Abstract
N-methyl-D-aspartic acid/glutamate receptor antagonists induce psychotomimetic effects in humans and animals, and much research has focused on the neurochemical and network-level effects that mediate those behavioral changes. For example, a reduction in NMDA-dependent glutamatergic transmission triggers increased release of the monoamine transmitters, and some of these changes are implicated in the cognitive, behavioral and neuroanatomical effects of phencyclidine (PCP). Alpha-2 adrenoceptor agonists (e.g., clonidine) are effective at preventing many of the behavioral, neurochemical and anatomical effects of NMDA antagonists. Evidence has indicated that a key mechanism of the clonidine-induced reversal of the effects of NMDA antagonists is an attenuation of enhanced dopamine release. We have pursued these findings by investigating the effects of alpha-2 agonists on PCP-evoked dopamine efflux in the prefrontal cortex of freely moving rats. Clonidine (0.003–0.1 mg/kg, i.p.) dose-dependently attenuated the ability of PCP (2.5 mg/kg, i.p.) to increase cortical dopamine output. The effects of clonidine were prevented by the alpha-2A subtype selective antagonist BRL-44408 (1 mg/kg, i.p.). Guanfacine, which is an alpha-2 agonist with a higher affinity for the 2A, compared with 2B or 2C, subtypes, also blocked the ability of PCP to increase dopamine efflux in the prefrontal cortex. These data indicate that alpha-2A agonists are effective at counteracting the hyperdopaminergic state induced by PCP and may play a role in their neurobehavioral effects in this putative animal model for schizophrenia.
Keywords: Phencyclidine, Dopamine, Norepinephrine, Adrenoceptor, Prefrontal cortex, Microdialysis
1. Introduction
Due to the robust psychotomimetic and psychotomimetic-like responses of humans and animals, respectively, to N-methyl-D-aspartic acid (NMDA)/glutamate receptor antagonists, such as phencyclidine (PCP) and ketamine, many studies have recently focused upon alterations in inter- and intra-cellular signaling after administration of these drugs to animals and people (Gerber and Tonegawa, 2004; Geyer et al., 2001; Javitt and Zukin, 1991; Jentsch and Roth, 1999; Krystal et al., 2002; Tsai and Coyle, 2002). PCP or ketamine, given as a single, acute dose, provokes the release of various neurotransmitters and neuromodulators, including the monoamines, acetylcholine, glutamate, cyclic adenosine monophosphate and nitric oxide (Hertel et al., 1995; Jentsch et al., 1997, 1998a; Kim et al., 1999; Klamer et al., 2005; Moghaddam and Adams, 1998). At the current time, a formal hypothesis linking individual neurochemical changes to the psychotomimetic effects of the NMDA receptor antagonists is unavailable.
These neurochemical changes, which are secondary to blockade of the NMDA receptor, appear, in many causes, to be necessary substrates for various behavioral effects of the drug. For example, serotonin 5-HT2A receptor antagonists, agonists of the group II metabotropic glutamate receptor and agonists for alpha-2 receptors are capable of preventing many of the cognitive, behavioral and neurochemical effects of PCP in animals (Cartmell et al., 2000; Jentsch et al., 1998c; Jentsch and Anzivino, 2004; Lorrain et al., 2003; Marrs et al., 2005; Swanson and Schoepp, 2003). Interestingly, alpha-2 agonists do not alleviate the PCP-induced block of the NMDA receptor (Coan and Collingridge, 1987), and in the frontal cortex clonidine does not alter extracellular glutamate levels (Robert et al., 1996), suggesting that alpha-2 agonists may affect certain forms of cognition “downstream” from the NMDA receptor.
An increase in brain dopamine efflux produced by NMDA antagonists is a component part of a complex set of neurochemical alterations that mediates the working memory deficits produced by the drugs, as demonstrated by efficacy of dopaminergic antagonists against these effects (Verma and Moghaddam, 1996). This hypothesis is further supported by our observation that alpha-2 agonists block the increase in dopamine turnover, measured post mortem, as well as prevent the cognitive deficits elicited by PCP (Jentsch et al., 1998c; Jentsch and Anzivino, 2004; Marrs et al., 2005). We therefore undertook the following studies to investigate whether alpha-2 agonists (clonidine and guanfacine) could prevent dopamine efflux, measured in vivo, produced by PCP.
