was significantly decreased by resveratrol treatment (4 fold) compared to cells treated with vehicle. The amount of intracellular ROS in resveratrol-treated cells under oxidative stress was similar to that of non-stressed control cells incubated at physiological oxygen concentration (5%) (Figure 2).
Figure 2.
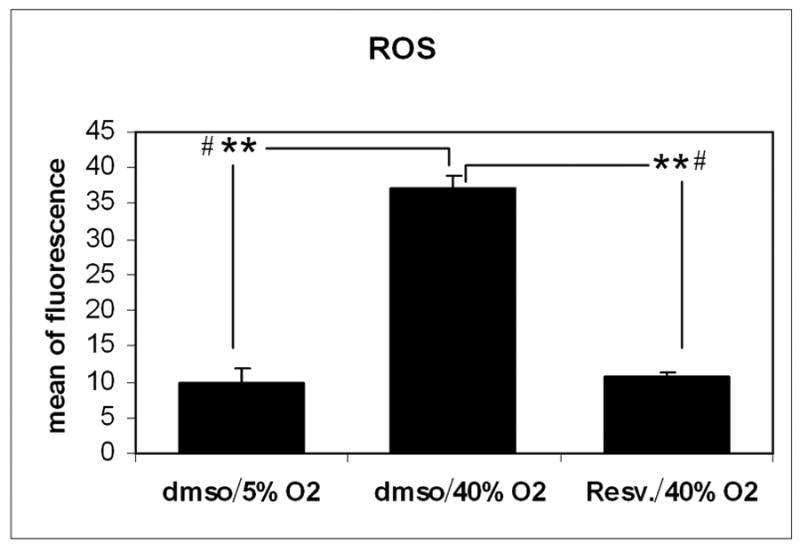
Effect of resveratrol treatment on the induction of iROS mediated by chronic oxidative stress in pTM cells. Levels of iROS were measured by H2DCFDA from cells incubated at 40% oxygen and treated with resveratrol or vehicle and compared to cells incubated at physiological oxygen conditions (5%) (N=3, t-test **P ≤ 0.001, # Kruskal-Wallis test P ≤ 0.049).
3.3. Resveratrol reduces the activation of inflammatory markers
The induction of mRNA expression of the inflammatory markers IL1α, IL6, IL8 and ELAM-1 after chronic oxidative stress was significantly inhibited by chronic treatment with resveratrol. As shown in figure 3, resveratrol inhibited the induction of these inflammatory markers in samples incubated at 40% oxygen.
Figure 3.
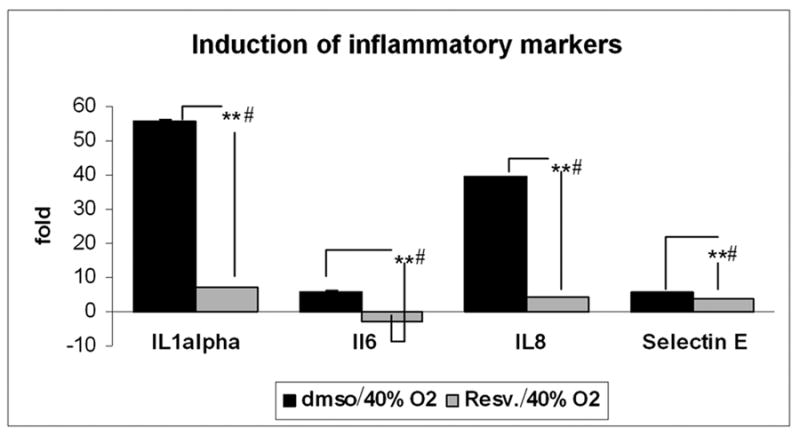
Effect of resveratrol treatment on the expression of inflammatory markers induced by chronic oxidative stress. The changes in mRNA expression of IL1α, IL6, IL8 and ELAM-1 induced by incubation at 40% oxygen compared to control cultures incubated at 5% oxygen were measured by Real Time- PCR in cells treated with either resveratrol or dmso. β-actin was used as an internal control (N=3, t-test ** P ≤ 0.001; # Kruskal-Wallis test P ≤ 0.049).
3.4. Resveratrol reduces the accumulation of senescence markers
Cells treated with vehicle and incubated under oxidative stress showed a significant increase in autofluorescence (2.4 fold) and sa-β-gal (1.8 fold) when compared to cells in the same condition treated with resveratrol. Cells under oxidative stress, treated with vehicle or resveratrol, showed higher levels of autofluorescence (3.1 and 1.2 fold respectively) and sa-β-gal (3.5 and 1.9 fold respectively) when compared to cells incubated at more physiological oxygen concentration (Figure 4A and 4B).
Figure 4.
