Abstract
Altered dietary choline availability early in life leads to persistent changes in spatial memory and hippocampal plasticity in adulthood. Developmental programming by early choline nutrition may determine the range of adult choline intake that is optimal for the types of neural plasticity involved in cognitive function. To test this, male Sprague-Dawley rats were exposed to a choline chloride deficient (DEF), sufficient (CON), or supplemented (SUP) diet during embryonic days 12-17 and then returned to a control diet (1.1 g choline chloride/kg). At 70 days of age, we found that DEF and SUP rats required fewer choices to locate 8 baited arms of a 12-arm radial maze than CON rats. When switched to a choline-deficient diet (0 g/kg), SUP rats showed impaired performance while CON and DEF rats were unaffected. In contrast, when switched to a choline-supplemented diet (5.0 g/kg), DEF rats' performance was significantly impaired while CON and SUP rats were less affected. These changes in performance were reversible when the rats were switched back to a control diet. In a second experiment, DEF, CON, and SUP rats were either maintained on a control diet, or the choline-supplemented diet. After 12 weeks, DEF rats were significantly impaired by choline supplementation on a matching-to-place water-maze task, which was also accompanied by a decrease in dentate cell proliferation in DEF rats only. IGF-1 levels were elevated by both prenatal and adult choline supplementation. Taken together, these findings suggest that the in utero availability of an essential nutrient, choline, causes differential behavioral and neuroplastic sensitivity to the adult choline supply.
Keywords: choline, hippocampus, memory, bromodeoxyuridine, growth factor, plasticity
1. Introduction
Choline (trimethyl-beta-hydroxy-ethylammonium) is a quaternary ammonium compound that is an essential nutrient for animals and humans (Zeisel and Blusztajn, 1994). Ten years ago, the Food and Nutrition Board of the Institute of Medicine – United States National Academy of Sciences issued a report on B vitamins that provided recommendations for the adequate intake of choline (Food and Nutrition Board of the Institute of Medicine, 1998). This recommendation was based, in part, on studies showing that the availability of choline during a specific prenatal period in rats, embryonic days (ED) 12 through 17 of the 22-day gestation period, has profound effects on cognitive performance throughout the lifespan (Blusztajn et al., 1998; McCann et al., 2006; Meck and Williams, 2003; Meck et al., 2008). In brief, prenatally choline-supplemented rats are characterized in adulthood by improved performance relative to control rats in tasks measuring spatial memory, temporal processing, and attention (Brandner, 2002; Meck et al., 1988, 1989; Meck and Williams, 1997a, b, c; Meck et al., 2008; Ricceri and Berger-Sweeney, 1998; Zeisel et al., 1991). In contrast, prenatally choline deficient rats show a different pattern of cognitive changes as adults. During the acquisition and maintenance of the standard radial-arm maze task, adult prenatal choline deficient rats sometimes show improvements in choice behavior similar to the prenatally choline supplemented rats. However, when the cognitive demands of the task are increased (e.g., by massing trials) dramatic impairments in spatial memory are observed in these rats (Meck and Williams, 1999). Similar improvements and decrements in performance for prenatal choline supplemented and deficient rats, respectively, have been reported for temporal processing tasks that require divided attention or increments in attention following a change in the predictiveness of a stimulus (e.g., Lamoureux et al., 2008; Meck and Williams, 1997a).
In addition to producing lasting changes in memory capacity and precision, altered dietary choline early in life leads to persistent changes in features of adult hippocampal plasticity known to influence learning and memory function. For example, prenatal choline supplementation leads to enhanced basal levels of neurogenesis, brain-derived neurotrophic factor (BDNF) (Glenn et al., 2007; Wong-Goodrich et al., 2008), nerve growth factor (NGF) (Sandstrom et al., 2002), and insulin-like growth factors- 1 and 2 (IGF-1 and -2) (Napoli et al., in press, this issue; Wong-Goodrich et al., 2008); a reduced stimulus threshold to induce long-term potentiation (LTP) (Jones et al., 1999; Pyapali et al., 1998); increased dendritic spine density in the CA1 and dentate gyrus (Meck et al., 2008); and enhanced depolarization-induced phosphorylation of mitogen-activated protein kinase (MAPK) and cAMP-response element binding protein (CREB) activation (Mellott et al., 2004). In contrast, when compared to control-fed rats, adult offspring of dams depleted of choline on ED12-17 display a higher threshold to induce LTP (Jones et al., 1999), show reduced MAPK and CREB activation (Mellot et al., 2004), and fail to increase dentate neurogenesis in response to an environmental enriching experience (Glenn et al., 2007). In the fetal mouse brain, dietary choline deficiency during ED 12-17 has also been shown to decrease progenitor cell proliferation in the developing hippocampus on ED 17 (Craciunescu et al., 2003).
If brain development is shaped adaptively by early nutrition, then one might predict that brains may function optimally when infant and adult diets are similar, and less well when diets are mismatched. This idea has been given much attention with regards to the developmental origins of disease and health (Bateson, 2007; Bateson et al., 2004; Gluckman et al., 2005), and there is some evidence to suggest that mismatches between the nutritional environment of the developing organism and the adult can place the organism at risk for disease (e.g., obesity, diabetes, heart disease) because the organism is not well adapted for its mature nutritional environment (Vickers et al., 2000; Vickers et al., 2005). Little is known, however, about the effects of developmental programming by maternal nutrition on cognitive function. We hypothesized that metabolic imprinting by early choline availability may make a brain differentially sensitive to choline supply in adulthood by setting the range of adult choline intake that is optimal for cognitive function and hippocampal plasticity.
The neurochemical mechanisms by which choline supplementation in utero leads to an improvement in memory and hippocampal plasticity are currently not well understood in part because choline serves several biological functions: it is the precursor of the neurotransmitter, acetylcholine (ACh); the structural phospholipids in biological membranes, phosphatidylcholine and sphingomyelin; and two signaling lipids, sphingosylphosphocholine and platelet-activating factor. It also serves as a methyl donor after its oxidization to betaine (Blusztajn et al., 1998; Blusztajn and Wurtman, 1983). It is possible that choline availability in utero affects one or more of these functions during brain development, resulting in changes in the brain's organization. We know, for instance, that prenatal choline availability produces long-term adaptations in the synthesis, storage and release of acetylcholine, and reuptake and recycling of choline in the adult hippocampus (Blusztajn et al., 1998; Cermak et al., 1999; Cermak et al., 1998; Meck et al., 2008) as well as alterations in the size and shape of basal forebrain cholinergic neurons (McKeon-O'Malley et al., 2003; Williams et al., 1998).
To test our hypothesis that there is “metabolic imprinting” of hippocampal plasticity and function by prenatal choline availability, we conducted two experiments that investigated the interactive effects of prenatal and adult choline availability on hippocampal-dependent tasks of spatial memory function and on dentate gyrus cell proliferation and hippocampal growth factor content as markers of hippocampal plasticity. For both studies, subjects were offspring of rat dams that consumed either a synthetic rat chow with 1.1 g/kg choline cloride (CON), were supplemented with choline such that they consumed about 4.5 times more choline (SUP), or were fed a diet deficient in choline (DEF) during embryonic days 12-17 (ED12-17). In Experiment 1, CON, SUP and DEF offspring were trained on a 12-arm radial maze beginning at 70 days of age while they were consuming a control diet, after being switched to a choline supplemented or deficient diet for 24 days, and again after a return to the standard control diet. In Experiment 2, we examined whether increases in choline availability for a longer time period, 16 weeks, in one-year-old CON, SUP and DEF offspring would affect hippocampally mediated spatial navigation, memory, and plasticity. In Experiment 2, spatial memory was evaluated using a matching-to-place water maze task; dentate cell proliferation and neurogenesis were assessed via immunohistochemistry of bromodeoxyuridine (BrdU) and doublecortin (DCX); and hippocampal BDNF, NGF, and IGF-1 were assayed via ELISA.
2. Results
2.1. Experiment 1
Figure 1 presents the timeline of procedures used in this experiment. Offspring of rat dams made deficient of choline (DEF), given a standard diet (CON), and supplemented with choline (SUP) during ED 12-17 were trained for one trial per day on a 12-arm radial maze with 8 baited arms beginning when they were 90 days of age. Rats from each prenatal treatment condition were trained for 14 days while continuing to consume the standard diet (Before phase). They were then switched to either the choline supplemented diet or the choline deficient diet for 24 days, and retrained on the radial arm maze for the last 14 of these days (During phase). Finally, they were switched back to the standard diet for 24 days and were retrained again for the last 14 days (After phase). Figure 2 presents the mean ± standard error of the mean (SEM) number of arms chosen to find all 8 food locations for the Before, During, and After phases for rats in the DEF, CON, and SUP prenatal treatment groups receiving adult choline deprivation (Fig. 2A) or adult choline supplementation (Fig. 2B). In general, these data indicated that a large mismatch in choline content between prenatal and adult diets led to impaired performance on a radial-arm maze task, which recovered when the adult diet was more closely matched in choline content to the prenatal choline diet.
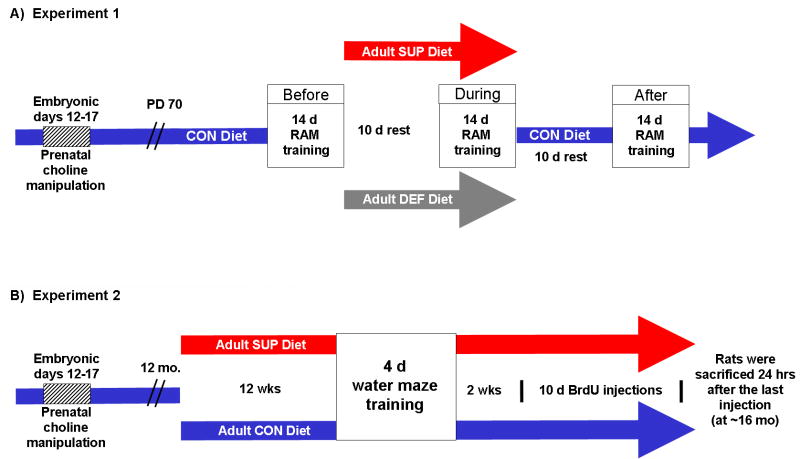
Figure 1.
