Abstract
Lateral interactions between hydrophobic transmembrane (TM) helices in membranes underlie the folding of multi-span membrane proteins and signal transduction by receptor tyrosine kinases (RTKs). Quantitative measurements of dimerization energetics in membranes are required to uncover the physical principles behind these processes. Here we overview how FRET measurements can be used to determine the thermodynamics of TM helix homo- and heterodimerization in vesicles and in supported bilayers. Such measurements can shed light on the molecular mechanism behind pathologies arising due to single-amino acid mutations in membrane proteins.
Introduction
The folding of membrane proteins is mediated by lateral interactions between adjacent TM helices (MacKenzie 2006,White and Wimley 1999). The lateral interactions between the TM domains of receptor tyrosine kinases (RTKs) are important for the process of signal transduction across the plasma membrane and for the regulation of cell growth, differentiation, motility, or death (Li and Hristova 2006). Therefore, free energy measurements of transmembrane (TM) helix dimerization are needed for understanding the physical principles behind membrane protein folding and signal transduction. Importantly, these measurements need to be carried out in quantitative manner in the native bilayer environment. Here we overview how FRET can be used to determine the concentrations of monomeric and dimeric TM helices in bilayers. Such measurements yield association constants and free energies of TM helix dimerization in vesicles and in supported bilayers, environments that mimic the biological membrane.
Challenges in quantitative measurements of interactions between TM helices in bilayers
First, there are challenges due to the chemical nature of the hydrophobic TM helices. The production of pure TM helices that are labeled with FRET dyes can be expensive and labor-intensive, due to low synthesis yields, low labeling yields, and low purification yields (Iwamoto et al 2005). Second, there are challenges associated with the incorporation of the TM helices in the bilayers, and in general, sample preparation and handling.
Each sample is prepared from stocks of lipids and donor- and acceptor-labeled proteins. Since there is no material exchange between the membranes and the aqueous medium, a new sample has to be prepared when an experimental parameter, such as peptide-to-lipid ratio, donor-to-acceptor ratio, or peptide concentration, is changed (discussed in details in (You et al 2005)). Furthermore, for each FRET measurement, three different samples are needed: (i) a sample containing both donor and acceptor, (ii) a “no FRET” control containing the donor only, and (iii) an acceptor-only control to monitor the direct excitation of the acceptor fluorophore.
Synthesis and purification protocols for TM helices are now available in the literature (Iwamoto et al 1994,Iwamoto et al 2005). Many hydrophobic TM domains contain aromatic residues such as Trp or Tyr, and their absorbance can be used to determine the concentration of the stock peptide solutions. Circular dichroism (CD) can be used to confirm that the peptides are helical. Some solvents, such as methanol, dissolve TM helices but do not support their helical structure. Such solvents should be avoided because they promote the misfolding and the aggregation of the TM domains into β-sheets. Furthermore, in liposomes, one must prove that the orientation of the helix is indeed transmembrane (Li et al 2005,You et al 2005). If the organic solvents used do not dissolve the two components (lipids and peptides) equally well, the helices may get “trapped” at the interface. Therefore, the tilt of the helices with respect to the bilayer normal should be measured using oriented circular dichroism (OCD) in oriented multilayers prior to hydration (Li et al 2005). TM helices exhibit characteristic OCD spectra with a single minimum around 230 nm and a maximum around 200 nm.
Furthermore, when peptides and lipids are mixed in organic solvents, they can either (i) form a homogeneous mixture (i.e., a single “phase”) or (ii) segregate into two or more distinct lipid- and peptide-rich phases. Therefore, homogeneity of peptide/lipid mixtures should be assessed using different methods such as X-ray diffraction, fluorescence microscopy, and FRET efficiency measurements (Li et al 2005).
Phase separation in lipid systems can be observed using X-ray diffraction. A homogeneous sample gives rise to a single set of Bragg peaks. A phase-separated sample shows either (i) two sets of Bragg peaks or (ii) a single set of Bragg peaks, identical to pure lipid samples, and one or several sharp lines due to protein aggregates. Since phase separation is particularly likely to occur in dry samples, X-ray diffraction of dry multilayers provides a very stringent test for possible phase separation (You et al 2005). Aggregation of the proteins due to their dissolution from the lipid matrix can be further detected by measuring FRET as a function of acceptor fraction (Li et al 2005,Li et al 2006,You et al 2006). If the helices form dimers but no higher order aggregates (such that only monomers and dimers are present), the FRET efficiency depends linearly on the acceptor ratio (Adair and Engelman 1994). However, larger peptide aggregates will show a nonlinear dependence on acceptor fraction.
Further experimental challenges can arise due to sample-to-sample variations in protein concentrations due to the very low solubility of the hydrophobic TM helices. These variations in concentration can introduce uncertainties in the calculated dimerization free energies. This problem can be resolved by using the EmEx-FRET method (Merzlyakov et al 2007) described below.
Before measuring FRET, one needs to investigate if the dyes affect (i) the secondary structure of the TM domains, by measuring the helicities of labeled and unlabeled peptides using circular dichroism (CD), and (ii) the dimerization propensities by running SDS-PAGE gels of labeled and unlabeled peptides (Iwamoto et al 2005). If the dyes are attached to the termini, or close to the termini, they generally have no effect on helicity or dimerization (Iwamoto et al 2005).