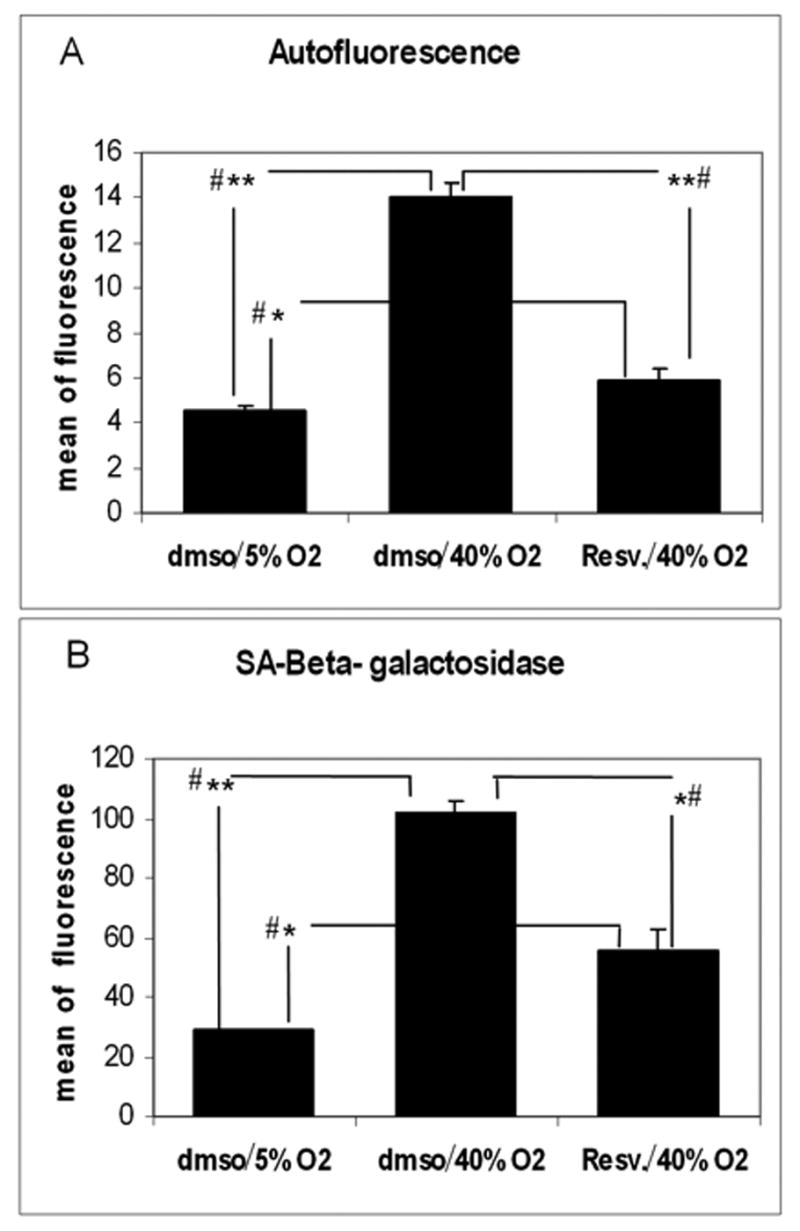
Effect of resveratrol treatment on the expression of the senescence markers autofluorescence (A) and sa-β-gal (B). Autofluorescence and sa-β-gal activity were determined by flow cytometry in cells subjected to chronic oxidative stress (40% oxygen) treated with either resveratrol or vehicle and compared to cells incubated at physiological oxygen concentration (N=3, t-test, * P ≤ 0.05, ** P ≤ 0.001; # Kruskal-Wallis test P ≤ 0.049).
3.5. Decreased accumulation of carbonylated proteins with no change in proteasomal activity after resveratrol treatment
The accumulation of carbonylated proteins induced by oxidative stress was significantly lower in resveratrol-treated samples compared to samples treated with vehicle under oxidative stress, as well as to that in cells incubated at physiological oxygen concentrations (Figures 5A, B and C). However, this decrease in oxidized proteins was not associated with an increase in proteasomal activity. The three proteolytic activities of the proteasome (chymotrypsin-like, trypsin-like and caspase-like activity) showed decreased activity in both resveratrol treated and non-treated cells incubated at 40% oxygen compared to the non-stressed controls incubated at physiologic oxygen concentrations (Figure 5D).
Figure 5.
