Abstract
Hypofractionated radiation therapy for prostate cancer has become of increasing interest with the recognition of a potential improvement in therapeutic ratio with treatments delivered in larger-sized fractions. In addition, the associated reduction in fraction number produces attractive cost and patient convenience advantages as well.
A still limited but growing number of hypofractionation trials have reported acceptable short-term levels of toxicity and biochemical control, but most have insufficient follow-up to assure the long-term safety and efficacy of this approach. This situation will improve as many currently active trials mature, particularly several high value randomized trials. In contrast, extreme hypofractionation, with schedules delivering only on the order of 5 fractions, is truly in its infancy for prostate cancer, with extremely limited tolerance and efficacy information currently available.
Several uncertainties in the radiobiology of hypofractionation mitigate for an organized, cautious investigational approach. The fractionation response (α/β ratio) of prostate cancers and, for that matter, late responding normal tissues, has yet to be rigorously defined. Additionally, the linear quadratic (LQ) model used in the design of hypofractionation schedules is subject to its own uncertainties, particularly with respect to the upper limit of fraction sizes for which it remains valid.
Contemporary dose escalated radiation therapy is already highly effective, making it imperative that ongoing and future studies of hypofractionation be carried out in carefully designed, randomized clinical trials. Clinical validation permitting, the adaptation of hypofractionation as a standard of care could profoundly influence future management of localized prostate cancer.
Introduction
There now is convincing evidence that biochemical control is improved with higher cumulative doses of radiation to the prostate. This dose escalation can be accomplished without undue complications by improving the physical delivery of the radiation using 3D conformal radiotherapy (3DCRT) or, better, intensity modulated radiation therapy (IMRT) techniques. However, an unusual prostate tumor radiobiology may allow a radically different approach to dose escalation that is radiobiological in nature. The unusual aspect of this radiobiology relates to prostate cancer’s suspected, uncharacteristically high sensitivity to large fractions of radiation.
Conventional fractionation schemes employing fraction sizes of 1.8–2.0 Gy are based upon the premise that tumors typically are less responsive to fraction size (have higher α/β ratios) than are late-responding normal tissues (with lower α/β ratios). An improved therapeutic ratio is therefore usually sought by using multiple, relatively small radiation fractions to leverage the lower α/β ratios that late responding normal tissues have relative to most classes of tumors.
In contrast, recent analyses and reviews of clinical tumor control data have argued for a low α/β ratio for prostate cancer on the order of 1–3 Gy, not the typical ratio of 8 Gy or higher that may apply to other tumors. This is also a lower value than is typically ascribed to the adjacent organs at risk, primarily bladder and rectum, putatively because the slow proliferation rate characteristic of most prostate tumors may be conducive to repair of radiation damage. This characteristic, if true, would imply a unique opportunity to improve the therapeutic ratio by treating prostate cancers with fewer but larger fractions of radiation, a hypofractionation approach.
This article will include: 1) a brief review of the evidence favoring or challenging an altered fractionation response for prostate cancer; 2) a description of the predicted impact of various hypofractionation approaches on tumor control and on normal tissue late toxicities; 3) a review of ongoing and completed clinical trials that employ hypofractionation; and 4) a description of ongoing treatment regimens that are extending the concept to extreme hypofractionation, a stereotactic body radiotherapy approach.
The opportunity will also be taken to emphasize that radiobiological uncertainties remain in the models applied to hypofractionation, uncertainties that necessitate caution and the need for well-defined clinical trials, particularly when extreme hypofractionation is being employed.
The Case for Hypofractionation
What is the fractionation response of prostate cancer?
Conventional fractionation schemes employing fraction sizes of 1.8–2.0 Gy are based upon the premise that tumors typically are less responsive to fraction size than are late-responding normal tissues. The α/β ratio is a measure of fractionation response, with low ratios (high α/β’s) associated with late responding normal tissues. A low α/β is consistent with a greater capacity for repair between fractions, with an accompanying greater relative sparing with small fraction sizes, than for tumors with their typically higher α/β ratios. Under these conditions, an improved therapeutic ratio is achieved with multiple small fractions for most types of tumors. The α/β ratios thought to be associated with tumors, however, are typically 8 or greater, whereas for late responding normal tissues, values on the order of 3 or 4 or somewhat less for CNS are suggested from the analyses of numerous experimental and some clinical outcome studies.
There appear to be exceptions to such typical tumor response to fractionation, however. Growth fraction (or effective cell cycle time) has often been associated with fractionation response, with slowly proliferating normal tissues (and some slowly proliferating tumors) generally displaying stronger than expected fraction size responses (low α/β ratios). This relationship has been demonstrated for melanomas1 and for some sarcomas2, for example. In the case of prostate cancer, there is ample evidence for slow proliferation, based both upon direct measurement of potential doubling times and labeling indices3 and upon analysis of the kinetics of rising PSA during tumor recurrence.4 Whether such is the case in all prostate cancers – those with high grade, for example, is not clear.
Recent analyses and reviews of clinical tumor response data do in fact argue for a low α/β ratio for prostate cancer.5,6 Brenner and Hall5, for example, analyzed dose response data for external beam radiation compared with I-125 brachytherapy data and estimated a very low α/β ratio of 1.5 Gy for prostate cancer. Duschene and Peters6 argued in analogous fashion that the α/β ratio for prostate cancer may be low and more similar to that expected for late responding normal tissue than for the typical, more rapidly proliferating tumor. These studies involved very simple calculational approaches comparing 145 Gy given at low dose rate to 70–74 Gy at fractionated high dose rates for patients with similar initial PSA levels and Gleason scores.
Fowler, Chappell and Ritter7 also conducted a comprehensive analysis of clinical outcome in patients treated with external beam radiotherapy only, I-125 implants only or Pd-103 implants only, in order to further test the above analyses. An α/β ratio for prostate cancer well below 2 Gy was also estimated.
Perhaps more convincing evidence for a low α/β is provided in the data of Martinez at al8. In this study, patients with prostate cancer were treated with a standard external beam course of treatment followed by high dose rate temporary implant boost doses which were escalated by decreasing fraction number from 3 to 2 and by increasing fraction size from 5.5 to 10.5 Gy. Patients were grouped according to prognostic factors and biochemical control was modeled versus equivalent dose, as calculated via a linear quadratic model. Higher biochemical control rates observed with escalation of hypofractionation were consistent with an α/β ratio of 1.2 (95% CI: 0.03, 4.1 Gy), again very low and in approximate agreement with values reported above using other estimation approaches. This study’s advantage was its ability to compare several high dose rate brachytherapy regimens that differed only in the radiation fraction size, rather than needing to compare two diverse treatments such as prostate seed brachytherapy and external beam radiotherapy. Its limitation was the uncertainty of accounting for the dose heterogeneity that is intrinsic to high dose rate brachytherapy, a limitation that that is present in the low dose rate implant analyses previously described as well.
