Abstract
P53 activity is controlled in large part by MDM2, an E3 ubiquitin ligase that binds p53 and promotes its degradation. The MDM2 antagonist Nutlin-3a stabilizes p53 by blocking its interaction with MDM2. Several studies have supported the potential use of Nutlin-3a in cancer therapy. Two different p53 wild-type cancer cell lines (U2OS and HCT116) treated with Nutlin-3a for 24 hrs accumulated with 2N and 4N DNA content, suggestive of G1 and G2-phase cell cycle arrest. This coincided with increased p53 and p21 expression, hypo-phosphorylation of pRb, and depletion of Cyclin B1, Cyclin A, and CDC2. Upon removal of Nutlin-3a, 4N cells entered S-phase and re-replicated their DNA without an intervening mitotic division, a process known as endoreduplication. P53-p21 pathway activation was required for the depletion of Cyclin B1, Cyclin A, and CDC2 in Nutlin-3a treated cells and for endoreduplication after Nutlin-3a removal. Stable tetraploid clones could be isolated from Nutlin-3a treated cells, and these tetraploid clones were more resistant to IR and cisplatin induced apoptosis than diploid counterparts. These data indicate that transient Nutlin-3a treatment of p53 wild-type cancer cells can promote endoreduplication and the generation of therapy-resistant tetraploid cells. These findings have important implications regarding the use of Nutlin-3a in cancer therapy.
Introduction
Wild-type p53 is a tumor suppressor and transcription factor activated by DNA damage and other stresses (1). P53 is normally maintained at low levels through the action of MDM2, an E3 ubiquitin-ligase that binds and ubiquitinates p53 and promotes its proteasomal degradation (2, 3). Stress (DNA damage) induced phosphorylations, particularly those in the p53 N-terminus, inhibit the binding between p53 and MDM2 and thus stabilize p53 and cause its levels to increase (4). The effect of increasing p53 is to stop proliferation, either through G1 and G2-phase cell cycle arrests or apoptosis (1). These effects are mediated by p53-responsive gene products such as p21 (G1/G2 arrest), bax, PUMA, and NOXA (apoptosis).
There is considerable interest in restoring wild-type p53 activity in cancer as a therapeutic strategy. This goal has led to the development of Nutlin-3a (hereafter referred to as Nutlin), a small molecule that binds MDM2 at the pocket used for interaction with p53. Nutlin prevents MDM2 from binding p53 and, consequently, stabilizes and activates p53 (5). At least two strategies have been proposed for Nutlin use in cancer therapy. In the first strategy, Nutlin would be used to treat p53 wild-type cancers due to its ability to trigger p53-dependent growth arrest or apoptosis. Support for this comes from various studies including reports that Nutlin could block the growth of p53 wild-type tumors grown as mouse xenografts, and studies in which Nutlin promoted apoptosis in p53 wild-type leukemia and lymphoma cells (5, 6). In the second strategy, Nutlin would be used to treat tumors that are null or mutant for p53. The notion here is that Nutlin would promote cell cycle arrest in normal tissues and cells that surround a p53-null or mutant tumor, while the tumor cells themselves would be unaffected and continue to proliferate. Subsequent treatment with drugs that target proliferating cells would then selectively kill the tumor cells while having no effect on the arrested normal cells. Support for this comes from studies in which p53 wild-type cells arrested in G1 or G2 phase by Nutlin pre-treatment were resistant to killing by the S-phase poison gemcitabine or microtubule poison taxol (7, 8).
In addition to its role in DNA damage and stress responses, p53 also functions in the “tetraploidy checkpoint”. Evidence for this comes from studies using microtubule inhibitors (MTIs) such as nocodazole and colcemid that block cells in metaphase. Cells arrested in metaphase by prolonged MTI exposure can eventually exit mitosis and enter a pseudo-G1 state with 4N DNA content (tetraploid G1) (9, 10). Endoreduplication refers to the case when these tetraploid cells re-replicate their DNA, giving rise to a polyploid 8N population. Cells lacking p53, p21, or pRb are more sensitive to MTI-induced endoreduplication than wild-type cells, supporting a p53-p21-pRb dependent “tetraploidy checkpoint” that prevents S-phase entry by 4N cells (9–13). Involvement of p21 in endoreduplication has also been revealed in over-expression studies. P21 over-expression arrests cells in G1 and G2 phases. Interestingly, cells released from p21-mediated G2 arrest underwent endoreduplication with an accumulation of polyploid 8N cells (11, 14, 15). It was suggested that endoreduplication and polyploidy resulted from p21-induced depletion at the mRNA level of G2/M regulators and checkpoint proteins, such as Cyclin B1, CDC2, mitotic control proteins MAD2, BubR1, PLK1, and cytokinesis-associated proteins such as PRC1, AIM1, and Citron kinase (15). Another report showed that p21 over-expression via adenovirus promoted endoreduplication, but only in cells that lacked pRb function (11). In that report, it was suggested that p21 expression in the absence of pRb may not fully inhibit Cyclin E-CDK2 activity, and that residual Cyclin E-CDK2 activity was likely driving G2-arrested cells into S-phase inappropriately. Nutlin-treated p53 wild-type cells express high levels of both p53 and p21. An effect of Nutlin on the tetraploidy checkpoint and endoreduplication has not been described.
There is mounting evidence that aneuploid cancer cells are generated from either asymmetric division or progressive chromosomal loss from tetraploid precursors (16, 17). For example, the appearance of tetraploid cells in the premalignant condition Barrett’s oesophagus correlated with p53 loss and preceded gross aneuploidy and carcinogeneis (18). Tetraploid or near-tetraploid cells have also been described in early-stage cancers, such as cervical cancer (19). Direct evidence for the tumorigenic potential of tetraploid cells was provided by Pellman and colleagues who isolated bi-nucleate, tetraploid mammary epithelial cells from p53-null mice (20). Remarkably, these cells were more susceptible to carcinogen-induced transformation (soft-agar growth) than normal diploid cells, and the tetraploid cells formed tumors in nude mice whereas diploid cells did not (20). In another study, Kroemer and colleagues used prolonged (48hr) nocodazole treatment to isolate tetraploid cells from two human colon cancer cell lines that express wild-type p53 (16). They found that most tetraploid cells died after attempting to divide, though some survived and gave rise to stable tetraploid clones. Surprisingly, these tetraploid clones were resistant to radiation and certain chemotherapy agents compared to normal diploid counterparts (16). These studies and others have sparked sharp interest in how tetraploid cells arise and their susceptibility to conventional therapies. Here we report that transient exposure of p53 wild-type cells to Nutlin can promote endoreduplication and the generation of therapy-resistant tetraploid clones.
