Abstract
In the course of the development of a potent series of nitrofuranylamide anti-tuberculosis agents, we investigated if the exceptional activity resulted in part from the isoxazoline core and if it possessed any intrinsic anti-tuberculosis activity. This led to the discovery of an isoxazoline ester with appreciable anti-tuberculosis activity. In this study we explored the anti-tuberculosis structure activity relationship of the isoxazoline ester compound through systematic modification of the 3,5-di-substituted isoxazoline core. Two approaches were used: (i) modification of the potentially metabolically labile ester functionality at the 3-position with acids, amines, amides, reverse amides, alcohols, hydrazides, and 1,3,4-oxadiazoles; (ii) substitution of the distal benzyl piperazine ring in the 5-position of the isoxazoline ring with piperazyl-ureas, piperazyl-carbamates, biaryl systems, piperidines and morpholine. Attempts to replace the ester group at C-3 position of isoxazoline with a variety of bioisosteric head groups led to significant loss of the tuberculosis inhibition indicating that an ester is required for anti-tuberculosis activity. Optimization of the isoxazoline C5-position produced compounds with improved anti-tuberculosis activity, most notably the piperazyl-urea and piperazyl-carbamate analogs.
Keywords: Anti-tuberculosis agents, antibacterial, nitrofurans, isoxazolines, lead optimization
1. Introduction
Tuberculosis is caused by Mycobacterium tuberculosis, a deadly obligate bacterial pathogen. The global effect of tuberculosis is immense [1–2]. According to the World Health Organization, currently one-third of world’s population is infected with latent tuberculosis [3]. Based on the trend over the past few years, a total of 225 million new cases and 79 million deaths are expected from tuberculosis between 1998 and 2030. The major concerns for current tuberculosis treatment are its latency, co-infection with HIV, poor patient compliance, and drug resistance issues caused by the emergence of multidrug resistant tuberculosis (MDR-TB) and the recent advent of extensively drug resistant tuberculosis (XDR-TB) [4]. Most of the drugs in the current tuberculosis regime result from research performed over 50 years ago [5]. At that time with the successful introduction of those agents it was widely believed that tuberculosis could be eliminated and this led to significant underinvestment in the development of new therapeutics to treat tuberculosis for many decades [6]. Today more people die from tuberculosis than ever before, and this has catalyzed a renewed effort to develop improved therapies [7]. Hence, there is an urgent need to develop potent and fast acting anti-tuberculosis drugs with new modes of action to overcome the cross resistance with current drugs and low toxicity profiles that can be tolerated for long treatment periods required for tuberculosis chemotherapy.
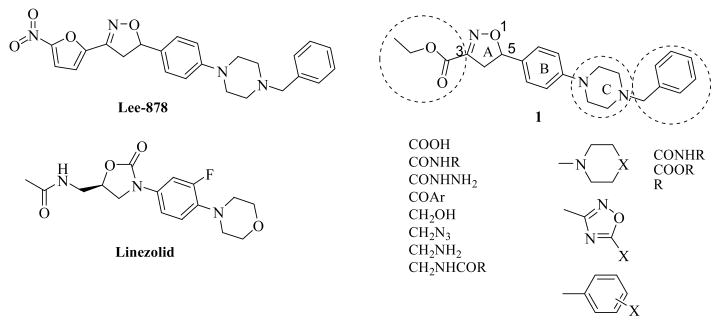
In an ongoing effort to develop novel anti-tuberculosis therapeutics, previously, we reported a series of nitrofuranyl compounds with potent inhibitory activity against M. tuberculosis [8–11]. The outstanding in vitro activity of Lee-878 from this series led us to explore if the isoxazoline core was itself privileged and if it had any intrinsic anti-tuberculosis activity. A series of non-nitrofuran isoxazolines was subsequently synthesized in which the nitrofuran head group was systematically replaced. This led to the discovery of isoxazoline ester 1, which showed appreciable anti-tuberculosis activity (MIC: 1.56 μg/mL) (Figure 1) [12]. Excitingly, this represents a new synthetically tractable anti-tuberculosis chemotype that has not been previously reported. The study described herein concerns our efforts to optimize this hit to increase its metabolic stability and to explore the structural similarity with the oxazolidinone class of antibiotics including Linezolid [13–15]. The outline of the approach is displayed in Figure 1 and features systematic modification and optimization of the isoxazoline ring to replace the 3 position with bioisosteric head groups and the 5 position with modified piperazyl C rings.
Figure 1.
2. Chemistry
a) Modifications of the C-5 side chain of the isoxazoline ring
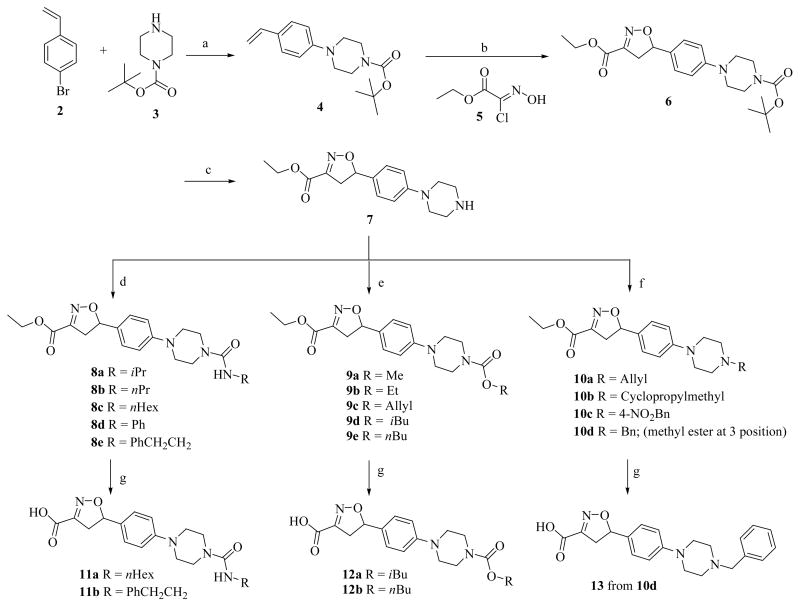
Four series of C-5 analogs were synthesized as described in Schemes 1 and 2. Our first target was the synthesis of a set of compounds possessing varying substitutions to the piperazine C ring of lead compound 1. N-Boc protected phenyl piperazine 4 was synthesized from 4-bromo styrene 2 and Boc-piperazine 3 using standard aromatic amination reaction conditions in 58% yield (Scheme 1) [16]. Olefin 4 was treated with commercially available oxime 5 in the presence of Et3N in dichloromethane at room temperature to afford isoxazoline 6 following a [3+2] regioselective cycloaddition mechanism in 71% yield [17]. The deprotection of Boc protected compound 6 was achieved by treating with trifluoroacetic acid in THF at room temperature to give 7 in quantitative yield. Compound 7 was used as key intermediate for the synthesis of several derivatives. Disubstituted urea derivatives 8a–e were synthesized by treating free amine 7 with a variety of alkyl and aryl isocyanates in greater than 80% yields. A set of carbamates derivatives 9a–e were then synthesized from amine 7 by reaction with the corresponding alkyl chloroformates also in 70–89% yields. N-alkylated derivatives 10a–d were synthesized from amine 7 by reaction with alkyl halides in moderate yields (48–58%).
Scheme 1.
Reagents and conditions: a) PdCl2[P(o-Tol)3]2, NaOtBu, Toluene, 100 °C; b) Et3N, CH2Cl2; c) TFA, THF, rt; d) Et3N, RNCO, CH2Cl2, rt; e) Et3N, ROCOCl, CH2Cl2, rt; f) K2CO3, RBr, DMF; g) LiOH, THF:H2O, rt.
Scheme 2.
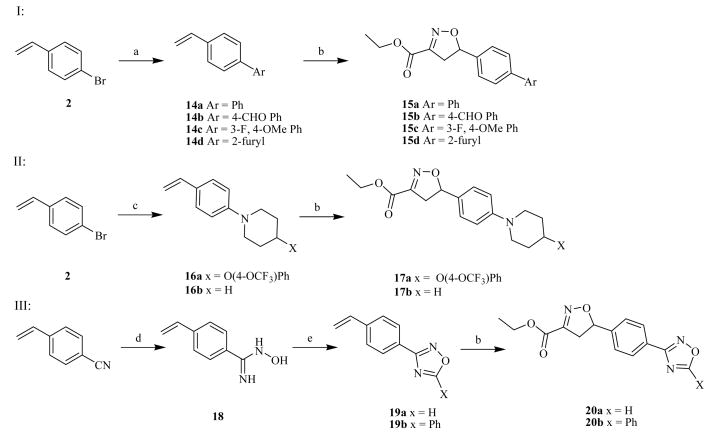
Reagents and conditions: a) aryl boronic acid, Pd(Ph3P)4, 1M aq. K2CO3, DME, reflux; b) ethylcholoroaximidoacetate, Et3N, CH2Cl2, rt; c) 4-(4-Trifluoromethoxy-phenoxy)-piperidine or piperidine, tBuONa, PdCl2[(oTol)3P]2, toluene, reflux; d) NH2OH.HCl, Et3N, EtOH, reflux; e) CH(OEt)3, BF3.OEt2, reflux or PhCOCl, Py, reflux
For the second series of analogs, the piperazine C-ring was completely replaced with a series aryl rings. (Scheme 2, I) The required biaryl systems were synthesized using the Suzuki chemistry. 4-bromo styrene 2 was treated with a variety of aryl boronic acids in the presence of Pd(PPh3)4 using 1M aqueous K2CO3 solution in DME at reflux to afford corresponding biaryl compounds 14a–d in 61–73% yields. These substituted olefins were then reacted to form their corresponding isoxazoline derivatives 15a–d using identical synthetic conditions described for the synthesis of compound 6.
For the third series of analogs, the piperazine C- ring was substituted with a piperidine system. (Scheme 2, II) These analogs were synthesized using the same aryl amination reaction conditions as described earlier for the synthesis of piperazine analog 4. Compounds 16a–b were prepared by nucleophilic aromatic amination of 2 with 4-(4-trifluoromethoxy-phenoxy)-piperidine and piperidine in 78% and 71% yields, respectively. The isoxazoline ring was then constructed on these cores using standard chemistries to afford target compounds 17a–b in 74% and 77% yields, respectively.