Another study utilizing both external beam and brachytherapy data is that of Williams et al9, who used a proportional hazards model to estimate the α/β ratio from data on 3756 external beam and 185 high dose rate brachytherapy boost patients. An estimated ratio of 2.6 Gy was determined, but with a wide 95% confidence interval of 0.9 to 4.8 Gy. The limited range of external beam fraction sizes as well as patient and prescription dose heterogeneity restricted the precision with which the α/β ratio could be estimated. Other efforts to estimate the α/β ratio from published clinical outcomes of purely external beam studies will be described in detail later.
Challenges to the concept that α/β for prostate cancer is low
Several authors have expressed concern that potential problems with analyses like those above could lead to substantial uncertainties in the values derived for α/β Some of these concerns, as previously described, relate to potential uncertainties with respect to the variable dose distributions and dose rates that occur within implants – factors not expected to play as significant a role with external beam radiotherapy - as well as in potential differences in the effective radiation quality of implants versus external beam radiation.10 While these are certainly issues that warrant discussion, it is also true that when clinical parameters used in the modeling are restricted to reasonable ranges, an α/β ratio of less than 3 usually results. In fact, adjusting for these factors in the most straightforward fashion tends in some cases to actually reduce rather than increase the calculated value of α/β for prostate tumors.
The potential impact of tumor clonogen repopulation during the protracted delivery of permanent seed brachytherapy has also been discussed by Wang et al as a potential confounding factor in the derivation of the α/β ratio from brachytherapy data.11 In their analysis, if repopulation occurred during treatment, the resultant calculation for α/β would yield a value of about 3 instead of the value of 1.5 found otherwise. While a thoughtful analysis, a potential question arises from the authors’ use of clonogen repopulation initiation times of 0 or 28 days. While 28 days may be appropriate for a rapidly proliferating tumor such as head and neck, it is likely to be too early for prostate cancer. Experience in other tumor sites has suggested that tumor repopulation starts at some multiple, perhaps 4 – 5×, of the potential doubling time (ref[rdt1]). Given prostate cancer’s very long average potential doubling time of about 35 days3, a repopulation kick-off time in excess of 100 days appears more likely, at which point repopulation would no longer significantly influence an α/β calculation.
Another challenge to the estimate of a low α/β ratio has been raised by Nahem et al 12, who hypothesize that the presence of hypoxia-related radioresistance in prostate cancer could produce significant underestimates of the α/β ratio, values of 3 or less, for example, instead of actual values of 8 or higher, if hypoxia is not taken into account. Several potential weaknesses in this hypothesis have been discussed13, however, including its reliance on survival parameters derived from in vitro prostate cancer cell survival curves. In addition, the modeling tumor control probabilities with hypoxia can be unreliable, particularly given the potentially confounding factors of reoxygenation or inhomogeneities.13
With all information, both pro and con, taken into account, a low α/β ratio for prostate cancer remains an attractive hypothesis that is supported by several lines of evidence, but uncertainties clearly remain that will ultimately not be resolved until biochemical control data from large, preferably randomized hypofractionation studies with 5 or more years of follow-up become available. As presented later, such data are now beginning to emerge.
The Theoretical Potential for Hypofractionation to Improve Tumor Control
While conventional fractionation schemes employing small fraction sizes of 1.8–2.0 Gy are based upon the premise that tumors typically have high α/β ratios that make them less responsive to fraction size than are late-responding normal tissues, the situation may well be reversed for prostate cancer, favoring the use of hypofractionation. These relationships, at least over a range of fraction sizes between 1 and 6 Gy, can best be illustrated through use of the linear quadratic equation, which calculates the biologically effective dose, BED, for a given total dose, D, dose per fraction, d, and alpha-beta ratio, α/β:
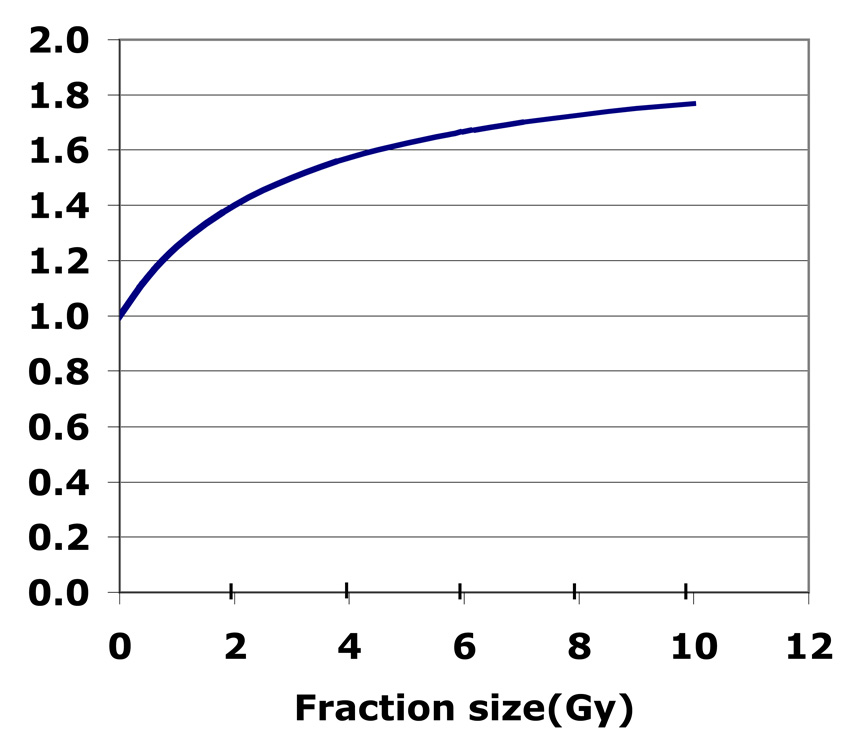
An α/β for tumor less than that for at-risk normal tissues predicts an improved therapeutic ratio with hypofractionation. For example, if the ratio of biologically effective dose at an α/β of 1.5 for tumor versus 3 for late tissue toxicity is taken as a form of therapeutic ratio, then this ratio, readily calculated using the linear quadratic equation, increases significantly with fraction size (Figure 1).
Figure 1.