The alpha-2 agonists clonidine and guanfacine do not discriminate between the three subtypes of alpha-2 receptors, although guanfacine has somewhat higher affinity for the 2A versus 2B and 2C subtypes (Arnsten and Leslie, 1991). The alpha-2A subtype is expressed highly throughout the cerebral cortex, where it is a post-synaptic target for norepinephrine (Ji et al., 2008), and within the locus coeruleus, where it acts as an autoreceptor (Callado and Stamford, 1999). The alpha-2B subtype is expressed most densely in the thalamus (Tavares et al., 1996), while the alpha-2C subtype is localized to the neocortex and basal ganglia (Lee et al., 1998; Uhlen et al., 1997). Electrophysiological data indicate that norepinephrine exerts a functionally excitatory influence on dopaminergic neurons through an alpha-1 receptor; (Shi et al., 2000), and therefore, clonidine and guanfacine may attenuate PCP-induced dopamine efflux by activating the alpha-2A type autoreceptors and suppressing noradrenergic effects. Therefore, we further hypothesized that the alpha-2A receptor subtype mediated the effects of clonidine against PCP-induced dopamine outflow. For these reasons, we examined whether an alpha 2A selective antagonist, BRL-44408 (Young et al., 1989), could block the effects of clonidine in this neurochemical assay.
2. Results
2.1. Clonidine+PCP effects on dopamine efflux
Consistent with previous studies (Hertel et al., 1995; Moghaddam and Adams, 1998), PCP (2.5 mg/kg, i.p.) produced ~250% increase in cortical dopamine output in the freely moving rat (Fig. 1). In this experiment, basal dopamine levels were 0.23± 0.03 fmol/μl and were increased to a maximal value of 0.87± 0.16 fmol/μl at 40 min post-PCP administration (Fig. 1). This change was statistically significant, as reflected by a main effect of PCP treatment (F(1,8) = 17.8, p <0.005). A significant PCP treatment×time interaction (F(5,40) =13.8, p <0.0001) supported pairwise comparisons demonstrating that, although there were no group differences in baseline dopamine levels (p>0.05), extracellular levels of the monoamine were higher in PCP-treated rats from 20–100 min post dosing (p<0.05 for each comparison).
Fig. 1.
Top panel: clonidine (0.003–0.05 mg/kg, i.p.) potently suppresses dopamine efflux in the prefrontal cortex induced by PCP (2.5 mg/kg, i.p.). Bottom panel: clonidine, only at the highest tested dose of 0.05 mg/kg, suppresses prefrontal cortical dopamine efflux on its own. Abbreviation: B, baseline.
The dopaminergic response to PCP was further assessed in four distinct groups: rats pre-treated with either saline or clonidine (0.003, 0.01 or 0.05 mg/kg; Fig. 1). A main effect of clonidine dose (F(3,18) = 12.4, p <0.0001) was detected by repeated measures ANOVA. Post hoc analyses confirmed that all three doses of clonidine significantly reduced PCP-induced dopamine release, as compared with the saline pre-exposed group; none of the three doses significantly differed from one another.
A control experiment involved comparing dopamine release after clonidine (0, 0.01 or 0.05 mg/kg) alone (Fig. 1). Repeated measures ANOVA revealed a nominally significant main effect of clonidine treatment (F(2,12) =4.3, p =0.04) that did not interact with time (p>0.05). Tukey post hoc tests indicated that this main effect was driven by a significant (p<0.05) difference of dopamine levels after the higher (0.05 mg/kg), but not lower (0.01 mg/kg), dose of clonidine.
2.2. Guanfacine+PCP effects on dopamine efflux
The alpha-2 agonist, guanfacine, with somewhat higher affinity for the 2A versus 2B or 2C subtypes, produced a set of effects qualitatively similar to those of clonidine (Fig. 2). A main effect of guanfacine treatment (F(2,14) =11.6, p=0.001) demonstrated a significant (p<0.05) difference between saline pre-treatment and administration of both the lower (0.05 mg/kg) and higher (0.5 mg/kg) doses of guanfacine. The two doses did not differ from one another, however. In addition, a control experiment (not shown) revealed that the highest dose of guanfacine (0.5 mg/kg) did not significantly affect dopamine efflux, as compared with saline treatment (F(1,8) =3.6, p =0.09).
Fig. 2.
Guanfacine (0.05–0.5 mg/kg, i.p.) inhibits PCP-evoked dopamine efflux in the prefrontal cortex.