Experimental timeline of procedures. Rats that were prenatally choline deficient (DEF), supplemented (SUP), or on a control (CON) diet on ED 12-17 were used as subjects either in Experiment 1 (panel A) and Experiment 2 (panel B). In Experiment 1, PD 70 rats from each prenatal treatment group were first trained on the radial arm maze (RAM) (Before) and then divided into two groups after 24 days of training. One group received a diet depleted of choline while the other group received a choline supplemented diet (5 g/kg choline chloride). After 10 days of the adult diet manipulation, rats were retrained for 14 days on the RAM (During). Rats were then put back on the choline control diet (1.1 g/kg) for 10 days and retrained on the RAM for 14 days (After). In Experiment 2, rats approximately 12 months of age from each prenatal treatment group were divided into two groups. One group was maintained on the standard control diet, while the other group was given a choline supplemented diet. After 12 weeks of the adult diet manipulations, rats were trained for 4 days on a water maze task, given 2 weeks of rest, administered 10 days of BrdU injections, and then sacrificed 24 hours after the last injection.
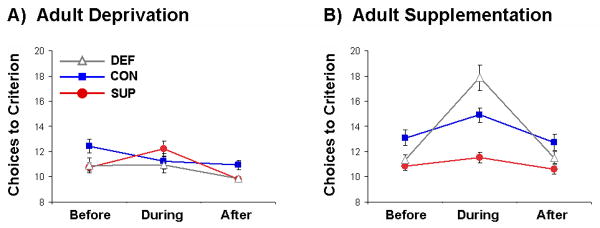
Figure 2.
Mean ± SEM choices to criterion as a function of the choline treatment phase (Before, During, and After) for rats in the prenatal DEF, CON, and SUP treatment groups tested under the treatment conditions of adult choline deprivation (panel A) or adult choline supplementation (panel B). Eight is the minimum number of choices required to locate the 8 baited arms of the 12-arm maze. During the Before phase, CON and SUP rats had significantly fewer choices than DEF rats (p < 0.05). Adult choline deprivation slightly impaired prenatal SUP rats (p < .05) in the During phase, whereas adult choline supplementation in prenatal CON and DEF rats lead to a robust impairment of maze performance in the During phase with prenatal DEF rats showing the greatest impairment (ps < 0.05).
Effect of adult choline deficient diet
For the adult choline-deficient condition (Fig. 2A), there was no main effect of Prenatal Treatment or Phase of Training in the Before vs. During comparison, but there was a significant Prenatal Treatment × Phase interaction, F(2,30) = 5.24, p < 0.05. Post-hoc comparisons indicated that both prenatal DEF and SUP rats performed better than CON rats in the Before phase, and that only the performance of prenatal SUP rats was significantly impaired by adult choline deprivation throughout the During phase, ps < 0.05. Performance of prenatal SUP rats was restored when they were fed the standard diet during the After phase (Fig. 2A). For the Before vs. After comparison, significant main effects of Prenatal Treatment, F(2,30) = 6.94, p < 0.05, and Phase, F(1,30) = 12.49, p < 0.05, were observed, but their interaction was non-significant. All groups appeared to improve slightly from the Before to After phases (Fig. 2A). Post-hoc comparisons indicated that both prenatal DEF and SUP rats performed better than CON rats in the After phase, ps < 0.05.
Effect of adult choline supplemented diet
When the prenatal DEF, CON, and SUP offspring were placed on a choline supplemented diet and trained on the radial arm maze (Fig. 2B), there were significant main effects of Prenatal Treatment, F(2,30) = 13.84, p < 0.05, and Phase of Training, F(1,30) = 57.64, p < 0.05, as well as their interaction, F(2,30) = 20.38, p < 0.05, for the Before vs. During comparison. For the Before vs. After comparison, only a significant effect of Prenatal Treatment on performance was observed, F(2,30) = 5.41, p < 0.05. Post-hoc comparisons indicated that both prenatal choline DEF and SUP rats performed better than CON rats during the Before phase, p < .05. In addition, both prenatal choline CON and DEF rats showed impaired performance from the Before to During phase as well as in comparison to SUP rats in the During phase, indicating that both CON and DEF rats were significantly impaired by adult choline supplementation in the During phase, with DEF rats showing significantly greater impairment than CON rats, ps < 0.05 (Fig. 2B). These impairments were not evident during the After phase, indicating that these impairments were reversible when rats were returned to the choline control diet.
2.2. Experiment 2
2.2.1. Water maze performance
Prenatal SUP, CON and DEF rats were reared to one year of age on the standard control diet and then half of each prenatal treatment group was switched to the choline supplemented diet for 16 weeks. After 12 weeks of altered diet, rats were trained on a matching-to-place water maze task where rats received 3 pairs of trials each day and the platform was positioned in a different pool location for each trial pair. The first trial in each pair served as the train trial where a rat would learn a novel platform location, and the second trial in each pair served as the test trial to determine whether the rat had a good short term memory of the platform location it had been exposed to on the preceding train trial. The duration of the retention interval between trials within a pair was varied (1-2 min vs. 60 min) to assess the effects of increasing cognitive demands on short term, episodic memory.
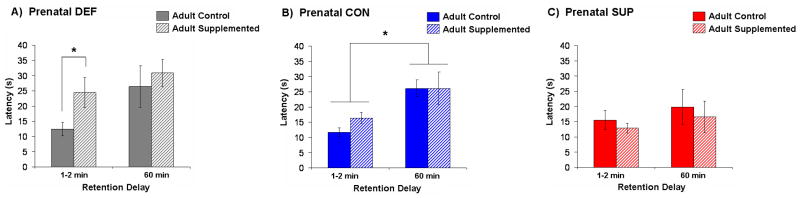
Mean latency to locate the hidden platform after a 1-2 min and 60 min delay for rats in each of the adult diet conditions are presented separately for prenatal DEF, CON, and SUP treatment groups in Figure 3. Prenatal CON rats consuming a standard adult diet and those consuming the choline supplemented diet found the platform rapidly when the delay between training and testing was 1-2 min. However, when a 60-min delay was imposed between training and testing, the performance of all prenatal CON rats was impaired regardless of adult diet. This was supported by a significant main effect of Delay for the prenatal CON group, F(1,8) = 7.97, p < 0.03 (Fig. 3B). Prenatal DEF rats showed a different pattern of results. While prenatal DEF rats maintained on the standard adult diet also found the platform rapidly at the shorter delay, a choline supplemented diet in adulthood significantly impaired their performance when the delay between training and testing was only 1-2 min: planned comparisons revealed that prenatal DEF rats that received choline supplementation in adulthood had significantly higher latencies at the 1-2 min delay (p < 0.05, Fig. 3A). The 60-min delay caused a large and significant decrement in their performance regardless of which adult diet they were consuming, as supported by a main effect of Delay, F(1, 7) = 5.29, p = 0.05 (Fig. 3A). In contrast, neither the 60-min delay nor the supplemented adult diet had any effect on the performance of prenatal SUP rats. Remarkably, SUP rats showed no decrement in memory retention when the delay between training and testing was increased to 60 min, and no impairment in memory by adult choline supplementation (Fig. 3C). Our observation that adult choline supplementation appears to selectively impair prenatal DEF rats is consistent with the results from Experiment 1, which showed the greatest impairment of radial-arm maze performance by adult choline supplementation in prenatal DEF rats.
Figure 3.
Mean ± SEM latencies to find the hidden platform after a 1-2 min. and 60 min. delay as a function of adult choline supply for prenatal DEF (panel A), CON (panel B), and SUP (panel C) rats. There was a significant effect of Delay for CON rats where rats exhibited longer latencies at the 60 min. delay, whereas SUP rats' latencies did not increase with the 60 min. delay. After adult choline supplementation, DEF rats had significantly longer latencies at the 1-2 min. delay while CON and SUP rats were not affected. * statistically significant at p < 0.05.
2.2.2. Hippocampal cell proliferation, neurogenesis, and growth factor levels
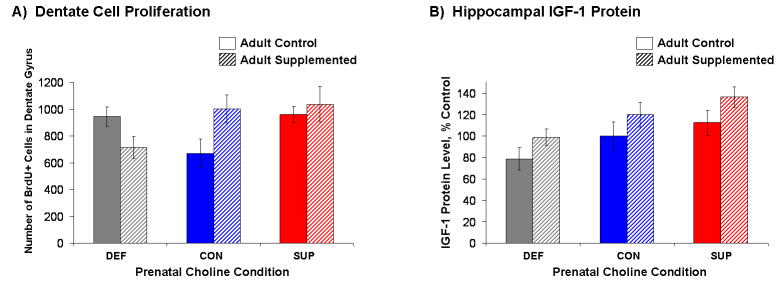
Using unbiased stereology, estimates of the number of BrdU+ cells in the dentate gyrus were generated for each rat and these data are shown in Figure 4A. The 3 (Prenatal Diet) × 2 (Adult Diet) ANOVA revealed a significant interaction, F (2, 24) = 4.01, p < 0.05. A careful observation of the group means led us to decompose our 3 (Prenatal Diet) × 2 (Adult Diet) design into two separate 2 (Prenatal Diet) × 2 (Adult Diet) ANOVAs for CON vs. SUP and CON vs. DEF groups. For CON vs. SUP, there were no significant main effects or interaction, but for CON vs. DEF, there was a significant Prenatal Diet × Adult Diet interaction, F (1, 15) = 8.44, p < 0.02, where adult choline supplementation caused an increase in the number of BrdU+ cells in the dentate gyrus for CON rats, but a decrease in DEF rats (Fig. 4A). SUP rats did not show an effect of adult choline supplementation on the number of new cells in the dentate gyrus, but an apriori comparison revealed that SUP rats maintained on the choline control diet had significantly more BrdU-labeled cells than CON rats maintained on the same control diet (p < 0.05). To determine whether a difference in overall size of structure could account for differences in the numbers of new cells detected, we estimated the volume of the dentate gyrus in each rat using Cavalleri's principle (Mouton, 2002) and did not detect any statistically significant differences between any treatment groups (Fs<1).