The ratio of biologically effective dose for prostate tumor (α/β = 1.5) to normal tissue late effects (α/β = 3). This parameter could be regarded as a crude estimator of therapeutic ratio and is shown as a function of fraction size. The maximum theoretical ratio equals the ratio of the normal tissue to tumor α/βratio, which in this case is 2.
While the relationship is mathematically independent of total dose, the total dose would of course need to be appropriately limited to prevent undue toxicities.
If, on the other hand, α/β ratios for prostate cancer and late normal tissue damage were equal, there then would be neither gain nor loss in predicted therapeutic gain from hypofractionation, although improvements in convenience and efficiency could still be useful benefits.
Some controversy exists regarding the upper range of fraction sizes for which the linear quadratic model remains valid, with some support for it being applicable to very large fraction sizes, even 20–30 Gy14,15, particularly with modifications as needed to account for processes such as reoxygenation and redistribution16, but other analyses suggesting that, while the linear quadratic model is likely to be sufficiently accurate for fraction size ranges up to 6 or 7 Gy, application of the model to larger fraction sizes could lead to an under prediction of the total dose required to produce a given effect.17 Such an under prediction of total required dose, if acted upon, could lead to a less toxic but also less effective treatment.
In spite of these remaining uncertainties, however, there is sufficient supporting evidence to justify continuing to test the hypothesis that larger radiation fraction sizes will selectively increase prostate tumor cell kill relative to the induction of late effects, offering the promise of an improved therapeutic ratio with hypofractionation. Although many were planned before an advantageous response of prostate cancer to large fractions was suspected, a number of prostate hypofractionation trials have been carried out and information regarding the efficacy and safety of such an approach is emerging. Such completed and ongoing efforts will be detailed later.
How best can hypofractionation be explored in a clinical setting?
Two types of hypofractionation designs could be considered that would exploit the hypothesized radiobiological advantages described above:
Normal tissue de-escalation of total dose while maintaining constant predicted tumor control.
Tumor biological dose escalation with constant predicted normal tissue late effects.
These two hypofractionation approaches seek to achieve different desirable objectives:
Approach 1: Normal tissue de-escalation of total dose with constant tumor control
This approach starts with the premise that a certain well-tested 1.8 – 2 Gy per fraction scheme and treatment technique provides an acceptably high tumor control rate for a given group of patients, but, at the same time, produces an undesirable level of late complications, say grade II or higher late rectal bleeding. Assuming a tumor α/β of 1.5 and a late tissue α/β of 3, a schema of dose-per-fraction escalation can be proposed, with linear quadratic modeling, that would predict a reduction in side effects while maintaining the same level of tumor control (Figure 2).
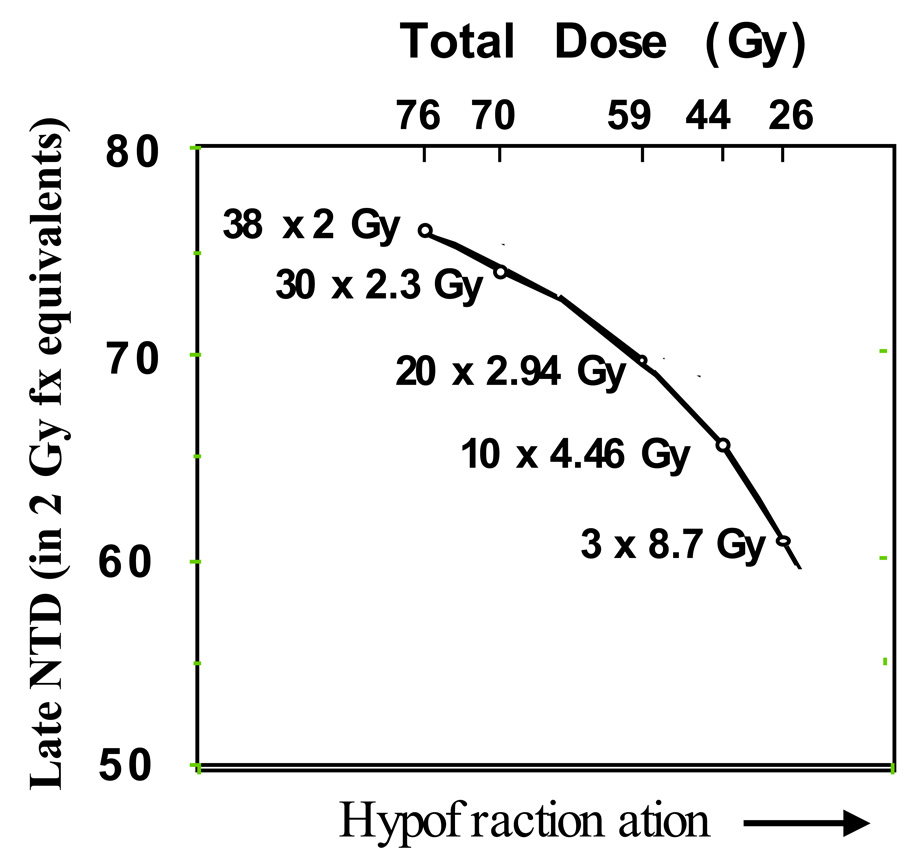
Figure 2.
An example of a dose-per-fraction escalation schemes that reduce the effective normal tissue dose for late effects while maintaining a constant predicted level of tumor control. The late normalized total dose (NTD) is the cumulative dose adjusted to its equivalent dose if delivered in 2 Gy fractions to late responding normal tissues. A tumor α/β of 1.5 Gy and a late tissue toxicity α/β of 3 Gy are assumed. The upper x-axis shows the actual cumulative dose delivered by each of the regimens.
For example, a hypofractionation step from 76 Gy in 38 fractions of 2 Gy each to 58.8 Gy in 20 fractions of 2.94 Gy each would predicts a late NTD dose (equivalent to a dose delivered in 2 Gy fractions) of only about 70 Gy without loss of tumor control (arrows). A potential advantage of such an approach would be that the late toxicity sparing achieved could allow use of a less sophisticated, less conformal treatment technique while attaining the same high level of uncomplicated tumor control. The actual outcome of a trial based upon this approach would of course be dependent on the accuracy of the assumed α/β ratios.
Approach 2: Tumor biological dose escalation with constant late effects
A potentially more interesting approach is to devise a fraction size escalation schedule that, as calculated using the linear quadratic equation, would predict a gain in tumor control while maintaining a constant, biologically effective Gy3 dose for late responding normal tissues (Figure 3).