For the fourth series of analogs, the piperazine C-ring was replaced with heteroaryl oxadiazole rings. (Scheme 2, III) The synthetic approach to this series utilized the synthetic utility of phenyl substituted cyano group as a synthon for introduction of heteroaryl rings [18]. Accordingly, 4-cyano styrene was treated with hydroxylamine hydrochloride in ethanol in the presence of Et3N to produce amidine intermediate 18. Amidine 18 was treated with triethyl orthoformate and a catalytic amount of boron trifluoride diethyl ether at 80 °C to give 3-phenyl 1,2,4-oxadiazole derivative 19a in 59% yield. Amidine 18 was treated with benzoyl chloride in pyridine at reflux temperature to afford 3,5-disubstituted 1,2,4-oxadiazole derivative 19b in 48% yield. The olefin moiety in both 19a and 19b was used for the synthesis of isoxazoline derivatives 20a and 20b respectively using standard procedures in 59% and 60% yields.
b) Modifications on C-3 side chain of isoxazoline ring
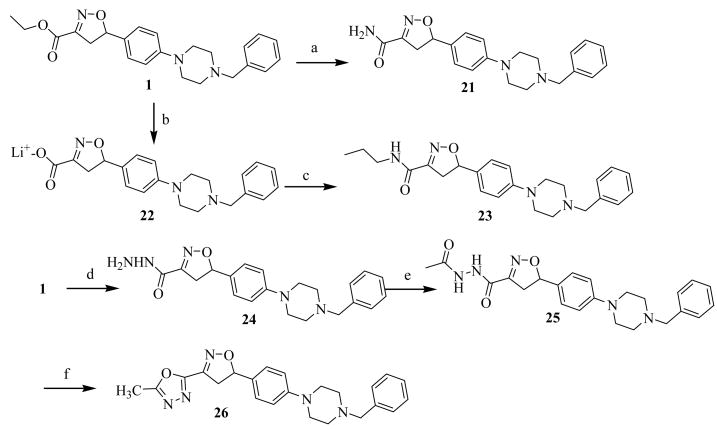
Synthetic modification to the C-3 side chain ester of the isoxazoline ring in 1 was approached by the synthesis of three series of compounds with acid, amine, amide, reverse amide, alcohol, hydrazide, and 1,3,4-oxadiazole substitutions at the 3 position. The rationale for these substitutions was to use a bioisosteric approach and synthesize derivatives with increased metabolic stability over labile ester functionality of 1 while retaining a similar pattern of heteroatoms. For the first series, free acids with a variety of 5 position substitutions were generated by hydrolysis of the esters 8c, 8e, 9d, 9e and 10d, using LiOH, in aqueous THF at room temperature to afford corresponding free acids 11a, 11b, 12a, 12b and 13 in 62–93% yields (Scheme 1). For the second series, modifications to the C-3 side chain were carried out keeping the isoxazoline 5-position unaltered as found in compound 1. The ester functionality in 1 was converted into amide 21 in 66% yield by treating with ammonium hydroxide in 1,4-dioxane at room temperature (Scheme 3). Propyl amide 23 was synthesized by hydrolysis of 1, followed by activation of the resulting free acid in situ as acid chloride and coupling to n-propyl amine in THF in 59% overall yield. The hydrazide derivative 24 was synthesized by treating the ester with hydrazine hydrate in ethanol at reflux temperature in 52% yield. Then 24 was used to construct a 1,3,4-oxadiazole ring at C-3 position of isoxazoline ring. Accordingly, diacyl hydrazine 25 was synthesized from 24 by treatment with acetyl chloride in dichloromethane using Et3N as a base in 61% yield. Subsequent treatment 25 with p-TsCl, Et3N in dichoromethane and 40% aq. K2CO3, sequentially afforded 2,5-disubstituted 1,3,4-oxadiazole analog 26 in 58% yield.
Scheme 3.
Reagents and conditions: a) NH4OH, 1,4-Dioxane, 12 h, rt; b) LiOH, H2O-THF; c) i) (COCl)2, DCM, cat. DMF; ii) n-PrNH2, THF; d) NH2NH2. H2O, EtOH, reflux; e) CH3COCl, Et3N, CH2Cl2, rt; f) i) p-TsCl2, Et3N, CH2Cl2, reflux; ii) 40% K2CO3
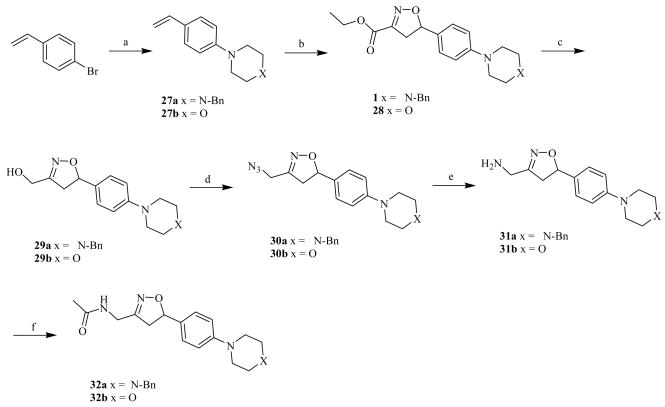
For the third series, free amines and reverse amides were substituted on the C-3 side chain of the isoxazoline ring producing compounds with structural resemblance to the oxazolidinone class of antibiotics. The esters in isoxazoline derivatives 1 and 28, with C-5 N-benzyl piperazine and morpholine moieties respectively, were reduced into corresponding primary alcohols 29a and 29b by DIBAL-H in THF at 0 °C in 56% and 60% yields. The primary alcohols 29a and 29b were then converted into their azides 30a and 30b by treating with CBr4, Ph3P and NaN3 in DMF at room temperature in 59% and 61% yields, followed by reduction with Ph3P in 1,4-dioxane to yield primary amines 31a and 31b in 67% and 60% yields. Amines 31a and 31b were finally treated with acetyl chloride using Et3N in CH2Cl2 to afford acetamides 32a and 32b in 78% and 71% yields (Scheme 4).
Scheme 4.
Reagents and conditions: a) morphiline or 1-benzyl piperazine, tBuONa, PdCl2[(oTol)3P]2, toluene, reflux; b) ethyl choloraximido acetate, Et3N, CH2Cl2, rt; c) DIBAL-H, THF, 0 oC; d) CBr4, Ph3P, NaN3, DMF, rt; e) Ph3P, 1,4-dioxane; f) CH3COCl, Et3N, CH2Cl2, rt.
3. Anti-tuberculosis activity
All compounds synthesized with the complete isoxazoline moiety were tested against M. tuberculosis (H37Rv) using microbroth dilution and MIC values were determined by visual inspection [19]. The anti-tuberculosis activities of the new isoxazolines esters bearing varying substitutions to the phenyl B in the C-5 position are shown in Table 1. Compounds with a piperazyl-ureas substitution 8a–e displayed varying anti-tuberculosis activity with MICs ranging from 0.4 to 25 μg/mL. Those with simple substitutions of i-propyl 8a (12.5 μg/mL) and n-propyl 8b (25 μg/mL) were least active with anti-tuberculosis activity inferior to the original hit compound 1 (1.56 μg/mL). Most active in this series were n-hexyl 8c (0.4 μg/mL) and phenethyl 8e (0.8 μg/mL), which had better MIC values than that of 1. Compounds in the piperazyl-carbamate series 6 and 9a–e all demonstrated equal or 1-fold more potent anti-tuberculosis activity than the lead compound 1. In a comparative analysis it was notable that the pharmacologically more desirable and smaller side chains were much better tolerated in the carbamate series than the urea series (e.g., 9c and 9d over 8a and 8b). In the alkylated piperazine series 10a–d and 1, larger lipophilic substituents were clearly favored by comparison 1, 10c and 10d over 10a and 10b. Ethyl ester lead compound 1 and it’s methyl ester 10d had the same MIC value (1.56 μg/mL). For the biphenyl systems 15a, 15b and 15c, functionalization of the outer phenyl ring was required for appreciable anti-tuberculosis activity and in general activity of this series was lower than that of the previous carbamate series. The biheteroaryl series 15d, 20a, and 20b showed that a furan substitution 15d was significantly favored over the isosteric oxadiazole ring 20a, phenyl substitution of the oxadizole ring 20b restored similar activity to that of furan 15d. Para-substitution of the C-5 phenyl with nitrogen-containing alkyl rings produced piperazine compound 7 with limited activity and morpholine compound 28 with no activity. In this series, the simple piperidine derivative 17b was the most active and it was notably more active than the more complex and lipophilic 4-(4-trifluoromethoxy-phenoxy)-piperidine substituition 17a that resembles the side chain found in the new anti-tuberculosis agent OPC-68638 [20].
Table 1.
In vitro anti-tuberculosis activity of C-5 modified isoxazoline ester analogs
| Compd | Structure | MIC (μg/mL) | Compd | Structure | MIC (μg/mL) |
|---|---|---|---|---|---|
| 1 |
|
1.56 | 10a |
|
6.25 |
| 6 |
|
0.8 | 10b |
|
12.5 |
| 7 |
|
50 | 10c |
|
0.8 |
| 8a |
|
12.5 | 10d |
|
1.56 |
| 8b |
|
25 | 15a |
|
100 |
| 8c |
|
0.4 | 15b |
|
6.25 |
| 8d |
|
6.25 | 15c |
|
3.125 |
| 8e |
|
0.8 | 15d |
|
3.25 |
| 9a |
|
1.56 | 17a |
|
25 |
| 9b |
|
1.56 | 17b |
|
6.25 |
| 9c |
|
0.8 | 20a |
|
50 |
| 9d |
|
0.8 | 20b |
|
3.125 |
| 9e |
|
0.8 | 28 |
|
>200 |
The anti-tuberculosis activity for the optimization of C-3 isoxazoline ester position is shown in Table 2. Hydrolysis of the esters to free acids 11a, 11b, 12a, 12b, 13, and 22 led to a complete loss in activity when compared to their corresponding esters 8c, 8e, 9d, 9e, 10d, and 1 regardless of C-5 substitution. The loss of activity is likely attributed to poor cell membrane penetration. Ester bioisoster replacements such as primary amide 21, n-propyl amide 23, hydrazide 24 and 1,3,4-oxadiazole 26 were also largely inactive. Potential metabolites alcohols 29a and 29b were inactive when compared to the corresponding esters. Finally, azides 30a, 30b, primary amines 31a, 31b and reverse amides 32a, 32b that mimic linezolid functionalization also all had negligible anti-tuberculosis activity. These findings strongly suggest that an ester functionality at the C-3 position of isoxazoline ring is absolutely required for anti-tuberculosis activity in this series.