Figure 4.
Mean (±SEM) numbers of BrdU-labeled cells detected in the dentate gyrus (panel A) and mean (± SEM) IGF-1 protein levels (expressed as percent of control levels) in the hippocampus hippocampal IGF-1 protein (panel B) of prenatal DEF, CON, and SUP rats that were either maintained on a standard control diet (open bars) or supplemented with choline for 16 weeks (hatched bars). A significant Prenatal Diet × Adult Diet interaction (p < .05) revealed that adult choline supplementation decreased the number of BrdU-labeled cells in DEF rats, increased the number of BrdU-labeled cells in CON rats, and had no effect on SUP rats (4A). Analysis of IGF-1 protein levels indicated a significant main effect of Prenatal Diet where SUP rats had the highest levels and prenatal DEF rats had the lowest levels of IGF-1 overall, and a significant main effect of Adult Diet where choline supplementation to adult rats in all prenatal diet groups increased IGF-1 expression (4B).
Numbers of DCX-labeled neurons for each rat were extremely low for all groups, making it difficult to detect any significant effects of prenatal and adult choline supply on the number of DCX-labeled neurons (all ps > 0.05).
Of all the growth factors examined (BDNF, NGF, and IGF-1), only analyses of IGF-1 levels produced a significant main effect of Prenatal Diet, F (2, 25) = 5.19, p < .02, which was driven by prenatal SUP rats having the highest, and prenatal DEF rats having the lowest levels of IGF-1 overall collapsed across adult diet conditions. There was also a significant main effect of Adult Diet, F (1, 25) = 5.41, p < .03. Choline supplementation to adult rats in all prenatal diet groups increased IGF-1 expression. Protein levels of hippocampal IGF-1, expressed as percent of control levels, are presented in Figure 4B.
3. Discussion
The results of Experiments 1 and 2 provide converging evidence that the in utero availability of an essential nutrient, choline, contributes to differential cognitive and hippocampal responses when subjects are exposed to changes in the adult choline supply. Although permanent adaptations of fat and carbohydrate metabolism resulting from variations of general caloric intake and the availability of these compounds through the pre-weaning period has previously been reported (Cleal et al., 2007; Lewis et al., 1979; Patel et al., 1993; Vickers et al., 2000; Vickers et al., 2005), to our knowledge this is the first report indicating that prenatal availability of a specific nutrient determines the effects of that nutrient's availability on cognitive function and hippocampal plasticity in the adult.
In Experiment 1, we found that young adult male rats that received sufficient levels of choline during development (CON) showed no reliable effect of adult choline deprivation on spatial memory performance during radial-arm maze training. In contrast, CON rats did show a small, but reliable decrement in performance during choline supplementation. Rats that were supplemented with choline during early development (SUP) showed significantly more accurate radial-arm maze performance as adults relative to CON rats when they were maintained on our standard control diet that is considered sufficient in choline. These data are consistent with many previous reports from our laboratory and others demonstrating that supplementation with choline during early development improves adult performance on a variety of spatial and temporal memory tasks (Brandner, 2002; Cheng et al., 2006, 2008; Cheng and Meck, 2007; Meck et al., 1988, 1989; Meck and Williams, 1997a, 1997b, 1997c; Meck et al., 2008; Ricceri and Berger-Sweeney, 1998; Schenk, 1995). Interestingly, when SUP rats were shifted to a choline-deficient diet during radial-arm maze training they performed significantly worse than CON rats that were consuming the same deficient diet. When choline levels were replenished, SUP rats returned to displaying performance superior to CON rats, suggesting that the sufficient diet provides an adequate level of choline for these rats to demonstrate superior maze performance.
Rats that were exposed to a brief period of choline deficiency during early development (DEF) in Experiment 1 also showed significantly more accurate radial-arm maze performance as adults relative to CON rats when they were maintained on a diet that is considered sufficient in choline. These findings are again consistent with previous studies that have reported that when trained on a standard radial-arm maze task (i.e., with relatively low memory demands), DEF rats show superior performance compared to CON rats (Meck and Williams, 1999). When DEF rats were shifted to a choline-deficient diet, their performance was no different from CON rats fed the same deficient diet. When choline levels were replenished, DEF rats returned to displaying performance superior to CON rats, suggesting that the choline sufficient adult diet is necessary for prenatal DEF rats to exhibit optimal spatial memory performance. In contrast, when DEF rats were shifted to a choline supplemented diet, their choice performance was dramatically impaired relative to their performance while consuming the choline sufficient diet while CON and SUP rats were less affected. The impaired performance of DEF rats could be rehabilitated by intake of a diet with sufficient levels of choline. Taken together, the results of Experiment 1 provide strong evidence that prenatal choline intake is a major factor in determining the level of choline intake that is needed in adulthood for optimal spatial memory performance.
In Experiment 2 we further explored the effects of supplementation with choline in adulthood on rats that had deficient, sufficient or supplemented choline intake prenatally. We found that the of the ability of one-year-old prenatal DEF rats to learn the location of a hidden platform in a water maze was significantly impaired by 12 weeks of adult choline supplementation, while water maze performance of age-matched CON and SUP rats was unaffected by this dietary manipulation. Together, the results of Experiment 1 and 2 provide converging evidence, using 2 different spatial memory tasks and both young and middle-aged rats, that prenatal choline nutrition imprints or organizes brain mechanisms for spatial cognition and influences the adult dietary choline intake necessary for optimal cognitive performance.
In Experiment 2 we also explored that possibility that prenatal diet modifies the hippocampal response to changes in the adult diet. We now report that supplementation of choline to the diet of adult rats had different effects on hippocampal dentate cell proliferation as a function of prenatal choline status. CON rats showed an increase in cell proliferation when placed on a choline-supplemented diet in adulthood, while DEF rats showed a decrease in proliferation. In contrast, SUP rats, which already had a higher basal rate of hippocampal cell proliferation than CON or DEF rats when they were consuming a standard diet, did not show a further increase in proliferation when placed for 12 weeks on a choline supplemented diet. One parsimonious explanation for why prenatal choline supplemented rats did not show increased hippocampal cell proliferation on a choline supplemented diet in adulthood is that we simply did not provide a sufficiently choline-enriched diet to drive cell proliferation beyond that achieved by prenatal choline supplementation alone. Alternatively, there may be a balance of plasticity in the hippocampus that prevents too much proliferation that may be disruptive to normal function. For example, seizures are known to produce abnormally high and disruptive levels of hippocampal neurogenesis (Bengzon et al., 1997; Parent et al., 1997; Scharfman et al., 2002). Prenatal choline supplementation combined with a standard adult diet may produce an optimal level of hippocampal cell proliferation in SUP rats. Interestingly, we have also reported that exploration/environmental enrichment does not add to the increase in hippocampal cell proliferation observed in prenatal choline supplemented rats (Glenn et al., 2007). This finding suggests that when rats are already producing a near optimal number of new cells in the hippocampus, it is difficult to drive cell proliferation higher with environmental stimulation. Though we did not examine the effects of adult choline deprivation on dentate cell proliferation in the current study, it is possible that choline deprivation in adulthood may differentially affect dentate cell proliferation based on prenatal choline supply. Previous work has shown that dietary deficiency of a related nutrient, folic acid, in adult mice that were exposed to sufficient levels of folic acid during development led to a marked decrease in dentate progenitor cell proliferation (Kruman et al., 2005). Thus, it is possible that mismatches between a sufficient prenatal choline diet (i.e., CON and/or SUP rats) and a deficient adult choline diet would elicit a similar decrease in dentate cell proliferation.
It should be noted that not all changes in hippocampal cell proliferation as a result of fetal and adult choline nutrition paralleled changes in cognitive function. In particular, while the spatial memory ability of CON rats was either slightly impaired (Experiment 1) or not influenced by a choline-supplemented diet in adulthood (Experiment 2), hippocampal cell proliferation was increased by this adult diet change. This is in stark contrast to the parallel changes in spatial memory and hippocampal proliferation seen in the prenatal choline supplemented and deficient rats placed on an adult supplemented diet. While our estimate of new neurons labeled with doublecortin was too low in our middle aged rats to reveal significant differences among treatment groups, it is possible that there might be a relationship between new neurons and cognitive function even in our control rats. Alternately, the cognitive changes that occurred as a result of alterations in the choline content of prenatal and adult diets may be due to other changes in hippocampal or neocortical plasticity (e.g., alterations in spine density, synapse number, dendritic arbor – Meck et al., 2008), or changes in cholinergic input to hippocampus (see below). We also found no relationship between hippocampal growth factor levels, cell proliferation, and spatial ability. While previous reports have shown that prenatal choline supplemented rats reared on control diet have increased hippocampal NGF (Sandstrom et al., 2002; Wong-Goodrich et al., 2008), BDNF (Glenn et al., 2007; Wong-Goodrich et al., 2008), IGF-1 (Wong-Goodrich et al., 2008), and IGF-2 (Napoli et al., in press-this issue) compared to rats reared on a control diet, we were only able to detect an increase in IGF-1 in our prenatal SUP rats reared on standard diet. We do not know why we were unable to see increases in other growth factors in our middle-aged rats, but since several of the prior reports (Glenn et al., 2007; Glenn et al., in press-this issue) used female, rather than male subjects, it is possible that prenatal choline's enhancement of some measures of hippocampal plasticity in middle to late adulthood may be more pronounced in females. Most surprising, is that we also found an increase in IGF-1 in adult choline supplemented rats regardless of prenatal diet. Thus, it is unlikely that this growth factor is an important mediating step in either the effects of adult choline availability on hippocampal cell proliferation or on spatial memory. Further work will be needed to understand the relationship between choline-induced changes in hippocampal growth factors, hippocampal neurogenesis, and hippocampally mediated behaviors.