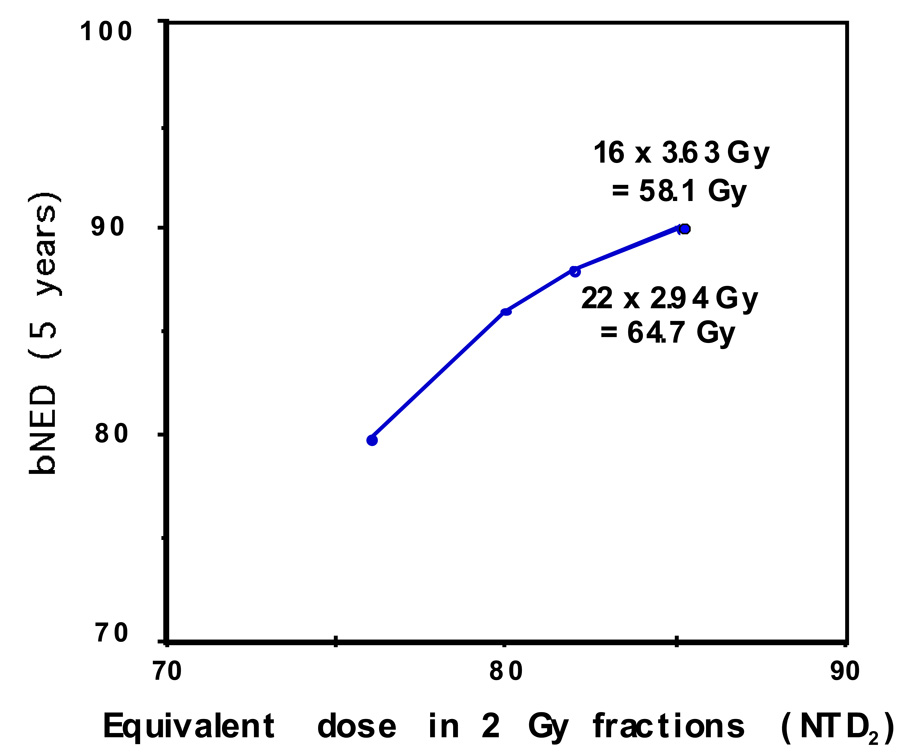
Figure 3.
Dose-per-fraction escalation regimens predicted to increase tumor control while maintaining constant late normal tissue toxicity. Biochemical freedom from disease (bNED) are estimated by calculating the equivalent doses if delivered in 2 Gy fractions (α/β = 1.5) and determining the corresponding bNED values from the biochemical control versus dose data in Fowler et al.18 Total dose delivered, are seen to decreases with increasing hypo-fractionation in order to maintain constant late effects.
Starting with an already dose escalated fractionation scheme of 38 × 2 Gy fractions and assuming a tumor α/β of 1.5, it is seen that the equivalent total dose normalized to 2 Gy fractions (NTD3) increases substantially with hypofractionation (x-axis) even as the actual total delivered dose decreases. The corresponding predicted tumor control probability, derived from Fowler et al 18, also increases. An α/β ratio for prostate cancer lower than that for normal tissues provides the basis for improving tumor control without increasing late effect risk. If tumor and normal tissue α/β’s were instead equal, tumor control would not improve with hypofractionation, but the cost and convenience benefit of delivering fewer fractions would remain. Of note, the 28 × 2.5 Gy schedule in figure 3 has been employed for some time by Cleveland Clinic investigators and has been extensively reported on.19,20 The recently opened, randomized RTOG trial - 0415 has adopted this regimen in its experimental, hypofractionation arm.
What is the Current State of Clinical Experience with Hypofractionation for Prostate Cancer?
Hypofractionated external beam radiotherapy has actually been used clinically for a number of years, particularly in the UK.21–23 While these treatments were generally well tolerated, overall efficacy is difficult to assess, given that these trials were carried out largely in the pre-PSA era. A number of more contemporary hypofractionation trials, either published or currently in progress, are shown in Table I. The most straightforward and intuitive way of estimating the predicted effectiveness and toxicities of these various approaches is to use the linear quadratic modeling to equate the various hypofractionation practices to the normalized equivalent dose (NED) if delivered in 2 Gy fractions. These are shown in Table 1 for assumed α/β of 1.5 and 3 for prostate cancer and late responding normal tissue, respectively. It is first of all apparent that, even for these only modestly hypofractionated schedules, normalized doses range between about 4 and 8 % higher for tumor than for normal tissue, illustrating the potential for therapeutic gain even with relatively modest hypofractionation should prostate cancer in fact have a lower α/β ratio than normal tissue.
Table 1.
Hypofractionation Trials: Schedules and Equivalent Total Doses in 2-Gy Fractions
| Fractionation (tot.dose/fx size/#fx) | Total Dose Equivalent if Given in 2-Gy Fractions (NTD2) | No. of PTS | Institution | References | |
|---|---|---|---|---|---|
| Alpha/Beta = 1.5 (tumor) | Alpha/Beta = 3 (late complications) | ||||
| 50 Gy/3.13 Gy/16 fx | 66 Gy | 61.3 Gy | 705 | Christie Hosp, Manchester | Livsey et al24 |
| 69 Gy/3 Gy/23 fx | 88.7 Gy | 82.8 Gy | 52 | Gunma, Japan | Akimoto et al25 |
| 66 GyE/3.3 GyE/20 fx (carbon ions) | 90.5 Gy | 83.1 Gy | 201 | NIRS, Chiba, Japan | Tsuji et al26 |
| 52.5 Gy/2.625 Gy/20 fx | 61.9 Gy | 59.1 Gy | 300 | Edenburgh | Higgins et al27 |
| 56 Gy/3.5 Gy/16 | 80 Gy | 72.8 Gy | 36 | Jette, Belgium | Soete et al28 |
| 60 Gy/3 Gy/20 fx | 77.2 Gy | 72 Gy | 92 | Princess Margaret | Martin et al29 |
| 70 Gy/2.5 Gy/28 fx | 80 Gy | 77 Gy | 770 | Cleveland Clinic | Kupelian et al[19] and [20] |
| 64.7 Gy/2.94 Gy/22 fx | 82.6 Gy | 77 Gy | 100 | Multi-institutional trial | Ritter et al30 |
| 58.1 Gy/3.63 Gy/16 fx | 85.1 Gy | 77 Gy | 100 | ||
| 51.6 Gy/4.3 Gy/12 fx | 85.5 Gy | 75 Gy | 58 (accruing) | ||
| 52.5/2.625 Gy/20 fx | 61.9 Gy | 59.1 Gy | 466 | NCI-Canada (phase III) | Lukka et al31 |
| 66 Gy/2 Gy/33 fx | 66 Gy | 66 Gy | 470 | ||
| 55 Gy/2.75 Gy/20 fx | 66.8 Gy | 63.2 Gy | 108 | Adelaid (phase III) | Yeoh et al32 |
| 64 Gy/2 Gy/32 fx | 64 Gy | 64 Gy | 109 | ||
| 70.2 Gy/2.7 Gy/26 fx | 84.2 Gy | 80 Gy | 150 | Fox Chase (phase III) | Pollack et al33 |
| 76 Gy/2 Gy/38 fx | 76 Gy | 76 Gy | 150 | ||
| 70 Gy/2.5 Gy/28 fx | 80 Gy | 77 Gy | Ongoing (goal of 1,067 pts) | RTOG 0415(phase III) | www.rtog.org/members/protocols/0415/0415.pdf |
| 73.8 Gy/1.8 Gy/41 fx | 69.6 Gy | 70.8 Gy | |||
| 57 Gy/3 Gy/19 fx | 73.3 Gy | 68.4 Gy | Ongoing (goal of 2,100 pts) | MRC (phase III) | Khoo et al34 |
| 60 Gy/3 Gy/20 fx | 77.2 Gy | 72 Gy | |||
Of those above trials that are contemporary, are completed and are reported, only the Princess Margaret29, Cleveland Clinic20, Manchester24, NCI-Canada31 and Chiba carbon ion26 trials have sufficient numbers of patients and sufficient albeit still relatively short follow-up to enable preliminary estimates of biochemical control and toxicity. Of these five, only the Princess Margaret, Cleveland Clinic and Chiba carbon ion trials deliver equivalent doses sufficiently large enough (assuming α/β = 1.5) to currently be considered adequately dose escalated. Additionally, only these three trials included sufficient patient numbers and follow-up to adequately estimate late toxicity.