Materials and Methods
Cell lines and culture conditions
U2OS cells were purchased from ATCC and grown in DMEM medium supplemented with 10% FBS. HCT116 cells and its derivatives (p53−/−, p21−/−) were gifts from Dr. Bert Vogelstein (John Hopkins University) and grown in McCoy’s 5A supplemented with 10% FBS. Cells were plated 24h before being treated with Nutlin-3a (5 μmol/L, Sigma), Irradiation (10Gy), Cisplatin (15 μmol/L, Bedford Laboratory) or as indicated.
Western analysis
Whole cell extracts were prepared by resuspending cell pellets in lysis buffer, resolved by SDS-PAGE and transferred to polyvinylidene difluoride membranes (NEN Life Science Products) (21). Antibodies to p21 (H168), Cyclin A (H432) were from Santa Cruz Biotechnology; antibodies to Cyclin B1 (V152), CDC2 (POH1), Tyr 15 Phospho-CDC2 were from Cell Signaling; antibodies to p53 (AB-2), pRb (Ab-5) were from Calbiochem; antibody to Cyclin E (HE-2) was from BD pharmingen. Primary antibodies were detected with goat anti-mouse or goat anti-rabbit conjugated to horseradish peroxidase (Jackson ImmunoResearch), using enhanced chemiluminescence (Perkin-Elmer).
FACS analysis and live cell sorting
For cell cycle analysis, cells were harvested and fixed in 25% ethanol overnight. The cells were stained with propidium iodide (10 μg/mL, Calbiochem). For BrdU incorporation assay, cells were incubated with 20 μM BrdU (BD pharmingen) for indicated time. The cells were then treated according to the manufacturer’s guidelines. FITC-conjugated mouse anti-BrdU monoclonal antibody (BD pharmingen) and propidium iodide were used to stain cells. For mitochondrial potential (ΔΨm) analysis, cells were harvested and stained with TMRE (Tetramethylrhodamine, 0.1 μmol/L). For Annexin-V staining, cells were stained with Annexin V-PE and 7-amino-actinomycin D (7-AAD) (BD Pharmingen, San Diego, CA, USA) according to manufacture’s instructions. FACS analysis was performed on FACS Canto (Becton Dickinson) and analyzed with CellQuest (Becton Dickinson) and FlowJo 8.7 (Treestar Inc). For each sample, 10,000 events were collected. For live cell sorting, cells were incubated with Hoechst 33342 (2 μmol/L, Invitrogen) for 30 minutes at 37°C and then harvested. DMEM or McCoy’s 5A medium were used to resuspend the pellet at 5×106 cells/mL. Cell sorting was performed based on DNA content using a FACSAria (Becton Dickinson) equipped with a 405nm excitation laser and a 450/50 bandwidth emission filter. The isolated cells were plated at low density and individual clones isolated.
Immunofluorescence microscopy
HCT116 and U2OS cells were grown on glass coverslips and treated as indicated. Cells were rinsed with PBS plus 0.1 mM CaCl2 and 1 mM MgCl2 and fixed with 4% paraformaldehyde for 30 min at 4°C. Coverslips were then incubated with 50 mM NH4Cl for 5 min, and cells were permeabilized with 0.1% Triton X-100 plus 0.2% BSA. Antibodies to α-tubulin and γ-tubulin (Santa Cruz) were used as the primary antibodies. Rhodamine red-conjugated anti-mouse antibody (Jackson ImmunoResearch) was used as the secondary antibody. Specimens were then examined under a Zeiss Axiovert 200m fluorescent microscope.
siRNA-mediated transient knock-down
p53 RNAi, p21 RNAi, pRb RNAi and Control RNAi (On-target plus siControl non-targeting pool) were purchased from Dharmacon and transfected according to the manufacturer’s guidelines using DharmaFECT I reagent. Treatments were applied 24 hours after transfection.
Results
G2/M (4N) cells arrested by Nutlin treatment re-replicate their DNA following Nutlin removal
Multiple in vitro studies have supported the potential for Nutlin in cancer treatment either as a single agent or in combination with radiation or chemotherapeutic drugs. We examined the combined effects of Nutlin and ionizing radiation (IR) in two commonly used p53 wild-type cancer cell lines, HCT116 and U2OS. Cells were treated with Nutlin alone (5 μM), IR alone (10 Gy), or combination of Nutlin and IR for 24 hrs. In some cases, the cells were then rinsed and refed with media lacking Nutlin (Nutlin removal) and cell cycle profiles determined at multiple time points. Treatment with Nutlin alone in both cell lines caused an accumulation of 2N and 4N cells with little or no S-phase, suggestive of G1 and G2-phase arrest (Fig 1). It should be noted that both cell lines treated with Nutlin plus the mitosis blocker colcemid accumulated almost entirely with 4N DNA (supplemental Fig 1), indicating Nutlin treated cells pass through one division cycle before arresting in G1 phase. In contrast to Nutlin only treatment, cells treated with IR alone or Nutlin plus IR arrested mostly with 4N DNA content, suggesting G2 arrest was the primary response to these treatments. Surprisingly, in cells that had been treated with Nutlin alone or Nutlin plus IR we observed the emergence of cells with >4N DNA content after Nutlin removal. In both cell lines treated with Nutlin alone, >4N cells became evident 12 hrs after Nutlin removal, accumulated in an 8N population at the 18 hr time point, and diminished by the 24 hr time point after Nutlin removal. With Nutlin plus IR treatment, >4N cells became apparent at 18 hrs after Nutlin removal and accumulated in an 8N population at the 24hr time point (Fig 1). In HCT116 cells treated with IR alone we also observed >4N cells at the +18h and +24h time points, whereas in U2OS cells treated with IR alone we did not observe a significant accumulation of >4N cells at any time points.
Figure 1.

