Abstract
Cocaine- and amphetamine-regulated transcript (CART) and its associated peptides have been implicated in a number of physiologic processes including modulation of the hypothalamo–pituitary–adrenal (HPA) axis and cardiovascular regulation. Recently, we reported that in isolated cerebral arterioles, CART peptide (CARTp) acts directly to produce endothelium-dependent constriction via the endothelin signaling pathway. We used the rat closed cranial window model to determine the in vivo effects of CARTp on pial arteriolar diameter. Intravenous administration of 30 μg/kg CARTp produced a significant pressor effect and constriction of pial arterioles. The pressor response to systemic CARTp was blocked by the β-adrenergic receptor antagonist propranolol (2 mg/kg IV). Direct application of 0.1 nM–1μM CARTp to pial arterioles produced a dose-dependent and long-lasting constriction to approximately 88% of baseline diameter. The constriction response to topically applied 100 nM CARTp was blocked by both the endothelin A (ETA) receptor antagonist BQ-123 (10 μM) and the inhibitor of endothelin-converting enzyme, phosphoramidon (100 nM). These results demonstrate for the first time that CARTp constricts cerebral vessels in vivo, an action mediated by its effects on the endothelin system, specifically via activation of ETA receptors. This supports the notion that CARTp plays a physiologic role in cerebrovascular regulation, particularly during times of HPA axis activation.
Keywords: cocaine- and amphetamine-regulated transcript, cerebral blood flow, endothelin, vasoconstriction
Introduction
Cocaine- and amphetamine-regulated transcript (CART) peptides have recently emerged as major regulators of a growing number of physiologic processes, including feeding behavior and drug reinforcement and reward.1–4 Our group recently reported that CART peptide (CARTp) exerts a direct effect on cerebral vessels, producing in isolated cerebral arterioles a potent and long-lasting constriction. This effect was determined to be endothelium-dependent and mediated via the endothelin (ET-1) signaling pathway.5 Although the exact significance of these in vitro findings remains unclear, circumstantial evidence suggests that CARTp may be involved in vascular regulation, particularly during times of hypothalamo–pituitary–adrenal (HPA) axis activation or increased sympathetic output.
In addition to being expressed throughout the HPA axis,6 CARTp immunoreactivity has been observed within major cardiovascular centers of the medulla.7 Intracerebroventricular (ICV) administration of CARTp modulates cardiovascular function, producing hypertension in conscious rabbits8 and attenuating phenylephrine-induced bradycardia in rats.9 CARTp was also observed to be released into the portal circulation in response to nitric oxide–induced hypotension.10 Such evidence, in light of the recently reported action of CARTp on cerebral microvessels,11 suggests that CARTp may act in the cerebral circulation of the intact animal, perhaps modulating cerebral blood flow during times of HPA axis activation and elevated cardiovascular output.
In considering the potential role for CARTp in such a cardiovascular regulatory process, we sought to determine whether the observed vascular effects are relevant in vivo. Using the closed cranial window preparation, we observed the pial arteriolar response to systemic or local administration of CARTp. We further investigated the involvement of the sympathetic nervous system and ET-1 signaling in mediating the in vivo effects of CARTp on cerebral vessels, characterizing pharmacologically their roles in the mediation of CARTp-induced cerebrovasoconstriction.
Methods
This study was conducted in accordance with the National Institutes of Health guidelines for the care and use of animals in research and under protocols approved by the Oregon Health & Science University Institutional Animal Care and Use Committee.
Closed Cranial Window Preparation
Forty-two 350-g male Sprague-Dawley rats were anesthetized with 2% isoflurane. The right femoral artery and vein were cannulated to allow for continuous monitoring of heart rate (HR), arterial blood pressure, intravenous (IV) drug administration, and blood sampling. The rat was tracheotomized, paralyzed with 1 mg/kg IV D-tubocurarine chloride, and placed on a mechanical ventilator to maintain physiologic blood gas values. Rectal temperature was maintained by means of a heating pad. The skull was placed in a stereotactic frame. To avoid the influence of the inhalation anesthesia on cerebral blood flow, isoflurane anesthesia was discontinued and anesthesia with intraperitoneal α-chloralose and urethane (50 and 500 mg/kg, respectively) was induced. The skull was fixed in a stereotactic frame and the pial vasculature was visualized through a closed cranial window implanted over the right somatosensory cortex, as previously described by Morii et al.12 One 40–80-μm diameter pial arteriole per animal was selected for study. Vessel diameter was monitored continuously by means of a microscope-mounted monochrome charge-coupled device (CCD) camera and video dimension analyzer (Living Systems Instrumentation, Burlington, Vermont).
Following installation of the closed cranial window, pial arteriolar responsiveness to mild hypercapnia was assessed to ensure the overall health of the vessels under study. The ventilator inflow tube was attached to a hypercapnic gas source composed of 5% CO2, 40% O2, and 55% N2; the resulting dilation response was measured. Blood gas values were determined immediately preceding and following mild hypercapnia. Vessel CO2 reactivity was expressed as the ratio between the percent increase from baseline diameter and the mm Hg change in PaCO2 values. Only vessels exhibiting a ratio above 1.0 were selected for further study.
Systemic Administration of CART Peptide
In all studies “CART peptide” (CARTp) refers to a biologically active CART 55-102 fragment of the rat pro-CART peptide. CARTp was purchased from the American Peptide Company (Sunnyvale, California), and 30 μg/kg was dissolved in 1.0 mL normal saline. The intravenous CARTp dosage was selected based on the study by Baranowska et al,13 in which the authors report that 30 μg/kg IV CARTp evoked pituitary hormone release in vivo.
