Abstract
Normal pressure hydrocephalus (NPH) is a syndrome of gait dysfunction and enlarged cerebral ventricles in the absence of another cause. It is frequently accompanied by frontal and subcortical cognitive deficits and bladder detrusor overactivity. NPH is rare relative to other potential causes of these symptoms in the elderly, but timely diagnosis can lead to reversal of symptoms through ventricular shunting. There are many tests used to predict possible response to surgery, such as MRI of the brain, formalized neuropsychological and gait testing, large-volume lumbar puncture, and prolonged lumbar drainage, but no one test has been validated to rule out potential response to surgery.
Introduction
Normal pressure hydrocephalus (NPH) is one of the few causes of dementia that is potentially reversible, as eloquently described by a physician who recovered sufficiently after treatment to publish a review drawn from his experiences as a patient [1]. NPH can occur with varying combinations or degrees of each of the elements of the classic clinical triad first described by Hakim and Adams [2] in 1965: gait disturbance, urinary incontinence, and dementia. Generally, gait disturbance plus one additional feature is required to consider the diagnosis. The term idiopathic adult hydrocephalus syndrome may be more accurate, because intracranial pressure is not always normal in NPH. Cases where earlier trauma, hemorrhage, infection, mass lesions, or aqueductal stenosis contribute to hydrocephalus are considered symptomatic or secondary forms of NPH, and the following discussion focuses on the idiopathic type (iNPH).
Epidemiology
Using a massive advertising campaign in Norway, Brean and Eide [3] found an incidence of 5.5 per 100,000 and prevalence of 21.9 per 100,000 for suspected iNPH. Prevalence ranged from 3.3 per 100,000 for people 50 to 59 years of age, to 49.3 per 100,000 for people 60 to 69 years of age, to 181.7 per 100,000 for people 70 to 79 years of age. A recent study of possible symptoms of NPH found that at least 21.2% of nursing home patients have gait impairment, 9.4% of whom also have dementia and 14.7% of whom also have incontinence [4]. The likelihood of identifying treatable NPH in those presenting with dementia is quite low. Of 560 cases of dementia seen at the Mayo Clinic from 1990 to 1994, 5 (1%) had suspected NPH, but none of the 3 treated with ventriculoperitoneal shunting (VPS) improved [5].
Neurologic Signs and Symptoms
Gait disturbance
Although no one feature is pathognomic of the gait disturbance in NPH, the most common descriptors include “shuffling,” “magnetic,” and “wide-based” [6••]. Disequilibrium and slowness of gait (due to short steps and gait apraxia) are common, and the latter feature is more likely to respond to shunting [7]. Slowness of both upper and lower extremities is common as well and can improve with shunting [8]. Appendicular tremor is present in 40% of NPH patients, is rarely of a parkinsonian (resting) quality, and does not respond to VPS [9].
Urinary incontinence
The bladder symptoms of iNPH are directly caused by detrusor overactivity, which can result in urinary frequency, urgency, or frank incontinence. Sakakibara et al. [10] found that 95% of 41 patients with possible iNPH had urodynamic evidence of detrusor overactivity.
Dementia
Frontal and subcortical deficits (psychomotor slowing and impaired attention, executive, and visuospatial dysfunction) can be the earliest cognitive signs of iNPH [11]. Significant improvement in these disturbances can occur after shunting [12]. More global cognitive deficits can be identified in individuals with suspected iNPH, even in those with Mini-Mental Status Examination (MMSE) scores greater than 25, and the severity of cognitive deficits appears to correlate with the presence of vascular risk factors [13]. Cerebrovascular disease is comorbid in over 60% of patients with iNPH [14•].
Asymmetric resting tremor, lead pipe rigidity, or visual hallucinations may suggest dementia with Lewy bodies (DLB), which causes similar cognitive deficits. Depression with pseudodementia is in the differential diagnosis as well. Early presence of cortical deficits such as aphasia, apraxia, or agnosia should raise suspicion for dementia with cortical pathology, such as Alzheimer’s disease (AD), multi-infarct dementia, or frontotemporal dementia. In patients with progressive dementia who lack gait dysfunction, a cause other than iNPH should be considered, regardless of ventriculomegaly.