One developmental mechanism for the enduring actions of prenatal choline availability on brain function is through an epigenetic process. Choline is the precursor to the methyl donor betaine and nutritional methyl status may influence the availability of methyl groups necessary for the methylation of CpG sites in DNA, and thus regulation of gene expression. For example, when pregnant rodents are fed a choline deficient diet, methylation of the CDKN3 gene promoter is decreased in fetal brain, which results in over expression of this gene, leading to decreased cell proliferation (Niculescu et al., 2004). Thus, a developmental change in gene promoter methylation by prenatal choline supplementation or deficiency could lead to the long-term alterations in hippocampal cell proliferation reported here. Epigenetic modifications of the brain can also continue to occur throughout life. For example, Porgribny and colleagues (in press-this issue) report that feeding a folate/methyl-deficient diet to adult rats resulted in global DNA and gene-specific hypermethylation and by an increase of methylation within unmethylated DNA domains. Thus, it seems possible that developmental and adult epigenetic mechanisms might interact to cause the patterns of change in hippocampal plasticity and cognitive function reported here after changes in fetal and adult choline availability.
While we do not know whether prenatal choline availability alters the brain and behavioral changes reported here via alterations in gene methylation or through some alternate mechanism that influences the development of cholinergic neurons, we do have considerable evidence that prenatal choline status causes long-term adaptations in hippocampal choline metabolism (Cermak et al., 1998, 1999). Because the cholinergic system has a central role in memory function (Bartus et al., 1982; Hasselmo and Giocomo, 2006) and appears to regulate adult hippocampal neurogenesis (Cooper-Kuhn et al., 2004; Kotani et al., 2006); Mohael et al., 2005) it seems likely that changes in basal forebrain cholinergic function likely underlie the diet induced changes in physiology and behavior reported here. Indeed, previous work has shown that choline availability in utero leads to multiple changes in its own metabolism (i.e., exerts a form of metabolic imprinting) later in life (Blusztajn et al., 1998). This work has revealed that prenatal choline-deficient rats compared to CON rats have elevated acetylcholinesterase (AChE) and choline acetyltransferase (ChAT) activity, and increased synthesis of acetylcholine (ACh) from choline transported by high-affinity choline uptake (HACU) (Blusztajn et al., 1998). These alterations in choline synaptic metabolism were accompanied by reduced hippocampal ACh content and a relative inability to sustain depolarization-evoked ACh release (Meck et al., 2008). Taken together, these results indicate that in the hippocampus of prenatally choline-deficient rats, ACh turnover is accelerated (i.e., there is more rapid synthesis, degradation, and choline reutilization). Efficient recycling of acetylcholine in the synapse may be one mechanism underlying the superior performance of prenatally choline-deficient rats when compared to control-fed rats in cognitive tasks with minimal cognitive demands. However, efficient recycling is not likely to be able to provide sufficient acetylcholine release when cognitive load is very high, which may explain why we and others have shown that prenatal choline-deficient rats show cognitive deficits only when task demands are increased (for review, see Meck & Williams, 2003). In contrast, in rats that were prenatally supplemented with choline, AChE and ChAT activities, and ACh synthesized from choline transported by HACU are low, while depolarization-evoked ACh release is very high (Blusztajn et al., 1998). The latter result, together with the reduced AChE activity, suggests that intrasynaptic ACh concentrations and dwell times are increased, possibly resulting in enhanced cholinergic neurotransmission in prenatal SUP rats. The observations that ACh turnover in prenatal choline-supplemented rats is relatively slow (as indicated by low specific radioactivity of ACh newly-synthesized from exogenous choline), but that cholinergic neurotransmission is well maintained (as evidenced by robust ACh release), suggest that the pool of choline used for the synthesis of ACh in these animals may include that stored in membrane phosphatidylcholine (PC) (Ulus et al., 1989; Wecker, 1986).
One way to describe the outcome of this metabolic imprinting is that prenatal DEF rats are efficient recyclers of choline, while prenatal SUP rats have reduced recycling and there appears to be greater reliance on PC-stored choline for ACh synthesis. These adaptations seem appropriate if prenatal choline intake is a good predictor of offspring's later choline availability. However, if the adult diet does not match the prenatal diet, the developmental changes in choline metabolism may be poor adaptations for optimal adult function. For example, the physiological and behavioral processes of DEF rats that are modulated by cholinergic function have been adapted to be relatively insensitive to choline deprivation due to the efficiency of their choline metabolism. In contrast, SUP rats exhibited a greater sensitivity to choline deprivation perhaps due to the relative inefficiency in their choline metabolism (Fig. 2A). For adult choline supplementation, it is possible that DEF rats showed hippocampal/behavioral disruption with surplus levels of choline because these levels exceeded those required for optimal spatial memory performance. In contrast, SUP rats, have synapses that are well adapted for high choline adult diet, but not for a deficient diet (e.g., slow recycling, lots of acetylcholine storage), and therefore their spatial memory is optimal when the adult diet is high in choline content, and they show some impairments in performance when choline is absent from the adult diet. Hence, DEF and SUP rats exhibited dose-response functions where too little and too much adult choline resulted in sub-optimal performance, but the mode of this function was centered on different choline levels. Differences in the range of choline levels that are optimal for the adult organism may thus be due to the metabolic imprinting of choline metabolism based upon in utero availability (Blusztajn et al., 1998; Meck and Williams, 2003; Meck et al., 2008).
It has been suggested that a fetus adaptively develops according to its nutritional environment during development in preparation to most efficiently operate in a predicted adult environment of similar nutritional availability (Bateson, 2007; Bateson et al., 2004; Gluckman and Hanson, 2004). That is, a fetus that develops in a nutritionally impoverished or supplemented environment would be best adapted for a similar nutritional environment in adulthood. Epigenetic influences from variations in maternal diet, however, can have long-term consequences for how the offspring responds to its nutritional environment later in life (Cottrell and Ozanne, 2008; Gluckman and Hanson, 2005). For example, mismatches between nutritional environments in early development and adulthood may yield deleterious physiological consequences for the organism (Bateson, 2007; Cleal et al., 2007; Vickers et al., 2000, 2005). Our pattern of behavioral results offer further support for this notion in that the most extreme diet mismatches elicited cognitive impairments: adult choline deprivation significantly impaired SUP rats' radial arm maze performance while adult choline supplementation significantly impaired DEF rats' radial arm maze and water maze performance. What is striking is that a mismatch between a prenatal choline deficient diet and adult choline supplemented diet caused the most robust memory impairment. This memory impairment was also accompanied by a decrease in dentate cell proliferation, one measure of hippocampal plasticity that has been associated with aspects of hippocampal learning and memory (for review, see Aimone et al., 2006; Bruel-Jungerman et al., 2007). Similarly, a recent study (Sellayah et al., in press-this issue) discovered that hypothalamic mRNA expression of genes that regulate appetite was most disrupted in mice that were prenatally exposed to a protein restricted diet, but were then maintained on a high fat diet in adulthood. Human epidemiological and global health research has highlighted that risk factors for poor health outcomes in adulthood (e.g., obesity, type 2 diabetes, coronary heart disease) are most exaggerated in individuals who were initially exposed to nutritional impoverishment during early development, but then were faced with over-nutrition later in life (Gluckman and Hanson, 2005; Neel, 1962; Prentice and Moore, 2005). The “thrifty genotype hypothesis” proposes that certain genes that increase the risk for developing a disease have persisted because they confer some survival advantage under conditions of poor nutrition that demand metabolic efficiency, but then are detrimental when faced with over-nutrition (Neel, 1962; Prentice and Moore, 2005). Thus, the maternal diet appears to have profound effects on the developmental programming of disease, particularly when a fetus is exposed to nutritional deprivation. While we did not evaluate the predisposition to any adult disease, our behavioral and dentate cell proliferation findings suggest that a similar phenomenon may also be present with regards to developmental programming of cognitive function by the gestational availability of a single nutrient.
4. Experimental procedures
4.1. Experiment 1
4.1.1. Animals and prenatal diet
Thirty timed-pregnant Charles River (Kingston, NY) Sprague-Dawley CD strain rats were individually housed in clear polycarbonate cages (27.9×27.9×17.8 cm) that were individually ventilated with water available ad libitum and the colony was maintained at 21°C on a 12-h light/dark cycle with lights on at 7 a.m. When the pregnant rats arrived in the laboratory on day 9 of gestation (ED 9), they were fed Dyets formula AIN-76A with 1.1g/kg choline chloride substituted for choline bitartrate ad libitum (Dyets Inc., Bethlehem, PA). On the evening of ED 11 through the morning of ED 18 (ED12-17) one group of dams (n = 10) was supplemented with choline in the drinking water (SUP), one group of dams (n = 10) was switched to a choline-deficient diet (AIN-76A with no added choline) (DEF), and one group of dams (n = 10) was maintained on the standard AIN-76A diet to serve as a control (CON). All dams received 50 mM saccharin in their drinking water, while the supplemented dams received 25 mM choline chloride in addition to the saccharin (resulting in approximately 4.5 times more choline intake than the control diet). There were no significant differences in the amount of food and water consumed or body weights on ED 12-17 among control, choline-supplemented, and choline-deficient dams (ps > .05; data not shown). These doses and timing of choline treatment were chosen because these alterations in dietary choline availability during this time frame have been shown to cause persistent changes in memory precision and hippocampal plasticity in offspring (Glenn et al., 2007; McCann et al., 2006; Meck et al., 2008; Meck and Williams, 2003; Wong-Goodrich et al., 2008), and have been shown to alter the concentration of choline metabolites in the brain of the fetus (Garner et al., 1995).