Table 2.
In vitro anti-tuberculosis activity of C-3 modified isoxazoline analogs
| Compd | Structure | MIC (μg/mL) | Compd | Structure | MIC (μg/mL) |
|---|---|---|---|---|---|
| 11a |
|
>200 | 26 |
|
200 |
| 11b |
|
>200 | 29a |
|
200 |
| 12a |
|
>200 | 29b |
|
>200 |
| 12b |
|
>200 | 30a |
|
50 |
| 13 |
|
>200 | 30b |
|
200 |
| 21 |
|
200 | 31a |
|
200 |
| 22 |
|
100 | 31b |
|
>200 |
| 23 |
|
200 | 32a |
|
>200 |
| 24 |
|
50 | 32b |
|
>200 |
4. Conclusions
There is a clear need to develop novel anti-tuberculosis agents that are chemically tractable and possess drug-like properties. In this study a new interesting chemotype with anti-tuberculosis activity was examined. Optimization of the isoxazoline C-5 position produced compounds with improved anti-tuberculosis activity, including most notably urea analog (8c) and carbamates (9c, 9d and 9e). Attempts to replace the metabolically labile ester group at the C-3 position of isoxazoline with a variety of bioisosteric head groups led to complete loss of the tuberculosis inhibition. This is unfortunate because the serum stability for these esters is extremely short, precluding the advancement of this class for testing of their efficacy in animal models of tuberculosis. Presumably, the active compounds are taken up as esters by the tubercle bacilli in vitro and then hydrolyzed to active free acids once the compounds are internalized in the bacteria. The free acids of the corresponding active esters are presumably inactive in vitro as they cannot penetrate the complex M. tuberculosis cell wall, perhaps in part due to their residual negative charge. The structural resemblance of this series to the oxazolidinone class of antibiotics was also explored through the synthesis of reverse amides 32b with high structural similarity to Linezolid (Figure 1). However, 32b was inactive in contrast to linezolid, which has good anti-tuberculosis activity.[21] This suggests that this series of compounds works through a different mechanism of action, a hypothesis that is further supported by the lack of antimicrobial activity of these compounds against other bacteria including S. aureus, S. pneumoniae and E. coli.
5. Experimental
All the anhydrous solvents and starting materials were purchased from Aldrich Chemical Co. (Milwaukee, WI). All reagent grade solvents used for chromatography were purchased from Fisher Scientific (Suwanee, GA) and flash column chromatography silica cartridges were obtained from Biotage Inc. (Lake Forest, VA). The reactions were monitored by thin-layer chromatography (TLC) on precoated Merck 60 F254 silica gel plates and visualized using UV light (254 nm). A Biotage FLASH column chromatography system was used to purify mixtures. All 1H NMR spectra were recorded on a Varian INOVA-500 spectrometer. Chemical shifts (δ) are reported in ppm relative to the residual solvent peak or internal standard (tetramethylsilane), and coupling constants (J) are reported in hertz (Hz). Mass spectra were recorded on a Bruker Esquire LCMS using ESI. Purity of the products was confirmed before testing by analytical RP-HPLC on Shimadzu HPLC system. Gradient conditions: solvent A (0.1% TFA in water) and solvent B (acetonitrile): 0–2.00 min 100% A, 2.00–7.00 min 0–100% B (linear gradient), 7.00–8.00 min 100% B, UV detection at 254 nm.
General procedure for aryl amination reactions
A mixture of 4-bromo styrene (1 mmol), amine (1.3 mmol), NaOtBu (1.5 mmol) and PdCl2[P(oTol)3]2 (0.03 mmol) in toluene was heated to reflux for 4 h. The solvent was evaporated under reduced pressure and the crude residue was purified by flash chromatography.
Representative aryl amination
4-(4-Vinyl-phenyl)-piperazine-1-carboxylic acid tert-butyl ester (4): A mixture of 4-bromostyrene (1.0 g, 5.46 mmol), 1-Boc-piperazine (1.32 g, 7.10 mmol), sodium ter-butoxide (0.78 g, 8.19 mmol) and PdCl2[P(oTol)3]2 (0.12 g, 0.16 mmol) was heated in anhydrous toluene (20 mL) at 100 °C under argon for 3 h. The reaction mixture was then concentrated under reduced pressure and subjected to flash column chromatography to give 4 (0.91 g) in 58% yield. 1H NMR (500 MHz, CDCl3): δ 1.51 (9H, s), 3.18 (4H, s), 3.61 (4H, s), 5.13 (1H, d, J = 10.7 Hz), 5.63 (1H, d, J = 17.5 Hz), 6.67 (1H, dd, J = 10.9, 17.5 Hz), 6.88 – 6.95 (2H, broad doublet), 7.36 (2H, d, J = 8.7 Hz); ESI MS: 311.8 (M+23).
General procedure for the synthesis of isoxazoline derivatives
To a cooled (0 °C) solution of olefin (1 mmol) and Et3N (2 mmol) in CH2Cl2, ethyl chlorooximido acetate (1.5 mmol) was added in portions and stirred at room temperature for overnight. The reaction mixture was washed with water, brine, dried (anhyd. Na2SO4), concentrated under reduced pressure and crude products were purified by flash chromatography.
4-[4-(3-Ethoxycarbonyl-4,5-dihydro-isoxazol-5-yl)-phenyl]-piperazine-1-carboxylic acid tert-butyl ester (6): To a stirred solution of olefin 4 (0.8 g, 2.77 mmol) in CH2Cl2 (10 mL), Et3N (0.77 mL, 5.55 mmol) and ethyl chlorooximido acetate (0.631 g, 4.16 mmol) were added at 0 °C and stirred at room temperature for overnight. The reaction mixture was diluted with excess CH2Cl2 (20 mL) and washed with water (20 mL), dried (anhyd. Na2SO4), concentrated under reduced pressure and the crude residue was purified by flash chromatography to afford 6 (0.788 g) in 71% yield. 1H NMR (300 MHz, CDCl3): δ 1.39 (3H, t, J = 7.1 Hz), 1.49–1.51 (9H, s), 3.13–3.27 (5H, m), 3.52–3.65 (5H, m), 4.38 (2H, q, J = 7.1 Hz), 5.72 (1H, dd, J = 9.3, 11.4 Hz), 6.92 (2H, d, J = 8.7 Hz), 7.24 (2H, d, J = 8.7 Hz). ESI MS: 426.3 (M+Na); HPLC purity: 97%, tR = 6.6 min.
5-(4-Piperazin-1-yl-phenyl)-4,5-dihydro-isoxazole-3-carboxylic acid ethyl ester (7): A solution of 6 (0.8 g, 1.98 mmol) in THF:TFA (1:1, 10 mL) was stirred at room temperature for 1h. The reaction mixture was concentrated under reduced pressure to give 7 (0.552 g) in 92% yield. 1H NMR (500 MHz, CDCl3): δ 1.41 (3H, t, J = 7.1 Hz), 3.22 (1H, dd, J = 9.0 and 17.8 Hz), 3.34–3.42 (4H, broad s), 3.43–3.52 (4H, broad s), 3.62 (1H, dd, J = 11.5 and 17.8 Hz), 4.39 (2H, q, J = 7.1 Hz), 5.75 (1H, dd, J = 9.3 and 11.5 Hz), 6.95 (2H, d, J = 8.8 Hz), 7.29 (2H, d, J = 8.5 Hz), 9.62 (2H, broad s); 13C NMR (500 MHz, CDCl3): δ 14.15, 41.08, 43.41, 46.71, 62.24, 84.83, 117.31, 127.45, 132.28, 150.23, 151.26, 160.65; ESI MS: 304.2 (M+1); HPLC purity: 98%, tR = 4.7 min.
General procedure for the synthesis of urea and carbamate derivatives
Piperazine TFA salt 7 (1 mmol) was dissolved in anhydrous dichloromethane, then triethylamine (4 mmol) was added, followed by isocyanate or chloroformate (3 mmol). The reaction mixture was stirred at room temperature for overnight and evaporated. The residue was purified by column chromatography.
5-[4-(4-Isopropylcarbamoyl-piperazin-1-yl)-phenyl]-4,5-dihydro-isoxazole-3-carboxylic acid ethyl ester (8a): Yield: 91.1%; 1H NMR (500 MHz, CDCl3): δ 1.20 (6H, d, J = 6.6 Hz), 1.41 (3H, t, J = 7.1 Hz), 3.21–3.26 (5H, m), 3.53–3.63 (5H, m), 4.02 (1H, m), 4.29 (1H, d, J = 6.6 Hz), 4.39 (2H, q, J = 7.1 Hz), 5.74 (1H, dd, J = 9.0 and 11.2 Hz), 6.95 (2H, d, J = 8.3 Hz), 7.27 (2H, d, J = 8.5 Hz); 13C NMR (500 MHz, CDCl3): δ 14.17, 23.49, 40.96, 42.71, 43.44, 48.92, 62.17, 85.07, 116.45, 127.36, 151.23, 156.97, 160.73; ESI MS: 411.3 (M+Na); HPLC purity: 99%, tR = 5.6 min.
