Abstract
The brain maintains high levels of ascorbic acid (AA) despite a concentration gradient favoring diffusion from brain to peripheral tissues. Dietary antioxidants, including AA, appear to modify the risk of Alzheimer’s disease (AD). The objective of this study was to test the hypothesis that neurodegeneration in AD is modified by brain levels of AA. Thirty-two patients with mild to moderate AD participated in a biomarker study involving standardized clinical assessments over one year. Cerebrospinal fluid (CSF) and serum were collected at baseline for AA and albumin content. Cognitive measures were collected at baseline and one year. CSF and plasma AA failed to predict cognitive decline independently, however, CSF: plasma AA ratio did. After adding CSF Albumin Index (an established marker of blood-brain barrier integrity) to the regression models the effect of CSF: plasma AA ratio as a predictor of cognitive decline was weakened. CSF: plasma AA ratio predicts rate of decline in AD. This relationship may indicate that the CSF: plasma AA ratio is an index of AA availability to the brain or may be an artifact of a relationship between blood-brain barrier impairment and neurodegeneration.
Keywords: Albumin, Alzheimer’s disease, antioxidants, ascorbic acid, blood-brain barrier, cerebrospinal fluid, cognitive decline, vitamin C
INTRODUCTION
The pathophysiology of Alzheimer’s disease (AD) remains incompletely understood, although the accrual of oxidative damage in areas of the brain responsible for memory and higher cognitive faculties is a consistent finding [1,2]. There are multiple lines of evidence supporting a role for antioxidants in reducing oxidative damage to neurons and reducing amyloid plaque formation [3–8]. Ascorbic acid (AA; vitamin C) is a potent water-soluble antioxidant without synthesis in the brain. Animal models have demonstrated 40% of AA content in the brain turn over per day and AA maintenance as high as 10 µM in neurons [9]. This concentration of AA reduces oxidative neuronal stress through protection of cell membranes and DNA, reducing amyloid-β toxicity and regenerating other antioxidants [10,11].
In humans, most plasma components are diluted about 100-fold in cerebrospinal fluid (CSF). Conversely, the content of AA in CSF is highly concentrated compared to plasma (CSF: plasma ratio of about 3–4:1) [12], supporting statements by some investigators calling AA “nourishing liquor” that constantly surrounds the brain [13]. At the choroid plexus, AA is actively transported across the basolateral membrane by a sodium dependent transporter (SVCT-2) into the epithelium and then released into the CSF [14]. The maintenance of highCSF levels of AA leans on both active “carrier” transport processes and “barrier” integrity of the blood-brain barrier (BBB). This physiology is necessary to prevent AA from diffusing out of the CNS according to the concentration gradient. Here, we assess the “barrier” integrity at the blood-CSF-barrier and choroid plexus in living patients utilizing a reliable and validated measure, the CSF Albumin Index [15–17]. Serum albumin levels are normally about 200 times higher than that found in the CSF [18]. In the setting of BBB disruption, the CSF Albumin Index is increased due to albumin’s greater access to the CSF from serum through the disturbed BBB.
Our primary hypothesis in this study was that brain AA concentrations predict cognitive decline in adults with mild to moderate AD. A secondary hypothesis was that BBB integrity modifies the CSF and plasma compartmentalization of this antioxidant.
METHODS
To test whether CSF and plasma AA predict rates of AD progression, we analyzed CSF and plasma for AA and serum albumin in overnight fasting subjects along with clinical risk factors, apolipoprotein E genotype, and cognitive measures in 32 subjects with mild to moderate AD followed for 1 year.
Study participants
The subject population for this longitudinal biomarker study included patients in the NIA-Layton Aging and Alzheimer’s Disease Center of Oregon Health & Science University (OHSU) with a diagnosis of probable AD. Participants were diagnosed by National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer’s Disease and Related Disorders Association criteria [19] and Clinical Dementia Rating (CDR) of 0.5 or 1.0, established mild to moderate AD. All patients from the biomarker study with available biochemical measures were included, yielding 32 mild to moderate probable AD subjects. All participants provided informed consent in accord with the Institutional Review Board for human study at OHSU.
