Abstract
A simple, easily measured surrogate outcome measure for use in early treatment trials for acute ischemic stroke therapies would be highly valued. We hypothesized that day 5 NIH stroke scale score (NIHSS) and day 5 diffusion weighted imaging (DWI) volume would predict clinical outcome better than either alone and could be considered as a possible surrogate outcome in early phase acute stroke trials.
Methods
The prospective Acute Stroke Accurate Prediction (ASAP) trial included a prespecified subgroup evaluated for early outcome. Logistic regression analysis was used to assess the prediction of modified Rankin (mRankin) of 0 or 1.
Results
A total of 204 subjects completed the substudy and 116 (57%) had excellent outcome at 3 months. The area under the ROC curve (AUC) for day 5 NIHSS predicting 3 month excellent outcome was 0.84; for DWI volume predicting outcome was 0.76 and for the multivariable model combining both was 0.84.
Conclusions
The results of the early outcome substudy of the ASAP trial suggest that early stroke severity and infarct volume measures are predictive of 3 month excellent outcome. In our data set the DWI volume does not add clinically relevant information in predicting 3 month outcome. Validation of these results is required.
Keywords: cerebral ischemia, prognosis, stroke outcome, models, statistical, surrogate
A simple, valid, reliable, sensitive and inexpensive tool to accurately measure patient outcome in stroke clinical trials would be highly valued1. A surrogate measure that identifies early outcomes has been proposed 2-4. Early NIHSS score as a measure of stroke severity, is highly predictive of 3 month outcome in acute ischemic stroke patients2 and acute diffusion weighted imaging (DWI) data adds to the prediction5. We hypothesized that the combination of day 5 NIHSS score and DWI volume in acute ischemic stroke (AIS) patients would be more predictive of 3 month clinical outcome than either alone.
Subject and Methods
As described previously, the ASAP trial was a prospective, single center, observational trial of AIS patients who underwent clinical and imaging assessment within 24 hours of stroke onset and were followed for 90 days5. A pre-specified early outcome sub-study of the ASAP trial included subjects who underwent additional imaging and clinical assessment at day 5 (+/-2) based on previous data6. All subjects were eligible for the sub-study until enrollment was complete. Of the total 266 from the ASAP trial, 209 were in the sub-study and 204 of these were included in this analysis. Five patients were excluded due to missing day 5 NIHSS scores. All subjects provided written informed consent and the protocol was IRB approved.
We used univariate regression analysis to assess the association of day 5 variables with 3 month mRankin score. We used multivariable logistic regression to estimate the multivariable relationships. Model performance was measured by AUC for discrimination with success defined as AUC >=0.87. The multivariable models were adjusted for age and tPA treatment to control for confounding.
Results
Baseline and day 5 characteristics and the 90 day clinical outcomes are shown in Table 1. The univariate logistic regression analysis of the day 5 NIHSS score had an AUC of 0.84. The univariate day 5 DWI volume had an AUC of 0.76. The combined model including both variables demonstrated an AUC of 0.84.
Table 1.
Patient Characteristics (N= 204)
| Baseline Characteristics | |
| Median Age years (IQ Range) | 68 (56-78) |
| Median NIHSS score (IQ range) | 5.5 (3-11) |
| Median DWI volume cc (IQ Range) | 3.1 (0.3-19.7) |
| Female Sex | 96 (47%) |
| White Race | 175 (85.8%) |
| Lacune | 62 (30%) |
| TPA treatment | 31 (15%) |
| Day 5 status median (IQ range) | |
| NIHSS score | 4 (1-9) |
| DWI Volume | 5.64 (0.76-33.8) |
| 3 month outcome | |
| Death | 15 (7.35%) |
| mRankin = 0,1 | 116 (57%) |
| mRankin = 5,6* | 21 (10.29%) |
mRankin of 5,6 represents nursing home level disability (5) and death (6).
An additional analysis adjusting for the potential effect of treatment with tPA did not change the model estimates (data not shown) or the relative statistical importance of NIHSS score or DWI volume.
Age was a statistically significant covariate at the 0.01 level in all models. The AUC increased to 0.82 for the DWI model and to 0.87 for the NIHSS score model and combined model when age was included.
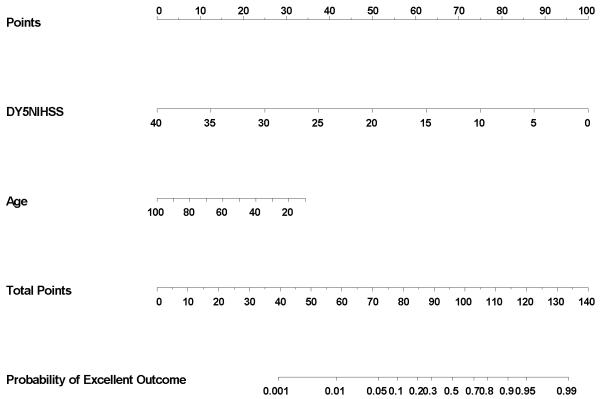
An accompanying on-line nomogram provides a means to determine the probability of an excellent 90-day clinical outcome for an individual patient using the age-adjusted day 5 NIHSS score
Discussion
Our data from the early outcome substudy of the ASAP trial demonstrate that day 5 imaging and clinical information are highly predictive of three month outcome in AIS patients. The AUC of the univariate model using early outcome NIHSS score was 0.84 which exceeded our prespecified definition of success (0.80), however, the addition of the early imaging data did not improve the accuracy of the prediction. A previous study using CT infarct volumes measured between days 6 and 11 demonstrated only modest correlation between infarct volume and 3-month clinical outcomes8. MRI adds information in the acute stroke setting but offers no clinically relevant improvement on 3 month outcome prediction5.