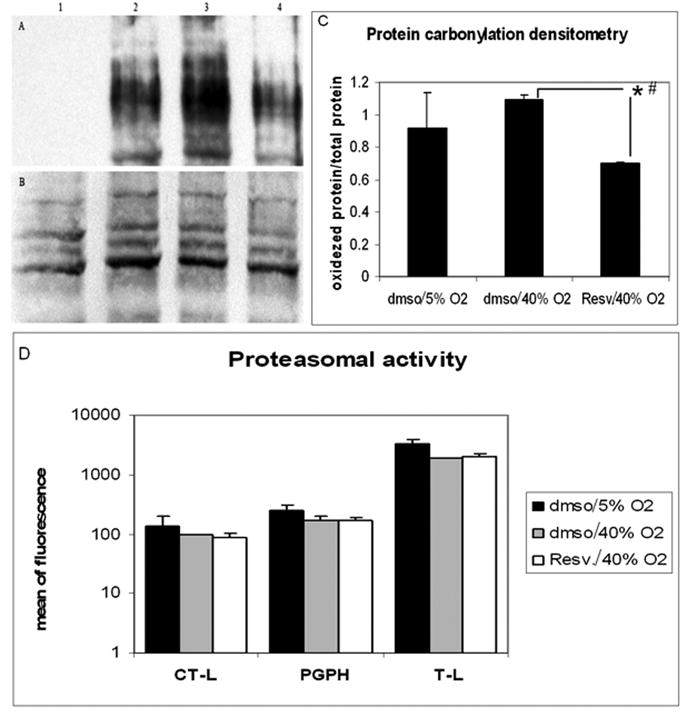
Effects of resveratrol treatment on the accumulation of carbonylated proteins and proteasome activity in pTM cells after chronic oxidative stress. Total protein carbonylation and proteolitic activity were measured in cells incubated at 40% oxygen treated with either resveratrol or vehicle and compared to cells incubated at 5% oxygen. Representative Western Blot from oxidized proteins is shown in panel A (1= negative control for carbonylation; 2= DMSO/ 5% oxygen; 3= DMSO/ 40% oxygen; 4= resveratrol/ 40% oxygen) and total proteins stained with Commasie Blue in panel B. Densitometry summarizing 3 different experiments is showed in panel C. Panel D shows the proteolytic activities of the proteasome subunits: CT-L (chymotrypsin like), T-L (trypsin like), and PGHP (caspase like) (N=3, t-test, * P ≤ 0.05; # Kruskal-Wallis test P ≤ 0.049).
3.6. Resveratrol protects against apoptosis in an acute model of oxidative stress
Cells treated with resveratrol showed protection against apoptosis after acute oxidative stress (200, 400, and 800μM of H2O2), when compared to cells treated with vehicle. Cells treated with vehicle showed a linear correlation between H2O2 concentration and apoptosis; cells treated with resveratrol exhibited protection against apoptosis in all H2O2 concentrations (Figure 6).
Figure 6.
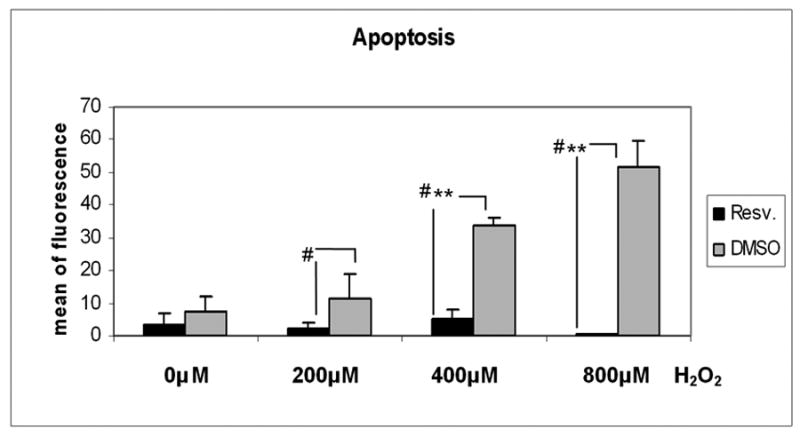
Protective effect of resveratrol against apoptosis induced by acute oxidative challenge. Cells treated with resveratrol or vehicle were subjected to different concentrations of H2O2 (200, 400, and 800μM) for 4 hours and assayed for apoptosis (N=3, t-test, ** P ≤ 0.001; # Kruskal-Wallis test P ≤ 0.049).
3.7. Proliferation and DNA damage are not significantly affected by resveratrol treatment in a chronic model of oxidative stress
Resveratrol treatment did not result in significant changes on proliferation measured by BrDU incorporation when compared to cells treated with vehicle (Figure 7A). Similarly, no significant changes were observed in the amount of DNA damage under oxidative stress. Cells treated with vehicle or resveratrol under oxidative stress showed similar levels of DNA damage that were higher than those in cells incubated at physiological oxygen concentration (Figure 7B).
Figure 7.