2.3. Effects of the alpha-2A selective antagonist
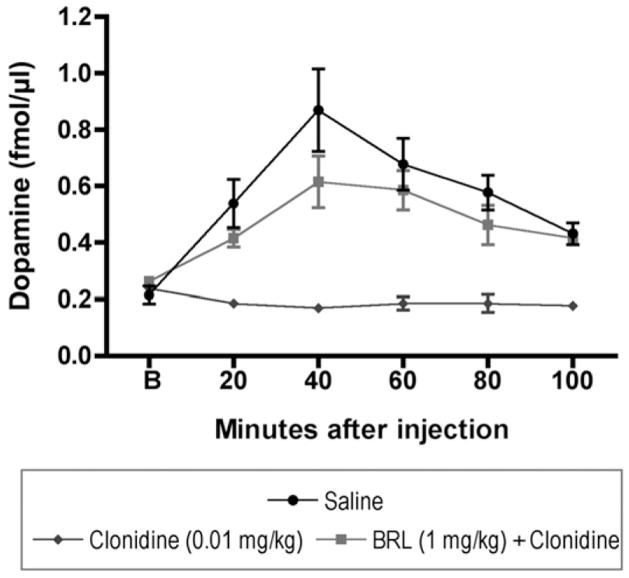
Pre-treatment with the alpha-2A receptor selective antagonist, BRL-44408, was utilized in order to determine whether clonidine-induced suppression of dopamine overflow evoked by PCP dependent critically upon the 2A receptor subtype (Fig. 3). A main effect of treatment was found (F(2,13) =16.6, p<0.001); post hoc Tukey’s tests revealed that the clonidine+PCP group differed significantly from saline+PCP (p<0.05) and that BRL + clonidine + PCP significantly differed from clonidine+PCP (p<0.05) but not saline+PCP (p>0.05). This dose of BRL (1 mg/kg) failed to significantly affect dopamine efflux when administered alone (data not shown, F(1,8) =0.08, p =0.86).
Fig. 3.
Pretreatment with the alpha-2A receptor antagonist (BRL-44408) prevents clonidine from suppressing PCP-induced dopamine overflow in the prefrontal cortex.
3. Discussion
The current results indicate that activation of alpha-2 receptors produces an inhibition of NMDA antagonist-induced dopamine release in freely moving rats. These data dovetail with other studies demonstrating that alpha-2 adrenoceptor agonists are effective at preventing the cognitive disruptions produced by PCP and related drugs (Jentsch and Anzivino, 2004; Marrs et al., 2005). The collective results underscore the importance of further elucidating the neural and pharmacological mechanisms of action of alpha-2 agonists in these behavioral and neurochemical assays.
In a recent study, it has been demonstrated that a partial lesion of the ascending noradrenergic system reduced PCP-induced deficits of working memory (Marrs et al., 2005), suggesting that hyperactivity of the locus coeruleus system may be a critical factor in eliciting the cognitive deficits produced by PCP. This hypothesis is supported by the effectiveness of clonidine and guanfacine to suppress locus coeruleus neuron activity (Kawahara et al., 1999; Redmond, 1981; Van Gaalen et al., 1997), an effect dependent upon the alpha-2A receptor subtype (Pudovkina et al., 2001). Collectively, this model also explains the current results. Dopaminergic hyperactivity within the cortex may be secondary to an effect of PCP on the locus coeruleus, either through a direct stimulation of the ventral tegmental area by ascending noradrenergic fibers (Grenhoff and Svensson, 1989; Grenhoff et al., 1993; Mathe et al., 1996) or through co-release of dopamine from noradrenergic terminals within the prefrontal cortex (Devoto et al., 2001).
Suppression of noradrenergic activity has proven effective in other pre-clinical models sensitive to NMDA receptor antagonists. For example, PCP-induced deficits of pre-pulse inhibition of the startle reflex are blocked by the alpha-1 adrenoceptor antagonist prazosin and mimicked by the alpha-1 agonist, cirazoline (Bakshi and Geyer, 1997; Carasso et al., 1998). Additionally, NMDA antagonist evoked hyperlocomotion depends, in part, upon noradrenergic hyperactivity (Harkin et al., 2001; Swanson and Schoepp, 2003). Therefore, several aspects of PCP-evoked behavior depend upon noradrenergic systems.
These actions of alpha 2 agonists on PCP-induced effects are not necessarily limited to catecholamine systems. Kim et al. demonstrated earlier that clonidine could also suppress the cholinergic hyperactivity elicited by another high potency NMDA antagonist, MK-801 (Kim et al., 1999). The broad effectiveness of clonidine against the neurochemical and behavioral effects of PCP tends to suggest that alpha-2 agonists are capable of acting against a central set of cellular effects of NMDA antagonists; notably, locally administered clonidine is not able to prevent the functional antagonism of the NMDA receptor produced by PCP (Coan and Collingridge, 1987), ruling out a simple drug-drug interaction in these effects. The locus nature of the interaction between alpha-2 and NMDA receptors in the present study is not known.