Reported toxicity in these three trials was quite acceptable, with the actuarial RTOG Grade ≥ 2 late rectal and genitourinary toxicity rates being only 2% and 2%, 4.5% and 5.3%, and 1% and 1% for the Princess Margaret, Cleveland Clinic and Chiba trials, respectively. Efficacy also has appeared to be satisfactory. An analysis of biochemical control in the Cleveland clinic trial yielded a 5 years overall rate of 82%, equivalent to or better than previously attained with the institutional standard of 78 Gy in 2 Gy fractions.20 The Chiba trial found an overall biochemical control rate of 83% at 5 years.
The Manchester study24 used a schedule delivering lower equivalent doses and accordingly reported low late toxicities and relatively poor biochemical control rates, consistent with results expected for a “conventional” doses of only 66 Gy in 2 Gy fractions. Similarly, the NCI-Canada trial employed a schedule equivalent to only about 62 Gy in 2 Gy fractions.31 Biochemical control rates and toxicities were also correspondingly low. In spite of the low equivalent doses these two trials delivered, comparisons of their reported outcomes can enable a useful testing of hypofractionation modeling. The Princess Margaret and Cleveland Clinic studies, with their higher delivered equivalent doses, are valuable for such testing as well.
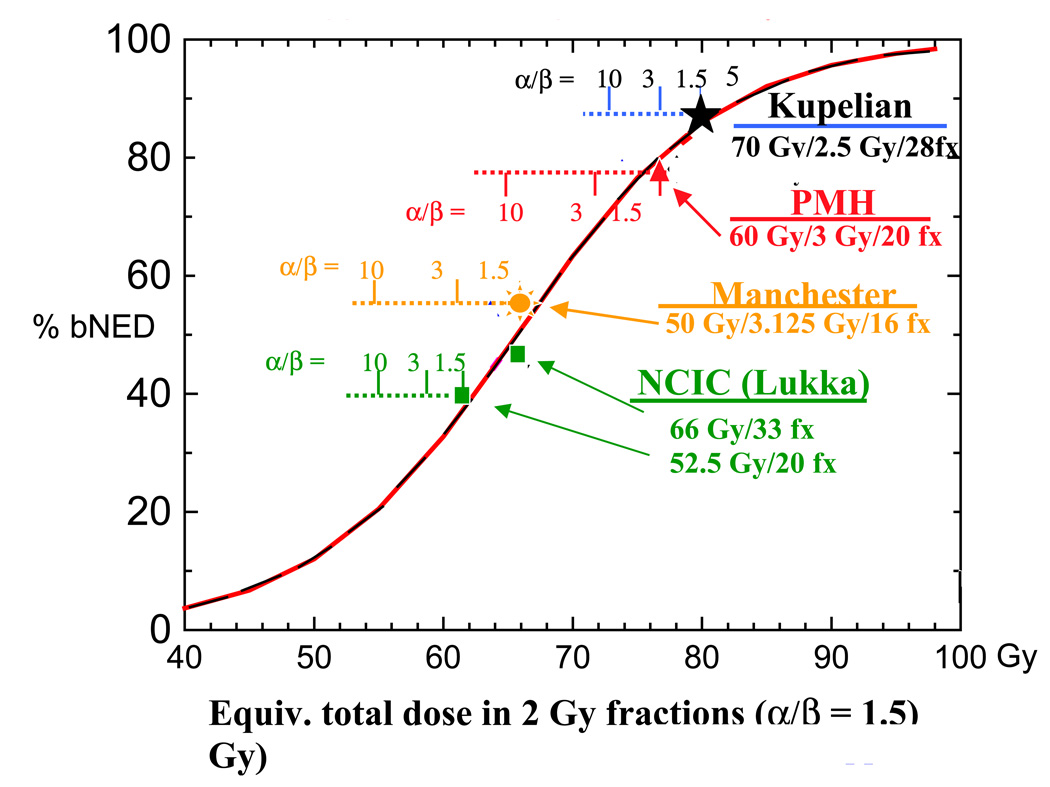
A graphical depiction of biochemical control rates versus equivalent dose from these four studies (for intermediate risk prostate cancer, when specifically reported) is presented in Figure 4. Represented are 3–5 year actuarial bDFS rates using the ASTRO definition, the only definition uniformly available in these reports. The solid line dose response curve for radiation delivered in 2 Gy fractions is adapted from Fowler et al 18 and is based upon 5 year biochemical control data for intermediate risk patients from 5 conventionally fractionated prostate cancer trials. Biochemical control points from the hypofractionation trials are plotted relative to their equivalent dose for three assumed α/β ratios of 1.5, 3 and 10. The ratio of 1.5 clearly produces the closest fit to the curve. While many unaccounted for variables render this a non-rigorous and strictly post hoc comparison, the degree of outcomes agreement between hypofractionated and conventional regimens when an α/β of 1.5 is chosen intriguingly suggests that prostate cancer response is indeed characterized by a low α/β ratio.