Transient Nutlin treatment induces the appearance of cells with greater than 4n DNA content. HCT116 (A) and U2OS (B) cells were treated with IR (10 Gy), Nutlin (Nut) (5 μmol/L) or combination of both treatments (Nut+IR) for 24 hours followed by Nutlin removal. (NT: untreated) The cells were harvested at the indicated time points (eg. +12h represents 12 hrs after 24 hrs treatment). Fixed cells were stained with propidium iodide and subjected to flow cytometry analysis. Shown are the representative DNA profiles, analyzed using FlowJo (Cell count vs. propidium iodide/DNA content). DNA content was indicated as 2n, 4n and 8n. C) Cell-cycle distribution was determined from DNA profiles of three independent experiments using FlowJo. (Mean ± s.e.m, n=3) Cells with greater than 4n DNA content (>4n) were gated as shown in A (Nut: +18h).
The appearance of cells with >4N DNA content and their accumulation in an 8N population suggested that 4N cells which had accumulated in Nutlin and Nutlin plus IR-treated cells were reinitiating DNA synthesis and replicating their DNA, a process known as endoreduplication. BrdU incorporation and propidium iodide staining (DNA content) was used to test whether 4N cells reinitiated DNA synthesis after Nutlin removal. U2OS and HCT116 cells were treated with Nutlin alone, IR alone, or Nutlin plus IR for 24 hrs. In some cases, cells were then rinsed and refed with media lacking Nutlin and allowed to incorporate BrdU for the first 12 hrs after Nutlin removal (+12h panels, Fig 2). In other cases, cells were rinsed and refed with media lacking Nutlin and grown for 12 hrs, and BrdU then added to the medium at this 12 hr time point. These cells were allowed to incorporate BrdU for 2 hrs (+14h, Fig 2). The results show that U2OS and HCT116 cells treated with Nutlin alone accumulate with 2N and 4N DNA content, and that both 2N and 4N cells reinitiate DNA synthesis (incorporate BrdU) within the first 12 hrs after Nutlin removal. U2OS cells treated with IR alone accumulated mostly with 4N DNA content, and these 4N cells did not reinitiate DNA synthesis (did not incorporate BrdU). In contrast, Nutlin plus IR treated U2OS cells behaved similarly to cells treated with Nutlin alone, with 4N cells reinitiating DNA synthesis following Nutlin removal. These results suggest Nutlin can drive IR-treated U2OS cells with 4N DNA content into a state from which they can re-replicate their DNA. In HCT116 cells treated with IR alone we observed 4N cells reinitiate DNA synthesis (incorporate BrdU), consistent with the appearance of >4N cells in IR-treated HCT116 in Fig 1A. In Nutlin plus IR treated HCT116 cells we also observed 4N cells reinitiating DNA synthesis following Nutlin removal. In total, the results of Figs 1 and 2 indicate that Nutlin treatment causes U2OS and HCT116 cells to accumulate with 2N and 4N DNA content, and the 4N cells can reinitiate DNA synthesis and replicate their DNA after Nutlin removal, giving rise to polyploid 8N cells.
Figure 2.

4n cells arrested with Nutlin treatment entered S phase after removal of Nutlin. HCT116 (A) and U2OS (B) cells were treated with IR (10 Gy), Nutlin (5 μmol/L) or combination of both treatments for 24 hours followed by Nutlin removal. (NT: untreated) For 24h time point, BrdU was added for 2 hrs without media refreshment. For +12h time point, BrdU was added with Nutlin removal at 24h for 12 hrs. For +14h time point, BrdU was added at +12h time point for 2 hrs. Cells were harvested at the indicated time points and stained with FITC-conjugated anti-BrdU antibody and propidium iodide and analyzed by two dimensional flow cytometry (BrdU vs. propidium iodide/DNA content). DNA content was indicated as 2n, 4n and 8n.
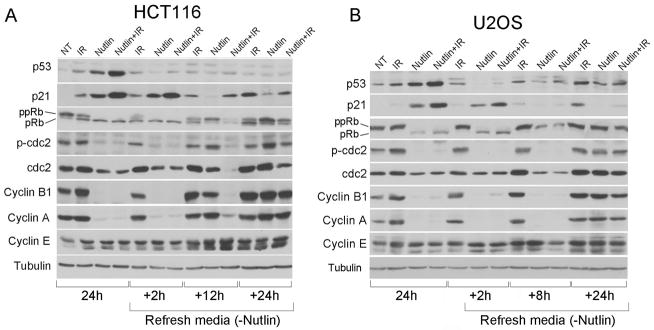
Nutlin treatment induces down-regulation of Cyclin B1, Cyclin A and CDC2
In order for endoreduplication to occur, 4N (G2/M) cells must first enter into a G1-like state, referred to as tetraploid G1 (17). This is often associated with decreased expression of Cyclin B, Cyclin A, and/or CDC2, such that a condition of low CDK activity resembling early G1 phase is established in 4N cells (21, 22). Indeed, depletion or conditional inactivation of Cyclin B, Cyclin A, and CDC2 has been shown to establish a tetraploid G1 state and subsequent endoreduplication in different cell systems (23–26). To investigate whether Nutlin promotes a tetraploid G1 arrest, we compared the levels of Cyclin B1, Cyclin A, and CDC2 in cells treated with Nutlin alone, IR alone, or combination Nutlin plus IR 24 hrs after treatment and at time points after Nutlin removal (Fig. 3). Cyclin A and Cyclin B1 accumulate during the cell cycle and are high in G2 phase, but then decrease rapidly as cells pass through mitosis (27–29). IR arrests cells in G2-phase, in part, through inhibitory phosphorylation of CDC2 at tyrosine-15 (Tyr-15). U2OS and HCT116 cells treated with IR alone expressed increased levels of Cyclin B1, Cyclin A, and Tyr-15 phosphorylated CDC2, indicative of G2 arrest. In contrast, cells treated with Nutlin alone or Nutlin plus IR expressed low/undetectable levels of Cyclin B1, Cyclin A, and Tyr-15 phosphorylated CDC2. Overall CDC2 levels were also modestly decreased in Nutlin and Nutlin plus IR treated cells. These results indicated that Nutlin alone or Nutlin plus IR treated cells were not undergoing a typical G2 arrest. Notably, the effects of Nutlin on Cyclin B1, Cyclin A, and CDC2 were specific since Cyclin E levels were not decreased in Nutlin or Nutlin plus IR treated cells. To examine whether these cells resembled G1 arrested cells, we monitored expression and phosphorylation of pRb. PRb levels were decreased in both cell lines treated with Nutlin or Nutlin plus IR. In Nutlin or Nutlin plus IR treated U2OS cells pRb was expressed only in the hypo-phosphorylated form that is typically associated with arrest in G1-phase, while in cells treated with IR alone pRb remained mostly in a hyper-phosphorylated form. HCT116 cells treated with Nutlin alone or Nutlin+IR also expressed hypo-phosphorylated pRb, while HCT116 cells treated with IR alone expressed pRb in both hyper- and hypo-phosphorylated forms. These results are consistent with 4N U2OS and HCT116 cells treated with Nutlin only or Nutlin plus IR being arrested in a tetraploid G1-like state. P53 levels decreased rapidly (within 2 hrs) after Nutlin removal, and p21 levels decreased 8–12 hrs after Nutlin removal. Decreased levels of p21 coincided with pRb accumulating in a hyper-phosphorylated form and the eventual return of Cyclin B1 and Cyclin A levels (Fig 3).