There are anatomical and electrophysiological data to support the hypothesis that the effect of NMDA receptor antagonism on dopamine release in the prefrontal cortex depends upon a relative decrease in GABA released from interneurons (Yonezawa et al., 1998). Administration of an NMDA receptor antagonist results in elevated glutamate release, with a consequential increased glutamate interaction at non-NMDA receptors (Moghaddam et al., 1997). At the functional level, clonidine can prevent the deficits of working memory triggered by an inverse agonist acting upon the GABA-A receptor (Birnbaum et al., 2000; Murphy et al., 1996), indicating that alpha-2 adrenoceptor agonists can balance the effects of GABA insufficiency. If a PCP-induced reduction in the activity of GABAergic neurons, presumably in the pre-frontal cortex (see Jentsch et al., 1998b), were counteracted by clonidine, this may represent a proximal interaction that attenuates downstream neurochemical events, including an increase in cortical glutamatergic tone (Moghaddam et al., 1997) that results in greater corticofugal outflow into the brainstem catecholaminergic cell groups. Supporting this model is the ability of benzodiazepine agonists to suppress catecholamine hyperactivity elicited by PCP (Bowers and Morton, 1992; Kubota et al., 1999).
3.1. Summary
Taken together, these current data add to the body of evidence that alpha-2 agonists are effective against the behavioral and neurochemical effects of NMDA receptor antagonists. Coupled to clinical findings of clonidine- and guanfacine-induced improvements in cognitive performance in patients with schizophrenia, these pre-clinical data are beginning to reveal the pharmacological routes to a novel method for cognitive enhancement in the disease. Further studies of the receptor subtypes contributing to the effects of clonidine and guanfacine in these systems are clearly the next logical step.
4. Experimental procedures
4.1. Animals
Male Sprague-Dawley rats (Charles River, Wilmington, MA) weighing 300–350 g were used in this study. Animals were maintained on a 12:12 light/dark schedule. All experiments were performed during the light cycle. Water and food was provided ad libitum. All animal use procedures were performed in an AAALAC accredited facility in accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Yale University Animal Care and Use Committee.
4.2. Materials
All reagents for the HPLC mobile phase and the perfusion fluid were of an analytical grade and were obtained from Sigma (St. Louis, MO). PCP hydrochloride was purchased from Sigma. Clonidine hydrochloride and guanfacine hydrochloride were obtained from RBI (Natick, MA), and BRL-44408 maleate was purchased from Tocris-Cookson (Ellisville, MO). All drugs were dissolved in 1 ml/kg of isotonic sterile saline.
4.3. Surgical preparation
Animals were given 1 week to acclimate after arrival in the vivarium before undergoing probe implantation. All rats were anesthetized with halothane (1–5%, as needed) and placed in a Stoelting stereotaxic instrument fitted with blunt ear bars. A thermostatically controlled electric heating pad was used to maintain the body temperature at 37 °C.
An incision (~10 mm) was made in the skin over the skull and the wound margin was infiltrated with 2% lidocaine. Burr holes were drilled for two skull screws and two concentric microdialysis probes. The probes were constructed using Hospal AN69 polyacrylonitrile dialysis tubing (Renal Care, Lakewood, CO). The outer diameter of the probes was 330 μm, and the exposed membrane tip was 3.0 mm.
The probes were positioned bilaterally in the right and left medial prefrontal cortex, respectively: AP, +3.2; L, 1.2 angled 10° towards midline; V, −5.6 and AP, +3.2; L=0.7 no angle; V, −5.0 with respect to bregma (in mm) according to the atlas of Paxinos and Watson (1982). Loctite Tak Pak 444 (Rocky Hill, CT) was used to initially secure probes and screws to the skull before removing the stereotaxic probe holders. Dental cement was then used to build up a protective cap around the microdialysis probes.
Immediately after surgery the animals were connected to a Harvard syringe pump (Harvard Apparatus, Holliston, MA) via fused-silica tubing (Polymicro Inc., Phoenix, AZ) encased in PE50 polyethylene intramedic tubing and a swivel connector that was attached to a liquid swivel/balance arm assembly (Instech Laboratory, Plymouth Meeting, PA). During the recovery period, probes were perfused at a rate of 0.5 μL/min with a Ringer’s solution containing 145 mM NaCl, 2.7 mM KCl, 1.0 mM MgCl2, and 1.2 mM CaCl2. The animals were allowed to recover in a modified home cage 24 h prior to collection of the dialysis samples with food and water available.