For quantitative FRET measurements, one needs to measure the labeling yields. The attachment of the fluorophores to the hydrophobic TM domains does not always change the elution time on the HPLC column. In this case, the labeled and unlabeled peptides cannot be separated, and the labeling yield is determined by comparing the concentration of the labeled peptides (determined via absorbance measurements of fluorophore concentrations) and the concentration of all peptides (measured using CD). Quantitative FRET measurements of dimerization eneregetics require high labeling yields (You et al 2005).
Bilayer platforms for measuring TM helix interactions using FRET
Three bilayer platforms have proven useful in the studies of TM helix dimerization: multilamellar vesicles (MLVs), large unilamellar vesicles (LUVs), and supported bilayers (Merzlyakov et al 2006,You et al 2005). In all cases, lipids and peptides are first co-dissolved in organic solvents to ensure complete mixing of the two components, followed by the removal of the organic solvents. We recommend that a mixture of solvents, such as HFIP/TFE/chloroform is used to co-dissolve peptides and lipids.
(i) Multilamellar vesicles
The hydration of a dry protein/lipid films leads to the formation of multilamellar vesicles (MLVs) containing TM helices (You et al 2005). MLVs can be used for FRET measurements of dimerization energetics after a single freeze–thaw cycle (You et al 2005). Just after one cycle, the turbidity of the MLVs is greatly reduced. This decrease in turbidity is surprising, given that it does not occur for lipid MLVs that do not contain the peptides. Therefore, it appears that the TM helices promote the formation of relatively small MLVs for peptide concentrations ranging from 0.01 to 1 mol%. Thus, the CD and fluorescence spectra can be measured in MLVs (You et al 2005).
We have observed that the TM peptides are homogeneously distributed when MLVs are prepared, and their distribution remains homogeneous over time. The fluorescence intensity and the FRET signal of such MLVs are very stable over a month (You et al 2005). Furthermore, the fluorescence intensity measured for the MLVs is not affected by light scattering. Finally, the FRET efficiency is determined only by the protein-to-lipid ratio, not by the total peptide and lipid concentrations, such that the measurements are relevant for processes occurring in cellular membranes (You et al 2005).
(ii) Extruded large unilamellar vesicles
The MLVs can be extruded using a 100-nm pore diameter membrane (Avanti) to produce large unilamellar vesicles (LUVs). There is no statistically significant change in FRET in the LUVs compared to MLVs. Therefore, FRET efficiencies and helix–helix interactions measured in MLVs and LUVs are comparable.
A potential problem with extrusion is the loss of peptides and lipids in the extrusion process. We therefore determined peptide and lipid concentrations before and after extrusion (You et al 2005). We found that typical losses of peptides and lipids were identical; that is, between 13 and 18% of both lipids and peptides were lost during the extrusion process. The peptide-to-lipid ratio does not change during the extrusion process, and therefore the FRET efficiency, which is determined solely by the peptide-to-lipid ratio, remains unchanged after extrusion (You et al 2005).
(iii) Small unilamellar vesicles
We have shown that the small unilamellar vesicles (SUVs) with TM peptides aggregate and come out of solution, such that the fluorescence signal gradually decreases over time (You et al 2005). Thus, SUVs are not an appropriate system for FRET measurements, particularly if Eq. (2) below is used to calculate FRET efficiencies.
(iv) Surface-supported bilayers
To study the interactions between TM films in supported bilayers, we have recently developed a surface-supported bilayer platform based on the so-called “directed assembly method” ((Merzlyakov et al 2006), see Fig. 1). In this assembly method, the peptides are incorporated into lipid monolayers at the air/water interface, and the monolayers are then transferred onto glass substrates using Langmuir-Blodgett (LB) deposition. The bilayers are completed via lipid vesicle fusion on top of the LB monolayers (see (Merzlyakov et al 2006) for detailed protocol). The novelty in the assembly is the incorporation of the peptides into the monolayer at the first step of the bilayer assembly, which allows control over the peptide concentration and orientation (Merzlyakov et al 2006). The transmembrane orientation of the peptides in the supported bilayer was confirmed directly by oriented circular dichroism (OCD) - both the shape and the amplitude of the OCD spectra showed that the peptides are transmembrane (Merzlyakov et al 2006). The lateral mobility of the peptides was assessed in fluorescence recovery after photobleaching (FRAP) experiments. Although the peptides were moving much slower than lipids, there was no immobile peptide fraction (Merzlyakov et al 2006), such that the energetics of TM helix dimarization can be studied.
Figure 1.
Illustration of the directed assembly method. Reprinted with permission from (Merzlyakov et al 2006). Copyright (2006) American Chemical Society.
The quartz slide, with the coverslip supporting the bilayer, was inserted into the home-built adapter shown in Fig. 2A. The adapter was designed to fit into the standard cuvette holder of a fluorometer. The emission and excitation spectra of labeled peptides in supported bilayers were measured in a Fluorolog-3 fluorometer (Jobin Yvon, Edison, NJ). To reduce the background from stray light, the angle between the excitation beam and the quartz slide was set to 35 degrees (Fig. 2B), such that the reflected excitation beam was not captured by the photodetector.
Figure 2.