Data collection
Thirty-two patients (10 females, mean age 71 ± 7 years) were assessed at baseline and 12 months. Clinical evaluation included medical history, physical exam, Mini-Mental Status Exam (MMSE) [20], CDR [21], Alzheimer’s Disease Assessment Scale-cognitive subscale (ADAS-Cog) [22], Hachinski ischemia score [23] as a measure of vascular burden, and Geriatric Depression Scale [24]. Lumbar CSF and peripheral blood were collected at baseline for analyses. Supplementation was assessed by interview with caregiver including examination of all containers from which subjects were regularly taking pills at baseline and 12 month visits.
Biochemical assays
Lumbar punctures were performed in the morning under standardized conditions at L3–L4 or L4–L5 interspace. CSF samples were immediately aliquoted and snap frozen at −70°C until assayed, at which point samples had normal cell count and glucose levels. Peripheral blood samples collected at the same visit as lumbar puncture were analyzed with the CSF samples to quantify albumin and AA [25]. CSF and serum albumin were quantified to calculate CSF Albumin Index discussed here and validated elsewhere [15,26]. Genotyping of apolipoprotein E isoforms was performed using DNA isolated for blood cells and described elsewhere [27]. All biochemical and genetic analyses were performed and the results recorded by staff blinded to subject’s clinical information.
Statistical methods
Our primary hypotheses were that CSF AA and plasma AA predicts the rate of AD progression over one year. CSF AA, plasma AA, and CSF: plasma AA ratio were primary predictor variables and cognitive measures (MMSE, ADAS-Cog, CDR) were designated dependent variables. Pearson correlations were obtained to describe the relationship between the primary predictor and primary dependent variables. Linear regression analysis was used to examine associations between AA and cognitive measures. Preliminary study demonstrated that neither CSF AA nor plasma AA had univariate association with rate of cognitive decline, but CSF: plasma AA ratio did. Therefore, we focused on CSF: plasma AA ratio as a primary predictor variable. Three models were fitted with predictors including: 1) CSF: plasma AA ratio; 2) CSF: plasma AA ratio plus age, gender, education years, APOE-4 carrier status, and baseline dependent cognitive measure; and 3) model #2 plus the addition of CSF Albumin Index. CSF Albumin Index was dichotomized at the a priori threshold of 9.0 (BBB intact <9.0; BBB impaired ⩾9.0) to examine the significance of BBB impairment on CSF: plasma AA ratio. Significance level was set at 0.05 for all analyses. All statistical analyses were performed using SPSS v.16.0 (Chicago, Ill).
RESULTS
The baseline characteristics of the study population are provided in Table 1.
Table 1.
Baseline demographic, genetic, biochemical and clinical characteristics in mild-to-moderate AD (n = 32)*
| Age, y | 71 (7) |
| Female, (%) | 10 (31) |
| Education years | 14 (3) |
| APOE ε4 carriers, (%) | 24 (74) |
| BP systolic, mm Hg | 144 (23) |
| BP diastolic, mm Hg | 77 (11) |
| Body Mass Index | 27 (5) |
| Hachinski Ischemia Score | 0.6 (1.0) |
| Ascorbic acid supplementation present, (%) | 34.4 |
| Ascorbic acid, CSF (µM) | 129 (52) |
| Ascorbic acid, plasma (µM) | 41 (30) |
| CSF-to-plasma ascorbic acid ratio | 4.0 (1.6) |
| Albumin, CSF (µM) | 30.5 (16) |
| Albumin, serum (µM) | 4052.5 (371.3) |
| CSF Albumin Index | 7.5 (3.8) |
| Mini Mental Status Exam | 19 (5) |
| Alzheimer’s Disease Assessment Scale – cognitive subscale | 24 (10) |
| Clinical Dementia Rating – sum of box score | 5.9 (1.4) |
| Annual change in Mini Mental State Exam | 3.3 (3.8) |
| Annual change in Alzheimer’s Disease Assessment Scale –cognitive subscale | 8.9 (9.1) |
| Annual change in Clinical Dementia rating – sum of box score | 6.8 (2.7) |
Mean (SD) unless denoted otherwise.