4.4. Microdialysis procedure
On the day of the experiment, the flow rate was increased to 2 μL/min approximately 2 h before beginning the collection of baseline samples. Dialysates were collected every 20 min; after 4 baseline samples were collected, animals were pretreated with an intra-peritoneal (i.p.) injection of either 0.9% saline (the vehicle), clonidine (0.0033, 0.01 or 0.05 mg/kg) or guanfacine (0.05 or 0.5 mg/kg), before receiving an injection of PCP (2.5 mg/kg, i.p.) 20 min later. In a separate study, BRL (1.0 mg/kg) was administered 20 min prior to clonidine. In addition, for some control experiments, the animals only received one injection of saline, clonidine (0.01 or 0.05 mg/kg), guanfacine (0.5 mg/kg) or BRL (1.0 mg/kg). The doses of clonidine, guanfacine and PCP were based on our previous studies (Jentsch et al., 1998a; Jentsch and Anzivino, 2004; Marrs et al., 2005). The dose of BRL was chosen following a preliminary dose-response study, in which 1 mg/kg was found to have no significant effect on extracellular PFC dopamine levels, yet to be effective at blocking the effect of clonidine on PCP-induced elevation of dopamine levels. In addition, a 1 mg/kg dose of BRL has been found by others to exert selective alpha2A antagonism (Galeotti et al., 2004).
After each 20 min sample was collected, the dialysate was injected immediately onto a high pressure liquid chromatography (HPLC) system equipped with electrochemical detection for analysis of dopamine content. Each HPLC system used a narrow-bore column (2.0 mm i.d.; 3 μm C-18 particles, Keystone, Bellefonte, PA) and Bioanalytical Systems LC-4C potentiostats (West Lafayette, IN). The Eapp was +0.55 V versus an Ag/AgCl reference electrode. The mobile phase consisted of 0.l M NaH2PO4, 450 mg/L of octylsulfonic acid, 7.2% (vol/vol) acetonitrile, 0.25% EDTA and 350 μl/L of triethylamine (pH 5.0). A detection limit of 5 fmol for dopamine was routinely achieved with this system.
4.5. Histology
After the termination of each experiment, animals were anesthetized with chloral hydrate (600 mg/kg, i.p.) and perfused transcardially with 0.9% saline. The brains were removed and post-fixed in 10% formalin before being placed in sucrose (30% in saline) for 2 days. Serial sections were cut on a freezing stage microtome at 250 μm intervals and stained with Cresyl Violet. Probe placement was verified for all the animals used in this study.
4.6. Data analysis
For the microdialysis data, a within-group analysis was performed by using one-way ANOVA, with time as the repeated measure. Comparisons between drug-treated and control groups were analyzed using a two-way ANOVA with time as the repeated measure. When a significant F value was obtained, further comparison of individual data at corresponding time points between groups were carried out using Tukey’s post hoc tests.
Acknowledgments
This work was supported by PHS grants MH-14092 and MH-57483 (RHR) and MH-69360 (JDJ).
Abbreviations
- HPLC
high pressure liquid chromatography
- NMDA
N-methyl-D-aspartic acid
- PCP
phencyclidine
Footnotes
Publisher's Disclaimer: This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues.
Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited.