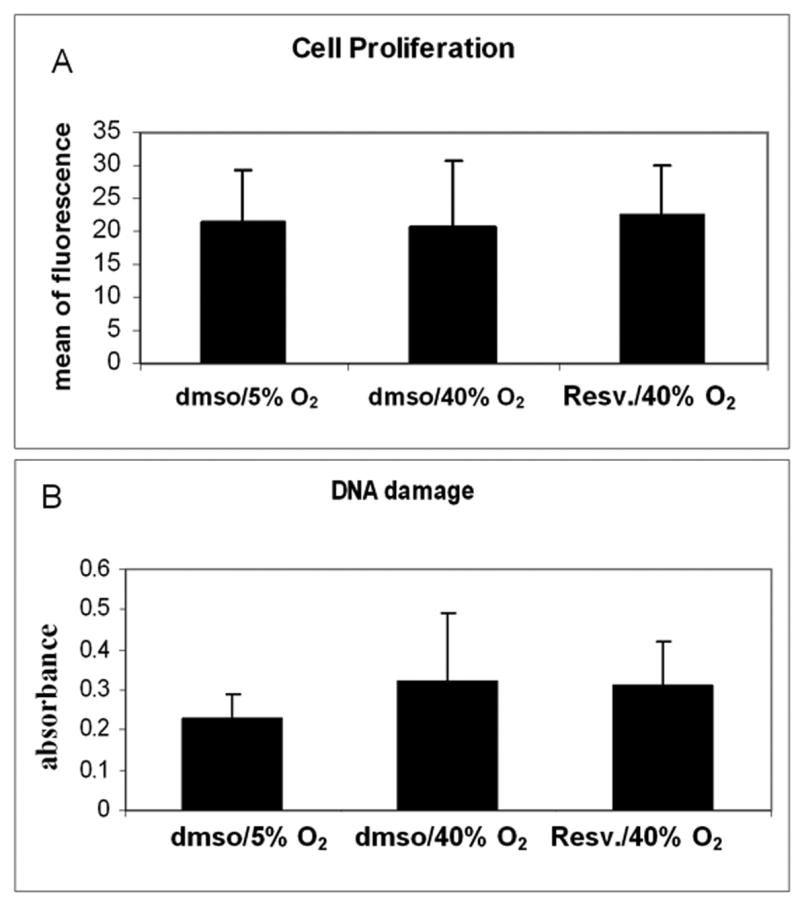
Effects of resveratrol treatment on cell proliferation and DNA damage. Cells incubated at 40% oxygen and treated with resveratrol did not show any significant difference in proliferation quantified by BrdU incorporation (A) or DNA damage analyzed by measuring the formation of aldehyde groups (B) (Panel A, N=5; panel B, N=3).
4. Discussion
Our results demonstrated that chronic treatment of cultured TM cells with resveratrol effectively prevented the upregulation of markers induced by oxidative stress.
Resveratrol has been shown to reduce inflammation in vivo via inhibition of cyclooxygenase-2 and NF-κB activities (Holmes-McNary and Baldwin, 2000; Martin, et al., 2006; Tsai, et al., 1999). However, we have recently reported that the induction of the inflammatory markers IL1α, IL-6, IL-8, and ELAM-1 in TM cells subjected to chronic oxidative stress is dependent on the activation of iROS generated by the mitochondria. This increase in iROS is not dependent on cyclooxygenase and is responsible for the chronic activation of NF-κB. One of the more clear effects of chronic treatment with resveratrol in TM cells was the inhibition of iROS production that results from oxidative stress. Therefore the inhibitory effect of resveratrol on the production of iROS may be responsible for the concomitant inhibition of the sustained stress response. Although resveratrol has been shown to induce the production of iROS in some cancer cell lines (Heiss, et al., 2007; Shankar, et al., 2007), our results are consistent with those reported in other primary cells including HUVEC cells (Chow, et al., 2007; Ungvari, et al., 2007), primary cortical astrocytes (de Almeida, et al., 2007; de Almeida, et al., 2007), RAW264.7 macrophages (Ciz, et al., 2007; Jang, et al., 1999), RPE cells (King, et al., 2005), and dopaminergic neurons (Okawara, et al., 2007).
Chronic treatment of TM cells with resveratrol also resulted in a significant decrease in the accumulation of fluorescent pigments, carbonylated proteins, and the expression of the cellular senescence marker sa-β-gal, that are induced by oxidative stress. A decrease in lipofuscin and sa-β-gal upon resveratrol treatment has also been observed in the short-lived fish N. furzeri. In this small vertebrate resveratrol treatment not only reduces the expression of senescence markers but increases life span and decreases aggregated protein in elderly fish brains (Valenzano and Cellerino, 2006; Valenzano, et al., 2006). In our model, treatment with resveratrol did not prevent the loss of proteasome activity induced by oxidative stress. Therefore, the observed decrease in accumulation of oxidized proteins and fluorescent pigments is not likely to result from increased proteasomal degradation of damaged proteins, but rather from other factors such as resveratrol ROS scavenging properties (Ciz, et al., 2007; Lorenz, et al., 2003); an increase in antioxidant enzymes (Baur and Sinclair, 2006; Karaoglan, et al., 2008; Robb, et al., 2007; Ungvari, et al., 2007); activation of autophagy pathways (Opipari, et al., 2004; Trincheri, et al., 2007); and/or the observed inhibition of iROS production (Chow, et al., 2007; de Almeida, et al., 2007; Jha, et al., 2008; King, et al., 2005).