(A) Schematic drawing of the adapter used for spectral FRET measurements of TM helix dimerization in surface supported bilayers. The adapter fits in the cuvette holder of a standard fluorometer. A fluid bilayer is supported on the coverslip, and is sandwiched between the coverslip and the quartz slide. The gap between the coverslip and the quartz slide is filled with buffer, such that the bilayer is fully hydrated. (B) Top view showing the bilayer, supported on the coverslip, and the quartz slide (not drawn to scale). Reprinted with permission from (Merzlyakov et al 2006). Copyright (2006) American Chemical Society.
The fluorescence spectra of labeled peptides in the supported bilayers are very reproducible: the typical standard deviation in fluorescence intensity is below a few percent (Merzlyakov et al 2006). Furthermore, the fluorescence intensity in these bilayers is proportional to the fluorophore concentration, as expected. Bleaching during spectral collection is negligible. Thus, measurements of fluorescence spectra in surface-supported bilayers are reproducible and reliable, even for dyes with poor photostability, such as fluorescein. Furthermore, the spectra of the surface supported bilayers (i.e. close to the surface) are very similar to spectra of suspended liposomes (Merzlyakov et al 2006).
We have recorded FRET spectra of fluorescein and rhodamine labeled peptides, while varying the donor-to-acceptor ratio and keeping the total peptide concentration fixed. The dependence of the FRET efficiency on acceptor fraction is linear, indicating that only monomers and dimers are present and that the peptides do not aggregate in the surface-supported bilayers (Merzlyakov et al 2006). Furthermore, we have shown that the FRET efficiencies, measured in surface supported bilayers, are similar to the ones in liposomes (Merzlyakov et al 2006).
There are advantages to performing the measurements in supported bilayers, as compared to free liposomes in suspension: (i) The amount of peptide required for an experiment in surface supported bilayers is 1/100 of the peptide amount typically used in vesicle solutions, thus substantially reducing the cost of research. (ii) Supported bilayer platforms could be adapted to parallel high-throughput measurements of lateral protein interactions in bilayers, paving the way for the development of novel sensing devices that utilize membrane proteins (Sackmann 1996,Sackmann and Tanaka 2000). (iii) The directed assembly method may offer a means to achieve unidirectional orientation of the peptides (Merzlyakov et al 2006) because the orientation of the peptides occurs at the air-water interface, and therefore asymmetry in the sequence of the flanking residues may ensure the unidirectionality of the peptides and may prevent non-biological lateral interactions.
FRET due to random co-localization of donors and acceptors (proximity FRET)
When measuring FRET in a fluid lipid bilayer, it is important to recognize that FRET can arise simply due to random proximity of the acceptors and donors. Therefore, it is desirable to use low peptide concentration, such that the average distances between the peptides always exceed the Förster radii for the donor/acceptor pair. It should be further taken into account that the peptides diffuse randomly in the bilayers, such that for any concentration some acceptors will come in close contact with donors and FRET will occur even without sequence-specific dimerization.
An indicator of what portion of the measured FRET efficiency is due to dimerization rather than random colocalization is the deviation of the measured FRET from the expected FRET calculated for randomly distributed fluorophores. In this calculation, carried out first by Wolber and Hudson (Wolber and Hudson 1979) and simulated by Wimley and White (Wimley and White 2000), FRET from randomly distributed peptides is determined by averaging the donor quenching by acceptors over a large number of acceptor configurations. The FRET efficiency (E) for a specific acceptor configuration is given by (Wolber and Hudson 1979):
| (1) |
where ri is the distance between the donor and the ith acceptor in the system and R0 is the Förster radius for the donor/acceptor FRET pair. The simulation of FRET from random colocalization in bilayers shows that this random proximity effect can contribute to the measured FRET efficiencies even at acceptor concentrations far less than 1 mol% (You et al 2005). This nonspecific FRET is higher for fluorophores with larger R0.
We have determined the Forster radii, R0, of the fluorescein/rhodamine and the BODIPY-fluorescein/rhodamine pairs, when the dyes are attached to the TM helices and are likely positioned in the bilayer interface as R0 = 55 Å using two different methods. First, R0 was calculated by measuring the fluorescent yields of the donor, QYDONOR, and the degree of overlap between donor emission and acceptor excitation, as described [27]. R0 was also calculated by fitting the measured FRET efficiencies to a sum of two constributions: a sequence specific dimerization contribution and a random co-localization contribution, via a two-parameter fit, by simultaneously varying K (or ΔG) and R0 (or the predicted FRET due to random co-localization of donors and acceptors) (Li et al 2006).
A control experiment that can distinguish between random colocalization and sequence-specific dimerization is to monitor the effect of “dilution” of labeled peptides with unlabeled peptides. If sequence-specific dimerization occurs, the addition of unlabeled peptide to donor and acceptor-labeled dimers will decrease the FRET signal. FRET that is due to random colocalization will not decrease in the presence of unlabeled peptide. This control experiment has demonstrated that FGFR3 TM domains form sequence-specific dimers in POPC bilayers (Li et al 2005). This experiment can identify sequence-specific interactions even for very weakly dimerizing helices. It should be kept in mind, however, that this experiment works only when high labeling yields can be achieved.