Figure 3.
Nutlin treatment induces repression of G2/M regulators. HCT116 (A) and U2OS (B) cells were treated with IR (10 Gy), Nutlin (5 μmol/L) or combination of both treatments for 24 hours followed by Nutlin removal. (NT: untreated) Cell lysates were collected at indicated time points and analyzed by Western Blot with indicated antibodies. Tubulin was loaded as loading control. pRb, hypophosphorylated Rb; ppRb, hyperphosphorylated Rb; p-cdc2: phospho-cdc2 (Tyr 15).
DNA endoreduplication after transient Nutlin treatment is p53 and p21 dependent
Previous studies support a p53, p21, and pRb-dependent checkpoint that blocks endoreduplication. In contrast, transient p21 expression can promote endoreduplication (14,15). We investigated whether p53, p21, and/or pRb are required for downregulation of CDC2, Cyclin B1, and Cyclin A in Nutlin treated cells and endoreduplication after Nutlin removal. First, HCT116 cells transfected with control siRNA or siRNAs targeting p53, p21, or pRb were treated with Nutlin or untreated for 24 hrs, and protein lysates examined by immunoblotting (Fig 4A). Cyclin A and Cyclin B1 expression was markedly decreased in both cell types and CDC2 expression also decreased after Nutlin treatment in control cells and cells with pRb knocked down, but not in cells with either p53 or p21 knocked down. This indicates p53-p21 pathway activation is required for decreased expression Cyclin A, Cyclin B1, and CDC2 in Nutlin-treated cells, while pRb is not required. Next, we tested whether p53, p21, and pRb are required for endoreduplication in Nutlin treated cells. First, U2OS and HCT116 cells transfected with control siRNA or siRNAs targeting p53, p21, or pRb were treated with Nutlin for 24 hrs, followed by Nutlin removal for different periods. FACS analysis was used to monitor the appearance of cells with >4N DNA content after Nutlin removal (Fig 4B and D). Cells with >4N DNA content were observed in control samples and cells with pRb knocked down at the 16 and 20 hr time points after Nutlin removal. In contrast, cells with >4N DNA content were not observed after Nutlin removal in cells with either p53 or p21 knocked down. This indicates that both p53 and p21 are also required for Nutlin-induced endoreduplication. Second, we compared the extent of endoreduplication in HCT116 cells with intact or deleted p53 and p21 alleles and obtained similar results (Fig 4C). Cells with an intact p53-p21 signaling pathway treated with Nutlin underwent endoreduplication after Nutlin removal, whereas p53 and p21 null cells did not. Taken together, the results suggest p53-p21 pathway activation in Nutlin-treated cells is required for the cells to undergo a tetraploid G1 arrest (indicated by changes in Cyclin A, Cyclin B1, CDC2, and pRb expression) and subsequent endoreduplication after Nutlin removal.
Figure 4.
DNA endoreduplication after Nutlin removal is p53 and p21 dependent. A) HCT116 and U2OS cells were transfected with siControl (siC), sip53, sip21, sipRb or no transfection (UT). These cells were treated with Nutlin (5 μmol/L) or untreated (NT) for 24 hrs. Cell lysates were collected and analyzed by Western Blot with indicated antibodies. B) HCT116 and U2OS cells were transfected as in A). These cells were treated with Nutlin (5 μmol/L) followed by Nutlin removal. Cells were harvested at the indicated time points and subject to flow cytometry for cell cycle analysis. C) HCT116 cells that are WT (with intact p53 and p21 genes), p53−/− and p21−/− were treated with Nutlin (5 μmol/L) followed by Nutlin removal. Cells were harvested at the indicated time points and stained with propidium iodide to measure DNA content. D) The percentage of cells in B) with greater than 4n DNA content (>4n) was quantified using Flowjo software (Mean ± s.e.m, n=2). >4n cells were gated as shown in B (UT: +16h).
Stable tetraploid clones isolated after transient Nutlin treatment show resistance to DNA damage induced apoptosis
U2OS and HCT116 cells with >4N DNA content that emerged after Nutlin removal gave rise to a transient 8N population that eventually diminished (Fig 1). This suggested these 8N cells might enter mitosis and divide. We considered two possible outcomes: first, the 8N cells could undergo an asymmetric division characterized by abnormal, multipolar mitoses and three or more centrosomes. Quadri-polar and tri-polar mitoses were readily apparent in HCT116 cells (observed in 25% of mitotic cells) that had been Nutlin treated for 24 hours followed by Nutlin removal for 16 more hours (Fig 5A), but not in cells maintained in Nutlin (not shown). Similarly, cells with three or more centrosomes were readily apparent in U2OS cells (observed in 8.5% of cells) that had been Nutlin treated for 24 hours followed by Nutlin removal for 24 more hours (Fig 5B). Abnormal mitoses and cells with supernumerary centrosomes were not observed in untreated cells (data not shown). These results are consistent with at least some 8N cells entering mitosis and undergoing asymmetric cell division. Notably, asymmetric divisions of this type can lead to whole chromosome aneuploidy in surviving daughter cells. We also considered that 8N cells might divide symmetrically and survive as stable tetraploid clones with twice the normal DNA content. To examine this possibility, U2OS and HCT116 cells were treated with Nutlin for 24 hrs, followed by Nutlin removal for an additional 16 hrs. The cells were then labeled with the live-cell DNA stain Hoechst 33342. 2N and 8N cells were isolated by flow-cytometry and replated at low density for isolation of individual clones (Fig 5C). A total of 29 HCT116 clones and 9 U2OS clones were obtained from isolated 8N populations that arose after Nutlin removal. 7 of the 29 HCT116 clones (24%) and 1 of the 9 U2OS clones (11%) grew as tetraploid (4N) cells. This was evidenced by the G1 peak of these clones overlapping the G2/M peak of normal diploid cells (Fig 5D), and by chromosome counting of metaphase spreads which supported these clones having twice the DNA content as diploid counterparts (supplemental Fig 2). In summary, the results indicate two possible fates for 8N cells that arise from endoreduplication after Nutlin treatment: 1) asymmetric mitotic division that could potentially yield aneuploidy in surviving cells, 2) survival as stable tetraploid clones.