Comorbid AD and iNPH is not uncommon, and the likelihood of each is increased with the presence of hypertension and advancing age. AD pathology is present in cortical biopsy of 75% of those iNPH patients with significant dementia at the time of shunt surgery [15]. Although gait can improve with shunting in such patients, dementia typically does not. Surgical treatment is generally discouraged for patients with severe dementia, even in the setting of gait dysfunction and incontinence, regardless of radiographic findings [16].
Pathophysiology
The majority of adult hydrocephalus cases are secondary to other causes, and previously compensated congenital hydrocephalus may account for a subset of NPH, as suggested by the finding that head circumference above the 90th and 97th percentiles appears to be more common in iNPH patients than the general population [17].
A leading theory to explain iNPH is that poor venous compliance, which has been demonstrated in the superior sagittal sinus of iNPH patients [18], impairs both cerebrospinal fluid (CSF) pulsations (and hence flow through the aqueduct) and CSF absorption through arachnoid granulations. Because hypertension is present in 83% of individuals with iNPH [19], and because both can frequently co-occur with cerebrovascular disease or AD, a link in the causative pathways of these three disorders has been suggested [14•].
Altered expression of molecules regulating CSF production and absorption could play a role in iNPH. Elevated CSF tumor necrosis factor-α, which is known to regulate CSF production, improves after shunting [20]. CSF transforming growth factor-β and related proteins are also elevated in iNPH [21]. It is not clear if cytokines accumulate secondary to impaired CSF flow and hence mediate symptoms of NPH, or whether increased production might occur as an adaptive response to hydrocephalus.
The neurologic symptoms of iNPH may be mediated in part by interstitial edema in periventricular white matter, leading to impaired blood flow or metabolism in vital prefrontal pathways. Poor perfusion of periventricular white matter and prefrontal regions has been suggested in nuclear imaging studies [22,23] and may improve after shunting in a manner that correlates with measures of cognitive improvement [24]. A recent positron emission tomography study suggested that disturbances in basal ganglia pathways may also have a role in some of the gait and cognitive abnormalities of NPH. Gait speed and MMSE scores improved after shunting in eight NPH patients whose low striatal dopamine D2 receptor density normalized after the surgery [25]. Compression of brainstem structures, such as the pedunculopontine nucleus, could also be a factor in gait dysfunction of NPH, as suggested by a finding that the degree of increase in midbrain volume following shunting correlates with clinical improvement [26].
Although iNPH is not generally considered to be hereditary, a kindred with essential tremor (ET) and iNPH has recently been identified. Of 13 family members with ET, 2 with concomitant NPH were studied. No linkage to known ET genetic loci could be found [27].
Diagnostic Criteria
Evidence-based guidelines for the diagnosis of iNPH have been established through systematic review of 653 references dating from 1966 to 2003 [28••]. Patients who have not yet been shunted can be assigned to one of three categories: probable, possible, or unlikely iNPH. Consideration of iNPH requires that other medical causes, including a structural lesion or congenital aqueductal stenosis, cannot account for clinical and radiographic findings. Patients with probable iNPH are older than 40 years of age with insidious (nonacute) progression of symptoms over a period of at least 3 months and have CSF opening pressures between 70 and 245 mm H2O. MRI or CT must show an Evan’s index of at least 0.3 (Fig. 1), as well as temporal horn enlargement, periventricular signal changes, periventricular edema, or an aqueductal/fourth ventricular flow void. Although callosal angle of greater than 40° was included in these guidelines, it is not a widely recognized criterion and we have not used it in our own experience. Clinically, patients must demonstrate gait dysfunction plus either urinary or cognitive dysfunction. Abnormal urinary urgency or frequency is sufficient to document urinary bladder dysfunction. To meet criteria for cognitive dysfunction, there must be impairments of two or more domains, such as psychomotor speed, fine motor speed or accuracy, attention, short-term recall, executive function, or behavioral/personality change. Supportive literature for this last domain was drawn only from case reports, and if these are the dominant cognitive findings then frontotemporal dementia must also be considered. Patients are classified as possible iNPH if they are below the age of 40 years or have symptoms for less than 3 months, unavailable or abnormal CSF opening pressures, nonprogressive symptoms, or cerebral atrophy severe enough to explain ventriculomegaly. Those unlikely to have iNPH usually have papilledema or symptoms explicable by other causes and have no ventriculomegaly or component of the iNPH clinical triad.