FRET efficiencies and energetics of TM helix dimerization
(i) Direct calculation of FRET efficiencies from donor quenching
FRET efficiencies can be calculated from the emission spectra of donor- and acceptor-labeled peptides of known concentration in MLVs, LUVs, and supported bilayers. Bilayers containing only donor-labeled peptides serve as the “no FRET control”. Energy transfer, E, is calculated from the donor emission in the absence (FD) and presence of the acceptor (FDA) according to:
| (2) |
where is the wavelength of the donor emission maximum.
The measured FRET efficiency has two contributions, one due to random colocalization of donors and acceptors (proximity effect) and one due to sequence-specific dimerization:
| (3) |
The FRET efficiency due to sequence-specific dimerization Edimer can be presented as:
| (4) |
where fD is the fraction of molecules in the dimeric state, fD = 2[D]/[T], [D] is the dimer concentration, [T] is the total peptide concentration, pD is the probability for donor quenching to occur in the dimer, and ER is the FRET efficiency in the dimer. If the distance between the FRET pair in the dimer is smaller than the Förster radius, then ER≃1. The probability of a donor-labeled peptide to dimerize with an acceptor-labeled peptide equals the fraction of acceptor-labeled molecules:
| (5) |
where [d] and [a] are the donor and acceptor concentrations, fd and fa are the donor and acceptor labeling yields. A donor will be quenched if it dimerizes with an acceptor, and therefore:
| (6) |
Since [d] and [a] are known as aliquoted (or can be determined using the EmEx-FRET method described below) for each sample, Eproximity and xA can be calculated too, such that the dimer concentration can be calculated as:
| (7) |
The mole fraction association constant, K, is given by
| (8) |
where [M] =[T] − 2[D] is the monomer concentration in the lipid vesicles.
K is usually determined from a plot of measured dimer fractions versus total peptide concentration (Li et al 2006,You et al 2006,You et al 2007). The free energy of dimerization is given by:
| (9) |
(ii) The EmEx-FRET method
As discussed above, sample-to-sample variations in protein concentrations (due to the very low solubility of the hydrophobic TM helices) can introduce uncertainties in the measured FRET efficiencies and the calculated dimerization free energies. Here we overview the EmEx-FRET method (Merzlyakov et al 2007) which reduces such experimental uncertainties. In addition, this method can be useful when the concentrations of the donor- and acceptor-tagged molecules cannot be controlled, such as in cellular studies.
The EmEx-FRET method relies on the acquisition of both excitation and emission spectra for “the FRET sample” which contains both donor-labeled and acceptor-labeled TM helices. Furthermore, both excitation and emission spectra need to be acquired for a donor-only and an acceptor-only “standards” of known donor and acceptor concentration (shown in Figure 3). The EmEx-FRET method uses these standard spectra to calculate not only the FRET efficiency, but also the actual donor and acceptor concentration, and therefore the equilibrium constants and the free energy of lateral dimerization with high experimental precision.
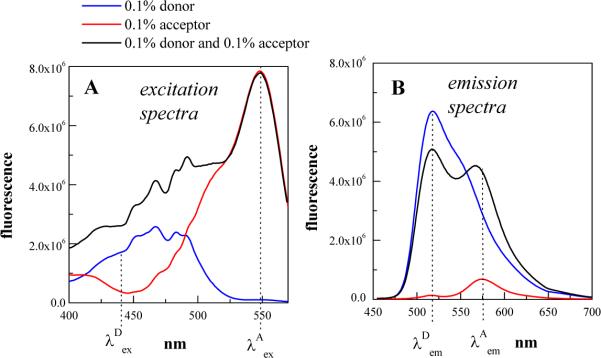
Figure 3.
Emission and excitation spectra of fluorescein (Fl) and rhodamine (Rhod), a common FRET pair, conjugated to wild-type FGFR3 TM domain. POPC concentration was 0.25 mg/ml; the concentration of the protein is reported in moles of protein per mole of lipid. (A) Excitation spectra, collected by recording emission at 595 nm while scanning the excitation from 400 to 570 nm. (B) Emission spectra, with excitation fixed at 439 nm, and emission scanned from 450 to 700 nm. In (A) and (B), the blue lines correspond to 0.1 mol% Fl-TMWT, while the red lines are for 0.1 mol% Rhod-TMWT. The blue and red spectra serve as standard excitation and emission spectra, to be used with the EmEx-FRET method. These spectra are averages, derived from measurements of multiple samples. Also shown are the excitation and emission spectra of 0.1 mol% fluorescein-labeled wild-type and 0.1 mol% rhodamine-labeled wild-type (the donor/acceptor sample, or “FRET sample”, black lines). The FRET spectrum (black) is the sum of three contributions: direct donor emission in the presence of the acceptor FDA, direct acceptor emission FA, and sensitized acceptor emission Fsen. Inspection of the excitation spectra in (A) reveals that at excitation wavelength the acceptor excitation reaches its maximum, while the donor excitation is negligible. As a result, the excitation of the donor/acceptor sample (black) at is contributed by the acceptor only (red). = 439 nm is the excitation wavelength used in the acquisition of the emission spectra. In (B), the emissions of the donor and the acceptor reach their maxima at and , respectively. Note that the emission of the acceptor is low, but not negligible, at . Reprinted from (Merzlyakov et al 2007), with permission from Springer Science and Business Media.