In addition to the protective effects in our chronic oxidative stress model, resveratrol also demonstrated a significant anti-apoptotic effect after acute oxidative injury. Resveratrol has received a great deal of attention because of its pro-apoptotic effects in cancer cells (Kaindl, et al., 2007; Kalra, et al., 2007; Park, et al., 2007; van Ginkel, et al., 2007). However, its anti-apoptotic effects in several primary cell lines have been well documented (Csaki, et al., 2008; Jha, et al., 2008; Kumar, et al., 2007). Interestingly, our results showed no negative effects of chronic resveratrol treatment on the proliferation of TM cells. This is somewhat surprising, since resveratrol has been reported to exert antiproliferative effects, mainly in cancer cells, but also in other non-cancerous cell types (Lee and Moon, 2005; Poussier, et al., 2005). RPE cells treated with resveratrol at pre-confluence levels (80%) showed a significant decrease in proliferation that was not observed when they were confluent (King, et al., 2005). In our experimental model, cells were treated at confluence levels and this difference might explain our observed effect of resveratrol on proliferation. Since the decrease in cellularity of the TM associated with aging and POAG has been hypothesized to result, at least in part, from oxidative damage, our results suggest that chronic treatment with resveratrol could be effective in delaying the loss of cells in the TM by acting to prevent apoptosis without inhibiting cell proliferation. One important point that deserves future investigation is whether resveratrol can reverse, at least in part, some of the effects of chronic oxidative stress. Preliminary data suggest that short-term treatment with resveratrol may not be helpful to reverse the damage that has already been produced (data not showed). However, a definitive answer to this question will require longer term experiments aimed at evaluating the effects of resveratrol treatment on the progression of oxidative damage for an extended period of time after chronic oxidative injury.
In conclusion, the inhibition of iROS production by resveratrol may help to prevent the induction of inflammatory and senescence markers in TM cells after chronic oxidative stress, as well as possibly prevent the decrease in cellularity observed in the TM of POAG patients. These results suggest that resveratrol could potentially have a novel role in preventing or delaying some of the abnormalities of the TM observed in POAG.
Acknowledgments
This work was supported by NEI EY01894, NEI EY016228, and NEI EY 014209NEI EY05722, and Research to Prevent Blindness.
The authors thank Thusita Dissanayake (Flow Cytometry Facility, Duke University) for his help in the flow cytometry analyses.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- Alvarado J, Murphy C, Polansky J, Juster R. Age-related changes in trabecular meshwork cellularity. Invest Ophthalmol Vis Sci. 1981;21:714–727. [PubMed] [Google Scholar]
- Alvarado J, Murphy C, Juster R. Trabecular meshwork cellularity in primary open-angle glaucoma and nonglaucomatous normals. Ophthalmology. 1984;91:564–579. doi: 10.1016/s0161-6420(84)34248-8. [DOI] [PubMed] [Google Scholar]
- Armaly MF, Krueger DE, Maunder L, Becker B, Hetherington J, Jr, Kolker AE, Levene RZ, Maumenee AE, Pollack IP, Shaffer RN. Biostatistical analysis of the collaborative glaucoma study. I. Summary report of the risk factors for glaucomatous visual-field defects. Arch Ophthalmol. 1980;98:2163–2171. doi: 10.1001/archopht.1980.01020041015002. [DOI] [PubMed] [Google Scholar]
- Bauer JH, Goupil S, Garber GB, Helfand SL. An accelerated assay for the identification of lifespan-extending interventions in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2004;101:12980–12985. doi: 10.1073/pnas.0403493101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- Caballero M, Liton PB, Epstein DL, Gonzalez P. Proteasome inhibition by chronic oxidative stress in human trabecular meshwork cells. Biochem Biophys Res Commun. 2003;308:346–352. doi: 10.1016/s0006-291x(03)01385-8. [DOI] [PubMed] [Google Scholar]