CARTp or saline vehicle was administered via the femoral venous catheter. Immediately following, an additional 0.5 mL of saline was flushed through the catheter for a total volume of 1.5 mL. Following CARTp or saline vehicle injection, changes in heart rate and mean arterial blood pressure (MABP) were observed at t = 0, 1, 2, 3, 4, 5, 10, 20, 30, 40, 50, 60, 90, and 120 minutes postadministration. In vehicle-administered animals, the fluid bolus did not result in a significant change in MABP (Fig. 1), suggesting that this volume did not present an excessive hydrostatic strain up the cardiovascular system of 350-g male Sprague-Dawley rats.
Figure 1.
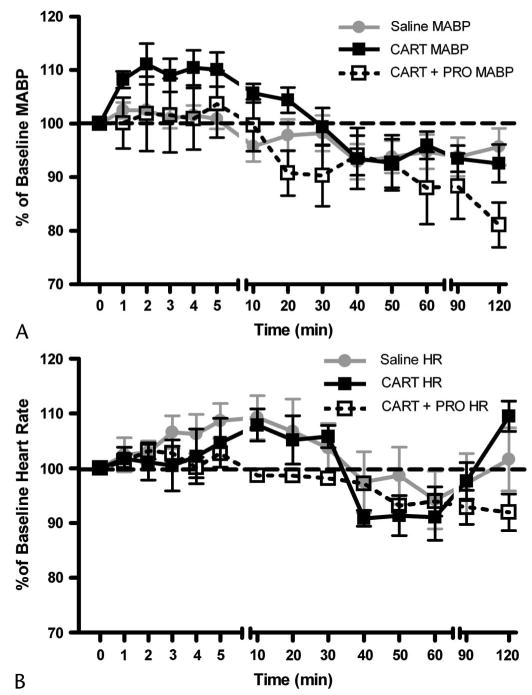
Effect of intravenous CARTp administration on mean arterial blood pressure and heart rate. A, Intravenous administration of 1.5-mL saline vehicle did not alter MABP compared with preinjection values. Administration of 30 μg/kg CARTp evoked a significant elevation in blood pressure (P < 0.05, 1-way ANOVA vs saline). Pretreatment of animal with propranolol (2 mg/kg IV) abolished the pressor response to systemic CARTp (1-way ANOVA; P > 0.05 vs saline, P < 0.05 vs CARTp alone). B, Neither the injection of saline vehicle, nor CARTp in the presence or absence of propranolol significantly altered heart rate from baseline levels.
To determine the effect of intravenous CARTp on changes in cerebral blood flow, CARTp (n = 6) or saline vehicle (n = 6) was administered via the femoral venous catheter and the resulting effects on HR, MABP and pial arteriolar diameter were measured at t = 0, 1, 2, 3, 4, 5, 10, 20, 30, 40, 50, 60, 90, and 120 minutes. The influence of CARTp-evoked changes in sympathetic tone on these vascular responses was delineated by pretreating a third set of animals (n = 6) with the β-adrenergic antagonist propranolol (2 mg/kg IV) 5 minutes prior to the IV administration of CARTp.
Local Administration of CART Peptide
To determine any direct effect on cerebral arterioles in vivo, CARTp was dissolved in artificial cerebrospinal fluid (aCSF) and perfused through the closed window, across the pial surface by means of a peristaltic perfusion pump, at a rate of 0.25 mL/min. The vascular responses to cumulative 100-fold increases in CARTp concentration were observed (n = 6). CARTp (100 pM) was perfused for 10 minutes, during which intracranial pressure was maintained at a constant level (∼4 mm Hg) by raising or lowering the cranial window outflow tube to ensure an anatomic profile of the cortical surface. The resulting changes in vessel diameter were observed during a 5-minute equilibration period following the cessation of transwindow flow. Consecutive 10-minute perfusions of 10 nM and 1 μM CARTp were each followed by a 5-minute equilibration period. The CARTp dosages selected were based on the findings of Larsen et al,10 in which the authors report concentrations of CARTp in the picomolar range in the systemic circulation and in the nanomolar range in the portal circulation. Further, we have previously demonstrated that these doses are vasoactive in cerebral microvessels in vitro.11
Involvement of Endothelin System in Vascular CART Peptide Response
The involvement of the ET-1 system in the pial arteriolar response to direct topical application of CARTp was assessed. The baseline vascular response to 100 nM CARTp was first determined as described previously. This was followed by a 30-minute washout period with aCSF, followed by a 5-minute equilibration period. Once the vessel had regained baseline diameter (±10% of resting diameter), CO2 reactivity was measured a second time to ensure normal vasoreactivity. The window was then perfused for 10 minutes with the respective inhibitor of the ET-1 system: the endothelin A receptor (ETAR) antagonist BQ-123 (10 μM, n = 6), the endothelin B receptor (ETBR) antagonist BQ-788 (1 μM, n = 6), or the endothelin-converting enzyme (ECE) inhibitor phosphoramidon (PHO, 100 nM, n = 6). These are concentrations at which the inhibitors of the ET-1 pathway are known to be specific, yet exert the minimal direct vascular effects in vitro.11,14–16 The direct effect of the inhibitors was observed during the 5-minute equilibration period. CARTp (100 nM) was then coperfused with the respective inhibitor for 10 minutes, and the resulting vascular response was observed during the 5-minute equilibration period that followed.