Figure 1.
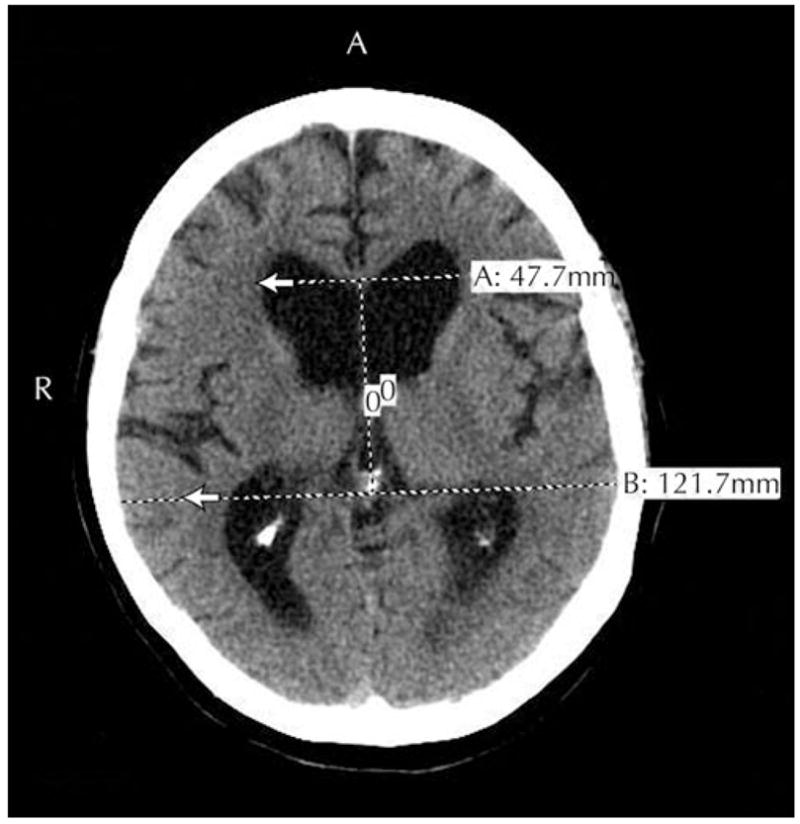
MRI of a patient with probable idiopathic normal pressure hydrocephalus. The Evans ratio is the maximal ventricular width divided by the largest biparietal distance between the inner tables of the skull. In this case, the Evans ratio was 0.39. Ventriculomegaly is defined as an Evans ratio of 0.30 or greater.
A universally accepted scale for rating severity of impairment in each of the symptom triad domains is currently lacking. Kubo et al. [29] recently proposed a 12-point iNPH scale similar to that utilized in earlier studies [30], assigning a 4-point subscale score for each domain of the clinical triad. They demonstrated good inter-rater reliability, with significant correlations between other measures (MMSE, Trails A test, timed up and go test, gait status scale, and an incontinence questionnaire) and the subscale scores.
Treatment
CSF shunting procedures, including ventriculoperitoneal, ventriculopleural, or ventriculoatrial shunting, can lead to significant clinical improvement in iNPH symptoms in approximately 60% of iNPH patients [31].
Predictors of Outcome
Age should not be considered an exclusionary criterion in those without other surgical risk factors. Older age only predicts a lower likelihood of improvement after VPS in those with the full triad [6••]. A variety of tests have been proposed to aid in the prediction of a good response to shunting.