EmEx-FRET theory
The efficiency of energy transfer E from an excited donor to an acceptor depends on the donor emission in the absence (FD) and presence (FDA) of the acceptor according to equation (2). It can be also determined from the acceptor emission in the absence (FA) and presence (FAD) of the donor. When both the donor and the acceptor are present, we can write:
| (10) |
| (11) |
where and are the extinction coefficients of the donor and the acceptor at (see Fig. 3), SD and SA are scaling factors which depend on the quantum yields of the donor and acceptor, the photodetector efficiencies at and , and the geometry of the experimental set-up. At zero FRET efficiency (E = 0), the donor and the acceptor emit due to direct excitation only, such that FDA = FD and FAD = FA. At non-zero FRET efficiency (E ≠ 0), part of the energy, absorbed by the donor, is transferred to the acceptor. The donor fluorescence is quenched (FDA < FD) and the acceptor fluorescence is enhanced (FAD > FA). The acceptor enhancement, termed “sensitized fluorescence” Fsen, can be calculated as:
| (12) |
The donor quenching is given by:
| (13) |
Therefore, the efficiency of donor quenching at is proportional to the sensitized acceptor fluorescence at , with SD/SA being the scaling factor (Merzlyakov et al 2007).
The scaling factor SD/SA depends on the dyes used and on the characteristics of the instrument, not on the particular dye concentration. SD/SA can be calculated from the FRET spectrum for well-defined dye concentrations and known FRET efficiency according to:
| (14) |
Alternatively, if the photodetector efficiency at and is the same (or if the fluorescence signal is normalized by the photodetector efficiency), then SD/SA equals the ratio of donor and acceptor quantum yields, ϕD/ϕA, which can be calculated from measured absorbance and emission spectra. Both methods yield SD/SA =1 ± 0.05 for the fluorescein-rhodamine FRET pair (Merzlyakov et al 2007).
Once SD/SA is determined, it can be used to calculate the donor fluorescence in the absence of the acceptor (i.e. the donor control, FD) from the measured donor fluorescence in the presence of the acceptor FDA according to:
| (15) |
EmEx-FRET Protocol
Here we show how to calculate the FRET efficiency E, the donor concentration [d], and the acceptor concentration [a] using (i) the excitation and emission spectra acquired in the presence of donors and acceptors of unknown concentrations [d] and [a], (ii) the donor emission standard spectrum (acquired for a known donor concentration), and (iii) the acceptor excitation and emission standard spectra (acquired for a known acceptor concentration).
Step 1. The excitation and emission spectra of bilayers containing donor-labeled and acceptor-labeled peptides are acquired in a fluorometer. Each FRET spectrum has three contributions: direct donor emission in the presence of the acceptor FDA, direct acceptor emission FA, and sensitized acceptor emission Fsen. Furthermore, FAD = FA + Fsen.
Step 2. The acquired FRET excitation spectrum (Fig. 4A, black line) is compared to the standard excitation spectrum of the acceptor (Fig. 3A, red line). The standard spectrum is multiplied by a coefficient, ξ, to produce the red line in Fig. 4A, such that the amplitude of the scaled standard (Fig. 4A, red line) is identical with the FRET excitation spectrum (Fig. 4A, black line) at . This step gives the concentration of the acceptor in the sample, [a], as ξ times the standard acceptor concentration, in this case ξ × 0.1 mol%.
Figure 4.
The EmEx-FRET method.
(A) An acquired FRET excitation spectrum (black line) is compared to the excitation standard spectrum of the acceptor (Fig. 3A, red line) This step gives the concentration of the acceptor in the sample.
(B) The emission standard of the acceptor (Fig. 3B, red line) is scaled according to the acceptor concentration determined in (A) (red line). The difference between the red line and the FRET emission spectrum (black line) is the sum FDA + Fsen.
(C) Dashed black line: FDA + Fsen, sum of the direct donor (Bell et al 2000) emission in the presence of the acceptor, FDA, and the sensitized acceptor emission, Fsen. The standard emission spectrum of the donor (Fig. 3B, blue line) is scaled, such that the amplitude of the scaled standard (blue dashed line) is identical to the amplitude of the dashed black line at . The difference between the black dashed and the blue dashed line is the sensitized acceptor emission, Fsen. The value of the sensitized emission at , , is related to the decrease in donor emission at , (Merzlyakov et al 2007)
(D) The value , determined in (C), is used to determine the donor emission in the absence of the acceptor FD (blue line), and the donor concentration.
Reprinted from (Merzlyakov et al 2007), with permission from Springer Science and Business Media.
Step 3. The emission standard of the acceptor (Fig. 3B, red line) is multiplied by ξ to obtain the direct emission contribution of the acceptor (Fig. 4B, red line). The direct acceptor contribution (red line) is subtracted from the FRET emission spectrum (Fig. 4B, black line), to reveal the sum of the direct donor emission and the sensitized acceptor emission, FDA + Fsen. This sum is plotted in Fig. 4C with the black dashed line.
Step 4. The emission standard spectrum of the donor (Fig. 3B, blue line) is multiplied by a coefficient to produce the blue dashed line in Fig. 4C, such that the amplitude of the scaled standard (blue dashed line) is identical with the black dashed line in Fig. 4C at . The difference between the black dashed and the blue dashed line is the sensitized acceptor emission, Fsen. The value of the sensitized emission at , , is related to the decrease in donor emission at , , given by Equation (13).