Drugs and Solutions
The composition of aCSF was as follows (in mEq/L): 156.5 Na+, 2.95 K+, 2.50 Ca2+, 1.33 Mg2+, and 24.6 HCO−3, and (in mg/dL) 66.5 dextrose and 40.2 urea. CARTp (rat CART 55-102), BQ-123, and BQ-788 were purchased from American Peptide Company (Sunnyvale, California). Phosphoramidon was purchased from Sigma-Aldrich Co. (St. Louis, Missouri). CARTp for intravenous administration was dissolved in 0.9% saline. CARTp, BQ-123, BQ-788, and phosphoramidon for topical administration were dissolved in aCSF.
Data Analysis
All data are expressed as means ± standard error (SE). Variations among animals in MABP, HR, and arteriolar diameter were normalized by reporting them as the percentage of the baseline value (100% × resulting value/baseline value). Because of variations in hypercapnic blood gas values between trials, the hypercapnic dilation response (CO2 reactivity) was normalized by dividing the percentage increase in vessel diameter by the increase in arterial PaCO2 (mm Hg). Data and statistical analysis were performed with GraphPad Prism® 4 (GraphPad Software Inc., San Diego, California). Comparisons of data were made with paired Student's t-test or with analysis of variance (ANOVA), followed by a post hoc Bonferroni procedure. A value of P <0.05 was considered significant.
Results
Cardiovascular Effects of CART Peptide
Saline vehicle, 30 μg/kg CARTp, or CARTp with pretreatment with 2 mg/kg propranolol were injected intravenously via the femoral vein catheter. None of these treatments resulted in any change in arterial blood gas values (pH, PaO2, PaCO2; Table 1) as assessed prior to and 60 minutes post-intervention. Intravenous injection of 1.5 mL saline vehicle did not significantly alter MABP or HR from baseline values (n = 15). Intravenous administration of CARTp (30 μg/kg, n = 10) evoked a significant (P < 0.05, 1-way ANOVA CARTp vs saline) pressor effect compared to saline vehicle. Following CARTp injection, MABP reached 111% of baseline value at t = 2 minutes, returning to baseline within 30 minutes postinjection. Neither the injection of saline vehicle nor that of CARTp significantly altered HR, although a mild and non-significant increase in rate was observed in both groups between t = 4 and t = 20 minutes postinjection. Pretreatment of animals with 2 mg/kg propranolol (n = 6) depressed basal HR (366 ± 9 beats/min to 332 ± 8 beats/min, P < 0.05) and MABP (104 ± 6 mm Hg to 91 ± 5 mm Hg, P = 0.16) and abolished the pressor response to intravenous CARTp administration (Fig. 1A). Within the propranolol pretreated group, HR was not appreciably elevated above baseline at any time point measured following CARTp administration (Fig. 1B).
Table 1.
Effect of Treatment on Physiologic Variables and Vascular Reactivity
| Treatment | MABP | CO2R | pH | PaCO2 | PaO2 | |
|---|---|---|---|---|---|---|
| IV Saline | ||||||
| 1.5-mL vehicle | Preadmininstration | 101 ± 8 | 2.5 ± 0.6 | 7.41 ± 0.04 | 32 ± 3 | 129 ± 10 |
| t = 60 min | 98 ± 10 | 1.9 ± 1.1 | 7.37 ± 0.08 | 36 ± 1 | 107 ± 14 | |
| IV propranolol | ||||||
| 2 mg/kg | Preadmininstration | 104 ± 6 | 1.6 ± 0.5 | 7.39 ± 0.05 | 31 ± 3 | 112 ± 11 |
| t = 60 | 94 ± 3 | 2.2 ± 0.8 | 7.40 ± 0.02 | 35 ± 5 | 124 ± 14 | |
| IV CARTp | ||||||
| 30 μg/kg | Preadmininstration | 105 ± 6 | 1.4 ± 0.2 | 7.36 ± 0.04 | 34 ± 4 | 119 ± 12 |
| t = 60 min | 107 ± 5 | 1.6 ± 0.4 | 7.35 ± 0.05 | 36 ± 4 | 108 ± 8 | |
| Topical CARTp | ||||||
| 100 nM | Preadmininstration | 113 ± 6 | 1.7 ± 0.5 | 7.34 ± 0.01 | 35 ± 2 | 103 ± 5 |
| Postadmininstration | 105 ± 6 | 2.1 ± 1.0 | 7.36 ± 0.03 | 34 ± 3 | 114 ± 12 | |
| Topical PHO | ||||||
| 100 nM | Preadmininstration | 97 ± 4 | 2.6 ± 1.5 | 7.34 ± 0.02 | 33 ± 2 | 131 ± 2 |
| Postadmininstration | 99 ± 4 | 3.0 ± 1.3 | 7.33 ± 0.01 | 37 ± 2 | 138 ± 14 | |
| Topical BQ-123 | ||||||
| 10 μM | Preadmininstration | 96 ± 6 | 1.6 ± 0.3 | 7.38 ± 0.01 | 35 ± 2 | 129 ± 3 |
| Postadmininstration | 100 ± 3 | 1.6 ± 0.6 | 7.37 ± 0.02 | 36 ± 1 | 118 ± 6 | |
| Topical BQ-788 | ||||||
| 1 μM | Preadmininstration | 109 ± 4 | 2.0 ± 0.4 | 7.35 ± 0.02 | 31 ± 3 | 115 ± 9 |
| Postadmininstration | 104 ± 6 | 2.5 ± 1.5 | 7.37 ± 0.01 | 34 ± 4 | 126 ± 12 |
All values are means ± SE.