- Cheung W, Guo L, Cordeiro MF. Neuroprotection in glaucoma: drug-based approaches. Optom Vis Sci. 2008;85:406–416. doi: 10.1097/OPX.0b013e31817841e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow SE, Hshu YC, Wang JS, Chen JK. Resveratrol attenuates oxLDL-stimulated NADPH oxidase activity and protects endothelial cells from oxidative functional damages. J Appl Physiol. 2007;102:1520–1527. doi: 10.1152/japplphysiol.00881.2006. [DOI] [PubMed] [Google Scholar]
- Ciz M, Pavelkova M, Gallova L, Kralova J, Kubala L, Lojek A. The influence of wine polyphenols on reactive oxygen and nitrogen species production by rat macrophages RAW 264.7. Physiol Res. 2007 doi: 10.33549/physiolres.931088. PMID: 17465695. [DOI] [PubMed] [Google Scholar]
- Csaki C, Keshishzadeh N, Fischer K, Shakibaei M. Regulation of inflammation signalling by resveratrol in human chondrocytes in vitro. Biochem Pharmacol. 2008;75:677–687. doi: 10.1016/j.bcp.2007.09.014. [DOI] [PubMed] [Google Scholar]
- Cucciolla V, Borriello A, Oliva A, Galletti P, Zappia V, Ragione FD. Resveratrol: From Basic Science to the Clinic. Cell Cycle. 2007;6:2495–2510. doi: 10.4161/cc.6.20.4815. [DOI] [PubMed] [Google Scholar]
- de Almeida LM, Pineiro CC, Leite MC, Brolese G, Leal RB, Gottfried C, Goncalves CA. Protective Effects of Resveratrol on Hydrogen Peroxide Induced Toxicity in Primary Cortical Astrocyte Cultures. Neurochem Res. 2007;33:8–15. doi: 10.1007/s11064-007-9399-5. [DOI] [PubMed] [Google Scholar]
- de Almeida LM, Pineiro CC, Leite MC, Brolese G, Tramontina F, Feoli AM, Gottfried C, Goncalves CA. Resveratrol increases glutamate uptake, glutathione content, and S100B secretion in cortical astrocyte cultures. Cell Mol Neurobiol. 2007;27:661–668. doi: 10.1007/s10571-007-9152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La Paz MA, Epstein DL. Effect of age on superoxide dismutase activity of human trabecular meshwork. Invest Ophthalmol Vis Sci. 1996;37:1849–1853. [PubMed] [Google Scholar]
- Fiering SN, Roederer M, Nolan GP, Micklem DR, Parks DR, Herzenberg LA. Improved FACS-Gal: flow cytometric analysis and sorting of viable eukaryotic cells expressing reporter gene constructs. Cytometry. 1991;12:291–301. doi: 10.1002/cyto.990120402. [DOI] [PubMed] [Google Scholar]
- Heiss EH, Schilder YD, Dirsch VM. Chronic Treatment with Resveratrol Induces Redox Stress- and Ataxia Telangiectasia-mutated (ATM)-dependent Senescence in p53-positive Cancer Cells. J Biol Chem. 2007;282:26759–26766. doi: 10.1074/jbc.M703229200. [DOI] [PubMed] [Google Scholar]
- Helbig H, Hinz JP, Kellner U, Foerster MH. Oxygen in the anterior chamber of the human eye. Ger J Ophthalmol. 1993;2:161–164. [PubMed] [Google Scholar]
- Holme AL, Pervaiz S. Resveratrol in cell fate decisions. J Bioenerg Biomembr. 2007;39:59–63. doi: 10.1007/s10863-006-9053-y. [DOI] [PubMed] [Google Scholar]
- Holmes-McNary M, Baldwin AS., Jr Chemopreventive properties of trans-resveratrol are associated with inhibition of activation of the IkappaB kinase. Cancer Res. 2000;60:3477–3483. [PubMed] [Google Scholar]
- Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- Jang DS, Kang BS, Ryu SY, Chang IM, Min KR, Kim Y. Inhibitory effects of resveratrol analogs on unopsonized zymosan-induced oxygen radical production. Biochem Pharmacol. 1999;57:705–712. doi: 10.1016/s0006-2952(98)00350-5. [DOI] [PubMed] [Google Scholar]
- Jha RK, Yong MQ, Chen SH. The protective effect of resveratrol on the intestinal mucosal barrier in rats with severe acute pancreatitis. Med Sci Monit. 2008;14:BR14–19. [PubMed] [Google Scholar]
- Kahn MG, Giblin FJ, Epstein DL. Glutathione in calf trabecular meshwork and its relation to aqueous humor outflow facility. Invest Ophthalmol Vis Sci. 1983;24:1283–1287. [PubMed] [Google Scholar]
- Kaindl U, Eyberg I, Rohr-Udilova N, Heinzle C, Marian B. The dietary antioxidants resveratrol and quercetin protect cells from exogenous pro-oxidative damage. Food Chem Toxicol. 2007 doi: 10.1016/j.fct.2007.09.002. PMID: 17936464. [DOI] [PubMed] [Google Scholar]