In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier's archiving and manuscript policies are encouraged to visit: http://www.elsevier.com/copyright
References
- Arnsten AF, Leslie FM. Behavioral and receptor binding analysis of the alpha 2-adrenergic agonist, 5-bromo-6 [2-imidazoline-2-yl amino] quinoxaline (UK-14304): evidence for cognitive enhancement at an alpha 2-adrenoceptor subtype. Neuropharmacology. 1991;30:1279–1289. doi: 10.1016/0028-3908(91)90024-6. [DOI] [PubMed] [Google Scholar]
- Bakshi VP, Geyer MA. Phencyclidine-induced deficits in prepulse inhibition of startle are blocked by prazosin, an alpha-1 noradrenergic antagonist. J Pharmacol Exp Ther. 1997;283:666–674. [PubMed] [Google Scholar]
- Birnbaum SG, Podell DM, Arnsten AF. Noradrenergic alpha-2 receptor agonists reverse working memory deficits induced by the anxiogenic drug, FG7142, in rats. Pharmacol Biochem Behav. 2000;67:397–403. doi: 10.1016/s0091-3057(00)00306-3. [DOI] [PubMed] [Google Scholar]
- Bowers MB, Jr, Morton JB. Diazepam antagonizes effects on dopamine metabolism produced by PCP receptor agonists. Prog Neuropsychopharmacol Biol Psychiatry. 1992;16:211–215. doi: 10.1016/0278-5846(92)90072-m. [DOI] [PubMed] [Google Scholar]
- Callado LF, Stamford JA. Alpha2A- but not alpha2B/C-adrenoceptors modulate noradrenaline release in rat locus coeruleus: voltammetric data. Eur J Pharmacol. 1999;366:35–39. doi: 10.1016/s0014-2999(98)00889-9. [DOI] [PubMed] [Google Scholar]
- Carasso BS, Bakshi VP, Geyer MA. Disruption in prepulse inhibition after alpha-1 adrenoceptor stimulation in rats. Neuropharmacology. 1998;37:401–404. doi: 10.1016/s0028-3908(98)00051-3. [DOI] [PubMed] [Google Scholar]
- Cartmell J, Monn JA, Schoepp DD. Attenuation of specific PCP-evoked behaviors by the potent mGlu2/3 receptor agonist, LY379268 and comparison with the atypical antipsychotic, clozapine. Psychopharmacology (Berl) 2000;148:423–429. doi: 10.1007/s002130050072. [DOI] [PubMed] [Google Scholar]
- Coan EJ, Collingridge GL. Effects of phencyclidine, SKF 10,047 and related psychotomimetic agents on N-methyl-D-aspartate receptor mediated synaptic responses in rat hippocampal slices. Br J Pharmacol. 1987;91:547–556. doi: 10.1111/j.1476-5381.1987.tb11248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devoto P, Flore G, Pani L, Gessa GL. Evidence for co-release of noradrenaline and dopamine from noradrenergic neurons in the cerebral cortex. Mol Psychiatry. 2001;6:657–664. doi: 10.1038/sj.mp.4000904. [DOI] [PubMed] [Google Scholar]
- Galeotti N, Bartolini A, Ghelardini C. Alpha-2 agonist-induced memory impairment is mediated by the alpha-2A-adrenoceptor subtype. Behav Brain Res. 2004;153:409–417. doi: 10.1016/j.bbr.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Gerber DJ, Tonegawa S. Psychotomimetic effects of drugs—a common pathway to schizophrenia? N Engl J Med. 2004;350:1047–1048. doi: 10.1056/NEJMcibr033201. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology (Berl) 2001;156:117–154. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- Grenhoff J, Svensson TH. Clonidine modulates dopamine cell firing in rat ventral tegmental area. Eur J Pharmacol. 1989;165:11 –18. doi: 10.1016/0014-2999(89)90765-6. [DOI] [PubMed] [Google Scholar]
- Grenhoff J, Nisell M, Ferre S, Aston-Jones G, Svensson TH. Noradrenergic modulation of midbrain dopamine cell firing elicited by stimulation of the locus coeruleus in the rat. J Neural Transm Gen Sect. 1993;93:11–25. doi: 10.1007/BF01244934. [DOI] [PubMed] [Google Scholar]
- Harkin A, Morris K, Kelly JPO, Donnell JM, Leonard BE. Modulation of MK-801-induced behaviour by noradrenergic agents in mice. Psychopharmacology (Berl) 2001;154:177–188. doi: 10.1007/s002130000630. [DOI] [PubMed] [Google Scholar]