MABP, mean arterial blood pressure; CO2R, pial arteriolar response to transient hypercapnia (% change in diameter/change in PaCO2); PaCO2, arterial PCO2; PaO2, arterial PO2.
In Vivo Cerebrovascular Response to CART Peptide
In cranial window-equipped animals, a single bolus of intravenous CARTp (n = 6) evoked a significant and long-lasting pial arteriolar constriction (P < 0.05, 1-way ANOVA CARTp vs saline; Fig. 2). During the first 10 minutes following CARTp injection, vessels constricted to ∼94% of diameter at t = 0 minutes. During the interval between 20 and 40 minutes, a constriction to ∼89% of baseline diameter was observed. The vessels tended to remain constricted through 50 to 90 minutes postinjection. Animals pretreated with propranolol (1 mg/kg IV, n = 6) exhibited a cerebrovascular constriction response to intravenous CARTp that was indistinguishable from those treated with intravenous CARTp alone (Fig. 2; P > 0.05, 1-way ANOVA CARTp/PRO vs CARTp alone). Thus, pretreatment with β-adrenergic blockade blocked the pressor but not the pial constrictor response to intravenous CARTp.
Figure 2.
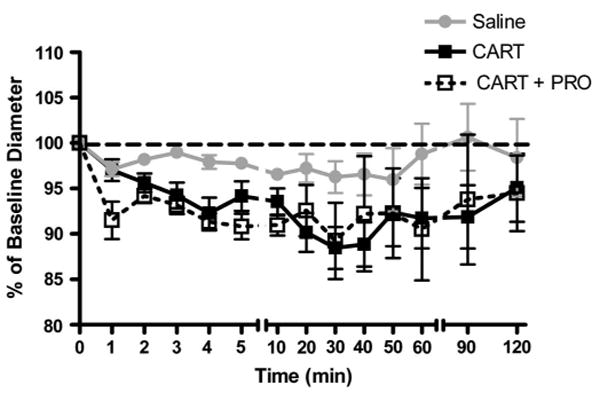
Effect of intravenous CARTp administration on pial arteriolar diameter. Intravenous injection of 1.5-mL saline vehicle produced a slight constriction in pial arterioles. CARTp administration (30 μg/kg) caused a significantly greater constriction effect (P <0.05, 1-way ANOVA CARTp vs saline). Blockade of β-adrenergic receptors with 2 mg/kg IV propranolol prior to systemic CARTp administration did not alter the magnitude or time course of CARTp-evoked cerebrovasoconstriction.
Direct perfusion of CARTp through the closed cranial window resulted in a long-lasting and dose-dependent constriction of pial arterioles (Fig. 3A). The constriction effect was slow to develop (taking several minutes to reach maximal values) and typically required at least 20 minutes of aCSF perfusion for washout. Cumulative administration of 0.1 nM, 10 nM, and 1 μM CARTp (n = 6) resulted in significant (P < 0.01 at all points, 1 sample t-test) constriction of vessels to 94.2 ± 0.5%, 91.1 ± 0.4% and 87.6 ± 0.6% of baseline diameter, respectively. The observed constriction response had an IC50 value of 7.2 × 10−7M. Direct CARTp administration did not alter arterial blood gas values, MABP, or the pial arteriolar dilation response to mild hypercapnia (Table 1).
Figure 3.
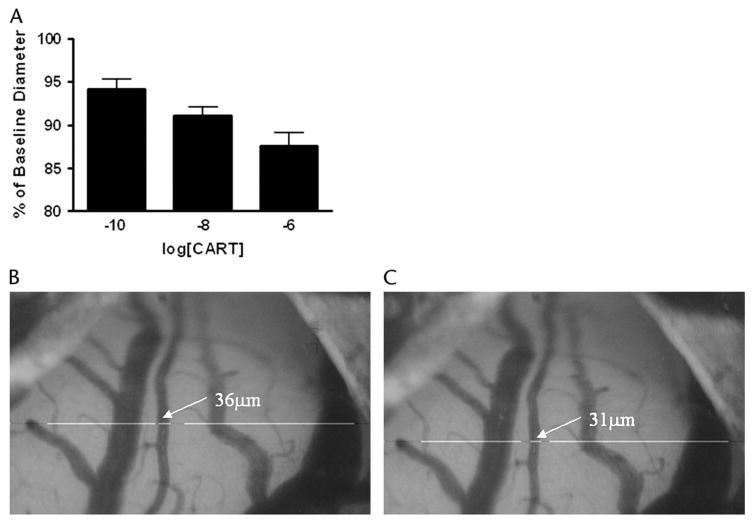
Changes in pial arteriolar diameter after topical CARTp application. A, Cumulative concentration-response for CARTp (0.1 nM to 1.0 μM) perfused through the closed cranial window. CARTp evoked a dose-dependent constriction of the arterioles. B–C, Images depict the constriction of pial arteriole from 36 μm to 31 μm during the course of cumulative dose response to CARTp.
Involvement of Endothelin System in CART Peptide Response
The involvement of the ETA receptor in the constriction response to topical CARTp administration was determined (Fig. 4A, n = 6). Topically applied 100 nM CARTp initially constricted the pial arterioles to 90.6 ± 0.6% of baseline diameter. This constriction effect washed out with aCSF, and the vessel regained basal tone. Topical administration of the ETA receptor-specific antagonist BQ-123 (10 μM) alone caused a significant (P < 0.01, 1 sample t-test) dilation of the vessel to 110.3 ± 1.0% of baseline diameter, suggesting the blockade of a tonic ET-1 evoked constrictor influence on basal tone. When 100 nM CARTp was then coapplied with BQ-123 (10 μM), the constriction response to CARTp was completely abolished (100.9 ± 1.6%, P < 0.05, t-test CARTp vs CARTp/BQ-123).