5-[4-(4-Propylcarbamoyl-piperazin-1-yl)-phenyl]-4,5-dihydro-isoxazole-3-carboxylic acid ethyl ester (8b): Yield: 97.8%; 1H NMR (500 MHz, CDCl3): δ 0.93 (3H, t, J = 7.1 Hz), 1.38 (3H, t, J = 7.1 Hz), 1.49–1.60 (2H, m), 3.10–3.28 (7H, m), 3.46–3.64 (5H, m), 4.37 (2H, q, J = 7.1 Hz), 4.55 (1H, broad s), 5.71 (1H, t, J = 10.0 Hz), 6.90 (2H, d, J = 8.1 Hz), 7.23 (2H, d, J = 8.1 Hz); 13C NMR (500 MHz, CDCl3): δ 11.42, 14.17, 23.46, 40.92, 42.71, 43.60, 48.71, 62.15, 85.16, 116.22, 127.34, 130.31, 151.23, 151.33, 157.71, 160.74; ESI MS: 411.3 (M+Na); HPLC purity: 100%, tR = 5.6 min.
5-[4-(4-Hexylcarbamoyl-piperazin-1-yl)-phenyl]-4,5-dihydro-isoxazole-3-carboxylic acid ethyl ester (8c): Yield: 100%; 1H NMR (500 MHz, CDCl3): δ 0.91–0.97 (3H, t), 1.22–1.41 (9H, m), 1.40–1.58 (2H, m), 3.16–3.27 (7H, m), 3.48–3.61 (5H, m), 4.36 (2H, q, J = 7.1 Hz), 4.58 (1H, t), 5.71 (1H, dd, J = 9.5 and 11.0 Hz), 6.90 (2H, d, J = 8.5 Hz), 7.23 (2H, d, J = 8.8 Hz); 13C NMR (300 MHz, CDCl3): δ 13.45, 13.58, 22.01, 26.08, 29.69, 31.01, 40.35, 40.50, 43.07, 48.15, 61.52, 84.57, 115.63, 126.74, 129.74, 150.66, 150.78, 157.17, 160.16; ESI MS: 453.3 (M+Na); HPLC purity: 98%, tR = 6.4 min.
5-[4-(4-Phenylcarbamoyl-piperazin-1-yl)-phenyl]-4,5-dihydro-isoxazole-3-carboxylic acid ethyl ester (8d): Yield: 95.5%; 1H NMR (500 MHz, CDCl3): δ 1.37 (3H, t, J = 7.1 Hz), 3.17–3.26 (5H, m), 3.54–3.66 (5H, m), 4.36 (2H, q, J = 7.1 Hz), 5.71 (1H, dd, J = 9.3 and 11.5 Hz), 6.67 (1H, s), 6.89 (2H, d, J = 8.5 Hz), 7.04 (1H, t, J = 7.3 Hz), 7.24 (2H, t, J = 8.8 Hz), 7.28 (2H, m), 7.37 (2H, d, J = 7.6 Hz); 13C NMR (300 MHz, CDCl3): δ 13.59, 40.36, 43.36, 48.16, 61.57, 84.57, 115.68, 119.63, 122.73, 126.78, 128.35, 129.87, 138.41, 150.65, 150.71, 154.54, 160.15; ESI MS: 445.2 (M+Na); HPLC purity: 96%, tR = 6.0 min.
5-[4-(4-Phenethylcarbamoyl-piperazin-1-yl)-phenyl]-4,5-dihydro-isoxazole-3-carboxylic acid ethyl ester (8e): Yield: 82.6%; 1H NMR (500 MHz, CDCl3): δ 1.38 (3H, t, J = 7.1 Hz), 2.85 (2H, t, J = 6.8 Hz), 3.17 (4H, t, J = 5.1 Hz), 3.21 (1H, dd, J = 9.3 and 17.8 Hz), 3.47 (2H, t, J = 4.9 Hz), 3.52 (2H, q, J = 6.6 Hz), 3.57 (1H, dd, J = 11.5 and 17.8 Hz), 4.37 (2H, q, J = 7.3 Hz), 4.48 (1H, t, J = 5.4 Hz), 5.71 (1H, dd, J = 9.3 and 11.2 Hz), 6.89 (2H, d, J = 8.8 Hz), 7.19–7.25 (5H, m), 7.32 (2H, t, J = 7.3 Hz); 13C NMR (500 MHz, CDCl3): δ 13.59, 35.75, 40.36, 41.47, 43.01, 48.12, 61.53, 84.55, 115.65, 125.91, 126.74, 128.08, 128.30, 129.82, 138.76, 150.64, 150.74, 156.90, 160.18; ESI MS: 473.2 (M+Na); HPLC purity: 98%, tR = 6.1 min.
4-[4-(3-Ethoxycarbonyl-4,5-dihydro-isoxazol-5-yl)-phenyl]-piperazine-1-carboxylic acid methyl ester (9a): Yield: 76.3%; 1H NMR (500 MHz, CDCl3): δ 1.38 (3H, t, J = 7.1 Hz), 3.10–3.25 (5H, m), 3.53–3.67 (5H, m), 3.73 (3H, s), 4.37 (2H, q, J = 7.1 Hz), 5.71 (1H, dd, J = 9.8 and 11.0 Hz), 6.91 (2H, d, J = 8.5 Hz), 7.24 (2H, d, J = 8.3 Hz); 13C NMR (500 MHz, CDCl3): δ 14.17, 40.93, 43.59, 49.01, 52.76, 62.15, 85.12, 116.54, 127.32, 130.54, 151.22, 151.48, 155.87, 160.74; ESI MS: 384.1 (M+Na); HPLC purity: 98%, tR = 5.8 min.
4-[4-(3-Ethoxycarbonyl-4,5-dihydro-isoxazol-5-yl)-phenyl]-piperazine-1-carboxylic acid ethyl ester (9b): Yield: 89.1%; 1H NMR (500 MHz, CDCl3): δ 1.27 (3H, t), 1.38 (3H, t), 3.16 (4H, t, J = 4.9 Hz), 3.21 (1H, dd, J = 9.3 and 17.8 Hz), 3.57 (1H, dd, J = 11.5 and 17.6 Hz), 3.63 (4H, t, J = 4.9 Hz), 4.17 (2H, q, J = 7.1 Hz), 4.37 (2H, q, J = 7.1 Hz), 5.71 (1H, dd, J = 9.3 and 11.2 Hz), 6.91 (2H, d, J = 8.8 Hz), 7.24 (2H, d, J = 8.8 Hz); 13C NMR (500 MHz, CDCl3): δ 14.17, 14.70, 40.93, 43.51, 49.03, 61.56, 62.15, 85.14, 116.52, 127.32, 130.49, 151.22, 151.52, 155.49, 160.75; ESI MS: 398.3 (M+Na); HPLC purity: 96%, tR = 6.1 min.
4-[4-(3-Ethoxycarbonyl-4,5-dihydro-isoxazol-5-yl)-phenyl]-piperazine-1-carboxylic acid allyl ester (9c): Yield: 69.6%; 1H NMR (500 MHz, CDCl3): δ 1.38 (3H, t, J = 7.1 Hz), 3.12–3.28 (5H, m), 3.57 (1H, dd, J = 11.5 and 17.8 Hz), 3.65 (4H, t, J = 4.9 Hz), 4.37 (2H, q, J = 7.1 Hz), 4.62 (2H, d, J = 5.4 Hz), 5.23 (1H, d, J = 10.5 Hz), 5.32 (1H, d, J = 17.1 Hz), 5.71 (1H, dd, J = 9.5 and 11.0 Hz), 5.96 (1H, m), 6.92 (2H, d, J = 8.5 Hz), 7.24 (2H, d, J = 8.3 Hz); 13C NMR (500 MHz, CDCl3): δ 14.15, 40.91, 43.60, 49.01, 62.13, 66.19, 85.10, 116.52, 117.60, 127.30, 130.54, 132.93, 151.20, 151.45, 155.04, 160.72; ESI MS: 410.3 (M+Na); HPLC purity: 98%, tR = 6.3 min.
4-[4-(3-Ethoxycarbonyl-4,5-dihydro-isoxazol-5-yl)-phenyl]-piperazine-1-carboxylic acid isobutyl ester (9d): Yield: 82.0%; 1H NMR (500 MHz, CDCl3): δ 0.95 (6H, d, J = 6.6 Hz), 1.38 (3H, t, J = 6.8 Hz), 1.96 (1H, m), 3.12–3.27 (5H, m), 3.53–3.68 (5H, m), 3.90 (2H, d, J = 6.6 Hz), 4.37 (2H, q, J = 6.8 Hz), 5.71 (1H, dd, J = 9.8 and 10.7 Hz), 6.92 (2H, d, J = 8.1 Hz), 7.24 (2H, d, J = 8.3 Hz); 13C NMR (500 MHz, CDCl3): δ 14.17, 19.14, 28.03, 40.93, 43.51, 49.02, 62.14, 71.71, 85.13, 116.51, 127.33, 130.50, 151.22, 151.50, 155.54, 160.74; ESI MS: 426.3 (M+Na); HPLC purity: 89%, tR = 6.6 min.
4-[4-(3-Ethoxycarbonyl-4,5-dihydro-isoxazol-5-yl)-phenyl]-piperazine-1-carboxylic acid butyl ester (9e): Yield: 84.9%; 1H NMR (500 MHz, CDCl3): δ 0.95 (3H, t, J = 6.8 Hz), 1.34–1.45 (5H, m), 1.60–1.68 (2H, m), 3.10–3.26 (5H, m), 3.53–3.68 (5H, m), 4.12 (2H, t, J = 5.9 Hz), 4.37 (2H, q, J = 6.8 Hz), 5.71 (1H, dd, J = 10.0 and 10.3 Hz), 6.92 (2H, d, J = 7.6 Hz), 7.24 (2H, d, J = 8.1 Hz); 13C NMR (500 MHz, CDCl3): δ 13.81, 14.17, 19.22, 31.09, 40.93, 43.52, 49.02, 62.14, 65.50, 85.13, 116.51, 127.32, 130.49, 151.22, 151.51, 155.57, 160.74; ESI MS: 426.3 (M+Na); HPLC purity: 91%, tR = 6.7 min.