- Hertel P, Mathe JM, Nomikos GG, Iurlo M, Mathe AA, Svensson TH. Effects of D-amphetamine and phencyclidine on behavior and extracellular concentrations of neurotensin and dopamine in the ventral striatum and the medial prefrontal cortex of the rat. Behav Brain Res. 1995;72:103–114. doi: 10.1016/0166-4328(96)00138-6. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148:1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Anzivino LA. A low dose of the alpha2 agonist clonidine ameliorates the visual attention and spatial working memory deficits produced by phencyclidine administration to rats. Psychopharmacology (Berl) 2004;175:76–83. doi: 10.1007/s00213-004-1772-3. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Roth RH. The neuropsychopharmacology of phencyclidine: from NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacology. 1999;20:201–225. doi: 10.1016/S0893-133X(98)00060-8. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Elsworth JD, Redmond DE, Jr, Roth RH. Phencyclidine increases forebrain monoamine metabolism in rats and monkeys: modulation by the isomers of HA966. J Neurosci. 1997;17:1769–1775. doi: 10.1523/JNEUROSCI.17-05-01769.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch JD, Dazzi L, Chhatwal JP, Verrico CD, Roth RH. Reduced prefrontal cortical dopamine, but not acetylcholine, release in vivo after repeated, intermittent phencyclidine administration to rats. Neurosci Lett. 1998a;258:175–178. doi: 10.1016/s0304-3940(98)00879-9. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Tran A, Taylor JR, Roth RH. Prefrontal cortical involvement in phencyclidine-induced activation of the mesolimbic dopamine system: behavioral and neurochemical evidence. Psychopharmacology (Berl) 1998b;138:89 –95. doi: 10.1007/s002130050649. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Wise A, Katz Z, Roth RH. Alpha-noradrenergic receptor modulation of the phencyclidine- and delta9-tetrahydrocannabinol-induced increases in dopamine utilization in rat prefrontal cortex. Synapse. 1998c;28:21–26. doi: 10.1002/(SICI)1098-2396(199801)28:1<21::AID-SYN3>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Ji XH, Ji JZ, Zhang H, Li BM. Stimulation of alpha(2)-adrenoceptors suppresses excitatory synaptic transmission in the medial prefrontal cortex of rat. Neuropsychopharmacology. 2008;33:2263–2271. doi: 10.1038/sj.npp.1301603. [DOI] [PubMed] [Google Scholar]
- Kawahara Y, Kawahara H, Westerink BH. Tonic regulation of the activity of noradrenergic neurons in the locus coeruleus of the conscious rat studied by dual-probe microdialysis. Brain Res. 1999;823:42–48. doi: 10.1016/s0006-8993(99)01062-8. [DOI] [PubMed] [Google Scholar]
- Kim SH, Price MT, Olney JW, Farber NB. Excessive cerebrocortical release of acetylcholine induced by NMDA antagonists is reduced by GABAergic and alpha2-adrenergic agonists. Mol Psychiatry. 1999;4:344–352. doi: 10.1038/sj.mp.4000529. [DOI] [PubMed] [Google Scholar]
- Klamer D, Palsson E, Fejgin K, Zhang J, Engel JA, Svensson L. Activation of a nitric-oxide-sensitive cAMP pathway with phencyclidine: elevated hippocampal cAMP levels are temporally associated with deficits in prepulse inhibition. Psychopharmacology (Berl) 2005;179:479–488. doi: 10.1007/s00213-004-2051-z. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Anand A, Moghaddam B. Effects of NMDA receptor antagonists: implications for the pathophysiology of schizophrenia. Arch Gen Psychiatry. 2002;59:663–664. doi: 10.1001/archpsyc.59.7.663. [DOI] [PubMed] [Google Scholar]
- Kubota T, Hirota K, Yoshida H, Takahashi S, Anzawa N, Ohkawa H, Kushikata T, Matsuki A. Effects of sedatives on noradrenaline release from the medial prefrontal cortex in rats. Psychopharmacology (Berl) 1999;146:335–338. doi: 10.1007/s002130051125. [DOI] [PubMed] [Google Scholar]
- Lee A, Wissekerke AE, Rosin DL, Lynch KR. Localization of alpha2C-adrenergic receptor immunoreactivity in catecholaminergic neurons in the rat central nervous system. Neuroscience. 1998;84:1085–1096. doi: 10.1016/s0306-4522(97)00578-2. [DOI] [PubMed] [Google Scholar]
- Lorrain DS, Schaffhauser H, Campbell UC, Baccei CS, Correa LD, Rowe B, Rodriguez DE, Anderson JJ, Varney MA, Pinkerton AB, Vernier JM, Bristow LJ. Group II mGlu receptor activation suppresses norepinephrine release in the ventral hippocampus and locomotor responses to acute ketamine challenge. Neuropsychopharmacology. 2003;28:1622–1632. doi: 10.1038/sj.npp.1300238. [DOI] [PubMed] [Google Scholar]