Figure 4.
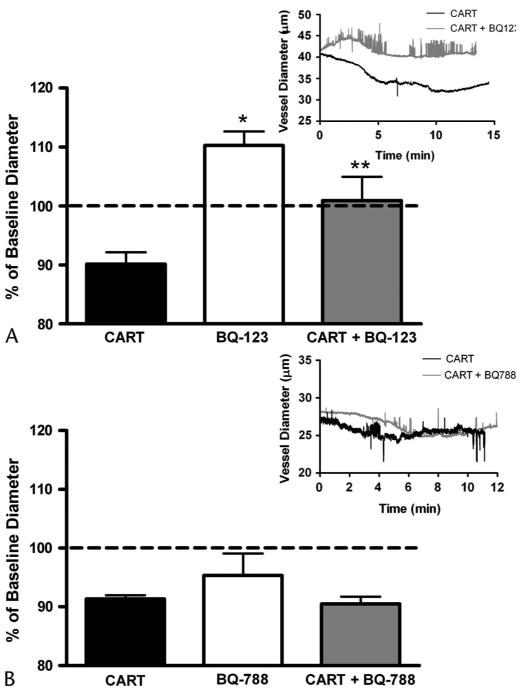
Endothelin ETA receptor antagonist blocks the constriction response to CARTp (100 nmol/L). A, Topical perfusion of the ETA-specific antagonist BQ-123 (10 μM) alone significantly dilated pial arterioles (*P <0.01, 1 sample t-test) from basal tone. Topical coadministration of CARTp with BQ-123 attenuated CARTp-evoked vasoconstriction (**P < 0.05, t-test CARTp vs CARTp/BQ-123) versus CARTp alone. B, Topical perfusion of the ETB-specific antagonist BQ-788 (1 μM) alone did not significantly alter baseline vascular tone. The constriction response to CARTp (100 nM) administration in the presence of BQ-788 was no different from the vascular response to CARTp alone. Insets depict representative traces of vessel diameter following administration of CARTp in the presence or absence of the respective receptor antagonist.
The role of the ETB receptor in the vascular response to topical CARTp application (n = 6) was assessed (Fig. 4B). CARTp (100 nM) initially constricted the vessel to 90.6 ± 0.6% of baseline diameter. This constriction effect washed out with aCSF, and the vessel regained basal tone. Perfusion of the ETB receptor-specific antagonist BQ-788 (1 μM) alone did not significantly alter pial arteriolar diameter. Coperfusion of 100 nM CARTp with 1 μM BQ-788 resulted in a constriction response of 90.5 ± 0.5%, which was not significantly altered from that of CARTp alone. Thus, block of the ETB receptor has no effect of CARTp-induced constriction of pial arteries.
The effect of ET-1 converting enzyme inhibition with 100 nM phosphoramidon of CARTp-induced vasoconstriction (n = 6) was tested (Fig. 5). CARTp (100 nM) caused pial arterioles to constrict to 91.3 ± 0.5% of baseline diameter. This effect washed out with aCSF, and vessels regained resting diameter. Topical administration of PHO alone significantly (P < 0.01, 1 sample t-test) dilated the vessels to 110.4 ± 0.7% of baseline diameter, suggesting that the de novo synthesis of ET-1 contributes a tonic constricting influence on resting tone. Subsequent coadministration of PHO with CARTp resulted in an abolition of the constriction response (97.4 ± 0.3% of baseline, P <0.01 t-test CARTp vs CARTp/PHO). The topical administration of BQ-123, BQ-788, or PHO did not alter MABP, the pial arteriolar dilation response to mild hypercapnia, or arterial blood gas values (Table 1).
Figure 5.
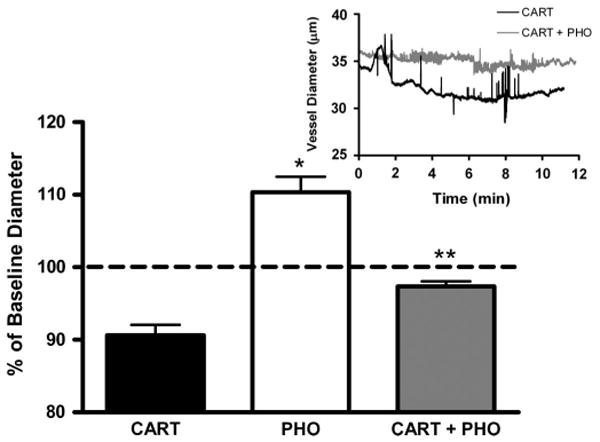
Attenuation of the CARTp (100 nmol/L) constriction response by inhibitor of ECE. Extraluminal administration of the ECE inhibitor phosphoramidon (100 nmol/L) significantly dilated isolated cerebral arterioles from baseline diameter (*P < 0.01, 1 sample t-test). Coadministration with extraluminal PHO markedly attenuated the constriction response to intraluminal CARTp (**P < 0.01, t-test CARTp vs CARTp/PHO). Inset depicts representative trace of vessel diameter following administration of CARTp in the presence of absence of PHO.