CSF and plasma AA are correlated and plasma AA drives the CSF/plasma AA ratio
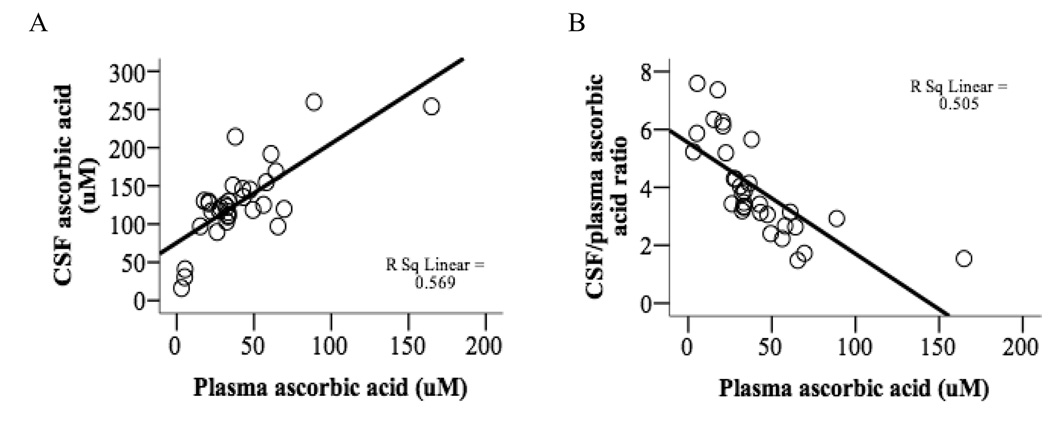
Fifty-six percent of the variance in CSF AA is attributable to plasma AA (p < 0.001) (Fig. 1A) and 50% of the variance in CSF: plasma AA ratio is attributable to plasma AA (p < 0.001) (Fig. 1B).
Fig. 1.
Relationship between CSF and plasma ascorbic acid. A) Correlation between plasma and CSF AA (r = 0.755, p < 0.001). B) Inverse correlation between plasma AA and CSF: plasma AA ratio (r = −0.710, p < 0.001).
Neither CSF nor plasma AA predict rate of cognitive decline in AD, but CSF: plasma AA ratio does
CSF and plasma AA, and CSF: plasma AA ratio, were independent of age, gender, APOE-4 genotype, and baseline measures of dementia severity (e.g., MMSE, ADAS cog; with the exception of CDR-SOB). A significant univariate association was demonstrated between CSF: plasma AA ratio and rate of cognitive decline over 1 year (p = 0.03 for MMSE, ADAS-cog and CDR-SOB) (Table 2, Model 1). This association remained after controlling for age, gender, education, APOE-4 carrier status, and baseline cognitive measure (p = 0.02 for MMSE, p = 0.03 for ADAS-cog, p = 0.01 for CDR-SOB) (Table 2, Model 2). Each unit increase in CSF: plasma AA ratio was associated with 1.1 units less point loss on MMSE and 2.7 unit less loss on ADAS-cog.
Table 2.