Figure 5.
Stable tetraploid clones were isolated after DNA endoreduplication. A, B) HCT116 and U2OS cells were treated as indicated. The cells were fixed and subject to anti-α-tubulin antibody, anti-γ-tubulin antibody and DAPI staining. α-tubulin was used to stain microtubules, γ-tubulin was used to stain centrosome, DAPI was used to stain DNA. The representative images were taken under 100x using a Zeiss axiovert200m fluorescence microscope. A) Abnormal mitoses were detected in 25% (51/200) mitotic cells counted. B) Supernumerary centrosomes (>2) were counted in 8.5% (17/200) cells. C) Shown is the procedure to isolate diploid and tetraploid clones from both untreated and Nutlin treated cells. 2n cells from untreated sample, 2n cells from Nutlin treated sample (6hrs after Nutlin removal (24h+6h)) and 8n cells from Nutlin treated sample (16hrs after Nutlin removal (24h+16h) were live sorted by FACS and individual clones were isolated. D) The comparison of the DNA profiles between a representative diploid clone and a tetraploid clone. Solid line: HCT diploid clone; Dashed line: HCT tetraploid clone.
We were particularly interested in the stable tetraploid clones that arose after transient Nutlin treatment because of the high frequency with which they appeared. Recent studies have suggested that tetraploid cells may be more resistant than diploid cells to certain anti-cancer agents but not others (16). We tested whether tetraploid HCT116 clones that arose after Nutlin treatment were more resistant to IR and cisplatin induced apoptosis than diploid counterparts. The 7 tetraploid and 7 diploid clones isolated from Nutlin treated cells, and the 10 diploid clones isolated from untreated cells, were treated with IR (10 Gy) or cisplatin (15 μM) doses that can effectively promote HCT116 cell killing (16). Cell death was monitored by sub-G1 DNA content and apoptosis by decreased mitochondrial membrane potential (low ΔΨm) and annexin-V staining 72 hrs after treatment. As shown in Fig 6, the tetraploid clones as a group were significantly more resistant to IR and cisplatin (CP) treatment by each criteria compared to the parental cells and diploid clones isolated from either untreated or Nutlin-treated cells (p<0.05). There was no significant difference between the parental cell line (P) and diploid clones from untreated cells (D) or Nutlin treated cells (ND). Long term clonogenic survival after treatment can depend on a combination of senescence, apoptosis, and non-apoptotic death (30). We therefore also examined clonogenic survival after IR in parental HCT116 cells, 3 diploid and 3 tetraploid clones (supplemental Fig 3). In these studies, the tetraploid clones were slightly, but not significantly, more resistant to IR treatment than diploid clones or parental cells suggesting clonogenic survival may depend on other pathways in addition to apoptosis. In summary, the data indicate endoreduplication after transient Nutlin treatment can give rise to tetraploid cells resistant to therapy induced apoptosis.
Figure 6.
Tetraploid clones show resistance to irradiation (IR) and cisplatin (CP) induced apoptosis. The 7 tetraploid clones (T) and 7 diploid clones isolated from Nutlin treated cells (ND), and the 10 diploid clones isolated from untreated cells (D), were exposed to 15 μmol/L cisplatin (CP) or 10Gy irradiation (IR) for 72 hours. The cells were then harvested, stained with indicated fluorophore dyes and subjected to flow cytometry analysis. A) The percentage of cells with low ΔΨm (TMRE staining) of tetraploid clones, diploid clones and parent cells 72 hours post treatment. B) The percentage of sub-G1 cells (PI staining) of tetraploid clones, diploid clones and parent cells 72 hours post treatment. C) The percentage Annexin V positive cells of tetraploid clones, diploid clones and parent cells 72 hours post treatment. Data in A, B, and C are the mean of three independent experiments ± s.e.m. Asterisks (*) indicate a significant difference (p<0.05) comparing tetraploid clones to diploid clones isolated from untreated cells (D)) or Nutlin-treated cells (ND) as well as to parental cells (P). No significant difference (p>0.05) was observed between diploid clones and parental cells. Statistical analysis was done using unpaired Student’s t-test (n=3).
Discussion
Genomic stability is maintained by elaborate checkpoint mechanisms that regulate mitotic entry and exit, as well as entry into S-phase. Defects in these checkpoints can result in numeric and structural chromosomal changes that promote tumorigenesis. In normal cells, entry into S-phase is dependent on the completion of mitosis and activation of G1-phase Cyclin-CDK complexes. Endoreduplication occurs when G2 or M-phase (4N) cells enter a G1-like state (tetraploid G1) and re-replicate their DNA without an intervening mitotic division. There is increasing evidence that aneuploid cancer cells can arise from tetraploid precursors. This has led to sharp interest in how tetraploid cells arise and their susceptibility to conventional therapies. The MDM2 antagonist Nutlin-3a (Nutlin) is being considered as a single or combination agent for cancer treatment. The current report demonstrates that p53 wild-type cancer cells exposed to Nutlin for 24 hrs undergo endoreduplication upon Nutlin removal, giving rise to therapy-resistant tetraploid clones.