CSF removal
Although a high-volume (> 30 mL) spinal tap (lumbar tap test) was the earliest method for establishing the diagnosis of iNPH and predicting response to shunting, external lumbar drainage (ELD) is gaining acceptance as a more sensitive predictor in patients who do not have a significant response to a tap test. A lumbar spinal catheter is inserted and CSF is drained at a rate of 10 to 15 cm3 per hour for 72 hours. Although a commercially available automated gait analysis system is available to quantify response to ELD [32], walking speed can also be measured using a timed 10-meter walk before and after ELD. Of 151 patients with possible iNPH as evidenced by gait disturbance and ventriculomegaly with or without dementia or urinary symptoms, 100 patients (66%) showed improvement after ELD [6••]. Although 84% of those with a positive test had significant improvement in walking speed after VPS placement, only 35% with a negative response to ELD had improvement after VPS placement. Positive predictive value, the chance of improvement after VPS given a positive ELD test, was 90%. Sensitivity, specificity, and negative predictive value were 95%, 64%, and 78%, respectively. Thus, even ELD does not have very good negative predictive value. Because patients with a negative test result can drop out of a study, it is unclear what proportion of these patients would have improved with shunting. Very similar results were reported previously [33], including a positive response by 4 of 18 patients to VPS in those who had negative ELD tests. Thus, a positive ELD test has sufficient positive predictive value to recommend VPS, whereas patients with a negative test should be advised in a manner that weighs the invasive nature and cost of the procedure plus the individual’s risk of complications against an approximately 20% chance of benefit. Therefore, the value of this test needs to be weighed against its invasive nature, cost, and associated risk of complications, including headache, radiculopathy, and bacterial meningitis [6••,34••].
MRI
The likelihood of a positive lumbar tap test declines with increasing white matter burden [35]; however, small vessel white matter disease does not reduce the likelihood of improvement with VPS in those who have a positive ELD test [36]. MRI is better able to detect periventricular white matter changes than CT. These contiguous T2/fluid-attenuated inversion recovery hyperintensities are thought to represent transependymal edema due to elevated CSF pressure but may have identical appearance to that commonly attributed to small vessel ischemic disease. A narrow CSF space at the high convexity/midline areas relative to Sylvian fissure size was recently shown to correlate with a diagnosis of probable or definite iNPH [37]. Volumetric MRI, including ventricular, brain, and pericerebral CSF volume ratios, has not shown value in predicting which patients will respond to VPS [38].
Cine phase-contrast MRI
Cine phase-contrast MRI quantifies CSF flow in terms of stroke volume, defined as mean volume of CSF passing through the cerebral aqueduct in systole and diastole. Stroke volume greater than 42 μL may predict the likelihood of response to VPS. Eighteen of 42 patients were selected for VPS based on results of the flow studies, and 12 of 12 patients with stroke volume greater than 42 μL improved versus 3 of 6 patients with stroke volume below 42 μL [39]. CSF stroke volume increases after onset of symptoms, reaches a plateau after 18 to 20 months and then declines, suggesting that increased flow associated with ventriculomegaly might cause shear stress on periventricular tissues that would be reversible with VPS [40]. There was no correlation between outcome of a high-volume lumbar puncture or VPS and CSF stroke volume as measured by cine phase-contrast MRI, even at a median duration of symptoms of 1 year [41]. At this time, there is insufficient evidence to determine the value of this imaging technique in predicting response to shunting in iNPH, but an elevated CSF stroke volume is considered a supportive criterion for diagnosis.
Treatment Outcomes
The perioperative and long-term morbidity and mortality of CSF shunting procedures are significant. A meta-analysis of 44 articles found that the pooled, mean rate of shunt complication (including death, infection, seizures, shunt malfunction, subdural hemorrhage or effusion) was 38% [31]. The need for additional surgery occurred in 22% and the combined rate of permanent neurologic deficit or death was 6%. Over a period of 10 years and 99 procedures, rates of death, subdural hematoma, infection, shunt infection, and need for shunt revision were 1%, 3%, 12%, 6.7%, and 33%, respectively [42]. The pooled mean response rate to shunting for iNPH was 59% in the meta-analysis [31]. In those with good long-term survival, sustained improvement is possible, with a rate of 39% documented after 5 years [43].