Figure 4.
Biochemical disease-free survival (bDFS) rates versus equivalent doses from four hypofractionation studies identified in Table 1 (for intermediate risk prostate cancer, when separately reported in the publications). Shown are 3–5 year actuarial bDFS rates using the ASTRO definition. The solid line dose response curve for radiation delivered in 2 Gy fractions is adapted from Fowler et al18 and is based upon 3–5 year biochemical control data for intermediate risk patients from 5 conventionally fractionated prostate cancer trials. Biochemical control points from the hypofractionation trials are plotted relative to their equivalent dose for three assumed α/β ratios of 1.5, 3 and 10.
A more formalized analysis by Bentzen and Ritter35 of one of these trials, the NCI-Canada study31, again but more rigorously yields a quite low α/β ratio estimate for prostate of 1.12 Gy with 95% confidence interval (−3.3, 5.6) Gy.
The final non-randomized study listed in Table 1 is a multi-institutional trial (University of Wisconsin, M.D. Anderson-Orlando, Wayne State University, Medical college of Wisconsin and JT Vucurevich Cancer Inst., Rapid City) and is a phase I/II study30 that escalates dose per fraction in three steps, with late rectal bleeding the escalation-limiting factor. The design results in predicted late effects expected to remain relatively constant (at a level consistent with about 76 Gy delivered in 2 Gy fractions) even as fraction size escalates. The trial design also includes a nested fractions-per-week escalation/de-escalation to monitor for and prevent unacceptable acute toxicities that might result from too extreme a shortening of treatment duration. Overly severe acute toxicities not only create intratreatment morbidity, but might also lead to so-called consequential late injuries in adjacent organs such as rectum or bladder.18
Preliminary results from this phase I/II trial30 have indicated acceptably low rates of GI and GU toxicity (2 years grade 2 GI and GU toxicity rates of 8.8 and 3%, respectively, even after five-day-per-week treatment, and, also, preliminary biochemical control rates that are high and in the expected range. The trial is nearing completion with 258 of a target 300 patients accrued. When mature, its three increasingly hypofractionated schedules will be evaluable in terms of their suitability for future trials and, in addition, collected and centrally analyzed dose-volume data together with the trial’s three significantly different doses per fraction should permit solid estimates to be made of α/β ratios both for prostate cancer as well as for adjacent organs at risk.
Thus, the outcomes of several hypofractionation trials support the hypothesis that the α/β ratio for prostate cancer is low and that further investigation via prospective, preferably randomized clinical trials is fully warranted. As is also evident from Table 1, several such randomized trials that deliver suitably high effective doses have either recently completed accrual or are currently underway. Thus the prospects for a comprehensive evaluation of this potentially clinically advantageous, cost effective and convenient treatment approach appear excellent. Ideally, these or future trials will also adequately collect delivered rather than planned dose-volume information, enabling the most accurate analysis of the fractionation response of prostate cancer and normal tissue. The methodology to do so has become available and its use should be encouraged.
Extreme Hypofractionation
Even fewer fractions are beginning to be employed in some clinical practices, although not always in the context of prospective trials. Called stereotactic body radiotherapy (SBRT) when only five total fractions are used, fraction sizes of between about 5.5 and 7 Gy are typically given, although higher dose fractions have been used as well. While this further reduction in fraction number might seem a logical extension of current, more modestly hypofractionated studies, there are numerous reasons why such an approach is even more in need of the monitoring and reporting guidelines that are integral to clinical trials. First, definitive results using more modest hypofractionation, while encouraging, are not yet mature. Also, unlike SBRT as used in treating lung cancer, for example, the intent of prostate SBRT is not ablative, a restriction related to the urethra’s intraprostatic location. More moderate but still large fraction sizes in the 5–7 Gy range or somewhat higher are therefore considered, but uncertainties remain over the validity of the linear quadratic model or variations thereof for predicting biological effectiveness of these higher fraction sizes. Radiation damage mechanisms could change with increasing fraction size, rendering predictions from such models unreliable.
Furthermore, the potential tumor control enhancing contributions of reoxygenation and redistribution could diminish as the number of fractions decreases and the duration of treatment shortens. In addition, the demand for treatment delivery accuracy surely intensifies with the delivery of fewer fractions, making it imperative that quality control issues such as immobilization, target motion and image guidance be given considerable attention. Only prospective trials can adequately address these requirements and ensure that patient safety and proper documentation of outcomes are provided for.
There are several studies of extreme hypofractionation, however, that are being carried out appropriately in prospective fashion. Four are listed in Table 2, of which only the Virginia Mason trial has been completed36 and has sufficient follow-up (median 48 months) to allow meaningful reporting. Reported outcomes were acceptable: Actuarial late GU and GI grade 2 toxicities at 48 months were 16.1 and 9.4% respectively, while the actuarial freedom from biochemical relapse at 48 months was 90% (nadir plus 2). The University of Toronto phase I/II trial has only reported on 30 patients and only on acute toxicities, which were acceptably low. The UTSW study listed in Table 2, is a recently initiated, ongoing phase I/II study notable for the higher dose fractions it employs, specifically 5 fractions of either 9.0, 9.5 or 10 Gy each. Were standard linear quadratic modeling to remain valid at these much larger fraction sizes, these regimens could produce very high tumor and normal tissue equivalent doses of 135 and 108 Gy, respectively, although modified linear quadratic models17 would predict significantly lower equivalent doses. Furthermore, normal tissue volume restrictions in place during treatment planning could reduce the potential for toxicity from these high dose fractions as well.
Table 2.
Phase I/II Ultrahypofractionation Trials: Schedules and Equivalent Total Doses in 2-Gy Fractions
| Fractionation (tot.does/fx size/#fx) | Total Dose Equivalent in 2Gy Fractions (NTD2) | No. of PTS | Institution | References | |
|---|---|---|---|---|---|
| Alpha/Beta = 1.5 (tumor) | Alpha/Beta = 3 (late effects) | ||||
| 33.5 Gy/6.7 Gy/5 fx | 78 Gy | 64.9 Gy | 40 | Virginia Mason | Madsen et al36 |
| 36.25 Gy/7.25 Gy/5 fx | 90.6 Gy | 74.3 Gy | 23 (ongoing) | Stanford | Pawlicki et al37 |
| 42.7 Gy/6.1 Gy/7 fx | 92.7 Gy | 77.7 Gy | 105 | Umea | Widmark (personal communication, 2008) |
| 35 Gy/7 Gy/5 fx | 85.1 Gy | 70 Gy | 30 (ongoing) | University of Toronto | Tang et al38 |
| 47.5 Gy/9.5 Gy/5 fx | 149 Gy* | 118 Gy | 15 | UTSW, Dallas | Timmerman (personal communication, 2008) |
| 50 Gy/10 Gy/5 fx | 164 Gy | 130 Gy | 10 (ongoing) | ||
| 52.5 Gy/10.5 Gy/5 fx | 180 Gy | 142 Gy | – | ||
NTD doses based on linear-quadratic modeling may overpredict NTDs for large fractions, as in the UTSW trial.