On the morning of ED 18, all dams were placed back on the standard AIN-76A diet with regular drinking water for the remainder of gestation. On the morning of postnatal day (PD) 1-2, offspring from all treatment groups were removed from their dams, weighed, sexed and mixed. Pups were then randomly chosen from these groups to make up litters of 10 pups, with each litter containing half males and half females and at least three offspring from each nutritional treatment group. Each litter was randomly assigned to a foster mother that had consumed the standard diet throughout pregnancy. At weaning on PD 24, pups were housed in same-sex pairs, fed the standard AIN-76A diet, and raised to adulthood. Sixty male offspring served as subjects in Experiment 1.
4.1.2. Apparatus
The behavioral testing apparatus was a radial-arm maze with 12 arms extending at equal angles from a central platform. The maze was raised approximately 80 cm above the ground with the central platform measuring 47 cm in diameter. Each arm was 83 cm in length, 7.6 cm in width, with raised edges 1.2 cm high. At the end of each arm was a food well 2.5 cm in diameter and 0.6 cm deep. The maze was constructed of plywood and was painted a flat gray. The maze was placed in the center of the test room that measured 7.2 × 4.4 meters. There were a variety of extramaze cues in the room (e.g., transport cart with rat cages, stool where experimenter sat, table with computer and printer to record data, and a trash can) that remained in fixed locations throughout the experiment.
4.1.3. Shaping
At approximately 70 days of age, behavioral evaluation of the rats began. Ten days prior to the beginning of training the rats were food restricted such that they were maintained at 85% of free-feeding body weight. Two days prior to training, these experimentally naive rats were introduced to the maze and shaped to run to the ends of the arms that were kept continuously baited with 45-mg food pellets (Bioserve, Frenchtown, NJ). The rats were placed on the maze in groups of 6-10 after 24 hrs of food deprivation and were allowed to explore the maze until they found the food at the ends of the arms. Rats failing to find the pellets were forced down the ends and allowed to eat. After 2 days of shaping all rats ran down arms and consumed pellets.
4.1.4. Radial-arm maze training and adult diet manipulations
Figure 1A illustrates the experimental design and timeline of procedures used in Experiment 1. Rats were initially trained every other day for the first 6 days, and then daily for another 10 days. Phase I training began when all rats were were completing the maze in less than 40 choices, but had not yet reached steady-state performance. During Phase I training when all rats were still consuming the standard diet (Before condition), rats were trained 7 days/week for 2 weeks with one trial/day. Each rat was randomly assigned one of 11 different baiting patterns (8 baited and 4 unbaited arms). These assignments were held constant throughout the experiment. The rats were all trained in the same order in an effort to ensure constant conditions between sessions. At the beginning of each trial the rat was placed on the central of the maze and allowed to either complete the maze by locating all of the baited arms or make 40 choices, whichever came first. Rats that did not complete the maze in 40 choices were forced down the remaining baited arms and allowed to eat. Rats were fed their daily ration of the AIN-76A diet following the completion of each day's trials. The arms selected and choice latencies were recorded via the computer, with a choice being defined as the rat proceeding more than halfway down the arm.
During Phase II training (During condition), half of the rats in each treatment group (n's = 11) was switched to an AIN-76A diet containing no choline, while the remaining rats in each treatment group (n's = 11) were switched to an AIN-76A diet containing 5 g/kg of choline. For the first 10 days after the diets were switched, no behavioral training was conducted. Rats were then retrained for 2 weeks (7 days/week) while continuing to be maintained on either the choline-deficient or -supplemented diet.
During Phase III training (After condition), all rats were returned to our standard AIN-76A diet with 1.1 g/kg choline for a period of 10 days during which no behavioral training was conducted. Rats were then retrained for 2 weeks (7 days/week) while continuing to be maintained on our standard diet.
4.1.5. Statistical analyses
Choice accuracy data for the Before vs. During and Before vs. After treatment phases were analyzed using separate repeated measures ANOVAs for the adult choline deprivation and adult choline supplementation treatment groups. An alpha level of 0.05 was set for all statistical tests. Values are reported in the text as means ± SEM. Note that subjects contained within each experimental condition were randomly selected from different litters (n of 1/litter). Thus, we have taken the necessary precautions to be sure that our findings are not contaminated by a lack of within-litter variability.
4.2. Experiment 2
4.2.1. Animals and prenatal diet
Procedures used to generate offspring of different prenatal diet conditions were the same as those used in Experiment 1 with the exception of the following changes: 1) rats used in Experiment 2 were the offspring of a separate cohort of 40 timed-pregnant Sprague-Dawley rats (CD strain, Charles River, Kingston, NY) that were obtained on ED 9, and 2) prenatal choline supplementation was administered via food rather than drinking water. Both methods of choline supplementation have been reported in the literature and lead to alterations in hippocampal plasticity and memory function (e.g., in water, Sandstrom et al., 2002; in food, Glenn et al., 2007). Choline supplemented dams were given a version of the AIN76-A diet that contained 5 g/kg of choline chloride substituted for choline bitartrate (approximately 4.5 times the amount in the control diet). There were no significant differences in the amount of food consumed or body weights on ED12-17 between CON (n = 24), SUP (n = 8), and DEF (n = 8) dams (ps > .05; data not shown). There were no significant differences between control and prenatally choline supplemented and deficient litter size or pup birth weights (ps > .05). For Experiment 2, 34 male offspring of mothers fed the choline-supplemented (SUP; n = 13), -control (CON; n = 11), or -deficient (DEF; n = 10) diet were selected from 24 CON foster mothers. All animal procedures were in compliance with the Institutional Animal Care and Use Committee of Duke University.
4.2.2. Adult diet manipulation
Figure 1B illustrates the experimental design and timeline of procedures used in Experiment 2. At approximately 12 months of age, rats either remained on the AIN76-A control diet or were switched to a choline-supplemented diet for the duration of the study (16 weeks). The diets used in adulthood were the same control and choline-supplemented diets used during ED12-17. The following treatment groups were generated: prenatal choline supplemented diet/adult control diet (n = 6), prenatal choline supplemented diet/adult choline-supplemented diet (n = 7), prenatal control diet/adult control diet (n = 5), prenatal control diet/adult choline-supplemented diet (n = 6), prenatal choline-deficient diet/adult control diet (n = 5), and prenatal choline-deficient diet/adult choline-supplemented diet (n = 5).
4.2.3. Water maze apparatus, design, and behavioral procedures
After 12 weeks of the adult diet manipulation, rats were trained for 4 days (2 days of pretraining and 2 days of training) on a matching-to-place version of the water maze. The maze consisted of a circular pool approximately 1.8 m in diameter and filled with room temperature water. A circular platform 10 cm in diameter was submerged 2 cm below the surface of the water, and the water was clouded by non-toxic powered tempera paint to ensure that the rats could not see the platform. The pool was located in a well-lit room (approximately 5.8 m × 2.6 m in dimension) with salient extramaze cues, such as a table with a computer, shelving that contained large objects, pictures of large black shapes adhered to a curtain, a large metal trash bin, and the experimenter who sat in a chair near the computer.
Rats were trained on 3 pairs of trials each day where the platform was positioned in a different pool location for each trial pair. The first trial in each pair served as the train trial where a rat would learn a novel platform location, and the second trial in each pair served as the test trial where a rat was tasked with locating the platform location it had been exposed to on the preceding train trial. The duration of the retention interval between trials within a pair was varied to assess the effects of increasing cognitive demands on working memory. During the retention interval, rats were placed in a holding cage lined with paper towel within the water maze room. Between each trial pair, rats were placed back in their home cage with standard bedding and with their cagemate outside of the water maze room (to signal the end of the trial pair) for approximately 2 hours until the next trial pair commenced. Different start locations were used for each trial pair within a day. Platform and start locations were randomly assigned to each trial pair.
On days 1 and 2 of pretraining, all rats were trained with 3 trial pairs per day with a 1-2 min. retention delay between train and test. On each train and test trial, rats were placed in the pool along the edge from one of 8 start locations around the pool and allowed to swim freely until the platform was located or until 60 seconds passed, whichever came first. If a rat did not find the platform by the end of 60 seconds, it was lead to the platform. Rats were allowed to sit on the platform for 30 seconds after climbing on to it. On days 3 and 4 of training, rats were subjected to 3 trial pairs per day with a 1-2 min. delay on one day and the 60-min. delay on the other day (counterbalanced across rats). Testing procedures were identical to days 1 and 2 with one alteration: during train trials, rats were not allowed to swim in the pool; instead, rats were placed directly on the platform location for 20 seconds. During the test trials, rats were placed in the pool in a random start location and were allowed to swim throughout the pool to locate the hidden platform. If the rat did not locate the platform within 60 s, it was gently guided to the platform and allowed to sit on the platform for 30 seconds.
Performance on the task was measured and recorded using a computerized tracking system (HVS Image). Upon close examination of the animal's performance, it was clear that a great majority of the animals became tired during the third trial pair of the day and the modal performance was 60 s. It is also possible that proactive interference from learning the previous platform locations on the same day contributed to poorer performance on the third trial. Thus, the data for the third trial pair did not accurately represent the rats' ability to complete the maze. We therefore calculated the mean latency (s) to locate the hidden platform across trial pairs 1 and 2 during training (days 3 and 4) for each rat to use for statistical analyses.