Step 5. The value , determined in Step 4, is added to (Fig. 4D) to give the value of the donor emission in the absence of acceptor at , i.e. . The donor emission standard (Fig. 3B, blue line) is multiplied by a coefficient ζ, such that the amplitude of the standard spectrum at equals . This step gives the concentration of the donor in the sample, [d], as ζ times the standard donor concentration, in this case ζ × 0.1 mol%. It also gives the complete emission spectrum of the donor in the absence of the acceptor FD (Fig. 4D, blue line) as the emission of the donor standard (Fig. 3B, blue line), multiplied by ζ.
Further data analysis is preformed using equations (2) through (9).
We have studied the dimerization energetics of the Gly382Asp TM domain of fibroblast growth factor receptor 3 (FGFR3) using the EmEx-FRET method (Merzlyakov et al 2007). The results are shown in Fig. 5, and each data point is derived from a single experiment. Yet, one can have high confidence in the data, because the actual donor and acceptor concentrations for each data point have been determined. Thus, errors associated with uncertainties in peptide concentrations due to low peptide solubility have been reduced. The free energy of dimerization is calculated as −2.78 ± 0.04 kcal/mol. On the other hand, the calculation of the dimerization free energy from donor quenching (i.e. not using the EmEx-FRET method) gives −2.9 ± 0.7 kcal/mol. Thus, the application of the EmEx-FRET method reduces the uncertainty in the free energy calculation from ± 0.7 to ± 0.04 kcal/mol, and therefore greatly improves the experimental precision.
Figure 5.

Dimer fraction [D]/[T] vs total peptide concentration [T] for TM382Asp. The symbols represent the dimer fractions calculated using the EmEx-FRET method, for different protein concentrations. The solid line is the theoretical equilibrium curve, obtained as described in details previously (Li et al 2005,Li et al 2006). The free energy of dimerization, as the average of the six experiments, is −2.78 ± 0.04 kcal/mol. For comparison, the average free energy value, obtained from donor quenching as previously described (You et al 2005) (i.e. not using the EmEx-FRET method), is −2.9 ± 0.7 kcal/mol. Thus, the EmEx-FRET method greatly improves the precision of TM helix dimerization energetics measurements. Reprinted from (Merzlyakov et al 2007), with permission from Springer Science and Business Media.
(iii) Energetics of TM helix heterodimerization
Measurements of TM helix heterodimerization energetics present a unique challenge if the helices have both homo- and hetero-dimerization propensities (Merzlyakov et al 2006). Here we overview the thermodynamics behind heterodimer formation and outline a method for calculating the free energy of heterodimerization using FRET.
Theory and protocol
In a lipid bilayer with two different TM helices, X and Y, three different types of dimers will form: XX, YY and XY. The monomer-dimer equilibrium is described by three equilibrium constants, KX, KY and KXY:
| (16) |
| (17) |
where [XX], [YY], [XY] are the concentrations of the XX homodimers, YY homodimers and XY heterodimers, respectively; [X] and [Y] are the concentrations of the X and Y monomers, respectively. The homodimer equilibrium constants KX and KY can be measured independently for helices X and Y as described above and in (Li et al 2005,Li et al 2006, You et al 2005).
The total concentrations of the X and Y helices, [TX] and [TY], are known as aliquoted and equal to:
| (18) |
| (19) |
Let X be labeled with a FRET donor, and Y be labeled with the appropriate acceptor. The FRET efficiency due to sequence specific heterodimerization is given by:
| (20) |
where [da] is the concentration of donor-acceptor dimers. [da] would be equal to [XY], and [d] would be equal to [Tx] if the labeling yield is 100% (i.e. the ratio of peptides to conjugated fluorescent dyes is 1). If the labeling yields, fd and fa, for the donor and the acceptor are lower than 100%, then:
| (21) |
Assuming that labeling does not affect the dimerization energetics, the concentration of donor-acceptor heterodimers relates to the total concentration of heterodimers XY as follows:
| (22) |
From Eqs. (20)-(22), we obtain:
| (23) |
Equations (18), (19) and (23) can be solved to determine the three unknowns: [X], [Y] and [XY]. The solution is:
| (24) |
| (25) |
| (26) |
The heterodimerization equilibrium constant KXY is calculated as:
| (27) |
where [X] and [Y] are given by Eqs. (26) and (27), respectively. Then, the free energy of heterodimerization can be calculated as:
| (28) |
Biological insights from FRET measurements
Using the described FRET methodology, we have studied the thermodynamic and structural principles that underlie signal transduction across biological membranes (Li and Hristova 2006). The application of these methods to wild-type and mutant RTK TM domains has yielded new information pertaining to the physical basis of complex biological processes, and to the mechanisms of pathogenesis in humans (Li et al 2006,Li and Hristova 2006,You et al 2006,You et al 2007).