Practice Guidelines
Recently suggested neurosurgical practice guidelines [44••] for determining whether or not to shunt a patient with iNPH include the following:
High CSF pressure should prompt investigation for a secondary cause of NPH
Response to a 40-mL to 50-mL (high-volume) lumbar tap suggests a potential benefit to shunting
An ELD may be used to evaluate those who do not respond to a high-volume tap
There is no substantial predictive value to MRI CSF flow studies
Given the lack of effective treatments for multi-infarct or Alzheimer’s disease dementia and the limited negative predictive value of even ELD, the dilemma facing clinicians evaluating patients for iNPH is what to do with the patient who has the full triad without a clear alternative explanation and who has generous ventricles on imaging studies but fails to respond to lumbar tap or ELD. Because the average 65-year-old patient with moderate dementia can look forward to only 1.4 quality-adjusted life-years, it has been argued that the threshold for placing shunts in such patients should be low [45••].
Conclusions
Treatment decisions for those with suspected iNPH need to be made on an individual basis, carefully weighing the surgical risks against the potential for significantly improving quality of life. Timely intervention may not only improve patient outcomes, but could save over $184 million in 5-year Medicare expenditures [46]. As the underlying cause of iNPH is elucidated, nonsurgical or even preventive treatments can be expected, but presently ventricular shunting is the only known treatment. Further clarification of guidelines for diagnosis of iNPH and identification of those most likely to respond to shunting will depend upon novel study designs that can account for flaws in previous studies while maintaining ethical integrity.
Footnotes
Disclosures
No potential conflicts of interest relevant to this article were reported.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Conn HO. Normal pressure hydrocephalus: new complications and concepts. Pract Neurol. 2007;7:252–258. doi: 10.1136/jnnp.2007.124404. [DOI] [PubMed] [Google Scholar]
- 2.Hakim S, Adams RD. The special clinical problem of symptomatic hydrocephalus with normal cerebrospinal fluid pressure. Observations on cerebrospinal fluid hydrodynamics. J Neurol Sci. 1965;2:307–327. doi: 10.1016/0022-510x(65)90016-x. [DOI] [PubMed] [Google Scholar]
- 3.Brean A, Eide PK. Prevalence of probable idiopathic normal pressure hydrocephalus in a Norwegian population. Acta Neurol Scand. 2008 doi: 10.1111/j.1600-0404.2007.00982.x. (in press) [DOI] [PubMed] [Google Scholar]
- 4.Marmarou A, Young HF, Aygok GA. Estimated incidence of normal pressure hydrocephalus and shunt outcome in patients residing in assisted-living and extended-care facilities. Neurosurg Focus. 2007;22:E1. doi: 10.3171/foc.2007.22.4.2. [DOI] [PubMed] [Google Scholar]
- 5.Knopman DS, Petersen RC, Cha RH, et al. Incidence and causes of nondegenerative nonvascular dementia: a population-based study. Arch Neurol. 2006;63:218–221. doi: 10.1001/archneur.63.2.218. [DOI] [PubMed] [Google Scholar]
- 6••.Marmarou A, Young HF, Aygok GA, et al. Diagnosis and management of idiopathic normal-pressure hydrocephalus: a prospective study in 151 patients. J Neurosurg. 2005;102:987–997. doi: 10.3171/jns.2005.102.6.0987. This is the largest study to date establishing the positive predictive value of ELD in predicting shunt responsiveness. [DOI] [PubMed] [Google Scholar]