- Kalra N, Roy P, Prasad S, Shukla Y. Resveratrol induces apoptosis involving mitochondrial pathways in mouse skin tumorigenesis. Life Sci. 2007;82:348–358. doi: 10.1016/j.lfs.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Karaoglan A, Akdemir O, Barut S, Kokturk S, Uzun H, Tasyurekli M, Colak A. The effects of resveratrol on vasospasm after experimental subarachnoidal hemorrhage in rats. Surg Neurol. 2008 doi: 10.1016/j.surneu.2007.07.031. PMID: 18207513. [DOI] [PubMed] [Google Scholar]
- King RE, Kent KD, Bomser JA. Resveratrol reduces oxidation and proliferation of human retinal pigment epithelial cells via extracellular signal-regulated kinase inhibition. Chem Biol Interact. 2005;151:143–149. doi: 10.1016/j.cbi.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Kumar A, Kaundal RK, Iyer S, Sharma SS. Effects of resveratrol on nerve functions, oxidative stress and DNA fragmentation in experimental diabetic neuropathy. Life Sci. 2007;80:1236–1244. doi: 10.1016/j.lfs.2006.12.036. [DOI] [PubMed] [Google Scholar]
- Lee B, Moon SK. Resveratrol inhibits TNF-alpha-induced proliferation and matrix metalloproteinase expression in human vascular smooth muscle cells. J Nutr. 2005;135:2767–2773. doi: 10.1093/jn/135.12.2767. [DOI] [PubMed] [Google Scholar]
- Leske MC, Heijl A, Hussein M, Bengtsson B, Hyman L, Komaroff E. Factors for glaucoma progression and the effect of treatment: the early manifest glaucoma trial. Arch Ophthalmol. 2003;121:48–56. doi: 10.1001/archopht.121.1.48. [DOI] [PubMed] [Google Scholar]
- Li G, Luna C, Liton P, Navarro I, Epstein DL, Gonzalez P. Sustained stress response after oxidative stress in trabecular meshwork cells. Mol Vis. 2007;13:2282–2288. [PMC free article] [PubMed] [Google Scholar]
- Liton PB, Challa P, Stinnett S, Luna C, Epstein DL, Gonzalez P. Cellular senescence in the glaucomatous outflow pathway. Exp Gerontol. 2005;40:745–748. doi: 10.1016/j.exger.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liton PB, Luna C, Challa P, Epstein DL, Gonzalez P. Genome-wide expression profile of human trabecular meshwork cultured cells, nonglaucomatous and primary open angle glaucoma tissue. Mol Vis. 2006;12:774–790. [PMC free article] [PubMed] [Google Scholar]
- Lorenz P, Roychowdhury S, Engelmann M, Wolf G, Horn TF. Oxyresveratrol and resveratrol are potent antioxidants and free radical scavengers: effect on nitrosative and oxidative stress derived from microglial cells. Nitric Oxide. 2003;9:64–76. doi: 10.1016/j.niox.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Maepea O, Bill A. Pressures in the juxtacanalicular tissue and Schlemm's canal in monkeys. Exp Eye Res. 1992;54:879–883. doi: 10.1016/0014-4835(92)90151-h. [DOI] [PubMed] [Google Scholar]
- Martin AR, Villegas I, Sanchez-Hidalgo M, de la Lastra CA. The effects of resveratrol, a phytoalexin derived from red wines, on chronic inflammation induced in an experimentally induced colitis model. Br J Pharmacol. 2006;147:873–885. doi: 10.1038/sj.bjp.0706469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses RA. The effect of intraocular pressure on resistance to outflow. Surv Ophthalmol. 1977;22:88–100. doi: 10.1016/0039-6257(77)90088-1. [DOI] [PubMed] [Google Scholar]
- Nguyen KP, Chung ML, Anderson PJ, Johnson M, Epstein DL. Hydrogen peroxide removal by the calf aqueous outflow pathway. Invest Ophthalmol Vis Sci. 1988;29:976–981. [PubMed] [Google Scholar]
- Okawara M, Katsuki H, Kurimoto E, Shibata H, Kume T, Akaike A. Resveratrol protects dopaminergic neurons in midbrain slice culture from multiple insults. Biochem Pharmacol. 2007;73:550–560. doi: 10.1016/j.bcp.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Opipari AW, Jr, Tan L, Boitano AE, Sorenson DR, Aurora A, Liu JR. Resveratrol-induced autophagocytosis in ovarian cancer cells. Cancer Res. 2004;64:696–703. doi: 10.1158/0008-5472.can-03-2404. [DOI] [PubMed] [Google Scholar]
- Park JW, Woo KJ, Lee JT, Lim JH, Lee TJ, Kim SH, Choi YH, Kwon TK. Resveratrol induces pro-apoptotic endoplasmic reticulum stress in human colon cancer cells. Oncol Rep. 2007;18:1269–1273. [PubMed] [Google Scholar]
- Poussier B, Cordova AC, Becquemin JP, Sumpio BE. Resveratrol inhibits vascular smooth muscle cell proliferation and induces apoptosis. J Vasc Surg. 2005;42:1190–1197. doi: 10.1016/j.jvs.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Robb EL, Page MM, Wiens BE, Stuart JA. Molecular mechanisms of oxidative stress resistance induced by resveratrol: Specific and progressive induction of MnSOD. Biochem Biophys Res Commun. 2007;367:406–412. doi: 10.1016/j.bbrc.2007.12.138. [DOI] [PubMed] [Google Scholar]