Two things must happen for endoreduplication to occur: First, 4N cells must exit G2 or M-phase prematurely and enter a pseudo-G1 state (tetraploid G1); Second, these tetraploid cells must reinitiate DNA synthesis, a process that requires activation of Cyclin E-CDK2 complexes (17, 31). The p53-p21 pathway inhibits endoreduplication as part of the “tetraploidy checkpoint”. Evidence for this comes largely from studies with microtubule inhibitors (MTIs), such as nocodazole or colcemid, that block cells in metaphase. Cells that are metaphase arrested in this way for prolonged periods can eventually exit mitosis and enter a tetraploid G1-state from which they can re-replicate their DNA (endoreduplication) (9, 10). Cells lacking p53, p21, or pRb are more prone to MTI-induced endoreduplication than normal cells, supporting a p53-p21-pRb checkpoint that inhibits endoreduplication (9–13). However, p21 was also reported to promote endoreduplication in different studies. For example, high p21 expression from an inducible promoter arrested cells in G1 and G2 states (14, 15). Cells released from p21-mediated G2 arrest by promoter shut-off underwent endoreduplication with an accumulation of 8N cells (14, 15). An explanation for these apparently discrepant findings is that high p21 levels drive 4N cells into a tetraploid G1 state, but subsequent DNA synthesis in the tetraploid cells requires p21 levels decrease so that CyclinE-CDK2 complexes can be activated.
Under normal conditions, pre-replication complexes (pre-RCs) assemble at DNA origins of replication in a process termed origin “licensing” only in late M and early G1 phase when CDK activity is low (32). This involves sequential binding of the origin recognition complex (ORC), CDC6 and Cdt1, and the replicative helicase MCM2-7 complex. Subsequent origin firing/S-phase entry occurs upon recruitment of the DNA synthesis machinery and activation of Cyclin E-CDK2. In G2 and early M-phase, origin licensing is prevented by Cyclin A/B-CDC2-dependent phosphorylation of factors including ORC, MCM4, and CDC6 that inhibits their origin binding (33–37). Thus, depletion of CDC2, Cyclin B, and/or Cyclin A can establish a “G1-like” state of low CDK activity in G2 or early M-phase cells, priming these cells for S-phase entry by allowing pre-RC formation. Indeed, depletion of CDC2 Cyclin A, and Cyclin B1 has been causally linked with tetraploid G1 arrest and endoreduplication in several studies (22, 24–26). In the current study, IR-treated cells accumulated with 4N DNA content and expressed elevated Cyclin A, Cyclin B1, and Tyr-15 phosphorylated CDC2, consistent with arrest in G2-phase. In contrast, cells treated with Nutlin alone or Nutlin plus IR accumulated with 4N DNA but had either lost or markedly decreased expression of CDC2, Cyclin B1, and Cyclin A. These results support Nutlin and Nutlin plus IR treated 4N cells being arrested in a tetraploid G1-like state. SiRNA knockdown studies revealed that p53 and p21 are required for Nutlin to cause decreased CDC2, Cyclin B1, and Cyclin A expression. In addition, p53/p21 knockdown or null cells were resistant to endoreduplication following Nutlin removal. In total, these results suggest that Nutlin drives 4N cells into a tetraploid G1 state via p53-p21 pathway activation, and this is required for endoreduplication after Nutlin removal. The tetraploid G1 arrest caused by Nutlin most likely results, at least in part, from downregulation of CDC2, Cyclin B1, and Cyclin A. We speculated these effects might be through p21-mediated activation of pRb, which could bind E2F complexes and potentially inhibit CDC2, Cyclin B1, and Cyclin A expression as a result. In this regard it is notable that pRb was not essential for the apparent tetraploid arrest and endoreduplication induced by Nutlin. For example, CDC2, Cyclin B1, and Cyclin A levels still decreased in Nutlin-treated cells in which pRb was knocked down, and pRb knockdown cells underwent endoreduplication after Nutlin removal. Previous studies demonstrated functional overlap between pRb-family members (pRb, p107, p130) in the cell cycle response to p53 (38). Specifically, cells lacking individual pRb family members were only partially resistant to p53-dependent G1 arrest, while cells lacking all three members were completely resistant to p53-dependent arrest. It remains to be seen whether p107 and p130 contribute to the p53/p21 dependent effects of Nutlin.
An interesting finding from the current report was that HCT116 cells were susceptible to endoreduplication following IR treatment while U2OS cells were not. This difference correlated with the extent of p21 induction in each cell line. In HCT116 cells, IR (10 Gy) induced p53 and p21 within 6 hrs of treatment, whereas in U2OS cells the induction of p21 was delayed (supplemental Fig 4). Previous studies found that IR can cause p53 and p21-dependent downregulation of CDC2, Cyclin A, and Cyclin B1, similar to what we observe for Nutlin (22, 39). This downregulation was at the mRNA level and, in at least one study, coincided with endoreduplication 1–6 days after treatment (22). It was suggested that endoreduplication after IR could also be explained in part by reduced CDC2 levels leading to tetraploid arrest and origin licensing in 4N cells (22, 24). CDC2, Cyclin A, and Cyclin B1 levels were not obviously decreased in IR-treated HCT116 cells in the current study. However, our ongoing studies suggest a fraction of IR-treated HCT116 cells with 4N DNA content lack or express low levels of Cyclin B1 (data not shown). We speculate these cells may undergo endoreduplication after IR treatment while high Cyclin B1 expressing cells may not. Notwithstanding a change in protein levels, it is also likely that inhibition of CDC2 activity after IR treatment contributed to a tetraploid arrest in HCT116 cells that preceded endoreduplication.
Endoreduplicating cells that emerged after Nutlin removal gave rise to a transient 8N population. A common consequence of endoreduplication is supernumerary centrosomes in 8N cells. These supernumerary centrosomes can lead to multi-polar mitoses and unequal chromosome segregation in daughter cells, a defect that directly causes whole chromosome aneuploidy. We observed supernumerary centrosomes and tri- and quadri-polar mitoses in U2OS and HCT116 cells after Nutlin removal. Thus, one apparent fate of 8N cells that arise following Nutlin treatment is to undergo asymmetric division, potentially giving rise to whole chromosome aneuploidy in surviving cells. It has also been observed that tetraploid cells can cluster supernumerary centrosomes in two poles, thereby promoting bipolar mitoses even in the presence of multiple chromosomes (17). Consistent with this, we were able to isolate stable tetraploid clones of HCT116 and U2OS cells that had been treated with Nutlin, and our results indicate that nearly all (97%) of these tetraploid cells divide in a bipolar fashion (not shown). One possibility is that these clones maintain their tetraploid DNA content through centrosome clustering and continuous bi-polar divisions.