- 7.Bugalho P, Guimaraes J. Gait disturbance in normal pressure hydrocephalus: a clinical study. Parkinsonism Relat Disord. 2007;13:434–437. doi: 10.1016/j.parkreldis.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Nowak DA, Topka HR. Broadening a classic clinical triad: the hypokinetic motor disorder of normal pressure hydrocephalus also affects the hand. Exp Neurol. 2006;198:81–87. doi: 10.1016/j.expneurol.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Krauss JK, Regel JP, Droste DW, et al. Movement disorders in adult hydrocephalus. Mov Disord. 1997;12:53–60. doi: 10.1002/mds.870120110. [DOI] [PubMed] [Google Scholar]
- 10.Sakakibara R, Kanda T, Sekido T, et al. Mechanism of bladder dysfunction in idiopathic normal pressure hydrocephalus. Neurourol Urodyn. 2007 doi: 10.1002/nau.20547. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 11.Iddon JL, Pickard JD, Cross JJ, et al. Specific patterns of cognitive impairment in patients with idiopathic normal pressure hydrocephalus and Alzheimer’s disease: a pilot study. J Neurol Neurosurg Psychiatry. 1999;67:723–732. doi: 10.1136/jnnp.67.6.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mataro M, Matarin M, Poca MA, et al. Functional and magnetic resonance imaging correlates of corpus callosum in normal pressure hydrocephalus before and after shunting. J Neurol Neurosurg Psychiatry. 2007;78:395–398. doi: 10.1136/jnnp.2006.096164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hellstrom P, Edsbagge M, Archer T, et al. The neuropsychology of patients with clinically diagnosed idiopathic normal pressure hydrocephalus. Neurosurgery. 2007;61:1219–1226. doi: 10.1227/01.neu.0000306100.83882.81. discussion 1227–1228. [DOI] [PubMed] [Google Scholar]
- 14•.Bech-Azeddine R, Hogh P, Juhler M, et al. Idiopathic normal-pressure hydrocephalus: clinical comorbidity correlated with cerebral biopsy findings and outcome of cerebrospinal fluid shunting. J Neurol Neurosurg Psychiatry. 2007;78:157–161. doi: 10.1136/jnnp.2006.095117. This was a biopsy study of iNPH patients receiving VPS. It showed a high rate of comorbid AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Golomb J, Wisoff J, Miller DC, et al. Alzheimer’s disease comorbidity in normal pressure hydrocephalus: prevalence and shunt response. J Neurol Neurosurg Psychiatry. 2000;68:778–781. doi: 10.1136/jnnp.68.6.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vanneste JA. Diagnosis and management of normal-pressure hydrocephalus. J Neurol. 2000;247:5–14. doi: 10.1007/s004150050003. [DOI] [PubMed] [Google Scholar]
- 17.Wilson RK, Williams MA. Evidence that congenital hydrocephalus is a precursor to idiopathic normal pressure hydrocephalus in only a subset of patients. J Neurol Neurosurg Psychiatry. 2007;78:508–511. doi: 10.1136/jnnp.2006.108761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bateman GA. Vascular compliance in normal pressure hydrocephalus. AJNR Am J Neuroradiol. 2000;21:1574–1585. [PMC free article] [PubMed] [Google Scholar]
- 19.Krauss JK, Regel JP, Vach W, et al. Vascular risk factors and arteriosclerotic disease in idiopathic normal-pressure hydrocephalus of the elderly. Stroke. 1996;27:24–29. doi: 10.1161/01.str.27.1.24. [DOI] [PubMed] [Google Scholar]
- 20.Tarkowski E, Tullberg M, Fredman P, et al. Normal pressure hydrocephalus triggers intrathecal production of TNF-alpha. Neurobiol Aging. 2003;24:707–714. doi: 10.1016/s0197-4580(02)00187-2. [DOI] [PubMed] [Google Scholar]