4.2.4. Bromodeoxyuridine injections and tissue harvesting
Two weeks after the last day of water maze testing, all rats were administered daily injections of 5-bromo-2-deoxyuridine (BrdU; 100mg/kg/day, i.p.; Sigma, St. Louis, MO) for 10 consecutive days to label dividing cells in the hippocampus. This injection regimen was based on past research designed to capture the impact of a variety of manipulations on cell proliferation and survival in the hippocampus (Glenn et al., 2007; Kempermann et al., 1997; Lee et al., 2002; Rao et al., 2005). Twenty-four hours after the last BrdU injection, rats were given an overdose of a ketamine/xylazine cocktail, decapitated, and brains were rapidly removed and midsagitally sectioned. The hippocampus from one half-brain was immediately dissected for protein analyses and stored at -80° C until assayed. The other half brains were immediately post-fixed in 4% paraformaldehyde for 72 hours at 4° C and then cryoprotected in a 30% sucrose solution in 1M phosphate buffer (PB). Brains were then sectioned coronally at 60 μm on a microtome through the rostral-caudal extent of the hippocampus and every fifth section was collected in 0.1% sodium azide in 1M PB to yield five series of 5-10 sections each. The first and second series were processed for BrdU and doublecortin (DCX) immunohistochemistry, respectively, for subsequent cell counting.
4.2.5. BrdU and doublecortin immunohistochemistry
Immunohistochemical procedures for BrdU-labeling were based on the methods of Kuhn et al. (1996). One series of free-floating sections was rinsed with tris-buffered saline (TBS: pH 7.3) followed by 30 minutes in 50% methanol and 30 minutes in 0.6% hydrogen peroxide in TBS at room temperature to reduce nonspecific staining. After rinsing again in TBS, tissue was treated for 2 hours in 50% Formamide/2× SSC (0.3 M NaCl, 0.03 M sodium citrate) at 65°C, rinsed in 2× SSC for 10 minutes, incubated in 2 N HCl for 30 minutes at 37°C, and rinsed in 0.1 M boric acid (pH 8.5) for 15 minutes. Sections were rinsed in TBS, incubated in 0.1% Triton X-100 (TTX; Sigma) and 3% normal horse serum (Vector Laboratories, Burlingame, CA) in TBS for 30 minutes at room temperature, and then incubated with the primary antibody (monoclonal mouse anti-BrdU, 1:400; Boehringer Mannheim, Indianapolis, IN) for 24 hours at 4 °C. Following this, the tissue was rinsed with TBS and incubated with the secondary antibody (biotinylated horse anti-mouse, 1:200; Vector Laboratories) for 2 hours at room temperature. The tissue was then rinsed in TBS, incubated in an avidin-biotinylated peroxidase complex (ABC, Vector Laboratories) for 1 hour at room temperature, rinsed again in TBS, and treated for peroxidase detection with diaminobenzidine (Vector Laboratories, nickel intensified) for 5 minutes. Stained sections were mounted on gelatin-coated slides, dehydrated, counterstained with cresyl violet, and coverslipped.
An adjacent series of tissue sets was processed for DCX immunohistochemistry. These procedures were based on previous methods (Rao and Shetty, 2004). Free-floating sections were rinsed in TBS, treated for 30 minutes in 50% methanol, treated for 30 minutes in 0.6% hydrogen peroxide, incubated in 0.1% TTX and 3% normal horse serum in TBS for 30 minutes at room temperature, and then incubated with the primary antibody (affinity purified polyclonal goat antibody raised against a peptide mapping at the carboxy terminus of human DCX, 1:200; Santa Cruz Biotechnology, Santa Cruz, CA) overnight at room temperature. Following this, the tissue was rinsed with TBS and incubated with the secondary antibody (biotinylated horse anti-goat, 1:200; Vector Laboratories) for 2 hours at room temperature. The tissue was then rinsed in TBS, incubated in an avidin-biotinylated peroxidase complex (ABC, Vector Laboratories) for 1 hour at room temperature, rinsed again in TBS, and developed with vector grey substrate (Vector Laboratories). Stained sections were mounted on gelatin-coated slides, dehydrated, and coverslipped.
4.2.6. Quantification of BrdU- and DCX-labeled cells using unbiased stereology
BrdU- and DCX-labeled cells in each dentate gyrus were counted using a modified optical fractionator method (Mouton, 2002; West, 1993, 1999). We sampled every fifth section through the rostral-caudal extent of the dentate gyrus. StereoInvestigator (Microbrightfield Inc., Williston, VT) was used to sample exhaustively throughout the entire dentate gyrus region and count numbers of labeled cells. For counting BrdU- and DCX-labeled cells, we used a 100 × 100 μm counting frame and ∼500-600 sites per section were analyzed in 5 sections per rat. The counting frame was moved throughout the entire extent of each contour surrounding the dentate gyrus region. Because of very sporadic labeling of BrdU- and DCX-labeled cells, these parameters ensured that we sampled exhaustively throughout the entire dentate gyrus such that every BrdU- and DCX-labeled cell was counted in each section for each rat. Brains were sectioned at 60 μm, but tissue sections shrank to ∼25 μm after being processed for immunohistochemistry and dehydrated. Thus, for analysis we set an optical dissector height of 20 μm with a 2-μm guard zone and counted stained cells in each frame using a 40× objective lens. The total number of cells counted was multiplied by 5, and then by 2 (to account for both hemispheres) to generate a total estimate. Finally, estimates of the volume of the region of dentate gyrus that was sampled for BrdU and DCX estimates were made using Cavalleri's principle (Mouton, 2002). For each section examined the area of the dentate gyrus was calculated by the StereoInvestigator software and was based on the boundaries of the contour tracings. Volume estimates were obtained by multiplying the section area estimates with the spacing between sampled areas. Spacing was derived by multiplying the measured, post-histology thickness of each sample by the number of sections examined.
4.2.7. ELISA for neurotrophic and growth factors
The entire hippocampus from the remaining half-brain from each rat was carefully dissected out and used for ELISA assays. All dissected samples were first weighed individually to get their wet weights. Whole tissue extracts were prepared by adding lysis buffer (50 mM Tris pH 7.5, 150 mM NaCl, 1% Nonidet NP-40, 10% glycerol, 2 mM 4-(2-aminoethyl)-benzenesulfonyl fluoride, 1 μg/ml leupeptin, 2 μg/ml aprotinin, 2 μg/ml pepstatin), followed by gentle sonication, incubation on ice for 15 min, and a brief centrifugation to clear. The supernatant from each sample was diluted 5 times with Dulbecco's PBS and acidified to pH 2.6. After 15 min of incubation at room temperature, the diluted supernatants were neutralized to pH 7.6, aliquoted and frozen for subsequent measurement of BDNF, NGF, and IGF-1 using ELISA. The above procedure was performed for all samples because a previous study indicated that acidification and subsequent neutralization with base increase the amount of detectable neurotrophins in extracts of CNS tissues (Okragly and Haak-Frendscho, 1997).
The ChemiKine™ BDNF sandwich ELISA kit (Chemicon Int., Inc.) was used to assay the BDNF levels in hippocampal lysates. BDNF levels were measured according to manufacturer's instructions. First, sample/standard diluent was added to each well of the microplate (100 μL/well). The standards were serially diluted (1:2) from 500-0 pg/mL. Standards and samples (100 μL/well) were added to wells in duplicate and were incubated overnight at 4°C. The wells were rinsed with wash buffer and then incubated with diluted biotinylated mouse anti-BDNF monoclonal antibody (1:1000) for 2 h at room temperature. After rinsing the plate again with wash buffer, diluted streptavidin-HRP conjugate solution (1:1000) was added to wells and incubated for 1 h at room temperature. Plates were again washed and then warm TMB buffer was added. After 15 min incubation at room temperature, stop solution was added.
The Quantikine® sandwich ELISA kits (R & D Systems) were used to assay IGF-1 levels in the samples. ELISAs were performed according to manufacture's instructions. Briefly, microplates were pre-coated with the first primary monocolonal antibody. Assay diluents were added to each well of the microplate (50 μL/well). Standard control samples were diluted serially (1:2) from 6–0 ng/mL or with the respective calibrator diluents and plated to two columns of wells (50 μL/well) designated for standard curve in every plate. The frozen ELISA samples (described above) were thawed on ice, and every sample plated in duplicate for measurement of each of the factors. Following a 2 h incubation at room temperature, wells were rinsed in wash buffer and treated with an enzyme-linked second primary antibody solution for 2 hours. The second primary antibody was a polyclonal IGF-1 antibody conjugated to HRP in IGF-1 detection kit. The wells were rinsed in wash buffer and a substrate solution was added to the wells and incubated in the dark for 30 min. The color reaction was stopped with 1M hydrochloric acid.
The Emax® immunoassay systems (Promega) were used to measure NGF in the samples. Flat-bottom 96 well plates (NUNC) were first coated with solution containing a polyclonal antibody against NGF (the first primary antibody solution) prepared in carbonate coating buffer (100 μL/well, 1:1000 dilution) and incubated for 16 h at 4°C. Following a wash in TBST (Tris-buffered saline solution containing Tween 20), the coated wells were incubated with block and sample buffer (1X) for 1 h at room temperature and washed again with TBST. Standard control samples for NGF were diluted serially (1:2) from 250–0 pg/mL, and plated in duplicate (100 μL/well). The frozen ELISA samples (described above) were thawed on ice, and every sample plated in duplicate for measurement of NGF. Following a 6 h incubation at room temperature, wells were washed in TBST. Diluted monoclonal antibody against either NGF (the second primary antibody solution; 1:4000) was added to each well and incubate overnight at 4°C. The wells were washed in TBST, incubated with appropriate secondary antibody conjugated to peroxidase for 2.5 h, washed again in TBST, and treated with tetramethyl benzidine (TMB) substrate for 10 min. The chromogen reaction was stopped by adding 100 μL of 1 N hydrochloric acid.
The optical density of each well was measured using the Victor3 microplate reader (PerkinElmer Life Sciences). The intensity of color was measured at a wavelength of 450 nm for all ELISAs. In order to correct for optical imperfections in the plate, readings at 540 nm were subtracted from readings at 450 nm. The standard curve was used to assess the validity of the protocol and to determine the relative concentrations of the growth factors. Values in all samples were normalized per gram of tissue assayed, and the average value for each sample was calculated separately before determining the group means.