The relationship between ploidy and DNA damage (radiation) sensitivity has been examined since the 1950s. Some studies have reported increased radioresistance in cells with higher ploidy (40, 41), while others reported cells with increased ploidy are equally sensitive or more sensitive than cells with lower ploidy to radiation killing (42, 43). Recently, Castedo et al compared radiation and chemosensitivity of diploid RKO and HCT116 cells with tetraploid clones that arose after prolonged nocodazole treatment (16). Tetraploid clones were significantly more resistant than diploid clones to certain agents (e.g. IR, cisplatin, campothecin), while being equally sensitive to other agents (staurosporine, etoposide). However, it is worth noting that it was not tetraploidy per se that afforded radiation and chemo-resistance in their study, but rather increased expression in tetraploid clones of certain DNA repair and anti-apoptotic factors that were identified by microarray analysis (16). We observed stable tetraploid HCT116 clones that arose following Nutlin treatment were more resistant to IR and cisplatin than diploid clones. It will be interesting to identify genes that are up/down-regulated in these tetraploid clones as potential mediators of this resistance phenotype. Regardless of the mechanism for this resistance, the results suggest a potentially adverse side-effect of any Nutlin-based therapy is endoreduplication and the generation of therapy-resistant tetraploid cells.
Supplementary Material
References
- 1.Vousden KH, Lu X. Live or let die: the cell’s response to p53. Nat Rev Cancer. 2002;2:594–604. doi: 10.1038/nrc864. [DOI] [PubMed] [Google Scholar]
- 2.Haupt Y, Maya R, Kazaz A, Oren M. Mdm2 promotes the rapid degradation of p53. Nature. 1997;387:296–9. doi: 10.1038/387296a0. [DOI] [PubMed] [Google Scholar]
- 3.Kubbutat MH, Jones SN, Vousden KH. Regulation of p53 stability by Mdm2. Nature. 1997;387:299–303. doi: 10.1038/387299a0. [DOI] [PubMed] [Google Scholar]
- 4.Toledo F, Wahl GM. Regulating the p53 pathway: in vitro hypotheses, in vivo veritas. Nat Rev Cancer. 2006;6:909–23. doi: 10.1038/nrc2012. [DOI] [PubMed] [Google Scholar]
- 5.Vassilev LT, Vu BT, Graves B, et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004;303:844–8. doi: 10.1126/science.1092472. [DOI] [PubMed] [Google Scholar]
- 6.Coll-Mulet L, Iglesias-Serret D, Santidrian AF, et al. MDM2 antagonists activate p53 and synergize with genotoxic drugs in B-cell chronic lymphocytic leukemia cells. Blood. 2006;107:4109–14. doi: 10.1182/blood-2005-08-3273. [DOI] [PubMed] [Google Scholar]
- 7.Kranz D, Dobbelstein M. Nongenotoxic p53 activation protects cells against S-phase-specific chemotherapy. Cancer Res. 2006;66:10274–80. doi: 10.1158/0008-5472.CAN-06-1527. [DOI] [PubMed] [Google Scholar]
- 8.Carvajal D, Tovar C, Yang H, Vu BT, Heimbrook DC, Vassilev LT. Activation of p53 by MDM2 antagonists can protect proliferating cells from mitotic inhibitors. Cancer Res. 2005;65:1918–24. doi: 10.1158/0008-5472.CAN-04-3576. [DOI] [PubMed] [Google Scholar]
- 9.Bunz F, Dutriaux A, Lengauer C, et al. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science. 1998;282:1497–501. doi: 10.1126/science.282.5393.1497. [DOI] [PubMed] [Google Scholar]
- 10.Stewart ZA, Leach SD, Pietenpol JA. p21(Waf1/Cip1) inhibition of cyclin E/Cdk2 activity prevents endoreduplication after mitotic spindle disruption. Mol Cell Biol. 1999;19:205–15. doi: 10.1128/mcb.19.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niculescu AB, 3rd, Chen X, Smeets M, Hengst L, Prives C, Reed SI. Effects of p21(Cip1/Waf1) at both the G1/S and the G2/M cell cycle transitions: pRb is a critical determinant in blocking DNA replication and in preventing endoreduplication. Mol Cell Biol. 1998;18:629–43. doi: 10.1128/mcb.18.1.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khan SH, Wahl GM. p53 and pRb prevent rereplication in response to microtubule inhibitors by mediating a reversible G1 arrest. Cancer Res. 1998;58:396–401. [PubMed] [Google Scholar]
- 13.Di Leonardo A, Khan SH, Linke SP, Greco V, Seidita G, Wahl GM. DNA rereplication in the presence of mitotic spindle inhibitors in human and mouse fibroblasts lacking either p53 or pRb function. Cancer Res. 1997;57:1013–9. [PubMed] [Google Scholar]
- 14.Bates S, Ryan KM, Phillips AC, Vousden KH. Cell cycle arrest and DNA endoreduplication following p21Waf1/Cip1 expression. Oncogene. 1998;17:1691–703. doi: 10.1038/sj.onc.1202104. [DOI] [PubMed] [Google Scholar]
- 15.Chang BD, Broude EV, Fang J, et al. p21Waf1/Cip1/Sdi1-induced growth arrest is associated with depletion of mitosis-control proteins and leads to abnormal mitosis and endoreduplication in recovering cells. Oncogene. 2000;19:2165–70. doi: 10.1038/sj.onc.1203573. [DOI] [PubMed] [Google Scholar]
- 16.Castedo M, Coquelle A, Vivet S, et al. Apoptosis regulation in tetraploid cancer cells. Embo J. 2006;25:2584–95. doi: 10.1038/sj.emboj.7601127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ganem NJ, Storchova Z, Pellman D. Tetraploidy, aneuploidy and cancer. Curr Opin Genet Dev. 2007;17:157–62. doi: 10.1016/j.gde.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 18.Galipeau PC, Cowan DS, Sanchez CA, et al. 17p (p53) allelic losses, 4N (G2/tetraploid) populations, and progression to aneuploidy in Barrett’s esophagus. Proc Natl Acad Sci U S A. 1996;93:7081–4. doi: 10.1073/pnas.93.14.7081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olaharski AJ, Sotelo R, Solorza-Luna G, et al. Tetraploidy and chromosomal instability are early events during cervical carcinogenesis. Carcinogenesis. 2006;27:337–43. doi: 10.1093/carcin/bgi218. [DOI] [PubMed] [Google Scholar]