- 21.Li X, Miyajima M, Jiang C, et al. Expression of TGF-betas and TGF-beta type II receptor in cerebrospinal fluid of patients with idiopathic normal pressure hydrocephalus. Neurosci Lett. 2007;413:141–144. doi: 10.1016/j.neulet.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 22.Sasaki H, Ishii K, Kono AK, et al. Cerebral perfusion pattern of idiopathic normal pressure hydrocephalus studied by SPECT and statistical brain mapping. Ann Nucl Med. 2007;21:39–45. doi: 10.1007/BF03033998. [DOI] [PubMed] [Google Scholar]
- 23.Momjian S, Owler BK, Czosnyka Z, et al. Pattern of white matter regional cerebral blood flow and autoregulation in normal pressure hydrocephalus. Brain. 2004;127:965–972. doi: 10.1093/brain/awh131. [DOI] [PubMed] [Google Scholar]
- 24.Klinge PM, Brooks DJ, Samii A, et al. Correlates of local cerebral blood flow (CBF) in normal pressure hydrocephalus patients before and after shunting-A retrospective analysis of [(15)O]H(2)O PET-CBF studies in 65 patients. Clin Neurol Neurosurg. 2008 doi: 10.1016/j.clineuro.2007.12.019. (in press) [DOI] [PubMed] [Google Scholar]
- 25.Nakayama T, Ouchi Y, Yoshikawa E, et al. Striatal D2 receptor availability after shunting in idiopathic normal pressure hydrocephalus. J Nucl Med. 2007;48:1981–1986. doi: 10.2967/jnumed.107.045310. [DOI] [PubMed] [Google Scholar]
- 26.Mocco J, Tomey MI, Komotar RJ, et al. Ventriculoperitoneal shunting of idiopathic normal pressure hydrocephalus increases midbrain size: a potential mechanism for gait improvement. Neurosurgery. 2006;59:847–850. doi: 10.1227/01.NEU.0000232655.78335.D5. discussion 850–851. [DOI] [PubMed] [Google Scholar]
- 27.Zhang J, Williams MA, Rigamonti D. Heritable essential tremor-idiopathic normal pressure hydrocephalus (ETINPH) Am J Med Genet. 2008;146:433–439. doi: 10.1002/ajmg.a.31958. [DOI] [PubMed] [Google Scholar]
- 28••.Relkin N, Marmarou A, Klinge P, et al. Diagnosing idiopathic normal-pressure hydrocephalus. Neurosurgery. 2005;57:S4–S16. doi: 10.1227/01.neu.0000168185.29659.c5. Evidence-based neurosurgical practice guidelines for predicting which patients are likely to benefit from shunting. [DOI] [PubMed] [Google Scholar]
- 29.Kubo Y, Kazui H, Yoshida T, et al. Validation of grading scale for evaluating symptoms of idiopathic normal-pressure hydrocephalus. Dement Geriatr Cogn Disord. 2008;25:37–45. doi: 10.1159/000111149. [DOI] [PubMed] [Google Scholar]
- 30.Krauss JK, Regel JP. The predictive value of ventricular CSF removal in normal pressure hydrocephalus. Neurol Res. 1997;19:357–360. doi: 10.1080/01616412.1997.11740825. [DOI] [PubMed] [Google Scholar]
- 31.Hebb AO, Cusimano MD. Idiopathic normal pressure hydrocephalus: a systematic review of diagnosis and outcome. Neurosurgery. 2001;49:1166–1184. doi: 10.1097/00006123-200111000-00028. discussion 1184–1186. [DOI] [PubMed] [Google Scholar]
- 32.Williams MA, Thomas G, de Lateur B, et al. Objective assessment of gait in normal-pressure hydrocephalus. Am J Phys Med Rehabil. 2008;87:39–45. doi: 10.1097/PHM.0b013e31815b6461. [DOI] [PubMed] [Google Scholar]
- 33.Walchenbach R, Geiger E, Thomeer RT, et al. The value of temporary external lumbar CSF drainage in predicting the outcome of shunting on normal pressure hydrocephalus. J Neurol Neurosurg Psychiatry. 2002;72:503–506. doi: 10.1136/jnnp.72.4.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34••.Burnett MG, Sonnad SS, Stein SC. Screening tests for normal-pressure hydrocephalus: sensitivity, specificity, and cost. J Neurosurg. 2006;105:823–829. doi: 10.3171/jns.2006.105.6.823. This is a cost-effectiveness analysis for shunting of iNPH that highlights the shortcomings of currently available predictive tests. [DOI] [PubMed] [Google Scholar]