Discussion
The present study describes 2 separate effects of CARTp in cardiovascular and cerebrovascular regulation. Our results demonstrate that a single intravenous CARTp dose causes a β-antagonist–sensitive systemic pressor effect and the β-antagonist–independent constriction of pial arterioles. Furthermore, direct application of CARTp to the pial surface results in the dose-dependent constriction of cerebral arterioles, an effect that was blocked by inhibitors of the ETA receptor and ECE enzyme but not an ETB receptor antagonist. Thus we propose that CARTp acts centrally to regulate the cardiovascular system via sympathetic modulation while acting peripherally within the cerebral circulation to constrict cerebral blood vessels in an ET-1/ETAR–dependent manner in vivo.
It has been reported that CARTp is involved in cardiovascular regulation in vivo, primarily through its modulatory effects on sympathetic outflow.8,9 The findings of the present study are in broad agreement with those of Matsumura et al,8 in which the authors report that the intracerebroventricular (ICV) injection of CARTp (1 nmol, 2.1 μg/kg) evokes a long-lasting pressor effect (MABP = 115% of baseline) that is mediated by heightened sympathetic outflow. Despite a 40-fold higher dose, herein we report a more mild (MABP = 110% of baseline) and transient (up to 20 minutes postinjection vs 90 minutes) effect of CARTp (Fig. 1A), a discrepancy that we attribute to an intravenous versus ICV route of CARTp administration in addition to the dampening effects of general anesthesia on sympathetic tone. Our finding that pretreatment with the β-adrenergic antagonist propranolol blocked the pressor response to intravenous CARTp administration confirms that this effect is attributable primarily to CARTp's action on central sympathetic outflow, rather than to a systemwide local vasoconstriction response.
In response to a single systemic dose of CARTp, cerebral microvessels constricted to ∼88% of baseline diameter over a period of 30 minutes postinjection (Fig. 2), representing a 40% reduction in regional blood flow. This constricted state tended to persist for up to 90 minutes postinjection. We attribute the cerebrovascular effects of intravenous CARTp administration to the mobilization of bioactive ET-1 produced locally in the endothelium and acting on adjacent vascular smooth muscle. Based on our previous in vitro findings11 and the present observations from the local administration of CARTp (Fig. 4), we presume that this represents the de novo local synthesis of bioactive ET-1 in the endothelium. It is unlikely that the observed constriction effect of systemic CARTp is the result of a secondary autoregulatory response to CARTp-evoked changes in blood pressure as β-adrenergic blockade prior to CARTp administration abolished the corresponding pressor response (Fig. 1) while leaving the cerebrovasoconstrictor response intact (Fig. 2).
Having defined the cerebrovascular effects of systemic elevations in CARTp, we used the direct application of CARTp to pial arterioles to better characterize the involvement of the ET-1 pathway in the in vivo cerebrovascular response to CARTp. As observed in vitro,11 CARTp directly constricted pial arterioles (Fig. 3); however, the magnitude of the effect (∼88% constriction) observed in the present study is slightly less than that noted in isolated cerebral microvessels (∼82% of baseline diameter). The vascular environment present within these 2 setups likely accounts for these differences. In the present in vivo preparation, cerebral arterioles are embedded in the pial membrane, exposed inferiorly to the glial limitans and intraluminally to freely circulating blood. These influences and their associated vasoactive factors are absent in the isolated cerebral vessel setup. The direct vascular effect of CARTp appeared to be a specific vasoconstrictor effect, given that pial arteriolar dilation in response to mild hypercapnia remained intact following CARTp exposure (Table 1).
Previously, we reported in vitro that CARTp acts in an endothelium-dependent manner to induce the synthesis of the potent vasoconstrictor ET-1 by ECE and ET-1 subsequently acts via the canonical ETA receptor to promote cerebrovasoconstriction.11 The present study confirms this mechanism of action in vivo, evidenced by the ability of the ECE inhibitor PHO (Fig. 5) and the ETA receptor–specific antagonist BQ-123 (Fig. 4), but not the ETB receptor-specific antagonist BQ-788, to block the local constriction response to CARTp perfusion. Although the ETB receptor is expressed on the cerebrovascular endothelium (unpublished data), the absence of its role in mediating the effects of CARTp-evoked ET-1 release are not surprising given the very negligible role that the ETB receptor plays in the normal physiologic response to ET-1 in the cerebral microcirculation (unpublished data). The specificity of the actions of the ET-1 pathway inhibitors at the concentrations used in the present study is supported by previous pharmacologic and physiologic studies from other groups.14–16 In vitro findings from our own laboratory, both published and unpublished, demonstrate that at these concentrations they act specifically to block the effects of exogenous ET-1 (unpublished data) and CARTp-evoked vasoconstriction).11
In the present study, we observed direct vascular responses to perturbations in the ET-1 signaling system. Blockade of ETA receptors with BQ-123 and ECE with PHO both resulted in a vasodilation from resting arteriolar tone (Figs. 4 and 5). Such findings indicate that ET-1 contributes to in vivo resting tone by the tonic stimulation of ETA receptors. The absence of such an effect by BQ-788 suggests that ETB receptors do not participate in modulating in vivo resting tone in the same manner. It is interesting that these effects are different from our previous in vitro results in which we found that the same concentration of BQ-123 (10 μM) did not alter baseline isolated vessel diameter, whereas 1 μM BQ-788 or 100 nM PHO alone produced mild vasoconstriction.11 They further conflict with previous in vitro and in vivo studies from other groups using different model species or experimental setups that have failed to demonstrate a tonic influence of ETA receptor signaling on basal cerebrovascular tone.17,18 Thus, although it is clear that in our hands ET-1 exerts a tonic influence on resting cerebrovascular tone in the rat, the specific roles of the 2 receptor subtypes, their respective interactions with differing experimental conditions, and any potential interplay with other modulators of cerebrovascular tone such as nitric oxide and prostacyclin remain to be defined.