- Marrs W, Kuperman J, Avedian T, Roth RH, Jentsch JD. Alpha-2 adrenoceptor activation inhibits phencyclidine-induced deficits of spatial working memory in rats. Neuropsychopharmacology. 2005;30:1500–1510. doi: 10.1038/sj.npp.1300700. [DOI] [PubMed] [Google Scholar]
- Mathe JM, Nomikos GG, Hildebrand BE, Hertel P, Svensson TH. Prazosin inhibits MK-801-induced hyperlocomotion and dopamine release in the nucleus accumbens. Eur J Pharmacol. 1996;309:1–11. doi: 10.1016/0014-2999(96)00315-9. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Adams BW. Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science. 1998;281:1349–1352. doi: 10.1126/science.281.5381.1349. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy BL, Arnsten AF, Jentsch JD, Roth RH. Dopamine and spatial working memory in rats and monkeys: pharmacological reversal of stress-induced impairment. J Neurosci. 1996;16:7768–7775. doi: 10.1523/JNEUROSCI.16-23-07768.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pudovkina OL, Kawahara Y, de Vries J, Westerink BH. The release of noradrenaline in the locus coeruleus and prefrontal cortex studied with dual-probe microdialysis. Brain Res. 2001;906:38–45. doi: 10.1016/s0006-8993(01)02553-7. [DOI] [PubMed] [Google Scholar]
- Redmond DE., Jr Clonidine and the primate locus coeruleus: evidence suggesting anxiolytic and anti-withdrawal effects. Prog Clin Biol Res. 1981;71:147–163. [PubMed] [Google Scholar]
- Robert F, Bert L, Lambas-Senas L, Denoroy L, Renaud B. In vivo monitoring of extracellular noradrenaline and glutamate from rat brain cortex with 2-min microdialysis sampling using capillary electrophoresis with laser-induced fluorescence detection. J Neurosci Methods. 1996;70:153–162. doi: 10.1016/s0165-0270(96)00113-6. [DOI] [PubMed] [Google Scholar]
- Shi WX, Pun CL, Zhang XX, Jones MD, Bunney BS. Dual effects of D-amphetamine on dopamine neurons mediated by dopamine and nondopamine receptors. J Neurosci. 2000;20:3504–3511. doi: 10.1523/JNEUROSCI.20-09-03504.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson CJ, Schoepp DD. A role for noradrenergic transmission in the actions of phencyclidine and the antipsychotic and antistress effects of mGlu2/3 receptor agonists. Ann N Y Acad Sci. 2003;1003:309–317. doi: 10.1196/annals.1300.019. [DOI] [PubMed] [Google Scholar]
- Tavares A, Handy DE, Bogdanova NN, Rosene DL, Gavras H. Localization of alpha 2A- and alpha 2B-adrenergic receptor subtypes in brain. Hypertension. 1996;27:449–455. doi: 10.1161/01.hyp.27.3.449. [DOI] [PubMed] [Google Scholar]
- Tsai G, Coyle JT. Glutamatergic mechanisms in schizophrenia. Annu Rev Pharmacol Toxicol. 2002;42:165–179. doi: 10.1146/annurev.pharmtox.42.082701.160735. [DOI] [PubMed] [Google Scholar]
- Uhlen S, Lindblom J, Tiger G, Wikberg JE. Quantification of alpha2A and alpha2C adrenoceptors in the rat striatum and in different regions of the spinal cord. Acta Physiol Scand. 1997;160:407–412. doi: 10.1046/j.1365-201X.1997.00175.x. [DOI] [PubMed] [Google Scholar]
- Van Gaalen M, Kawahara H, Kawahara Y, Westerink BH. The locus coeruleus noradrenergic system in the rat brain studied by dual-probe microdialysis. Brain Res. 1997;763:56–62. doi: 10.1016/s0006-8993(97)00416-2. [DOI] [PubMed] [Google Scholar]
- Verma A, Moghaddam B. NMDA receptor antagonists impair prefrontal cortex function as assessed via spatial delayed alternation performance in rats: modulation by dopamine. J Neurosci. 1996;16:373–379. doi: 10.1523/JNEUROSCI.16-01-00373.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonezawa Y, Kuroki T, Kawahara T, Tashiro N, Uchimura H. Involvement of gamma-aminobutyric acid neurotransmission in phencyclidine-induced dopamine release in the medial prefrontal cortex. Eur J Pharmacol. 1998;341:45 –56. doi: 10.1016/s0014-2999(97)01435-0. [DOI] [PubMed] [Google Scholar]
- Young P, Berge J, Chapman H, Cawthorne MA. Novel alpha 2-adrenoceptor antagonists show selectivity for alpha 2A- and alpha 2B-adrenoceptor subtypes. Eur J Pharmacol. 1989;168:381–386. doi: 10.1016/0014-2999(89)90801-7. [DOI] [PubMed] [Google Scholar]