Regression analysis of CSF-to-plasma ascorbic acid ratio and rates of cognitive decline in mild-to-moderate Alzheimer’s disease at 1 year
| Model | Baseline measure | Regression coefficients for rate of cognitive decline over 1 year |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MMSE |
ADAS cog |
CDR-SOB |
||||||||
| Mean r | SE | Sig. | Mean r | SE | Sig. | Mean r | SE | Sig. | ||
| 1 | CSF: plasma AA ratio | −0.88 | 0.39 | 0.033* | −2.19 | 0.98 | 0.033* | −0.637 | 0.285 | 0.033* |
| 2 | CSF: plasma AA ratio | −1.112 | 0.466 | 0.025* | −2.676 | 1.159 | 0.030* | −0.589 | 0.233 | 0.018* |
| Age | 0.043 | 0.100 | 0.669 | −0.052 | 0.244 | 0.833 | 0.030 | 0.051 | 0.567 | |
| Gender | −2.972 | 1.713 | 0.095 | −3.501 | 4.118 | 0.404 | −1.985 | 0.852 | 0.028* | |
| Education years | 0.089 | 0.247 | 0.722 | 0.711 | 0.575 | 0.228 | −0.064 | 0.118 | 0.589 | |
| ApoE-4 carrier status | −0.399 | 1.315 | 0.764 | 4.191 | 3.180 | 0.200 | 0.990 | 0.662 | 0.147 | |
| Cognitive measure | −0.061 | 0.136 | 0.656 | −0.110 | 0.176 | 0.537 | 1.229 | 0.234 | 0.000* | |
| 3 | CSF: plasma AA ratio | −0.845 | 0.523 | 0.119 | −2.707 | 1.299 | 0.048* | −0.340 | 0.244 | 0.177 |
| Age | 0.053 | 0.100 | 0.601 | −0.054 | 0.252 | 0.831 | 0.042 | 0.048 | 0.384 | |
| Gender | −2.124 | 1.871 | 0.267 | −3.623 | 4.699 | 0.449 | −1.154 | 0.877 | 0.201 | |
| Education years | 0.062 | 0.247 | 0.803 | 0.716 | 0.592 | 0.239 | −0.079 | 0.110 | 0.481 | |
| ApoE-4 carrier status | −0.618 | 1.324 | 0.645 | 4.229 | 3.310 | 0.214 | 0.821 | 0.620 | 0.198 | |
| Cognitive measure | −0.029 | 0.138 | 0.836 | −0.104 | 0.204 | 0.614 | 1.093 | 0.226 | 0.000* | |
| CSF Albumin Index | 0.226 | 0.205 | 0.281 | −0.033 | 0.563 | 0.954 | 0.216 | 0.098 | 0.037* | |
MMSE, Mini Mental Status Exam; ADAS cog, Alzheimer’s Disease Assessment Scale cognitive subscale, CSF, cerebral spinal fluid, AA, ascorbic acid
p ⩽ 0.05.
The association between CSF: plasma AA ratio and rate of cognitive decline in AD is modified by BBB integrity
When CSF Albumin Index is added to the regression analysis in Model 3 (Table 2), the significance of the association between CSF: plasma AA ratio and MMSE (p = 0.11) and CDR-SOB (p = 0.17) is lost, although maintained in ADAS-cog (p = 0.04). These data indicate that the effect of CSF: plasma AA ratio on cognitive decline in AD is partially attributed to underlying BBB integrity.
CSF: plasma AA ratio is modulated by BBB integrity
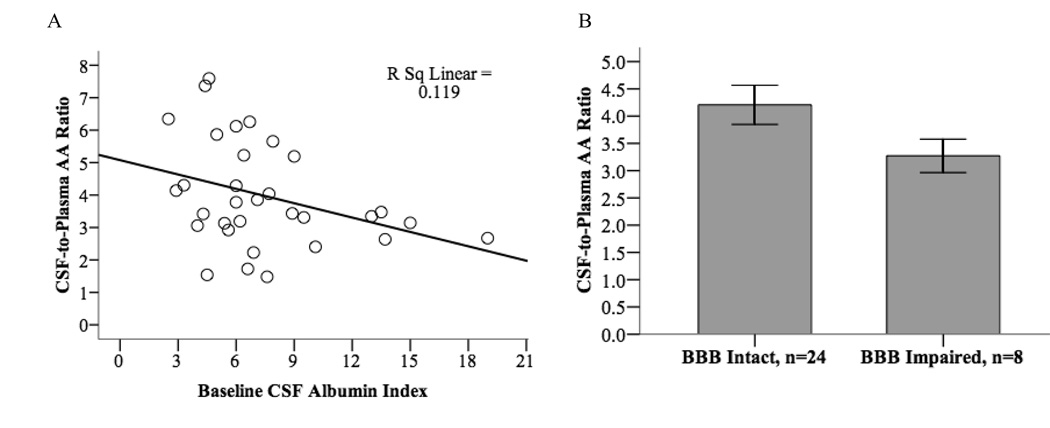
Scatter plot demonstrates an inverse linear correlation between CSF: plasma AA ratio and CSF Albumin Index (p = 0.05, n = 32) that is maintained in partial correlation analysis controlling for AA supplementation, age, gender, education years and APOE-4 carrier status (r = −0.414, p = 0.03) (Fig. 2A). After dichotomizing subjects by BBB integrity and comparing the mean CSF: plasma AA ratio in those with intact (n = 24) and impaired (n = 8) BBB, a marginal difference of 0.95 units was observed (p = 0.059) (Fig. 2B). The inverse correlation between CSF: plasma AA ratio and CSF Albumin Index provides some explanation as to why the association between AA ratio and rates of cognitive decline is attenuated in the regression analysis of Table 2.