4.2.8. Statistical analyses
Latency data were subjected to separate 2 (Adult Diet: Control vs. Supplemented) × 2 (Delay: 1-2 min. vs. 60 min.) mixed ANOVAs for prenatal SUP, CON, and DEF groups. Apriori comparisons were made between the two adult diet conditions within each delay. Numbers of BrdU-labeled and DCX-labeled cells estimated with the optical fractionator, volumes of dentate gyrus that were estimated using Cavalleri's principle, and growth factor protein levels (expressed as percent of control levels) were subjected to 3 (Prenatal Diet: SUP vs. CON vs. DEF) × 2 (Adult Diet: Control vs. Supplemented) ANOVAs. Where appropriate 3 × 2 ANOVAs were decomposed into separate two-way ANOVAs (see Results). Apriori comparisons were made between the two adult diet conditions within each prenatal diet treatment. An alpha level of 0.05 was set for all statistical tests. Values are reported in the text as means ± SEM. Again, subjects contained within each experimental condition were randomly selected from different litters (n of 1/litter). Thus, we have taken the necessary precautions to be sure that our findings are not contaminated by a lack of within litter variability.
Acknowledgments
We would like to thank John Jones, Jason Oak, Madeline Pfau, Sophia Cao, and Jeptha Johnson for assistance with data collection. This work was supported by AG009525 to J.K.B., W.H.M, and C.L.W.
Abbreviations
- CON
prenatal choline control
- SUP
prenatal choline supplemented
- DEF
prenatal choline deficient
- BrdU
bromodeoxyuridine
- DCX
doublecortin
- BDNF
brain derived neurotrophic factor
- NGF
nerve growth factor
- IGF-1
insulin-like growth factor-1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aimone JB, Wiles J, Gage FH. Potential role for adult neurogenesis in the encoding of time in new memories. Nat Neurosci. 2006;9:723–7. doi: 10.1038/nn1707. [DOI] [PubMed] [Google Scholar]
- Albright CD, Tsai AY, Friedrich CB, Mar MH, Zeisel SH. Choline availability alters embryonic development of the hippocampus and septum in the rat. Dev Brain Res. 1999;113:13–20. doi: 10.1016/s0165-3806(98)00183-7. [DOI] [PubMed] [Google Scholar]
- Bartus RT, Dean RL, 3rd, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217:408–14. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- Bateson P. Developmental plasticity and evolutionary biology. J Nutr. 2007;137:1060–62. doi: 10.1093/jn/137.4.1060. [DOI] [PubMed] [Google Scholar]
- Bateson P, Barker D, Clutton-Brock T, Deb D, D'Udine B, Foley RA, Gluckman P, Godfrey K, Kirkwood T, Lahr MM, McNamara J, Metcalfe NB, Monaghan P, Spencer HG, Sultan SE. Developmental plasticity and human health. Nature. 2004;430:419–21. doi: 10.1038/nature02725. [DOI] [PubMed] [Google Scholar]
- Bengzon J, Kokaia Z, Elmer E, Nanobashvili A, Kokaia M, Lindvall O. Apoptosis and proliferation of dentate gyrus neurons after single and intermittent limbic seizures. Proc Natl Acad Sci USA. 1997;94:10432–7. doi: 10.1073/pnas.94.19.10432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blusztajn JK, Cermak JM, Holler T, Jackson DA. Imprinting of hippocampal metabolism of choline by its availability during gestation: implications for cholinergic neurotransmission. J Physiol Paris. 1998;92:199–203. doi: 10.1016/s0928-4257(98)80010-7. [DOI] [PubMed] [Google Scholar]
- Blusztajn JK, Wurtman RJ. Choline and cholinergic neurons. Science. 1983;221:614–20. doi: 10.1126/science.6867732. [DOI] [PubMed] [Google Scholar]
- Brandner C. Perinatal choline treatment modifies the effects of a visuo-spatial attractive cue upon spatial memory in naive adult rats. Brain Res. 2002;928:85–95. doi: 10.1016/s0006-8993(01)03363-7. [DOI] [PubMed] [Google Scholar]
- Bruel-Jungerman E, Rampon C, Laroche S. Adult hippocampal neurogenesis, synaptic plasticity and memory: facts and hypotheses. Rev Neurosci. 2007;18:93–114. doi: 10.1515/revneuro.2007.18.2.93. [DOI] [PubMed] [Google Scholar]
- Cermak JM, Blusztajn JK, Meck WH, Williams CL, Fitzgerald CM, Rosene DL, Loy R. Prenatal availability of choline alters the development of acetylcholinesterase in the rat hippocampus. Dev Neurosci. 1999;21:94–104. doi: 10.1159/000017371. [DOI] [PubMed] [Google Scholar]
- Cermak JM, Holler T, Jackson DA, Blusztajn JK. Prenatal availability of choline modifies development of the hippocampal cholinergic system. FASEB J. 1998;12:349–57. doi: 10.1096/fasebj.12.3.349. [DOI] [PubMed] [Google Scholar]
- Cheng RK, MacDonald CJ, Williams CL, Meck WH. Prenatal choline supplementation alters the timing, emotion, and memory performance (TEMP) of adult male and female rats as indexed by differential reinforcement of low-rate schedule behavior. Learn Mem. 2008;15:153–162. doi: 10.1101/lm.729408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng RK, Meck WH. Prenatal choline supplementation increases sensitivity to time by reducing non-scalar sources of variance in adult temporal processing. Brain Res. 2007;1186:242–254. doi: 10.1016/j.brainres.2007.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng RK, Meck WH, Williams CL. α7 nicotinic acetylcholine receptors and temporal memory: synergistic effects of combining prenatal choline and nicotine on reinforcement-induced resetting of an interval clock. Learn Mem. 2006;13:127–134. doi: 10.1101/lm.31506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleal JK, Poore KR, Boullin JP, Khan O, Chau R, Hambidge O, Torrens C, Newman JP, Poston L, Noakes DE, Hanson MA, Green LR. Mismatched pre- and postnatal nutrition leads to cardiovascular dysfunction and altered renal function in adulthood. Proc Natl Acad Sci USA. 2007;104:9529–33. doi: 10.1073/pnas.0610373104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper-Kuhn CM, Winkler J, Kuhn HG. Decreased neurogenesis after cholinergic forebrain lesion in the adult rat. J Neurosci Res. 2004;77:155–65. doi: 10.1002/jnr.20116. [DOI] [PubMed] [Google Scholar]
- Cottrell EC, Ozanne SE. Early life programming of obesity and metabolic disease. Physiol Behav. 2008;94:17–28. doi: 10.1016/j.physbeh.2007.11.017. [DOI] [PubMed] [Google Scholar]
- Craciunescu CN, Albright CD, Mar MH, Song J, Zeisel SH. Choline availability during embryonic development alters progenitor cell mitosis in developing mouse hippocampus. J Nutr. 2003;133:3614–8. doi: 10.1093/jn/133.11.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Nutrition Board of the Institute of Medicine. Dietary reference intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, panthotenic acid, biotin, and choline. National Academy Press; Washington, DC: 1998. [PubMed] [Google Scholar]
- Garner SC, Mar MH, Zeisel SH. Choline distribution and metabolism in pregnant rats and fetuses are influenced by the choline content of the maternal diet. J Nutr. 1995;125:2851–8. doi: 10.1093/jn/125.11.2851. [DOI] [PubMed] [Google Scholar]
- Glenn MJ, Gibson EM, Kirby ED, Mellott TJ, Blusztajn JK, Williams CL. Prenatal choline availability modulates hippocampal neurogenesis and neurogenic responses to enriching experiences in adult female rats. Eur J Neurosci. 2007;25:2473–82. doi: 10.1111/j.1460-9568.2007.05505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, Pinal C. The developmental origins of adult disease. Matern Child Nutr. 2005;1:130–41. doi: 10.1111/j.1740-8709.2005.00020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME, Giocomo LM. Cholinergic modulation of cortical function. J Mol Neurosci. 2006;30:133–5. doi: 10.1385/JMN:30:1:133. [DOI] [PubMed] [Google Scholar]
- Holmes-McNary MQ, Loy R, Mar MH, Albright CD, Zeisel SH. Apoptosis is induced by choline deficiency in fetal brain and in PC12 cells. Dev Brain Res. 1997;101:9–16. doi: 10.1016/s0165-3806(97)00044-8. [DOI] [PubMed] [Google Scholar]
- Jones JP, Meck WH, Williams CL, Wilson WA, Swartzwelder HS. Choline availability to the developing rat fetus alters adult hippocampal long-term potentiation. Dev Brain Res. 1999;118:159–67. doi: 10.1016/s0165-3806(99)00103-0. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–5. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- Kotani S, Yamauchi T, Teramoto T, Ogura H. Pharmacological evidence of cholinergic involvement in adult hippocampal neurogenesis in rats. Neurosci. 2006;142:505–14. doi: 10.1016/j.neuroscience.2006.06.035. [DOI] [PubMed] [Google Scholar]
- Kruman II, Mouton PR, Emokpae R, Jr, Cutler RG, Mattson MP. Folate deficiency inhibits proliferation of adult hippocampal progenitors. Neuroreport. 2005;16:1055–9. doi: 10.1097/00001756-200507130-00005. [DOI] [PubMed] [Google Scholar]
- Lamoureux JA, Williams CL, Meck WH. Availability of prenatal dietary choline alters the context sensitivity of Pavlovian conditioning in adult rats. Learn Mem. 2008 doi: 10.1101/lm.1058708. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Seroogy KB, Mattson MP. Dietary restriction enhances neurotrophin expression and neurogenesis in the hippocampus of adult mice. J Neurochem. 2002;80:539–47. doi: 10.1046/j.0022-3042.2001.00747.x. [DOI] [PubMed] [Google Scholar]