Conclusions
Results from several mostly phase I/II, prostate radiotherapy studies have indicated a gain in therapeutic ratio with increases in radiation fraction size beyond the 1.8 to 2 Gy typical of standard practice. However, given that uncertainties exist in extrapolating biological effects to larger fractions and that dose-escalated radiation therapy with standard fractionation is already highly effective, it is imperative that ongoing and future studies of hypofractionation be carried out in a randomized controlled trial setting. Ideally, delivered rather than planned dose-volume information would be recorded in such studies to maximize the quality of fractionation response information obtained.
Several large, randomized trials are underway or are completed and awaiting sufficient follow-up for analysis and it is studies such as these that will ultimately define the utility of prostate hypofractionation. Hypofractionation has the potential for significant therapeutic gain as well as economic and logistic advantage. Should the approach be clinically validated, its acceptance as a standard of care will profoundly influence the future management of localized prostate cancer.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bentzen SM, Overgaard J, Thames HD, et al. Clinical radiobiology of malignant melanoma. Radiother Oncol. 1989;16:169–182. doi: 10.1016/0167-8140(89)90017-0. [DOI] [PubMed] [Google Scholar]
- 2.Thames HD, Suit HD. Tumor radioresponsiveness versus fractionation sensitivity. Int J Radiat Oncol Biol Phys. 1986;12:687–691. doi: 10.1016/0360-3016(86)90081-7. [DOI] [PubMed] [Google Scholar]
- 3.Haustermans KM, Hofland I, VP H, et al. Cell kinetic measurements in prostate cancer. Int J Radiat Oncol Biol Phys. 1997;37:1067–1070. doi: 10.1016/s0360-3016(96)00579-2. [DOI] [PubMed] [Google Scholar]
- 4.Pollack A, Zagars GK, Kavadi VS. Prostate specific antigen doubling time and disease relapse after radiotherapy for prostate cancer. Cancer. 1994;74:670–678. doi: 10.1002/1097-0142(19940715)74:2<670::aid-cncr2820740220>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 5.Brenner DJ, Hall EJ. Fractionation and protraction for radiotherapy of prostate carcinoma. Int J Radiat Oncol Biol Phys. 1999;43:1095–1101. doi: 10.1016/s0360-3016(98)00438-6. [DOI] [PubMed] [Google Scholar]
- 6.Duchesne GM, Peters LJ. What is the alpha/beta ratio for prostate cancer? Rationale for hypofractionated high-dose-rate brachytherapy [editorial] Int J Radiat Oncol Biol Phys. 1999;44:747–748. doi: 10.1016/s0360-3016(99)00024-3. [DOI] [PubMed] [Google Scholar]
- 7.Fowler J, Chappell R, Ritter M. Is alpha/beta for prostate tumors really low? Int J Radiat Oncol Biol Phys. 2001;50:1021–1031. doi: 10.1016/s0360-3016(01)01607-8. [DOI] [PubMed] [Google Scholar]
- 8.Brenner DJ, Martinez AA, Edmundson GK, et al. Direct evidence that prostate tumors show high sensitivity to fractionation (low alpha/beta ratio), similar to late-responding normal tissue. Int J Radiat Oncol Biol Phys. 2002;52:6–13. doi: 10.1016/s0360-3016(01)02664-5. [DOI] [PubMed] [Google Scholar]
- 9.Williams SG, Taylor JM, Liu N, et al. Use of individual fraction size data from 3756 patients to directly determine the alpha/beta ratio of prostate cancer. Int J Radiat Oncol Biol Phys. 2007;68:24–33. doi: 10.1016/j.ijrobp.2006.12.036. [DOI] [PubMed] [Google Scholar]
- 10.D’Souza WD, Thames HD. Is the a/b ratio for prostate cancer low? Int J Radiat Biol Oncol Phys. 2001;51:1–3. doi: 10.1016/s0360-3016(01)01650-9. [DOI] [PubMed] [Google Scholar]
- 11.Wang JZ, Guerrero M, Li XA. How low is the alpha/beta ratio for prostate cancer? Int J Radiat Oncol Biol Phys. 2003;55:194–203. doi: 10.1016/s0360-3016(02)03828-2. [DOI] [PubMed] [Google Scholar]
- 12.Nahum AE, Movsas B, Horwitz EM, et al. Incorporating clinical measurements of hypoxia into tumor local control modeling of prostate cancer: implications for the alpha/beta ratio. Int J Radiat Oncol Biol Phys. 2003;57:391–401. doi: 10.1016/s0360-3016(03)00534-0. [DOI] [PubMed] [Google Scholar]
- 13.Fowler JF, Nahum AE, Orton CG. Point/Counterpoint. The best radiotherapy for the treatment of prostate cancer involves hypofractionation. Med Phys. 2006;33:3081–3084. doi: 10.1118/1.2179008. [DOI] [PubMed] [Google Scholar]
- 14.Douglas BG, Fowler JF. The effect of multiple small doses of x rays on skin reactions in the mouse and a basic interpretation. Radiat Res. 1976;66:401–426. [PubMed] [Google Scholar]
- 15.Hall EJ, Brenner DJ. Extrapolating hypofractionated radiation schemes from radiosurgery data: regarding Hall et al., IJROBP 21:819-824; 1991 and Hall and Brenner, IJROBP 25:381-385; 1993. Int J Radiat Oncol Biol Phys. 1995;32:275–276. doi: 10.1016/0360-3016(95)91425-R. [DOI] [PubMed] [Google Scholar]
- 16.Brenner DJ, Hlatky LR, Hahnfeldt PJ, et al. A convenient extension of the linear-quadratic model to include redistribution and reoxygenation. Int J Radiat Oncol Biol Phys. 1995;32:379–390. doi: 10.1016/0360-3016(95)00544-9. [DOI] [PubMed] [Google Scholar]
- 17.Guerrero M, Li XA. Extending the linear-quadratic model for large fraction doses pertinent to stereotactic radiotherapy. Phys Med Biol. 2004;49:4825–4835. doi: 10.1088/0031-9155/49/20/012. [DOI] [PubMed] [Google Scholar]
- 18.Fowler JF, Ritter MA, Chappell RJ, et al. What hypofractionated protocols should be tested for prostate cancer? Int J Radiat Oncol Biol Phys. 2003;56:1093–1104. doi: 10.1016/s0360-3016(03)00132-9. [DOI] [PubMed] [Google Scholar]