Fig. 2.
Relationship between CSF-to-Plasma Ascorbic Acid Ratio and CSF Albumin Index (as a measure of blood-brain barrier integrity). AA, ascorbic acid; BBB, blood-brain barrier; BBB intact = < 9.0, BBB Impaired = ⩾ 9.0.
DISCUSSION
In these 32 adults followed for one year with mild-to-moderate AD, we found that rates of cognitive decline were not explained by CSF or plasma AA independently, but the ratio of CSF: plasma AA did predict cognitive decline.
The absence of a relationship between rate of cognitive decline and CSF or plasma AA may be readily explained. Plasma AA levels are dependent on recent dietary intake, so they are not very reproducible and also not representative of long-term consumption, which is probably more relevant to neurologic outcome than short-term exposure. Plasma AA is also not in direct contact with the brain. On the other hand, CSF AA may be a better reflection of the AA that the brain “sees”, but the drive to maintain a homeostatic level of vitamin C is so strong that low variability of AA content in CSF may actually prevent detection of a relationship between AA and rate of cognitive decline. This notion is exemplified by observations in overt scurvy where brain levels of AA are relatively maintained through depletion of peripheral tissues [28].
We consequently examined the relationship between CSF: plasma AA ratio and rate of decline in light of previous observations that CSF: plasma AA ratio discriminates AD patients from controls more reliably than CSF or plasma levels alone [12,29,30]. We and others have noted that the CSF: plasma AA ratio is higher in AD patients than in controls and hypothesized that this was a reflection of the increased “consumption” of AA by the oxidatively stressed brain, leading to lower plasma levels. Based on this experience, we would predict that AD patients with higher CSF: plasma AA ratio would have more rapid rates of decline, but we observe the opposite in this sample where the slowest rates of cognitive decline are seen in subjects with the highest CSF: plasma AA ratio. This may be interpreted as evidence that either a high CSF: plasma AA ratio is causally related to rate of cognitive decline, or there is a non-causal correlation due to the fact that the ability to maintain a high CSF: plasma AA ratio is a marker of a “healthier” brain, more able to cope with the neurodegenerative process of AD. We argue that the latter explanation is more plausible, and we use data on BBB integrity to test this possibility. CSF Albumin Index, a marker of BBB integrity, attenuated the association of CSF: plasma AA ratio with cognitive outcomes, suggesting that the observed effect of CSF: plasma AA ratio on cognitive decline is partially attributed to underlying “barrier” function of the BBB. An inverse correlation between CSF: plasma AA ratio and CSF Albumin Index further supports the hypothesis that lower AA ratios in some patients may be due to BBB dysfunction, impairing the ability of the brain to maintain high CSF: plasma AA ratio due to diffusion of AA out of the CNS according to its concentration gradient.
To our knowledge, this is the first prospective study of cognitive decline in AD that quantifies CSF and plasma AA content and BBB integrity as modifiers of disease progression. Its strengths lie in its prospective design, consensus diagnosis of mild-to-moderate AD, and simultaneous collection of CSF and plasma AA and serum albumin. Its limitations are that CSF and plasma AA was measured only at baseline and our study population was relatively small (n = 32). Nevertheless, these findings support the hypothesis that BBB integrity is relevant to the progression of AD, and raise the possibility that BBB dysfunction accelerates the rate of degeneration not only by potentially exposing brain parenchyma to peripheral toxins, but by impairing the ability of the brain to concentrate AA and perhaps other nutrients with neuroprotective properties.