- Lewis PD, Patel AJ, Balazs R. Effect of undernutrition on cell generation in the rat hippocampus. Brain Res. 1979;168:186–9. doi: 10.1016/0006-8993(79)90137-9. [DOI] [PubMed] [Google Scholar]
- McCann JC, Hudes M, Ames BN. An overview of evidence for a causal relationship between dietary availability of choline during development and cognitive function in offspring. Neurosci Biobehav Rev. 2006;30:696–712. doi: 10.1016/j.neubiorev.2005.12.003. [DOI] [PubMed] [Google Scholar]
- McKeon-O'Malley C, Siwek D, Lamoureux JA, Williams CL, Kowall NW. Prenatal choline deficiency decreases the cross-sectional area of cholinergic neurons in the medial septal nucleus. Brain Res. 2003;977:278–83. doi: 10.1016/s0006-8993(03)02599-x. [DOI] [PubMed] [Google Scholar]
- Meck WH, Smith RA, Williams CL. Pre- and postnatal choline supplementation produces long-term facilitation of spatial memory. Dev Psychobiol. 1988;21:339–53. doi: 10.1002/dev.420210405. [DOI] [PubMed] [Google Scholar]
- Meck WH, Smith RA, Williams CL. Organizational changes in cholinergic activity and enhanced visuospatial memory as a function of choline administered prenatally or postnatally or both. Behav Neurosci. 1989;103:1234–41. doi: 10.1037//0735-7044.103.6.1234. [DOI] [PubMed] [Google Scholar]
- Meck WH, Williams CL. Characterization of the facilitative effects of perinatal choline supplementation on timing and temporal memory. Neuroreport. 1997a;8:2831–5. doi: 10.1097/00001756-199709080-00005. [DOI] [PubMed] [Google Scholar]
- Meck WH, Williams CL. Perinatal choline supplementation increases the threshold for chunking in spatial memory. Neuroreport. 1997b;8:3053–9. doi: 10.1097/00001756-199709290-00010. [DOI] [PubMed] [Google Scholar]
- Meck WH, Williams CL. Simultaneous temporal processing is sensitive to prenatal choline availability in mature and aged rats. Neuroreport. 1997c;8:3045–51. doi: 10.1097/00001756-199709290-00009. [DOI] [PubMed] [Google Scholar]
- Meck WH, Williams CL. Choline supplementation during prenatal development reduces proactive interference in spatial memory. Dev Brain Res. 1999;118:51–9. doi: 10.1016/s0165-3806(99)00105-4. [DOI] [PubMed] [Google Scholar]
- Meck WH, Williams CL. Metabolic imprinting of choline by its availability during gestation: implications for memory and attentional processing across the lifespan. Neurosci Biobehav Rev. 2003;27:385–99. doi: 10.1016/s0149-7634(03)00069-1. [DOI] [PubMed] [Google Scholar]
- Meck WH, Williams CL, Cermak JM, Blusztajn JK. Developmental periods of choline sensitivity provide an ontogenetic mechanism for regulating memory capacity and age-related dementia. Front Integr Neurosci. 2008;1:7. doi: 10.3389/neuro.07.007.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellott TJ, Williams CL, Meck WH, Blusztajn JK. Prenatal choline supplementation advances hippocampal development and enhances MAPK and CREB activation. FASEB J. 2004;18:545–7. doi: 10.1096/fj.03-0877fje. [DOI] [PubMed] [Google Scholar]
- Mohapel P, Ekdahl CT, Lindvall O. Status epilepticus severity influences the long-term outcome of neurogenesis in the adult dentate gyrus. Neurobiol Dis. 2004;15:196–205. doi: 10.1016/j.nbd.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Mouton P. Principles and practices of unbiased stereology. The Johns Hopkins University Press; Baltimore: 2002. vol. [Google Scholar]
- Neel JV. Diabetes mellitus: a “thrifty” genotype rendered detrimental by “progress”? Am J Hum Genet. 1962;14:353–62. [PMC free article] [PubMed] [Google Scholar]
- Niculescu MD, Yamamuro Y, Zeisel SH. Choline availability modulates human neuroblastoma cell proliferation and alters the methylation of the promoter region of the cyclin-dependent kinase inhibitor 3 gene. J Neurochem. 2004;89:1252–9. doi: 10.1111/j.1471-4159.2004.02414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okragly AJ, Haak-Frendscho M. An acid-treatment method for the enhanced detection of GDNF in biological samples. Exp Neurol. 1997;145:592–6. doi: 10.1006/exnr.1997.6500. [DOI] [PubMed] [Google Scholar]
- Parent JM, Yu TW, Leibowitz RT, Geschwind DH, Sloviter RS, Lowenstein DH. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J Neurosci. 1997;17:3727–38. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel MS, Vadlamudi SP, Johanning GL. Overview of pup in a cup model: hepatic lipogenesis in rats artificially reared on a high-carbohydrate formula. J Nutr. 1993;123:373–7. doi: 10.1093/jn/123.suppl_2.373. [DOI] [PubMed] [Google Scholar]
- Prentice AM, Moore SE. Early programming of adult diseases in resource poor countries. Arch Dis Child. 2005;90:429–32. doi: 10.1136/adc.2004.059030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyapali GK, Turner DA, Williams CL, Meck WH, Swartzwelder HS. Prenatal dietary choline supplementation decreases the threshold for induction of long-term potentiation in young adult rats. J Neurophysiol. 1998;79:1790–6. doi: 10.1152/jn.1998.79.4.1790. [DOI] [PubMed] [Google Scholar]
- Rao MS, Hattiangady B, Abdel-Rahman A, Stanley DP, Shetty AK. Newly born cells in the ageing dentate gyrus display normal migration, survival and neuronal fate choice but endure retarded early maturation. Eur J Neurosci. 2005;21:464–76. doi: 10.1111/j.1460-9568.2005.03853.x. [DOI] [PubMed] [Google Scholar]
- Rao MS, Shetty AK. Efficacy of doublecortin as a marker to analyse the absolute number and dendritic growth of newly generated neurons in the adult dentate gyrus. Eur J Neurosci. 2004;19:234–46. doi: 10.1111/j.0953-816x.2003.03123.x. [DOI] [PubMed] [Google Scholar]
- Ricceri L, Berger-Sweeney J. Postnatal choline supplementation in preweanling mice: sexually dimorphic behavioral and neurochemical effects. Behav Neurosci. 1998;112:1387–92. [PubMed] [Google Scholar]
- Sandstrom NJ, Loy R, Williams CL. Prenatal choline supplementation increases NGF levels in the hippocampus and frontal cortex of young and adult rats. Brain Res. 2002;947:9–16. doi: 10.1016/s0006-8993(02)02900-1. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, Sollas AL, Goodman JH. Spontaneous recurrent seizures after pilocarpine-induced status epilepticus activate calbindin-immunoreactive hilar cells of the rat dentate gyrus. Neurosci. 2002;111:71–81. doi: 10.1016/s0306-4522(01)00599-1. [DOI] [PubMed] [Google Scholar]
- Schenk F, Brandner C. Indirect effects of peri- and postnatal choline treatment on place-learning abilities in rat. Psychobiology. 1995;23:302–313. [Google Scholar]
- Ulus IH, Wurtman RJ, Mauron C, Blusztajn JK. Choline increases acetylcholine release and protects against the stimulation-induced decrease in phosphatide levels within membranes of rat corpus striatum. Brain Res. 1989;484:217–27. doi: 10.1016/0006-8993(89)90364-8. [DOI] [PubMed] [Google Scholar]
- Vickers MH, Breier BH, Cutfield WS, Hofman PL, Gluckman PD. Fetal origins of hyperphagia, obesity, and hypertension and postnatal amplification by hypercaloric nutrition. Am J Physiol Endocrinol Metab. 2000;279:E83–7. doi: 10.1152/ajpendo.2000.279.1.E83. [DOI] [PubMed] [Google Scholar]
- Vickers MH, Gluckman PD, Coveny AH, Hofman PL, Cutfield WS, Gertler A, Breier BH, Harris M. Neonatal leptin treatment reverses developmental programming. Endocrinology. 2005;146:4211–6. doi: 10.1210/en.2005-0581. [DOI] [PubMed] [Google Scholar]
- Wecker L. Neurochemical effects of choline supplementation. Can J Physiol Pharmacol. 1986;64:329–33. doi: 10.1139/y86-054. [DOI] [PubMed] [Google Scholar]
- West MJ. New stereological methods for counting neurons. Neurobiol Aging. 1993;14:275–85. doi: 10.1016/0197-4580(93)90112-o. [DOI] [PubMed] [Google Scholar]
- West MJ. Stereological methods for estimating the total number of neurons and synapses: issues of precision and bias. Trends Neurosci. 1999;22:51–61. doi: 10.1016/s0166-2236(98)01362-9. [DOI] [PubMed] [Google Scholar]
- Williams CL, Meck WH, Heyer DD, Loy R. Hypertrophy of basal forebrain neurons and enhanced visuospatial memory in perinatally choline-supplemented rats. Brain Res. 1998;794:225–38. doi: 10.1016/s0006-8993(98)00229-7. [DOI] [PubMed] [Google Scholar]
- Wong-Goodrich SJ, Mellott TJ, Glenn MJ, Blusztajn JK, Williams CL. Prenatal choline supplementation attenuates neuropathological response to status epilepticus in the adult rat hippocampus. Neurobiol Dis. 2008;30:255–69. doi: 10.1016/j.nbd.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisel SH, Blusztajn JK. Choline and human nutrition. Ann Rev Nutr. 1994;14:269–96. doi: 10.1146/annurev.nu.14.070194.001413. [DOI] [PubMed] [Google Scholar]
- Zeisel SH, Da Costa KA, Franklin PD, Alexander EA, Lamont JT, Sheard NF, Beiser A. Choline, an essential nutrient for humans. FASEB J. 1991;5:2093–8. [PubMed] [Google Scholar]