- 35.Bugalho P, Alves L. Normal-pressure hydrocephalus: white matter lesions correlate negatively with gait improvement after lumbar puncture. Clin Neurol Neurosurg. 2007;109:774–778. doi: 10.1016/j.clineuro.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 36.Spagnoli D, Innocenti L, Bello L, et al. Impact of cerebrovascular disease on the surgical treatment of idiopathic normal pressure hydrocephalus. Neurosurgery. 2006;59:545–552. doi: 10.1227/01.NEU.0000230259.49167.95. discussion 545–552. [DOI] [PubMed] [Google Scholar]
- 37.Sasaki M, Honda S, Yuasa T, et al. Narrow CSF space at high convexity and high midline areas in idiopathic normal pressure hydrocephalus detected by axial and coronal MRI. Neuroradiology. 2008;50:117–122. doi: 10.1007/s00234-007-0318-x. [DOI] [PubMed] [Google Scholar]
- 38.Palm WM, Walchenbach R, Bruinsma B, et al. Intracranial compartment volumes in normal pressure hydrocephalus: volumetric assessment versus outcome. AJNR Am J Neuroradiol. 2006;27:76–79. [PMC free article] [PubMed] [Google Scholar]
- 39.Bradley WG, Jr, Scalzo D, Queralt J, et al. Normal-pressure hydrocephalus: evaluation with cerebrospinal fluid flow measurements at MR imaging. Radiology. 1996;198:523–529. doi: 10.1148/radiology.198.2.8596861. [DOI] [PubMed] [Google Scholar]
- 40.Scollato A, Tenenbaum R, Bahl G, et al. Changes in aqueductal CSF stroke volume and progression of symptoms in patients with unshunted idiopathic normal pressure hydrocephalus. AJNR Am J Neuroradiol. 2008;29:192–197. doi: 10.3174/ajnr.A0785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kahlon B, Annertz M, Stahlberg F, et al. Is aqueductal stroke volume, measured with cine phase-contrast magnetic resonance imaging scans useful in predicting outcome of shunt surgery in suspected normal pressure hydrocephalus? Neurosurgery. 2007;60:124–129. doi: 10.1227/01.NEU.0000249208.04344.A3. discussion 129–130. [DOI] [PubMed] [Google Scholar]
- 42.McGirt MJ, Woodworth G, Coon AL, et al. Diagnosis, treatment, and analysis of long-term outcomes in idiopathic normal-pressure hydrocephalus. Neurosurgery. 2005;57:699–705. doi: 10.1093/neurosurgery/57.4.699. discussion 699–705. [DOI] [PubMed] [Google Scholar]
- 43.Kahlon B, Sjunnesson J, Rehncrona S. Long-term outcome in patients with suspected normal pressure hydrocephalus. Neurosurgery. 2007;60:327–332. doi: 10.1227/01.NEU.0000249273.41569.6E. discussion 332. [DOI] [PubMed] [Google Scholar]
- 44••.Marmarou A, Bergsneider M, Klinge P, et al. The value of supplemental prognostic tests for the preoperative assessment of idiopathic normal-pressure hydrocephalus. Neurosurgery. 2005;57:S17–S28. doi: 10.1227/01.neu.0000168184.01002.60. These are evidence-based neurosurgical practice guidelines for predicting which patients are likely to benefit from shunting. [DOI] [PubMed] [Google Scholar]
- 45••.Stein SC, Burnett MG, Sonnad SS. Shunts in normal-pressure hydrocephalus: do we place too many or too few? J Neurosurg. 2006;105:815–822. doi: 10.3171/jns.2006.105.6.815. Published as a parallel article with that of Burnett et al. [34••], this decision analysis found that, despite the complication rates of shunt surgery, the quality-of-life outcome in an iNPH patient with moderate dementia may be better than that expected in the natural history of the disease. [DOI] [PubMed] [Google Scholar]
- 46.Williams MA, Sharkey P, van Doren D, et al. Influence of shunt surgery on healthcare expenditures of elderly fee-for-service Medicare beneficiaries with hydrocephalus. J Neurosurg. 2007;107:21–28. doi: 10.3171/JNS-07/07/0021. [DOI] [PubMed] [Google Scholar]