ACKNOWLEDGMENTS
NCCAM T32 AT002688 Ruth L. Kirschstein Scholar (GLB), NCRR UL1 RR024140 (GLB), VA Advanced Research Development Award (JFQ), NCCAM P01 AT002034 (BF), NIA AG08017 (JAK), Dana Foundation (JFQ).
References
- 1.Markesbery WR, Carney JM. Oxidative alterations in Alzheimer’s disease. Brain Pathol. 1999;9:133–146. doi: 10.1111/j.1750-3639.1999.tb00215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lovell MA, Markesbery WR. Oxidative damage in mild cognitive impairment and early Alzheimer’s disease. J Neurosci Res. 2007;85:3036–3040. doi: 10.1002/jnr.21346. [DOI] [PubMed] [Google Scholar]
- 3.Bourdel-Marchasson I, Delmas-Beauvieux MC, Peuchant E, Richard-Harston S, Decamps A, Reignier B, Emeriau JP, Rainfray M. Antioxidant defences and oxidative stress markers in erythrocytes and plasma from normally nourished elderly Alzheimer patients. Age Ageing. 2001;30:235–241. doi: 10.1093/ageing/30.3.235. [DOI] [PubMed] [Google Scholar]
- 4.Butterfield DA, Griffin S, Munch G, Pasinetti GM. Amyloid beta-peptide and amyloid pathology are central to the oxidative stress and inflammatory cascades under which Alzheimer’s disease brain exists. J Alzheimers Dis. 2002;4:193–201. doi: 10.3233/jad-2002-4309. [DOI] [PubMed] [Google Scholar]
- 5.Engelhart MJ, Geerlings MI, Ruitenberg A, van Swieten JC, Hofman A, Witteman JC, Breteler MM. Dietary intake of antioxidants and risk of Alzheimer disease. JAMA. 2002;287:3223–3229. doi: 10.1001/jama.287.24.3223. [DOI] [PubMed] [Google Scholar]
- 6.Martin A, Cherubini A, Andres-Lacueva C, Paniagua M, Joseph J. Effects of fruits and vegetables on levels of vitamins E and C in the brain and their association with cognitive performance. J Nutr Health Aging. 2002;6:392–404. [PubMed] [Google Scholar]
- 7.Meydani M. Antioxidants and cognitive function. Nutr Rev. 2001;59:S75–S80. doi: 10.1111/j.1753-4887.2001.tb05505.x. discussion S80–72. [DOI] [PubMed] [Google Scholar]
- 8.Resende R, Moreira PI, Proenca T, Deshpande A, Busciglio J, Pereira C, Oliveira CR. Brain oxidative stress in a tripletransgenic mouse model of Alzheimer disease. Free Radic Biol Med. 2008;44:2051–2057. doi: 10.1016/j.freeradbiomed.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 9.Rice ME. Ascorbate regulation and its neuroprotective role in the brain. Trends Neurosci. 2000;23:209–216. doi: 10.1016/s0166-2236(99)01543-x. [DOI] [PubMed] [Google Scholar]
- 10.Floyd RA, Hensley K. Oxidative stress in brain aging. Implications for therapeutics of neurodegenerative diseases. Neurobiol Aging. 2002;23:795–807. doi: 10.1016/s0197-4580(02)00019-2. [DOI] [PubMed] [Google Scholar]
- 11.Drew KL, Toien O, Rivera PM, Smith MA, Perry G, Rice ME. Role of the antioxidant ascorbate in hibernation and warming from hibernation. Comp Biochem Physiol C Toxicol Pharmacol. 2002;133:483–492. doi: 10.1016/s1532-0456(02)00118-7. [DOI] [PubMed] [Google Scholar]
- 12.Quinn J, Suh J, Moore MM, Kaye J, Frei B. Antioxidants in Alzheimer’s disease-vitamin C delivery to a demanding brain. J Alzheimers Dis. 2003;5:309–313. doi: 10.3233/jad-2003-5406. [DOI] [PubMed] [Google Scholar]
- 13.Davson H, Segal HB. Physiology of the CSF and Blood-Brain Barriers. Boca Raton: CRC; 1996. [Google Scholar]
- 14.Spector R, Johanson C. Micronutrient and urate transport in choroid plexus and kidney: implications for drug therapy. Pharm Res. 2006;23:2515–2524. doi: 10.1007/s11095-006-9091-5. [DOI] [PubMed] [Google Scholar]