- Rozen S, S H. Primer3 on the WWW for general users and for biologist programmers. In: Krawetz S, M S, editors. Bioinformatics Methods and Protocols: Methods in Molecular Biology. Totowa, NJ: Humana Press; 2000. pp. 365–386. [DOI] [PubMed] [Google Scholar]
- Sacca SC, Pascotto A, Camicione P, Capris P, Izzotti A. Oxidative DNA damage in the human trabecular meshwork: clinical correlation in patients with primary open-angle glaucoma. Arch Ophthalmol. 2005;123:458–463. doi: 10.1001/archopht.123.4.458. [DOI] [PubMed] [Google Scholar]
- Shankar S, Siddiqui I, Srivastava RK. Molecular mechanisms of resveratrol (3,4,5-trihydroxy-trans-stilbene) and its interaction with TNF-related apoptosis inducing ligand (TRAIL) in androgen-insensitive prostate cancer cells. Mol Cell Biochem. 2007;304:273–285. doi: 10.1007/s11010-007-9510-x. [DOI] [PubMed] [Google Scholar]
- Stamer WD, Seftor RE, Snyder RW, Regan JW. Cultured human trabecular meshwork cells express aquaporin-1 water channels. Curr Eye Res. 1995;14:1095–1100. doi: 10.3109/02713689508995815. [DOI] [PubMed] [Google Scholar]
- Trincheri NF, Follo C, Nicotra G, Peracchio C, Castino R, Isidoro C. Resveratrol-induced Apoptosis Depends on the Lipid Kinase Activity of Vps34 and on the Formation of Autophagolysosomes. Carcinogenesis. 2007;29:381–389. doi: 10.1093/carcin/bgm271. [DOI] [PubMed] [Google Scholar]
- Tsai SH, Lin-Shiau SY, Lin JK. Suppression of nitric oxide synthase and the down-regulation of the activation of NFkappaB in macrophages by resveratrol. Br J Pharmacol. 1999;126:673–680. doi: 10.1038/sj.bjp.0702357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Orosz Z, Rivera A, Labinskyy N, Xiangmin Z, Olson S, Podlutsky A, Csiszar A. Resveratrol increases vascular oxidative stress resistance. Am J Physiol Heart Circ Physiol. 2007;292:H2417–2424. doi: 10.1152/ajpheart.01258.2006. [DOI] [PubMed] [Google Scholar]
- Valenzano DR, Cellerino A. Resveratrol and the pharmacology of aging: a new vertebrate model to validate an old molecule. Cell Cycle. 2006;5:1027–1032. doi: 10.4161/cc.5.10.2739. [DOI] [PubMed] [Google Scholar]
- Valenzano DR, Terzibasi E, Genade T, Cattaneo A, Domenici L, Cellerino A. Resveratrol prolongs lifespan and retards the onset of age-related markers in a short-lived vertebrate. Curr Biol. 2006;16:296–300. doi: 10.1016/j.cub.2005.12.038. [DOI] [PubMed] [Google Scholar]
- van Ginkel PR, Sareen D, Subramanian L, Walker Q, Darjatmoko SR, Lindstrom MJ, Kulkarni A, Albert DM, Polans AS. Resveratrol inhibits tumor growth of human neuroblastoma and mediates apoptosis by directly targeting mitochondria. Clin Cancer Res. 2007;13:5162–5169. doi: 10.1158/1078-0432.CCR-07-0347. [DOI] [PubMed] [Google Scholar]
- Wang CY, Shen YC, Lo FY, Su CH, Lee SH, Lin KH, Tsai HY, Kuo NW, Fan SS. Polymorphism in the IL-1alpha (-889) locus associated with elevated risk of primary open angle glaucoma. Mol Vis. 2006;12:1380–1385. [PubMed] [Google Scholar]
- Wang N, Chintala SK, Fini ME, Schuman JS. Activation of a tissue-specific stress response in the aqueous outflow pathway of the eye defines the glaucoma disease phenotype. Nat Med. 2001;7:304–309. doi: 10.1038/85446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber A, Harman CD, Viswanathan S. Effects of optic nerve injury, glaucoma, and neuroprotection on the survival, structure, and function of ganglion cells in the mammalian retina. J Physiol. 2008 doi: 10.1113/jphysiol.2008.156729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Schroeder A, Callahan EM, Coyle BM, Wang N, Erickson KA, Schuman JS, Fini ME. Constitutive signalling pathway activity in trabecular meshwork cells from glaucomatous eyes. Exp Eye Res. 2006;82:968–973. doi: 10.1016/j.exer.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Liu YQ, Zhao JL, Zhang H. Effects of oxidative stress on the expression of endothelial-leukocyte adhesion molecule-1 in porcine trabecular meshwork cells. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2007;29:394–397. [PubMed] [Google Scholar]