General procedure for the synthesis of alkylated derivatives
Piperazine TFA salt 7 (1 mmol) was dissolved in anhydrous dimethylformamide, then K2CO3 (3 mmol) and alkyl bromide (1.2 mmol) were added. The reaction mixture was stirred at room temperature for overnight and evaporated, the residue was purified by column chromatography on silica gel.
5-[4-(4-Allyl-piperazin-1-yl)-phenyl]-4,5-dihydro-isoxazole-3-carboxylic acid ethyl ester (10a): Yield: 48.3%; 1H NMR (500 MHz, CDCl3): δ 1.38 (3H, t, J = 7.3 Hz), 2.60 (4H, t, J = 5.1 Hz), 3.06 (2H, d, J = 6.6 Hz), 3.18–3.25 (5H, m), 3.56 (1H, dd, J = 11.5 and 17.6 Hz ), 4.36 (2H, q, J = 7.3 Hz), 5.19 (1H, dd, J = 0.7 and 10.0 Hz), 5.23 (1H, dd, J = 1.7 and 17.3 Hz), 5.70 (1H, dd, J = 9.5 and 11.5 Hz), 5.85–5.92 (1H, m), 6.91 (2H, d, J = 8.8 Hz), 7.22 (2H, d, J = 8.8 Hz); 13C NMR (300 MHz, CDCl3): δ 13.59, 40.28, 48.19, 52.38, 61,18, 61.49, 84.71, 115.26, 117.64, 126.68, 129.05, 134.26, 150.65, 151.10, 160.22; ESI MS: 366.3 (M+Na); HPLC purity: 98%, tR = 4.9 min.
5-[4-(4-Cyclopropylmethyl-piperazin-1-yl)-phenyl]-4,5-dihydro-isoxazole-3-carboxylic acid ethyl ester (10b): Yield: 57.8%; 1H NMR (500 MHz, CDCl3): δ 0.14 (2H, q, J = 4.6 Hz), 0.53–0.57 (2H, m), 0.86 – 0.94 (1H, m), 1.38 (3H, t, J = 7.1 Hz), 2.31 (2H, d, J = 6.4 Hz), 2.68 (4H, t, J = 5.1 Hz), 3.18–3.28 (5H, m), 3.56 (1H, dd, J = 11.5 and 17.8 Hz ), 4.36 (2H, q, J = 7.1 Hz), 5.70 (1H, dd, J = 9.5 and 11.5 Hz), 6.92 (2H, d, J = 8.8 Hz), 7.22 (2H, d, J = 8.8 Hz); 13C NMR (500 MHz, CDCl3): δ 4.19, 8.59, 14.41, 41.06, 48.96, 53.38, 62.34, 64.02, 85.55, 116.05, 127.51, 129.79, 151.46, 151.96, 161.03; ESI MS: 380.2 (M+Na); HPLC purity: 96%, tR = 5.0 min.
5-{4-[4-(4-Nitro-benzyl)-piperazin-1-yl]-phenyl}-4,5-dihydro-isoxazole-3-carboxylic acid ethyl ester (10c): Yield: 57.9%; 1H NMR (500 MHz, CDCl3): δ 1.38 (3H, t, J = 7.1 Hz), 2.62 (4H, t, J = 4.4 Hz), 3.18–3.28 (5H, m), 3.56 (1H, dd, J = 11.7 and 17.8 Hz ), 3.65 (2H, s), 4.37 (2H, q, J = 7.1 Hz), 5.71 (1H, dd, J = 9.8 and 10.7 Hz), 6.90 (2H, d, J = 8.5 Hz), 7.22 (2H, d, J = 8.5 Hz), 7.55 (2H, d, J = 8.3 Hz), 8.20 (2H, d, J = 8.5 Hz); 13C NMR (500 MHz, CDCl3): δ 14.41, 41.09, 49.03, 53.32, 62.37, 85.49, 116.17, 123.86, 127.54, 129.76, 130.07, 146.34, 151.47, 151.78, 161.02; ESI MS: 461.1 (M+Na); HPLC purity: 99%, tR = 5.3 min.
5-[4-(4-Benzyl-piperazin-1-yl)-phenyl]-4,5-dihydro-isoxazole-3-carboxylic acid methyl ester (10d): (Complete ester transformation occurred during the post-workup evaporation process in methanol): Yield: 61.2%; 1H NMR (500 MHz, CDCl3): δ 2.60 (4H, t, J = 4.2 Hz), 3.18–3.26 (5H, m), 3.51–3.60 (3H, m), 3.90 (3H, s), 5.70 (1H, dd, J = 10.0 and 10.7 Hz), 6.89 (2H, d, J = 8.5 Hz), 7.20 (2H, d, J = 8.5 Hz), 7.24–7.38 (5H, m); 13C NMR (500 MHz, CDCl3): δ 40.71, 48.75, 52.83, 52.97, 63.08, 85.45, 115.81, 127.20, 127.29, 128.32, 129.22, 129.39, 137.96, 150.98, 151.75, 161.21; ESI MS: 402.2 (M+Na); HPLC purity: 98%, tR = 5.0 min.
General procedures for the synthesis of free acids
Ethyl ester (1 mmol) was dissolved in tetrahydrofuran/water (8 mL), then lithium hydroxide hydrate (1 mmol) was added. The reaction mixture was stirred at room temperature for 3 h, and the solvent was evaporated in vacuo at ambient temperature, then H2O (10 mL) was added and extracted with ethyl acetate (40 mL). The organic phase was washed with H2O (3 × 10 mL), dried (anhyd. Na2SO4) and evaporated to give free acid. Acid 13 was obtained by the evaporation of the combined aqueous phases, analytical sample was obtained as white powder after the solid residue was washed with minimum amount of ice-H2O, filtered, and dried.
5-[4-(4-Hexylcarbamoyl-piperazin-1-yl)-phenyl]-4,5-dihydro-isoxazole-3-carboxylic acid (11a): Yield: 62.7%; 1H NMR (500 MHz, CDCl3): δ 0.87 (3H, t, J = 6.0 Hz), 1.22–1.36 (6H, m), 1.46–1.54 (2H, m), 3.10–3.30 (7H, m), 3.48–3.64 (5H, m), 5.69 (1H, dd, J = 9.5 and 9.8 Hz), 6.90 (2H, d, J = 7.6 Hz), 7.21 (2H, d, J = 7.3 Hz); 13C NMR (500 MHz, CDCl3): δ 14.09, 22.60, 26.62, 30.10, 30.34, 31.56, 34.25, 40.92, 41.22, 43.49, 48.96, 84.57, 116.57, 125.54, 127.37, 131.14, 135.79, 150.74, 158.18, 162.18; ESI MS: 425.3 (M+Na); HPLC purity: 98%, tR = 5.7 min.
5-[4-(4-Phenethylcarbamoyl-piperazin-1-yl)-phenyl]-4,5-dihydro-isoxazole-3-carboxylic acid (11b): Yield: 86.0%; 1H NMR (500 MHz, DMSO-d6): δ 2.74 (2H, t, J = 7.5 Hz), 3.08–3.15 (5H, m), 3.23–3.28 (2H, m), 3.43 (2H, t, J = 5.0 Hz), 3.57 (1H, dd, J = 11.5 and 17.8 Hz), 5.68 (1H, dd, J = 10.0 and 11.0 Hz), 6.73 (1H, t, J = 5.4 Hz), 6.78 (2H, d, J = 8.8 Hz); 7.18–7.32 (7H, m); 13C NMR (500 MHz, DMSO-d6): δ 36.49, 40.58, 40.84, 42.40, 43.67, 48.45, 84.87, 116.00, 126.43, 128.04, 128.76, 129.13, 130.14, 140.30, 151.59, 153.02, 157.77, 162.17; ESI MS: 445.1 (M+Na); HPLC purity: 98%, tR = 5.4 min.
5-[4-(4-Isobutoxycarbonyl-piperazin-1-yl)-phenyl]-4,5-dihydro-isoxazole-3-carboxylic acid (12a): Yield: 92.6%; 1H NMR (500 MHz, CDCl3): δ 0.95 (6H, d, J = 6.6 Hz), 1.91–2.00 (1H, m), 3.10–3.26 (5H, m), 3.50–3.72 (5H, m), 3.91 (2H, d, J = 6.6 Hz), 5.74 (1H, t, J = 9.8 Hz), 6.97 (2H, d, J = 7.6 Hz), 7.24 (2H, d, J = 6.8 Hz); 13C NMR (500 MHz, CDCl3): δ 19.12, 27.99, 30.35, 43.38, 49.47, 72.05, 85.80, 117.07, 125.54, 127.44, 131.36, 150.88, 155.82, 162.66; ESI MS: 376.3 (M+H); HPLC purity: 98%, tR = 5.8 min.
5-[4-(4-Butoxycarbonyl-piperazin-1-yl)-phenyl]-4,5-dihydro-isoxazole-3-carboxylic acid (12b): Yield: 83.2%; 1H NMR (500 MHz, CDCl3): δ 0.94 (3H, t, J = 7.3 Hz), 1.35–1.45 (2H, m), 1.60–1.68 (2H, m), 3.12–3.26 (5H, m), 3.51–3.72 (5H, m), 4.13 (2H, t, J = 6.6 Hz), 5.72 (1H, dd, J = 9.3 and 9.5 Hz), 6.97 (2H, d, J = 7.8 Hz), 7.24 (2H, d, J = 7.3 Hz); 13C NMR (500 MHz, CDCl3): δ 13.79, 19.19, 30.35, 31.01, 40.70, 43.34, 49.49, 65.90, 85.55, 117.09, 127.42, 131.44, 150.82, 155.86, 162.64; ESI MS: 376.3 (M+H); HPLC purity: 99%, tR = 5.9 min.