- 15.Tibbling G, Link H, Ohman S. Principles of albumin and IgG analyses in neurological disorders. I. Establishment of reference values. Scand J Clin Lab Invest. 1977;37:385–390. doi: 10.1080/00365517709091496. [DOI] [PubMed] [Google Scholar]
- 16.Reiber H. Flow rate of cerebrospinal fluid (CSF)–a concept common to normal blood-CSF barrier function and to dysfunction in neurological diseases. J Neurol Sci. 1994;122:189–203. doi: 10.1016/0022-510x(94)90298-4. [DOI] [PubMed] [Google Scholar]
- 17.Bowman GL, Quinn JF. Alzheimer’s disease and the blood-brain barrier: past, present and future. Aging Health. 2008;4:47–57. doi: 10.2217/1745509X.4.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reiber H, Peter JB. Cerebrospinal fluid analysis: disease-related data patterns and evaluation programs. J Neurol Sci. 2001;184:101–122. doi: 10.1016/s0022-510x(00)00501-3. [DOI] [PubMed] [Google Scholar]
- 19.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 20.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 21.Dooneief G, Marder K, Tang MX, Stern Y. The Clinical Dementia Rating scale: community-based validation of “profound” and “terminal” stages. Neurology. 1996;46:1746–1749. doi: 10.1212/wnl.46.6.1746. [DOI] [PubMed] [Google Scholar]
- 22.Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer’s disease. Am J Psychiatry. 1984;141:1356–1364. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- 23.Moroney JT, Bagiella E, Desmond DW, Hachinski VC, Molsa PK, Gustafson L, Brun A, Fischer P, Erkinjuntti T, Rosen W, Paik MC, Tatemichi TK. Meta-analysis of the Hachinski Ischemic Score in pathologically verified dementias. Neurology. 1997;49:1096–1105. doi: 10.1212/wnl.49.4.1096. [DOI] [PubMed] [Google Scholar]
- 24.Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 25.Frei B, England L, Ames BN. Ascorbate is an outstanding antioxidant in human blood plasma. Proc Natl Acad Sci U S A. 1989;86:6377–6381. doi: 10.1073/pnas.86.16.6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bowman GL, Kaye JA, Moore M, Waichunas D, Carlson NE, Quinn JF. Blood-brain barrier impairment in Alzheimer disease: stability and functional significance. Neurology. 2007;68:1809–1814. doi: 10.1212/01.wnl.0000262031.18018.1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greenberg SM, Briggs ME, Hyman BT, Kokoris GJ, Takis C, Kanter DS, Kase CS, Pessin MS. Apolipoprotein E epsilon 4 is associated with the presence and earlier onset of hemorrhage in cerebral amyloid angiopathy. Stroke. 1996;27:1333–1337. doi: 10.1161/01.str.27.8.1333. [DOI] [PubMed] [Google Scholar]
- 28.Spector R. Vitamin homeostasis in the central nervous system. N Engl J Med. 1977;296:1393–1398. doi: 10.1056/NEJM197706162962409. [DOI] [PubMed] [Google Scholar]
- 29.Reiber H, Ruff M, Uhr M. Ascorbate concentration in human cerebrospinal fluid (CSF) and serum. Intrathecal accumulation and CSF flow rate. Clin Chim Acta. 1993;217:163–173. doi: 10.1016/0009-8981(93)90162-w. [DOI] [PubMed] [Google Scholar]
- 30.Paraskevas GP, Kapaki E, Libitaki G, Zournas C, Segditsa I, Papageorgiou C. Ascorbate in healthy subjects, amyotrophic lateral sclerosis and Alzheimer’s disease. Acta Neurol Scand. 1997;96:88–90. doi: 10.1111/j.1600-0404.1997.tb00245.x. [DOI] [PubMed] [Google Scholar]