(i) Thermodynamics of protein homodimerization in lipid bilayer membranes wild-type FGFR3 TM domain, and the pathogenic Ala391Glu, Gly380Arg and Gly382Asp mutants
Mutations in the TM domain of FGFR3 have been linked to growth disorders and cancers. One example is the Ala391→Glu mutation, causing Crouzon syndrome with acanthosis nigricans, as well as bladder cancer (Li and Hristova 2006,Meyers et al 1995,van Rhijin et al 2002). To gain insight into the molecular mechanism behind the pathology, we have determined the free energy of dimerization of the wild-type and the Ala391→Glu mutant in lipid bilayers. The change in the free energy of dimerization due to the Ala391→Glu pathogenic mutation is −1.3 kcal/mole (Li et al 2006). This seemingly modest value can lead to a large increase in receptor dimer fraction and thus profoundly affect FGFR3-mediated signal transduction (Li et al 2006). The pathogenic stabilization is most-likely due to Glu-mediated hydrogen bonds (Li and Hristova 2006).
We have also determined the energetics of dimerization of the Gly380→Arg FGFR3 variant linked to achondroplasia, the most common form of human dwarfism. The molecular mechanism of pathogenesis in achondroplasia is under debate, and two different mechanisms have been proposed to contribute to pathogenesis: (i) Arg380-mediated FGFR3 dimer stabilization (Webster and Donoghue 1996) and (ii) slow downregulation of the activated mutant receptors (Cho et al 2004). We have shown that the Gly380→Arg mutation does not alter the dimerization energetics of FGFR3 transmembrane domain in detergent micelles or in lipid bilayers (You et al 2006). This result indicates that pathogenesis in achondroplasia cannot be explained simply by a higher dimerization propensity of the mutant FGFR3 TM domain, thus highlighting the potential importance of the observed slow downregulation in phenotype induction (Cho et al 2004).
We have also investigated the effect of the Gly382Asp mutation, identified in the KSM-18 myeloma cell line (Otsuki et al 1998), on the dimerization propensity of FGFR3 TM domain. The measured free energy of dimerization, −2.78 ± 0.04 kcal/mol (Merzlyakov et al 2007), is the same as the value determined for wild-type, −2.8 ± 0.1 kcal/mol (Li et al 2006), indicating that the Gly382Asp mutation does not stabilize the FGFR3 TM domain dimer. Thus, the mechanism of pathogenesis in multiple myeloma is not associated with dimer stabilization due to Asp-mediated hydrogen bonds.
(ii) Thermodynamics of protein hetero-dimerization in lipid bilayer membranes
We have determined the propensity for heterodimer formation between wild-type FGFR3 TM domain and the Ala391→Glu mutant, linked to the autosomal dominant Crouzon syndrome with acanthosis nigricans (Merzlyakov et al 2006). The cells of the affected heterozygotes express both wild-type and mutant proteins, and thus measurements of heterodimer stabilities are critical for understanding the induction of the phenotype. The free energy of heterodimerization was determined as −3.37 ± 0.25 kcal/mol. Comparison of this value to the homodimerization free energies for the wild-type, −2.8± 0.2 kcal/mol, and the mutant, −4.1 ± 0.2 kcal/mol, reveals that the heterodimer stability is the average of the two homodimer stabilities. This finding may indicate that the mutant homodimer is stabilized by two hydrogen bonds, while a single hydrogen bond forms in the heterodimer.
Conclusion
Quantitative FRET measurements of TM helix dimerization energetics in lipid bilayers can be carried out to determine the homo- and heterodimerization propensities with high precision. Such experiments have yielded new insights into the role of receptor tyrosine kinase TM domains in cell signaling and human pathologies.
References
- White SH, Wimley WC. Membrane protein folding and stability: Physical principles. Annual Review of Biophysics and Biomolecular Structure. 1999;28:319–365. doi: 10.1146/annurev.biophys.28.1.319. [DOI] [PubMed] [Google Scholar]
- MacKenzie KR. Folding and stability of alpha-helical integral membrane proteins. Chemical Reviews. 2006;106:1931–1977. doi: 10.1021/cr0404388. [DOI] [PubMed] [Google Scholar]
- Li E, Hristova K. Role of receptor tyrosine kinase transmembrane domains in cell signaling and human pathologies. Biochemistry. 2006;45:6241–6251. doi: 10.1021/bi060609y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto T, You M, Li E, Spangler J, Tomich JM, Hristova K. Synthesis and initial characterization of FGFR3 transmembrane domain: Consequences of sequence modifications. Biochimica et Biophysica Acta. 2005;1668:240–247. doi: 10.1016/j.bbamem.2004.12.012. [DOI] [PubMed] [Google Scholar]
- You M, Li E, Wimley WC, Hristova K. FRET in liposomes: measurements of TM helix dimerization in the native bilayer environment. Analytical Biochemistry. 2005;340:154–164. doi: 10.1016/j.ab.2005.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto T, Grove A, Montal MO, Montal M, Tomich JM. Chemical Synthesis and Characterization of Peptides and Oligomeric Proteins Designed to Form Transmembrane. International Journal of Peptide and Protein Research. 1994;43:597–607. doi: 10.1111/j.1399-3011.1994.tb00562.x. [DOI] [PubMed] [Google Scholar]