5-[4-(4-Benzyl-piperazin-1-yl)-phenyl]-4,5-dihydro-isoxazole-3-carboxylic acid (13): Yield: 61.9%; 1H NMR (500 MHz, DMSO-d6): δ 2.83 (4H, broad s), 3.30 (4H, overlapped with H2O peak), 3.13 (1H, dd, J = 9.8 and 17.8 Hz), 3.59 (1H, dd, J = 11.2 and 17.8 Hz), 3.90 (2H, s), 5.71 (1H, dd, J = 10.0 and 10.7 Hz), 6.99 (2H, d, J = 8.5 Hz), 7.27 (2H, d, J = 8.5 Hz), 7.36–7.52 (5H, m); 13C NMR (300 MHz, DMSO-d6): δ 40.36, 46.53, 51.29, 60.36, 84.23, 115.38, 127.48, 128.13, 128.42, 129.86, 130.04, 150.31, 152.46, 161.59; ESI MS: 366.3 (M+H); HPLC purity: 100%, tR = 4.8 min.
General procedure for the synthesis of biaryl compounds 14a–d and isoxazoline compounds 15a–d
A mixture of 4-bromo styrene (1 mmol), aryl boronic acid (1.1 mmol), Pd(Ph3P)4 (0.03 mmol) and 1M K2CO3 solution in dimethoxy ethane was degassed and purged with argon twice and heated to reflux for overnight. The solvent was evaporated and ethyl acetate was added, the organic phase was washed with water, brine, dried (anhyd. Na2SO4) and concentrated under reduced pressure. The crude residue was purified by flash chromatography to afford biaryl compounds (14a–14d) in 50–70% yields. These olefins were converted into corresponding isoxazolines (15a–15d) following the general procedure discussed earlier.
5-Biphenyl-4-yl-4,5-dihydro-isoxazole-3-carboxylic acid ethyl ester (15a): Yield: 72.6%; 1H NMR (500 MHz, CDCl3): δ 1.39 (3H, t, J = 7.0 Hz), 3.27 (1H, dd, J = 8.7, 17.5 Hz), 3.66 (1H, dd, J = 8.7, 17.5 Hz), 4.37 (2H, q, J = 7.0 Hz), 5.83 (1H, dd, J = 8.7, 11.4 Hz), 7.34–7.46 (5H, m), 7.57–7.62 (4H, m); ESI MS: 296 (M+1); HPLC purity: 98.6%, tR = 6.8 min.
5-(4′-Formyl-biphenyl-4-yl)-4,5-dihydro-isoxazole-3-carboxylic acid ethyl ester (15b): Yield: 61%; 1H NMR (500 MHz, CDCl3): δ 1.39 (3H, t, J = 7.1 Hz), 3.28 (1H, dd, J = 8.8, 17.5 Hz), 3.66 (1H, dd, J = 8.7, 17.5 Hz), 4.36 (2H, q, J = 7.2 Hz), 5.83 (1H, dd, J = 8.7, 11.4 Hz), 7.42–7.45 (2H, m), 7.59–7.67 (4H, m), 7.74 (1H, d, J = 8.0 Hz), 7.96 (1H, d, J = 8.0 Hz), 10.06 (1H, s); ESI MS: 346 (M+Na); HPLC purity: 99.2%, tR = 6.8 min.
5-(3′-Fluoro-4′-methoxy-biphenyl-4-yl)-4,5-dihydro-isoxazole-3-carboxylic acid ethyl ester (15c): Yield: 70%; 1H NMR (500 MHz, CDCl3): δ 1.39 (3H, t, J = 7.3 Hz), 3.25 (1H, dd, J = 8.7, 17.5 Hz), 3.66 (1H, dd, J = 11.4, 17.8 Hz), 3.93 (3H, s), 4.37 (2H, q, J = 7.0 Hz), 5.81 (1H, dd, J = 8.7, 11.4 Hz), 7.02 (1H, t, J = 8.3 Hz), 7.29–7.33 (2H, m), 7.38 (2H, d, J = 8.3 Hz), 7.54 (2H, d, J = 8.3 Hz); ESI MS: 366.1 (M+Na); HPLC purity: 99.0%, tR = 6.8 min.
5-(4-Furan-2-yl-phenyl)-4,5-dihydro-isoxazole-3-carboxylic acid ethyl ester (15d): Yield: 58%; 1H NMR (500 MHz, CDCl3): δ 1.38 (3H, t, J = 7.0 Hz), 3.22 (1H, dd, J = 9.0, 17.8 Hz), 3.64 (1H, dd, J = 11.7, 17.8 Hz), 4.36 (2H, q, J = 6.3 Hz), 5.78 (1H, dd, J = 9.0, 11.4 Hz), 6.47–6.48 (1H, m), 6.66 (1H, d, J = 3.4 Hz), 7.33 (2H, d, J = 8.0 Hz), 7.47 (1H, d, J = 0.7 Hz), 7.67 (2H, d, J = 8.3 Hz); ESI MS: 286.2 (M+1); HPLC purity: 99.1%, tR = 6.6 min.
5-{4-[4-(4-Trifluoromethoxy-phenoxy)-piperidin-1-yl]-phenyl}-4,5-dihydro-isoxazole-3-carboxylic acid ethyl ester (17a): Yield: 74.3%; 1H NMR (500 MHz, CDCl3): δ 1.38 (3H, t, J = 7.3 Hz), 1.89–1.95 (2H, m), 2.06–2.11 (2H, m), 3.12–3.16 (2H, m), 3.21 (1H, dd, J = 9.2, 17.5 Hz), 3.49–3.59 (3H, m), 4.36 (2H, q, J = 7.0 Hz), 4.43–4.46 (1H, m), 5.70 (1H, dd, J = 9.2, 11.2 Hz), 6.90–6.94 (4H, m), 7.14 (2H, d, J = 8.7 Hz), 7.22 (2H, d, J = 8.7 Hz); ESI MS: 501.1 (M+Na); HPLC purity: 98.8%, tR = 6.4 min.
5-(4-Piperidin-1-yl-phenyl)-4,5-dihydro-isoxazole-3-carboxylic acid ethyl ester (17b): Yield: 77%; 1H NMR (500 MHz, CDCl3): δ 1.38 (3H, t, J = 7.0 Hz), 1.55–1.62 (2H, m), 1.66–1.72 (4H, m), 3.17 (4H, t, J = 5.6 Hz), 3.21 (1H, dd, J = 10.7, 19.0 Hz), 3.55 (1H, dd, J = 11.4, 17.5 Hz), 4.36 (2H, q, J = 7.0 Hz), 5.68 (1H, dd, J = 9.2, 11.2 Hz), 6.90 (2H, d, J = 8.7 Hz), 7.19 (2H, d, J = 8.7 Hz); ESI MS: 303.2 (M+1); HPLC purity: 99.0%, tR = 4.7 min.
General procedure for the synthesis of 20a and 20b
A solution of 4-cyano styrene (1 mmol), NH2OH.HCl (2 mmol) and Et3N (1.5 mmol) in EtOH was refluxed for 4 h. The reaction mixture was concentrated under reduced pressure and the crude amidine 18 was used as such for the next reaction without further purification.
To a solution of amidine 18 (1 mmmol) in triethyl orthoformate (5 mL), BF3.OEt2 (cat)/pyridine (5 mL), or benzoyl chloride (1.2 mmol) were added and the reaction mixtures were heated at 80 °C for 2 h, concentrated under reduced pressure and purified by flash chromatography to afford 19a and 19b in 51 and 45% yields respectively.
Compounds 19a and 19b were converted into corresponding isoxazolines 20a and 20b following the synthetic procedure discussed earlier.
5-(4-[1,2,4]Oxadiazol-3-yl-phenyl)-4,5-dihydro-isoxazole-3-carboxylic acid ethyl ester (20a): Yield: 59%; 1H NMR (500 MHz, CDCl3): δ 1.38 (3H, t, J = 7.0 Hz), 3.24 (1H, dd, J = 8.5, 17.8 Hz), 3.70 (1H, dd, J = 11.7, 17.8 Hz), 4.37 (2H, q, J = 7.0 Hz), 5.85 (1H, dd, J = 8.5, 11.7 Hz), 7.47 (2H, d, J = 8.3 Hz), 8.14 (2H, d, J = 8.0 Hz), 8.77 (1H, s); ESI MS: 288.1 (M+1).
5-[4-(5-Phenyl-[1,2,4]oxadiazol-3-yl)-phenyl]-4,5-dihydro-isoxazole-3-carboxylic acid ethyl ester (20b): Yield: 60%; 1.39 (3H, t, J = 7.0 Hz), 3.25 (1H, dd, J = 8.5, 17.5 Hz), 3.70 (1H, dd, J = 11.7, 17.8 Hz), 4.37 (2H, q, J = 7.3 Hz), 5.86 (1H, dd, J = 8.7, 11.7 Hz), 7.48 (2H, d, J = 8.0 Hz), 7.54–7.64 (3H, m), 8.21 (4H, t, J = 8.5 Hz); ESI MS: 362.3 (M−1); HPLC purity: 98.4%, tR = 7.1 min.
5-[4-(4-Benzyl-piperazin-1-yl)-phenyl]-4,5-dihydro-isoxazole-3-carboxylic acid amide (21): To a stirred solution of ester 1 in 1,4-dioxane, ammonium hydroxide (2 eq) was added and stirred at room temperature for overnight. The reaction mass was concentrated under reduced pressure to give amide 21 in 66% yield. 1H NMR (500 MHz, CDCl3): δ 2.61–2.69 (4H, m), 3.2–3.3 (5H, m), 3.53–3.63 (3H, m), 5.49–5.56 (1H, broad s), 5.69 (1H, dd, J = 9.5, 11.2 Hz), 6.56–6.63 (1H, broad s), 6.9 (2H, d, J = 8.7 Hz), 7.21 (2H, d, J = 8.7 Hz), 7.27–7.3 (1H, m), 7.32–7.38 (4H, m); ESI MS: 387.2 (M+Na); HPLC purity: 99%, tR = 4.6 min.