Based on our data, a scoring system using age-adjusted day 5 NIHSS score to predict functional outcomes is most likely to have the greatest clinical utility. Age-adjustment of the day 5 NIHSS score maximizes predictive accuracy because of the strong independent association of age and mRS. A simple nomogram can provide the adjustment for younger patients with mild to moderate strokes, but should be used cautiously prior to external validation.
Our study is limited by small sample size, single site of enrollment, and young population with mild strokes. A larger sample may have demonstrated a significant contribution by DWI. The additional predictive power added by imaging was much smaller than estimated and may have resulted from the use an of a single volume measure that did not capture information on infarct location or evolution6. Incorporation of perfusion MR sequences9, or clinical covariates such as diabetes or prestroke disability may have improved predictive power.
The relationships identified in this study have not been externally validated. As the sample size is small and our cohort was young with mild to moderately severe strokes, these data are only hypothesis generating requiring validation in a more robust data set.
Early clinical status is a strong predictor of 3 month outcome and may be useful in clinical and research settings. For proof of concept studies, utilization of a day 5 outcome may substantially reduce the time, cost and frequency of subjects lost to follow up while allowing an accurate determination of the appropriateness of proceeding to phase III trials. Additionally, this information may provide an imputation method for trials with early outcome information and a small number of patients missing final outcome data. The strong prediction supports a potential role for day 5 outcome. Once validated, our simple nomogram may be valuable in similar populations and may be useful in trials with adaptive designs and rapid accrual as they may facilitate early adjustment of pre trial estimates of event rates. These potential benefits may, in some trials, outweigh the disadvantages of an imperfect but highly predictive estimate of 3 month outcome.
Figure. Nomogram for Probability of Excellent Outcome.
Simple nomogram demonstrating likelihood of excellent outcome using day 5 NIHSS score and age. The day 5 NIHSS score is determined and a vertical line up demonstrates the points earned. The age is determined and a vertical line up demonstrates the points earned for age. The sum of the two point scores is found on the total score line and a vertical line down provides the predicted probability of excellent outcome at 90 days.
Acknowledgments and Funding
This research was funded by the National Institutes of Health- National Institute of Neurological Disorders and Stroke (NIH-NINDS) (K23NS02168).
The ASAP study was funded by the NIH-NINDS (K23NS02168) and Drs. Johnston and Wagner received support from this grant.
The authors gratefully acknowledge the contribution of the ASAP trial investigators and patients, without whose efforts, this work would not have been possible. Special gratitude is expressed to Dr. David Kallmes at the Mayo Clinic Rochester, Minnesota for his contributions in leading the imaging evaluation team.
References
- 1.Lees Kennedy R, Hankey Graeme J, Hacke Werner. Design of future acute-stroke treatment trials. Lancet Neurology. 2003;2:54–61. doi: 10.1016/s1474-4422(03)00267-9. [DOI] [PubMed] [Google Scholar]
- 2.Johnston Karen C, Wagner Douglas P, Haley EC, Jr, Connors Alfred F. Combined clinical and imaging information as an early stroke outcome measure. Stroke. 2002;33:466–472. doi: 10.1161/hs0202.102881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Higashida Randall T, Furlan AJ. Trial Design and Reporting Standards for Intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke. 2003;34:e109–e137. doi: 10.1161/01.STR.0000082721.62796.09. [DOI] [PubMed] [Google Scholar]
- 4.Brown Devin L, Johnston Karen C, Wagner Douglas P, Haley EC., Jr Predicting major neurological improvement with intravenous recombinant tissue plasminogen activator treatment of stroke. Stroke. 2004;35:147–150. doi: 10.1161/01.STR.0000105396.93273.72. [DOI] [PubMed] [Google Scholar]
- 5.Johnston KC, Wagner DP, Wang Xin-Qun, Newman George C, Hijs Vincent, Sen Souvik, Warach Steven for the GAIN, Citicoline and ASAP Investigators Validation of an Acute Ischemic Stroke Model. Does Diffusion-Weighted Imaging Lesion Volume Offer a Clinically Significant Improvement in Prediction of Outcome? Stroke. 2007;38:1820–1825. doi: 10.1161/STROKEAHA.106.479154. [DOI] [PubMed] [Google Scholar]
- 6.Baird AE, Benfield A, Schlaug G, Siewert B, Lovblad KO, Edelman RR, Warach S. Enlargement of human cerebral ischemic lesion volumes measured by diffusion-weighted magnetic resonance imaging. Ann Neurol. 1997;41:581–589. doi: 10.1002/ana.410410506. [DOI] [PubMed] [Google Scholar]
- 7.Harrell FE. Regression Modeling Strategies. Springer; New York: 2001. [Google Scholar]
- 8.Saver JL, Johnston KC, Homer D, Wityk R, Koroshetz W, Truskowski LL, Haley EC, the RANTTAS Investigators Infarct volume as a surrogate or auxiliary outcome measure in ischemic stroke clinical trials. Stroke. 1999;30:293–298. doi: 10.1161/01.str.30.2.293. [DOI] [PubMed] [Google Scholar]
- 9.Thijs VN, Adami A, Neumann-Haefelin T, Moseley ME, Marks MP, Albers GW. Relationship between severity of MR perfusion deficit and DWI lesion evolution. Neurology. 2001;57:1205–11. doi: 10.1212/wnl.57.7.1205. [DOI] [PubMed] [Google Scholar]