It is currently unclear what, if any, contribution CARTp makes in the regulation of cerebral blood flow under physiologic or pathophysiologic conditions. The lack of an identified CARTp receptor and, hence, any pharmacologic agonists or antagonists hampers meaningful exploration of these questions. Although recent reports have begun to elucidate cellular mechanisms of CARTp action,19–21 conclusions concerning the relevance of its vasoactive properties remain speculative. Most compelling, we believe, are the findings that CARTp immunoreactivity has been localized to the cardiovascular control centers of the medulla,7 that CARTp administration modulated cardiovascular activity,8,9 and that CARTp is observed to be released into the portal circulation in response to sodium nitroprusside–induced systemic hypotension.10 It is possible that the pressor effects of CARTp may be functionally linked to the observed cerebrovasoconstrictor effects. During times of stress and HPA axis activation, CARTp may act to modulate cerebral vascular tone, aiding in the maintenance of constant cerebral blood flow and the prevention of autoregulatory breakthrough under such conditions.
The initial report describing CART22 showed that CARTp was upregulated by cocaine and amphetamine administration. It is well-known that both cocaine and amphetamine use is associated with hemorrhagic and ischemic strokes. The underlying mechanism of cocaine- and amphetamine-induced stroke are not known, but the cause has been thought to be multifactorial, including acute hypertension, cardiac arrhythmia, vasospasm, vasculitis, and others.23 With regard to the direct vascular effects of cocaine and amphetamine, animal studies have shown that both agents, alone or in combination, can cause vasoconstriction of cerebral vessels.24,25 Thus, it is possible that one mechanism of vasoconstriction of cocaine and amphetamine may involve the release of CARTp. It remains to be determined whether CARTp is involved in pathologic diseases such as cocaine- and amphetamine-related stroke.
Conclusions
In the present study, we report on the cerebrovascular effects of systemically and locally administered CARTp in vivo. We demonstrate that intravenous administration of CARTp evokes a marked sympathetically mediated pressor response and the constriction of cerebral pial arterioles. These effects observed in the intact cerebral circulation are mediated by the de novo synthesis of ET-1, which in turn activates smooth muscle–bound ETA receptors to produce vasoconstriction. Such vasoactive effects of CARTp may contribute to the regulation of cerebral blood flow, particularly during activation of the HPA axis and stress response, and may be linked to pathologic states resulting from cerebrovasoconstriction.
Acknowledgments
The authors thank Yong-Feng Yang, PhD, for technical assistance; Shirley McCartney, PhD, for editing support; and Andy Rekito, MS, for graphic support. This work was supported in part by National Institutes of Neurological Disorders and Stroke Grant NA043997 to GAW, RO1 NS049210 to NJA, and NS 020020 to RJT.
This work was supported in part by National Institutes of Neurological Disorders and Stroke Grant NA043997 to GAW, RO1 NS049210 to NJA, and NS 020020 to RJT; Swedish Medical Center Neurotrauma Research to GAW.
References
- 1.Jaworski JN, Vicentic A, Hunter RG, et al. CART peptides are modulators of mesolimbic dopamine and psychostimulants. Life Sci. 2003;73:741–747. doi: 10.1016/s0024-3205(03)00394-1. [DOI] [PubMed] [Google Scholar]
- 2.Kristensen P, Judge ME, Thim L, et al. Hypothalamic CART is a new anorectic peptide regulated by leptin. Nature. 1998;393:72–76. doi: 10.1038/29993. [DOI] [PubMed] [Google Scholar]
- 3.Kuhar MJ, Dall Vechia SE. CART peptides: Novel addiction- and feeding-related neuropeptides. Trends Neurosci. 1999;22:316–320. doi: 10.1016/s0166-2236(98)01377-0. [DOI] [PubMed] [Google Scholar]
- 4.Lambert PD, Couceyro PR, McGirr KM, et al. CART peptides in the central control of feeding and interactions with neuropeptide Y. Synapse. 1998;29:293–298. doi: 10.1002/(SICI)1098-2396(199808)29:4<293::AID-SYN1>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 5.Iliff JJ, Alkayed NJ, Gloshani KJ, et al. Cocaine- and amphetamine-regulated transcript (CART) peptide: A vasoactive role in the cerebral circulation. J Cereb Blood Flow Metab. 2005;25:1376–1385. doi: 10.1038/sj.jcbfm.9600136. [DOI] [PubMed] [Google Scholar]
- 6.Koylu EO, Couceyro PR, Lambert PD, et al. Immunohistochemical localization of novel CART peptides in rat hypothalamus, pituitary and adrenal gland. J Neuroendocrinol. 1997;9:823–833. doi: 10.1046/j.1365-2826.1997.00651.x. [DOI] [PubMed] [Google Scholar]