Lithium; 5-[4-(4-benzyl-piperazin-1-yl)-phenyl]-4,5-dihydro-isoxazole-3-carboxylate (22): To a stirred solution of ester 1 in THF:H2O, LiOH (5 eq) was added and stirred at room temperature for 2 h. The reaction mass was concentrated under reduced pressure to give lithium salt 22 in 90% yield. 1H NMR (500 MHz, D2O): δ 2.54–2.6 (4H, m), 3.03–3.13 (5H, m), 3.46–3.54 (3H, m), 5.6 (1H, dd, J = 8.5, 10.9 Hz), 7.0 (2H, d, J = 9.0 Hz), 7.24 (2H, d, J = 9.0 Hz), 7.26–7.34 (5H, m); HPLC purity: 100%, tR = 4.7 min.
5-[4-(4-Benzyl-piperazin-1-yl)-phenyl]-4,5-dihydro-isoxazole-3-carboxylic acid propyl amide (23): To a stirred solution of 22 (1 eq) in THF, oxaloyl chloride (2 eq) and DMF (catalytic) was added and stirred at room temperature for 4 h. Then added n-propyl amine (6 eq) to the acid chloride and again stirred at room temperature for another 2 h. The reaction mixture was diluted with excess ethyl acetate and washed with 1N NaOH solution, water, brine, dried (anhyd. Na2SO4), concentrated under reduced pressure and purified by flash chromatography to give 23 in 59% yield; 1H NMR (500 MHz, CDCl3): δ 0.93 (3H, t, J = 7.3 Hz), 1.62 (2H, m), 2.6–2.7 (4H, broad s), 3.21–3.32 (5H, m), 3.35 (2H, dq, J = 1.9, 7.0 Hz), 3.56–3.66 (3H, m), 5.68 (1H, dd, J = 9.5, 11.2 Hz), 6.68–6.74 (1H, m), 6.92 (2H, d, J = 8.7 Hz), 7.23 (2H, d, J = 8.7 Hz), 7.29–7.33 (1H, m), 7.35–7.42 (4H, m); ESI MS: 429.3 (M+Na); HPLC purity: 100%, tR = 5.1 min.
5-[4-(4-Benzyl-piperazin-1-yl)-phenyl]-4,5-dihydro-isoxazole-3-carboxylic acid hydrazide (24): To a stirred solution of ethyl ester 1 (0.2 g, 0.508 mmol) in ethanol (10 mL), hydrazine hydrate (0.05 mL, 1.017 mmol) was added and the mixture was refluxed for overnight. The reaction mixture was concentrated under reduced pressure and the crude residue was purified by flash chromatography to afford 17 (0.1 g) in 52% yield. 1H NMR (500 MHz, CDCl3): δ 2.60 (4H, t, J = 5.1 Hz), 3.18–3.30 (5H, m), 3.54–3.62 (3H, m), 3.96 (2H, d), 6.89 (2H, d, J = 8.7 Hz), 7.19 (2H, d, J = 8.7 Hz), 7.24–7.29 (3H, m), 7.31–7.38 (4H, m), 7.78 (1H, broad s); 13C NMR (500 MHz, CDCl3): δ 40.60, 48.66, 52.89, 63.05, 84.63, 115.87, 127.26, 128.31, 129.36, 151.62, 152.42; ESI MS: 380.4 (M+1); HPLC purity: 99.3%, tR = 5.4 min.
1-Benzyl-4-{4-[3-(5-methyl-[1,3,4]oxadiazol-2-yl)-4,5-dihydro-isoxazol-5-yl]-phenyl}-piperazine (26): To a solution of 24 (0.85 g, 2.24 mmol) in CH2Cl2 (15 mL), Et3N (0.93 mL, 6.728 mmol) and acetyl chloride (0.23 mL, 3.36 mmol) were added at 0 °C and stirred at room temperature for 2 h. The reaction mixture was concentrated under reduced pressure and purified by flash chromatography to afford 5-[4-(4-Benzyl-piperazin-1-yl)-phenyl]-4,5-dihydro-isoxazole-3-carboxylic acid N′-acetyl-hydrazide (25) (0.58 g) in 61% yield. Compound 25 (0.47 g, 1.11 mmol) was dissolved in CH2Cl2 (20 mL) and Et3N (0.31 mL, 2.23 mmol) and p-TsCl (0.424 g, 2.23 mmol) were added at 0 °C and allowed to reflux gently for 4 h. The reaction mixture was cooled to room temperature and washed vigorously with 40% K2CO3 solution, dried (anhyd. Na2SO4), concentrated under reduced pressure and purified by flash chromatography to afford 26 (0.26 g) in 58% yield. 1H NMR (500 MHz, CDCl3): δ 2.54 (3H, s), 2.60 (4H, t, J = 5.1 Hz), 3.18–3.30 (5H, m), 3.54–3.62 (3H, m), 5.52 (1H, dd, J = 9.5, 11.2 Hz), 6.84 (2H, d, J = 8.7 Hz), 7.04 (2H, d, J = 8.7 Hz), 7.26–7.35 (3H, m), 7.93 (2H, d, J = 8.6 Hz); ESI MS: 426.4 (M+Na); HPLC purity: 98.5%, tR = 5.8 min.
5-(4-Morpholin-4-yl-phenyl)-4,5-dihydro-isoxazole-3-carboxylic acid ethyl ester (28): Compound 28 was synthesized following the general procedure described earlier in 78% yield. 1H NMR (500 MHz, CDCl3): δ 1.38 (3H, t, J = 7.0 Hz), 3.17 (4H, t, J = 4.8 Hz), 3.21 (1H, dd, J = 9.7, 18.3 Hz), 3.56 (1H, dd, J = 11.4, 17.8 Hz), 3.85 (4H, t, J = 4.8 Hz), 4.36 (2H, q, J = 7.0 Hz), 5.71 (1H, dd, J = 9.2, 11.4 Hz), 6.90 (2H, d, J = 8.5 Hz), 7.23 (2H, d, J = 8.7 Hz); ESI MS: 327.1 (M+Na); HPLC purity: 99.7%, tR = 5.6 min.
General procedure for the synthesis of 29a and 29b
To a stirred solution of ester 1 or 28 (1 mmol) in anhydrous THF, DIBAL-H (2 mmol) was added dropwise at 0 °C and stirred at the same temperature for 4 h. The reaction mass was quenched with saturated solution of potassium sodium tartrate and the product was extracted with EtOAc, washed with water, brine, dried (anhyd. Na2SO4) and concentrated. The crude residue was purified by flash chromatography to give pure compounds.
{5-[4-(4-Benzyl-piperazin-1-yl)-phenyl]-4,5-dihydro-isoxazol-3-yl}-methanol (29a): Yield: 56%; 1H NMR (500 MHz, CDCl3): δ 2.60 (4H, t, J = 5.1 Hz), 3.01 (1H, q, J = 8.7 Hz), 3.19 (4H, t, J = 5.1 Hz), 3.37 (1H, q, J = 8.7 Hz), 3.56 (2H, s), 4.43 (2H, s), 5.53 (1H, t, J = 9.2 Hz), 6.88 (2H, d, J = 8.7 Hz), 7.21 (2H, d, 8.7 Hz), 7.24–7.29 (1H, m), 7.30–7.39 (4H, m); 13C NMR (500 MHz, CDCl3): δ 42.59, 48.89, 52.96, 58.00, 63.06, 82.34, 115.95, 127.10, 127.27, 128.34, 129.32, 130.94, 137.71, 151.34, 158.61; ESI MS:374.4 (M+23); HPLC purity: 99%, tR = 4.6 min.
[5-(4-Morpholin-4-yl-phenyl)-4,5-dihydro-isoxazol-3-yl]-methanol (29b): Yield: 60%; 1H NMR (500 MHz, CDCl3): δ 3.02 (1H, dd, J = 8.7, 17.0 Hz), 3.16 (4H, t, J = 4.6 Hz), 3.41 (1H, dd, J = 10.7, 17.0 Hz), 3.86 (4H, t, J = 4.8 Hz), 4.46 (2H, d, J = 5.8 Hz), 5.57 (1H, dd, J = 8.7, 10.5 Hz), 6.90 (2H, d, J = 8.7 Hz), 7.25 (2H, d, J = 6.3 Hz); ESI MS: 263.1 (M+1); HPLC purity: 98.7%, tR = 4.2 min.
General procedure for the synthesis of 30a and 30b
To a stirred solution of alcohols 29a or 29b (1 mmol) in anhydrous DMF, CBr4 (1 mmol), Ph3P (1 mmol) and NaN3 (5 mmol) were added sequentially and the resulting mixture was stirred at room temperature for 2 h. The reaction mixtures were quenched with MeOH, filtered and concentrated. The crude residues were purified by flash chromatography.
1-[4-(3-Azidomethyl-4,5-dihydro-isoxazol-5-yl)-phenyl]-4-benzyl-piperazine (30a): Yield: 59%; 1H NMR (500 MHz, CDCl3): δ 2.60 (4H, t, J = 4.8 Hz), 3.01 (1H, q, J = 7.8 Hz), 3.20 (4H, t, J = 5.1 Hz), 3.37 (1H, q, J = 7.8 Hz), 3.56 (2H, s), 4.13 (2H, s), 5.57 (1H, t, J = 5.1 Hz), 6.89 (2H, d, J = 8.7 Hz), 7.21 (2H, d, J = 8.5 Hz), 7.24–7.29 (1H, m), 7.30–7.37 (4H, m); 13C NMR (500 MHz, CDCl3): δ 42.66, 42.71, 47.49, 48.85, 48.89, 52.99, 63.06, 82.91, 115.90, 126.99, 127.06, 128.36, 129.28, 130.45, 151.52, 153.79; ESI MS: 377.4 (M+1); HPLC purity: 98.5%, tR = 5.1 min.
4-[4-(3-Azidomethyl-4,5-dihydro-isoxazol-5-yl)-phenyl]-morpholine (30b): Yield: 61%; 1H NMR (500 MHz, CDCl3): δ 3.02 (1H, dd, J = 8.7, 17.0 Hz), 3.16 (4H, t, J = 4.6 Hz), 3.40 (1H, dd, J = 10.9, 17.3 Hz), 3.85 (4H, t, J = 4.8 Hz), 4.15 (2H, s), 5.59 (1H, dd, J = 9.0, 10.5 Hz), 6.90 (2H, d, J = 8.5 Hz), 7.24 (2H, d, J = 8.5 Hz); ESI MS: 288.3 (M+1); HPLC purity: 98.8%, tR = 5.3 min.