- 20.Fujiwara T, Bandi M, Nitta M, Ivanova EV, Bronson RT, Pellman D. Cytokinesis failure generating tetraploids promotes tumorigenesis in p53-null cells. Nature. 2005;437:1043–7. doi: 10.1038/nature04217. [DOI] [PubMed] [Google Scholar]
- 21.Gizatullin F, Yao Y, Kung V, Harding MW, Loda M, Shapiro GI. The Aurora kinase inhibitor VX-680 induces endoreduplication and apoptosis preferentially in cells with compromised p53-dependent postmitotic checkpoint function. Cancer Res. 2006;66:7668–77. doi: 10.1158/0008-5472.CAN-05-3353. [DOI] [PubMed] [Google Scholar]
- 22.Badie C, Itzhaki JE, Sullivan MJ, Carpenter AJ, Porter AC. Repression of CDK1 and other genes with CDE and CHR promoter elements during DNA damage-induced G(2)/M arrest in human cells. Mol Cell Biol. 2000;20:2358–66. doi: 10.1128/mcb.20.7.2358-2366.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brito DA, Rieder CL. Mitotic checkpoint slippage in humans occurs via cyclin B destruction in the presence of an active checkpoint. Curr Biol. 2006;16:1194–200. doi: 10.1016/j.cub.2006.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Itzhaki JE, Gilbert CS, Porter AC. Construction by gene targeting in human cells of a “conditional’ CDC2 mutant that rereplicates its DNA. Nat Genet. 1997;15:258–65. doi: 10.1038/ng0397-258. [DOI] [PubMed] [Google Scholar]
- 25.Mihaylov IS, Kondo T, Jones L, et al. Control of DNA replication and chromosome ploidy by geminin and cyclin A. Mol Cell Biol. 2002;22:1868–80. doi: 10.1128/MCB.22.6.1868-1880.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bellanger S, de Gramont A, Sobczak-Thepot J. Cyclin B2 suppresses mitotic failure and DNA re-replication in human somatic cells knocked down for both cyclins B1 and B2. Oncogene. 2007;26:7175–84. doi: 10.1038/sj.onc.1210539. [DOI] [PubMed] [Google Scholar]
- 27.Geley S, Kramer E, Gieffers C, Gannon J, Peters JM, Hunt T. Anaphase-promoting complex/cyclosome-dependent proteolysis of human cyclin A starts at the beginning of mitosis and is not subject to the spindle assembly checkpoint. J Cell Biol. 2001;153:137–48. doi: 10.1083/jcb.153.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Furuno N, den Elzen N, Pines J. Human cyclin A is required for mitosis until mid prophase. J Cell Biol. 1999;147:295–306. doi: 10.1083/jcb.147.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fung TK, Poon RY. A roller coaster ride with the mitotic cyclins. Semin Cell Dev Biol. 2005;16:335–42. doi: 10.1016/j.semcdb.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 30.Okada H, Mak TW. Pathways of apoptotic and non-apoptotic death in tumour cells. Nat Rev Cancer. 2004;4:592–603. doi: 10.1038/nrc1412. [DOI] [PubMed] [Google Scholar]
- 31.Ganem NJ, Pellman D. Limiting the proliferation of polyploid cells. Cell. 2007;131:437–40. doi: 10.1016/j.cell.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 32.Fujita M. Cdt1 revisited: complex and tight regulation during the cell cycle and consequences of deregulation in mammalian cells. Cell Div. 2006;1:22. doi: 10.1186/1747-1028-1-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fujita M, Yamada C, Tsurumi T, Hanaoka F, Matsuzawa K, Inagaki M. Cell cycle- and chromatin binding state-dependent phosphorylation of human MCM heterohexameric complexes. A role for cdc2 kinase J Biol Chem. 1998;273:17095–101. doi: 10.1074/jbc.273.27.17095. [DOI] [PubMed] [Google Scholar]
- 34.Jiang W, Wells NJ, Hunter T. Multistep regulation of DNA replication by Cdk phosphorylation of HsCdc6. Proc Natl Acad Sci U S A. 1999;96:6193–8. doi: 10.1073/pnas.96.11.6193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fujita M, Yamada C, Goto H, et al. Cell cycle regulation of human CDC6 protein. Intracellular localization, interaction with the human mcm complex, and CDC2 kinase-mediated hyperphosphorylation. J Biol Chem. 1999;274:25927–32. doi: 10.1074/jbc.274.36.25927. [DOI] [PubMed] [Google Scholar]
- 36.Hendrickson M, Madine M, Dalton S, Gautier J. Phosphorylation of MCM4 by cdc2 protein kinase inhibits the activity of the minichromosome maintenance complex. Proc Natl Acad Sci U S A. 1996;93:12223–8. doi: 10.1073/pnas.93.22.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nguyen VQ, Co C, Li JJ. Cyclin-dependent kinases prevent DNA re-replication through multiple mechanisms. Nature. 2001;411:1068–73. doi: 10.1038/35082600. [DOI] [PubMed] [Google Scholar]
- 38.Sage J, Mulligan GJ, Attardi LD, et al. Targeted disruption of the three Rb-related genes leads to loss of G(1) control and immortalization. Genes Dev. 2000;14:3037–50. doi: 10.1101/gad.843200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Toledo SM, Azzam EI, Keng P, Laffrenier S, Little JB. Regulation by ionizing radiation of CDC2, cyclin A, cyclin B, thymidine kinase, topoisomerase IIalpha, and RAD51 expression in normal human diploid fibroblasts is dependent on p53/p21Waf1. Cell Growth Differ. 1998;9:887–96. [PubMed] [Google Scholar]
- 40.Lucke WH, Sarachek A. X-ray inactivation of polyploid Saccharomyces. Nature. 1953;171:1014–5. doi: 10.1038/1711014a0. [DOI] [PubMed] [Google Scholar]
- 41.Schwartz JL, Murnane J, Weichselbaum RR. The contribution of DNA ploidy to radiation sensitivity in human tumour cell lines. Br J Cancer. 1999;79:744–7. doi: 10.1038/sj.bjc.6690119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mortimer RK. Radiobiological and genetic studies on a polyploid series (haploid to hexaploid) of Saccharomyces cerevisiae. Radiat Res. 1958;9:312–26. [PubMed] [Google Scholar]
- 43.Nunes de Langguth E, Beam CA. Letter: The effects of ploidy upon cell cycle dependent changes in x-ray sensitivity of Saccharomyces cerevisiae. Radiat Res. 1973;55:501–6. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.