- 7.Koylu EO, Couceyro PR, Lambert PD, et al. Cocaine- and amphetamine-regulated transcript peptide immunohistochemical localization in the rat brain. J Comp Neurol. 1998;391:115–132. [PubMed] [Google Scholar]
- 8.Matsumura K, Tsuchihashi T, Abe I. Central human cocaine- and amphetamine-regulated transcript peptide 55-102 increases arterial pressure in conscious rabbits. Hypertension. 2001;38:1096–1100. doi: 10.1161/hy1101.092968. [DOI] [PubMed] [Google Scholar]
- 9.Scruggs P, Dun SL, Dun NJ. Cocaine- and amphetamine-regulated transcript peptide attenuates phenylephrine-induced bradycardia in anesthetized rats. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1496–1503. doi: 10.1152/ajpregu.00183.2003. [DOI] [PubMed] [Google Scholar]
- 10.Larsen PJ, Seier V, Fink-Jensen A, et al. Cocaine- and amphetamine-regulated transcript is present in hypothalamic neuroendocrine neurones and is released to the hypothalamic-pituitary portal circuit. J Neuroendocrinol. 2003;15:219–226. doi: 10.1046/j.1365-2826.2003.00960.x. [DOI] [PubMed] [Google Scholar]
- 11.Iliff JJ, Alkayed NJ, Gloshani KJ, et al. Cocaine- and amphetamine-regulated transcript (CART) peptide: A vasoactive role in the cerebral circulation. J Cereb Blood Flow Metab. 2005;25:1376–1385. doi: 10.1038/sj.jcbfm.9600136. [DOI] [PubMed] [Google Scholar]
- 12.Morii S, Ngai AC, Winn HR. Reactivity of rat pial arterioles and venules to adenosine and carbon dioxide: With detailed description of the closed cranial window technique in rats. J Cereb Blood Flow Metab. 1986;6:34–41. doi: 10.1038/jcbfm.1986.5. [DOI] [PubMed] [Google Scholar]
- 13.Baranowska B, Wolinska-Witort E, Chmielowska M, et al. Direct effects of cocaine-amphetamine-regulated transcript (CART) on pituitary hormone release in pituitary cell culture. Neuro Endocrinol Lett. 2003;24:224–226. [PubMed] [Google Scholar]
- 14.Ishikawa K, Ihara M, Noguchi K, et al. Biochemical and pharmacological profile of a potent and selective endothelin B-receptor antagonist, BQ-788. Proc Natl Acad Sci USA. 1994;91:4892–4896. doi: 10.1073/pnas.91.11.4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vatter H, Mursch K, Zimmermann M, et al. Endothelin-converting enzyme activity in human cerebral circulation. Neurosurgery. 2002;51:445–451. discussion 51–52. [PubMed] [Google Scholar]
- 16.Vatter H, Schilling L, Schmiedek P, et al. Evidence for functional endothelin-converting enzyme activity in isolated rat basilar artery: Effect of inhibitors. J Cardiovasc Pharmacol. 1998;31:S64–67. doi: 10.1097/00005344-199800001-00021. [DOI] [PubMed] [Google Scholar]
- 17.Patel TR, Galbraith S, McAuley MA, et al. Endothelin-mediated vascular tone following focal cerebral ischaemia in the cat. J Cereb Blood Flow Metab. 1996;16:679–687. doi: 10.1097/00004647-199607000-00019. [DOI] [PubMed] [Google Scholar]
- 18.Feger GI, Schilling L, Ehrenreich H, et al. Endothelin-induced contraction and relaxation of rat isolated basilar artery: Effect of BQ-123. J Cereb Blood Flow Metab. 1994;14:845–852. doi: 10.1038/jcbfm.1994.106. [DOI] [PubMed] [Google Scholar]
- 19.Vicentic A, Lakatos A, Kuhar MJ. CART (cocaine- and amphetamine-regulated transcript) peptide receptors: Specific binding in AtT20 cells. Eur J Pharmacol. 2005;528:188–189. doi: 10.1016/j.ejphar.2005.11.041. [DOI] [PubMed] [Google Scholar]
- 20.Lakatos A, Prinster S, Vicentic A, et al. Cocaine- and amphetamine-regulated transcript (CART) peptide activates the extracellular signal-regulated kinase (ERK) pathway in AtT20 cells via putative G-protein coupled receptors. Neurosci Lett. 2005;384:198–202. doi: 10.1016/j.neulet.2005.04.072. [DOI] [PubMed] [Google Scholar]
- 21.Dominguez G, Lakatos A, Kuhar MJ. Characterization of the cocaine- and amphetamine-regulated transcript (CART) peptide gene promoter and its activation by a cyclic AMP-dependent signaling pathway in GH3 cells. J Neurochem. 2002;80:885–893. doi: 10.1046/j.0022-3042.2002.00775.x. [DOI] [PubMed] [Google Scholar]
- 22.Douglass J, McKinzie AA, Couceyro P. PCR differential display identifies a rat brain mRNA that is transcriptionally regulated by cocaine and amphetamine. J Neurosci. 1995;15:2471–2481. doi: 10.1523/JNEUROSCI.15-03-02471.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daras M, Tuchman AJ, Marks S. Central nervous system infarction related to cocaine abuse. Stroke. 1991;22:1320–1325. doi: 10.1161/01.str.22.10.1320. [DOI] [PubMed] [Google Scholar]
- 24.Wang AM, Suojanen JN, Colucci VM, et al. Cocaine- and methamphetamine-induced acute cerebral vasospasm: An angiographic study in rabbits. AJNR Am J Neuroradiol. 1990;11:1141–1146. [PMC free article] [PubMed] [Google Scholar]
- 25.Fandino J, Sherman JD, Zuccarello M, et al. Cocaine-induced endothelin-1-dependent spasm in rabbit basilar artery in vivo. J Cardiovasc Pharmacol. 2003;41:158–161. doi: 10.1097/00005344-200302000-00002. [DOI] [PubMed] [Google Scholar]