- Li E, You M, Hristova K. SDS-PAGE and FRET suggest weak interactions between FGFR3 TM domains in the absence of extracellular domains and ligands. Biochemistry. 2005;44:352–360. doi: 10.1021/bi048480k. [DOI] [PubMed] [Google Scholar]
- Li E, You M, Hristova K. FGFR3 dimer stabilization due to a single amino acid pathogenic mutation. Journal of Molecular Biology. 2006;356:600–612. doi: 10.1016/j.jmb.2005.11.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You M, Li E, Hristova K. The achondroplasia mutation does not alter the dimerization energetics of FGFR3 transmembrane domain. Biochemistry. 2006;45:5551–5556. doi: 10.1021/bi060113g. [DOI] [PubMed] [Google Scholar]
- Adair BD, Engelman DM. Glycophorin a helical transmembrane domains dimerize in phospholipid bilayers - a resonance energy transfer study. Biochemistry. 1994;33:5539–5544. doi: 10.1021/bi00184a024. [DOI] [PubMed] [Google Scholar]
- Merzlyakov M, Chen L, Hristova K. Studies of receptor tyrosine kinase transmembrane domain interactions: The EmEx-FRET method. Journal of Membrane Biology. 2007;215:93–103. doi: 10.1007/s00232-007-9009-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merzlyakov M, Li E, Casas R, Hristova K. Spectral Forster resonance energy transfer detection of protein interactions in surface-supported bilayers. Langmuir. 2006;22:6986–6992. doi: 10.1021/la061038d. [DOI] [PubMed] [Google Scholar]
- Merzlyakov M, Li E, Hristova K. Directed assembly of surface-supported bilayers with transmembrane helices. Langmuir. 2006;22:1247–1253. doi: 10.1021/la051933h. [DOI] [PubMed] [Google Scholar]
- Merzlyakov M, Li E, Gitsov I, Hristova K. Surface-supported bilayers with transmembrane proteins: role of the polymer cushion revisited. Langmuir. 2006;22:10145–10151. doi: 10.1021/la061976d. [DOI] [PubMed] [Google Scholar]
- Sackmann E. Supported membranes: Scientific and practical applications. Science. 1996;271:43–48. doi: 10.1126/science.271.5245.43. [DOI] [PubMed] [Google Scholar]
- Sackmann E, Tanaka M. Supported membranes on soft polymer cushions: Fabrication, characterization and applications. Trends in Biotechnology. 2000;18:58–64. doi: 10.1016/s0167-7799(99)01412-2. [DOI] [PubMed] [Google Scholar]
- Wolber PK, Hudson BS. An analytic solution to the Förster energy transfer problem in two dimensions. Biophysical Journal. 1979;28:197–210. doi: 10.1016/S0006-3495(79)85171-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimley WC, White SH. Designing transmembrane α-helices that insert spontaneously. Biochemistry. 2000;39:4432–4442. doi: 10.1021/bi992746j. [DOI] [PubMed] [Google Scholar]
- You M, Spangler J, Li E, Han X, Ghosh P, Hristova K. Effect of pathogenic cysteine mutations on FGFR3 transmembrane domain dimerization in detergents and lipid bilayers. Biochemistry. 2007;46:11039–11046. doi: 10.1021/bi700986n. [DOI] [PubMed] [Google Scholar]
- Merzlyakov M, You M, Li E, Hristova K. Transmembrane helix heterodimerization in lipids bilayers: probing the energetics behind autosomal dominant growth disorders. Journal of Molecular Biology. 2006;358:1–7. doi: 10.1016/j.jmb.2006.01.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers GA, Orlow SJ, Munro IR, Przylepa KA, Jabs EW. Fibroblast-Growth-Factor-Receptor-3 (Fgfr3) Transmembrane Mutation in Crouzon-Syndrome with Acanthosis Nigricans. Nature Genetics. 1995;11:462–464. doi: 10.1038/ng1295-462. [DOI] [PubMed] [Google Scholar]
- van Rhijin B, van Tilborg A, Lurkin I, Bonaventure J, de Vries A, Thiery JP, van der Kwast TH, Zwarthoff E, Radvanyi F. Novel fibroblast growth factor receptor 3 (FGFR3) mutations in bladder cancer previously identified in non-lethal skeletal disorders. European Journal of Human Genetics. 2002;10:819–824. doi: 10.1038/sj.ejhg.5200883. [DOI] [PubMed] [Google Scholar]
- Webster MK, Donoghue DJ. Constitutive activation of fibroblast growth factor receptor 3 by the transmembrane domain point mutation found in achondroplasia. EMBO Journal. 1996;15:520–527. [PMC free article] [PubMed] [Google Scholar]
- Cho JY, Guo CS, Torello M, Lunstrum GP, Iwata T, Deng CX, Horton WA. Defective lysosomal targeting of activated fibroblast growth factor receptor 3 in achondroplasia. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:609–614. doi: 10.1073/pnas.2237184100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuki T, Nakazawa N, Taniwaki M, Yamada O, Sakaguchi H, Wada H, Yawata Y, Ueki A. Establishment of a new human myeloma cell line, KMS-18, having t(4;14)(p16.3;q32.3) derived from a case phenotypically transformed from Ig A-lambda to BJP-lambda, and associated with hyperammonemia. International Journal of Oncology. 1998;12:545–552. doi: 10.3892/ijo.12.3.545. [DOI] [PubMed] [Google Scholar]
- Bell CA, Tynan JA, Hart KC, Meyer AN, Robertson SC, Donoghue DJ. Rotational coupling of the transmembrane and kinase domains of the Neu receptor tyrosine kinase. Molecular Biology of the Cell. 2000;11:3589–3599. doi: 10.1091/mbc.11.10.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]