General procedure for the synthesis of 31a and 31b
To a stirred solutions of azides 30a or 30b (1 mmol) in 1,4-dioxane (5 mL), Ph3P (1.6 mmol) was added and stirred at room temperature for overnight. The reaction mixtures were concentrated under reduced pressure and the crude residues were purified by flash chromatography to afford 31a or 31b.
C-{5-[4-(4-Benzyl-piperazin-1-yl)-phenyl]-4,5-dihydro-isoxazol-3-yl}-methylamine (31a): Yield: 67%; 1H NMR (500 MHz, CDCl3): δ 1.67 (2H, broad s), 2.59 (4H, t, J = 4.8 Hz), 2.95 (1H, q, J = 8.5 Hz), 3.19 (4H, t, J = 5.1 Hz), 3.32 (1H, q, J = 8.5 Hz), 3.56 (2H, s), 3.61 (2H, s), 5.51 (1H, t, J = 8.7 Hz), 6.88 (2H, d, J = 8.5 Hz), 7.21 (2H, d, J = 8.5 Hz), 7.27–7.28 (1H, m), 7.30–7.36 (4H, m); 13C NMR (500 MHz, CDCl3): δ 43.33, 48.90, 53.00, 63.06, 63.15, 82.13, 115.89, 127.02, 128.33, 129.23, 131.08, 137.90, 151.33; ESI MS: 373.1 (M+23); HPLC purity: 98.8%, tR = 4.3 min.
C-[5-(4-Morpholin-4-yl-phenyl)-4,5-dihydro-isoxazol-3-yl]-methylamine (31b): Yield: 60%; 1H NMR (500 MHz, CDCl3): δ 2.97 (1H, dd, J = 8.7, 17.0 Hz), 3.15 (4H, t, J = 4.8 Hz), 3.35 (1H, dd, J = 6.3, 17.0 Hz), 3.63 (2H, s), 3.85 (4H, t, J = 4.8 Hz), 5.53 (1H, dd, J = 9.0, 10.5 Hz), 6.89 (2H, d, J = 8.5 Hz), 7.24 (2H, d, J = 8.5 Hz); ESI MS: 284.2 (M+23); HPLC purity: 100%, tR = 4.0 min.
General procedure for the synthesis of 32a and 32b
To a stirred solutions of amines 31a and 31b (1 mmol) in CH2Cl2 (5 mL), Et3N (3 mmol) and acetyl chloride (1.5 mmol) were added at 0 °C and allowed to stir at room temperature for 1 h. The reaction mixtures were diluted with excess CH2Cl2 (15 mL) and washed with aq. NaHCO3 (10 mL), dried (anhyd. Na2SO4), concentrated under reduced pressure and the crude residues were purified by flash chromatography.
N-{5-[4-(4-Benzyl-piperazin-1-yl)-phenyl]-4,5-dihydro-isoxazol-3-ylmethyl}-acetamide (32a): Yiled: 78%; 1H NMR (500 MHz, CDCl3): δ 2.00 (3H, s), 2.59 (4H, t, J = 4.8 Hz), 2.96 (1H, q, J = 8.5 Hz), 3.19 (4H, t, J = 5.1 Hz), 3.30 (1H, q, J = 8.5 Hz), 3.55 (2H, s), 4.18 (2H, t, J = 4.8 Hz), 5.51 (1H, t, J = 5.1 Hz), 6.30 (1H, broad s), 6.87 (2H, d, J = 8.5 Hz), 7.18 (2H, d, J = 8.5 Hz), 7.24–7.28 (1H, m), 7.30–7.36 (4H, m); 13C NMR (500 MHz, CDCl3): δ 22.91, 22.96, 36.95, 37.00, 43.35, 48.85, 48.87, 52.97, 53.03, 62.95, 63.05, 82.41, 82.44, 115.85, 127.03, 127.08, 127.20, 128.31, 128.33, 129.24, 130.52, 137.91, 151.44, 156.15, 170.53; ESI MS: 415 (M+23); HPLC purity: 98.7%, tR = 4.6 min.
N-[5-(4-Morpholin-4-yl-phenyl)-4,5-dihydro-isoxazol-3-ylmethyl]-acetamide (32b): Yiled: 71%; 1H NMR (500 MHz, CDCl3): δ 2.97 (1H, dd, J = 9.0, 17.3 Hz), 3.15 (4H, t, J = 4.8 Hz), 3.34 (1H, dd, J = 10.7, 17.0 Hz), 3.85 (4H, t, J = 4.8 Hz), 4.20 (2H, q, J = 3.4 Hz), 5.54 (1H, dd, J = 9.2, 10.2 Hz), 6.19 (1H, br s), 6.89 (2H, d, J = 8.5 Hz), 7.22 (2H, d, J = 8.5 Hz); ESI MS: 326.1 (M+Na); HPLC purity: 99.1%, tR = 4.2 min.
Acknowledgments
We thank National Institutes of Health grant AI062415 for financial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dye C, Scheele S, Dolin P, et al. Global Burden of Tuberculosis. Estimated Incidence, Prevalence, and Mortality by Country. J Am Med Assoc. 1999;282(7):677–686. doi: 10.1001/jama.282.7.677. [DOI] [PubMed] [Google Scholar]
- 2.Murray JF. Tuberculosis and HIV Infection: A Global Perspective. Respiration. 1998;65(2):335–342. doi: 10.1159/000029291. [DOI] [PubMed] [Google Scholar]
- 3.“Tuberculosis” WHO Fact Sheet No 104. Geneva: Health Communications, WHO; 2006. http://www.who.int/mediacentre/factsheets/fs104/en/index.html. [Google Scholar]
- 4.Bloom BR, editor. Tuberculosis: Pathogenesis, Protection, and Control. ASM PRESS; Washington, DC: 1994. [Google Scholar]
- 5.Sacchettini JC, Rubin EJ, Freundlich JS. Nat Rev Microbiol. 2008;6(1):41–52. doi: 10.1038/nrmicro1816. [DOI] [PubMed] [Google Scholar]
- 6.Burman WJ, Jones BE. Am J Respir Crit Care Med. 2001;164:7–12. doi: 10.1164/ajrccm.164.1.2101133. [DOI] [PubMed] [Google Scholar]
- 7.Spigelman MK. J Infect Dis. 2007;196:S28–S34. doi: 10.1086/518663. [DOI] [PubMed] [Google Scholar]
- 8.Tangallapally RP, Yendapally R, Lee RE, Hevener K, Jones VC, Lenaerts AJM, McNeil MR, Wang Y, Franzblau S, Lee RE. J Med Chem. 2004;47:5276–5283. doi: 10.1021/jm049972y. [DOI] [PubMed] [Google Scholar]
- 9.Tangallapally RP, Yendapally R, Lee REB, Lenaerts AJM, Lee RE. J Med Chem. 2005;48:8261–8269. doi: 10.1021/jm050765n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tangallapally RP, Lee REB, Lenaerts AJM, Lee RE. Bioorg Med Chem Lett. 2006;16:2584–2589. doi: 10.1016/j.bmcl.2006.02.048. [DOI] [PubMed] [Google Scholar]
- 11.Tangallapally RP, Yendapally R, Daniels AJ, Lee REB, Lee RE. Curr Top Med Chem. 2007;7:509–526. doi: 10.2174/156802607780059772. [DOI] [PubMed] [Google Scholar]
- 12.Tangallapally RP, Sun D, Budha Rakesh N, Lee REB, Lenaerts AJM, Meibohm B, Lee RE. Bioorg Med Chem Lett. 2007;17:6638–6642. doi: 10.1016/j.bmcl.2007.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.For recent reviews related to oxazolidinone and oxazolidine antibacterial agents, see: Renslo AR, Luehr GW, Gordeev MF. Bioorg Med Chem. 2006;14:4227–4240. doi: 10.1016/j.bmc.2006.01.068.
- 14.Sood R, Bhadauriya T, Rao M, Gautam R, Malhotra S, Barman TK, Upadhyay DJ, Rattan A. Infect Disord Drug Targets. 2006;6:343–354. doi: 10.2174/187152606779025860. [DOI] [PubMed] [Google Scholar]
- 15.Zappia G, Menendez P, Delle Monache G, Misiti D, Nevola L, Botta B. Mini Rev Med Chem. 2007;7:389–409. doi: 10.2174/138955707780363783. [DOI] [PubMed] [Google Scholar]
- 16.Guram AA, Rennels RA, Buchwald SL. Angew Chem Int Ed Engl. 1995;34:1348–1350. [Google Scholar]
- 17.Padwa A, editor. 1,3-Dipolar Cycloaddition Chemistry. Wiley; New York: 1984. [Google Scholar]
- 18.Diana GD, Rudewicz P, Pevear DC, Nitz TJ, Aldous SC, Aldous DJ, Robinson DT, Draper T, Dutko FJ, Aldi C, Gendron G, Oglesby RC, Volkots DL, Reuman M, Bailey TR, Czerniak R, Block T, Roland R, Oppermann J. J Med Chem. 1995;38:1355–1371. doi: 10.1021/jm00008a014. [DOI] [PubMed] [Google Scholar]
- 19.Lee RE, Protopopova M, Crooks E, Slayden RA, Terrot M, Barry CE., III J Comb Chem. 2003;5:172–187. doi: 10.1021/cc020071p. [DOI] [PubMed] [Google Scholar]
- 20.Sasaki H, Haraguchi Y, Itotani M, Kuroda H, Hashizume H, Tomishige T, Kawasaki M, Matsumoto M, Komatsu M, Tsubouchi H. J Med Chem. 2006;49:7854–7860. doi: 10.1021/jm060957y. [DOI] [PubMed] [Google Scholar]
- 21.Fortun J, Davila PM, Navas E, Perez-Elias MJ, Cobo J, Tato M, De la Pedrosa EG, Gomez-Mampaso E, Moreno S. J Antimicrob Chemother. 2005;56:180–185. doi: 10.1093/jac/dki148. [DOI] [PubMed] [Google Scholar]