- 19.Kupelian PA, Reddy CA, Carlson TP, et al. Preliminary observations on biochemical relapse-free survival rates after short-course intensity-modulated radiotherapy (70 Gy at 2.5 Gy/fraction) for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2002;53:904–912. doi: 10.1016/s0360-3016(02)02836-5. [DOI] [PubMed] [Google Scholar]
- 20.Kupelian PA, Willoughby TR, Reddy CA, et al. Hypofractionated intensity-modulated radiotherapy (70 Gy at 2.5 Gy per fraction) for localized prostate cancer: Cleveland Clinic experience. Int J Radiat Oncol Biol Phys. 2007;68:1424–1430. doi: 10.1016/j.ijrobp.2007.01.067. [DOI] [PubMed] [Google Scholar]
- 21.Collins CD, Lloyd-Davies RW, Swan AV. Radical external beam radiotherapy for localized carcinoma of the prostate using a hypofractionation technique. Clin Oncol (R Coll Radiol) 1991;3:127–132. doi: 10.1016/s0936-6555(05)80831-3. [DOI] [PubMed] [Google Scholar]
- 22.Duncan W, Warde P, Catton CN, et al. Carcinoma of the prostate: results of radical radiotherapy (1970–1985) Int J Radiat Oncol Biol Phys. 1993;26:203–210. doi: 10.1016/0360-3016(93)90198-5. [DOI] [PubMed] [Google Scholar]
- 23.Read G, Pointon RC. Retrospective study of radiotherapy in early carcinoma of the prostate. Br J Urol. 1989;63:191–195. doi: 10.1111/j.1464-410x.1989.tb05163.x. [DOI] [PubMed] [Google Scholar]
- 24.Livsey JE, Cowan RA, Wylie JP, et al. Hypofractionated conformal radiotherapy in carcinoma of the prostate: five-year outcome analysis. Int J Radiat Oncol Biol Phys. 2003;57:1254–1259. doi: 10.1016/s0360-3016(03)00752-1. [DOI] [PubMed] [Google Scholar]
- 25.Akimoto T, Muramatsu H, Takahashi M, et al. Rectal bleeding after hypofractionated radiotherapy for prostate cancer: correlation between clinical and dosimetric parameters and the incidence of grade 2 or worse rectal bleeding. Int J Radiat Oncol Biol Phys. 2004;60:1033–1039. doi: 10.1016/j.ijrobp.2004.07.695. [DOI] [PubMed] [Google Scholar]
- 26.Tsuji H, Yanagi T, Ishikawa H, et al. Hypofractionated radiotherapy with carbon ion beams for prostate cancer. Int J Radiat Oncol Biol Phys. 2005;63:1153–1160. doi: 10.1016/j.ijrobp.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 27.Higgins GS, McLaren DB, Kerr GR, et al. Outcome analysis of 300 prostate cancer patients treated with neoadjuvant androgen deprivation and hypofractionated radiotherapy. Int J Radiat Oncol Biol Phys. 2006;65:982–989. doi: 10.1016/j.ijrobp.2006.02.029. [DOI] [PubMed] [Google Scholar]
- 28.Soete G, Arcangeli S, De Meerleer G, et al. Phase II study of a four-week hypofractionated external beam radiotherapy regimen for prostate cancer: report on acute toxicity. Radiother Oncol. 2006;80:78–81. doi: 10.1016/j.radonc.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 29.Martin JM, Rosewall T, Bayley A, et al. Phase II trial of hypofractionated image-guided intensity-modulated radiotherapy for localized prostate adenocarcinoma. Int J Radiat Oncol Biol Phys. 2007;69:1084–1089. doi: 10.1016/j.ijrobp.2007.04.049. [DOI] [PubMed] [Google Scholar]
- 30.Ritter MA, Forman JD, Kupelian PA, et al. A phase I/II trial of dose-per-fraction escalation for prostate cancer. Int J Radiat Oncol Biol Phys. 2007;69:S174. [Google Scholar]
- 31.Lukka H, Hayter C, Julian JA, et al. Randomized trial comparing two fractionation schedules for patients with localized prostate cancer. J Clin Oncol. 2005;23:6132–6138. doi: 10.1200/JCO.2005.06.153. [DOI] [PubMed] [Google Scholar]
- 32.Yeoh EE, Holloway RH, Fraser RJ, et al. Hypofractionated versus conventionally fractionated radiation therapy for prostate carcinoma: updated results of a phase III randomized trial. Int J Radiat Oncol Biol Phys. 2006;66:1072–1083. doi: 10.1016/j.ijrobp.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 33.Pollack A, Hanlon AL, Horwitz EM, et al. Dosimetry and preliminary acute toxicity in the first 100 men treated for prostate cancer on a randomized hypofractionation dose escalation trial. Int J Radiat Oncol Biol Phys. 2006;64:518–526. doi: 10.1016/j.ijrobp.2005.07.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khoo VS, Dearnaley DP. Question of Dose, Fractionation and Technique: Ingredients for Testing Hypofractionation in Prostate Cancer - the CHHiP Trial. Clin Oncol (R Coll Radiol) 2007 doi: 10.1016/j.clon.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 35.Bentzen SM, Ritter MA. The alpha/beta ratio for prostate cancer: what is it, really? Radiother Oncol. 2005;76:1–3. doi: 10.1016/j.radonc.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 36.Madsen BL, Hsi RA, Pham HT, et al. Stereotactic hypofractionated accurate radiotherapy of the prostate (SHARP), 33.5 Gy in five fractions for localized disease: first clinical trial results. Int J Radiat Oncol Biol Phys. 2007;67:1099–1105. doi: 10.1016/j.ijrobp.2006.10.050. [DOI] [PubMed] [Google Scholar]
- 37.Pawlicki T, Cotrutz C, King C. Prostate cancer therapy with stereotactic body radiation therapy. Front Radiat Ther Oncol. 2007;40:395–406. doi: 10.1159/000106049. [DOI] [PubMed] [Google Scholar]
- 38.Tang C, Loblaw DA, Cheung P, et al. Early results of pHART3. San Francisco, CA: Genitourinary Cancer Symposium; 2008. Condensing external beam radiotherapy to five fractions for low-risk localized prostate cancer; p. 148a. [Google Scholar]