Abstract
Although a critical role of COX-2 in bone repair has been established, the mechanism involved remains unclear. During early inflammatory phase of bone healing, COX-2 is produced by the surrounding inflammatory cells as well as bone/cartilage progenitors. Based on the temporal and spatial expression of COX-2 during the early phase of fracture healing, we hypothesize that COX-2 from both sources is critical for progenitor cell activation, proliferation and differentiation. To directly test this we utilized a murine femoral grafting model, in which live segmental grafts from the same strains were transplanted and donor versus host cell involvement in healing was assessed. Specifically, fresh femur cortical bone grafts of 4-mm in length from COX-2-/- (KO) mice were transplanted into wild type (WT) mice with the same sized segmental defect in femurs. Similarly, grafts from WT were transplanted into the defects in KO mice. As controls, transplantations between wild types, and transplantations between KO were also performed. Histologic analyses showed that WT-to-WT transplantation resulted in normal endochondral bone healing as evidenced by markedly induction of neovascularization and periosteal bone formation on donor graft. In contrast, transplantation of KO graft into KO host led to 96 % reduction of bone formation and near elimination of donor cell-initiated periosteal bone formation. Similarly, transplantation of WT graft into a KO host resulted in 87% reduction of bone formation (n=8, p>0.05), indicating that KO host impaired WT donor progenitor cell expansion and differentiation. When a KO graft was transplanted into WT host, KO donor periosteal cell-initiated endochondral bone formation was restored. Histomorphometric analyses demonstrated 10-fold increase in bone formation and 3-fold increase in cartilage formation compared to KO-to-KO transplantation (n=8, p<0.05), suggesting that COX-2 deficient donor cells were capable to differentiate and form bone when placed in a WT host. Taken together, our data strongly suggest that COX-2 is critical for initiation of periosteal cortical bone healing. The early induction of COX-2 constitutes a crucial host-healing environment for activation and differentiation of donor periosteal progenitors. Elimination of COX-2 at the early stage of healing could lead to detrimental effects on periosteal progenitor cell-initiated cortical bone repair.
Keywords: cyclooxygenase-2 (COX-2), periosteum, bone graft transplantation
Introduction
Periosteum contains a population of stem/progenitor cells that play key roles in cortical bone repair (1, 26, 28, 35). The activation and initiation of periosteal progenitor proliferation and differentiation at the time of cortical injury are not well understood, although, it has been suggested that mechanical stability and an early inflammatory response may play key roles (8, 9, 21). Following cortical bone fracture or osteotomy, the local progenitor cells residing in periosteum are activated, enabling them to respond to biological or biophysical stimuli produced within the local injury milieu. During the first few days of fracture, hematoma is formed followed by infiltration of inflammatory cells and release of growth factors. These factors direct the recruitment and activation of progenitor cells and initiate a cascade of cellular responses that eventually leads to endochondral and intramembraneous bone formation along the surface of the cortical bone (3, 10).
To better understand the origins of the bone forming cells and the critical factors responsible for cortical bone healing, we establish a live segmental bone graft transplantation model in mice that permits molecular and quantitative analyses of periosteal bone repair. Using live femoral isograft harvested from Rosa 26A-LacZ mice, we showed that 70% of bone and cartilage formation overlying the graft were derived from periosteal stem/progenitor cells residing on the donor graft. Removal of periosteum effectively eliminated donor periosteal bone formation and further impaired donor graft integration into host bone (18, 33, 39). This model could be further utilized to better understand the nature and origin of the factors that direct the expansion and differentiation of these periosteal progenitor cells during the early phases of cortical bone healing. The purpose of the current study is to use this established bone graft transplantation model to understand the role of COX-2 in the initiation of cortical bone healing.
COX-2 is the inducible isoform of cyclooxygenase, the key rate-limiting enzyme in the prostaglandin biosynthesis pathway (15, 32). COX-2 and its metabolite prostaglandins are important modulators of inflammatory response following tissue injury. COX-2 is also involved in diseases associated with dysregulated inflammatory conditions, such as rheumatoid and osteoarthritis, cardiovascular disease, and the carcinogenesis process (19, 34). Previous data from our laboratory as well as others demonstrate that fracture healing is markedly delayed and impaired in COX-2-/- mice (30, 38). The defective fracture healing phenotype is characterized by marked reduction of bone formation, persistence of undifferentiated mesenchyme and residual cartilaginous tissue in the callus. Further studies show that COX-2 is markedly induced during initiation stage of fracture healing, and its mRNA is localized around periosteal envelop in both inflammatory cells as well as in early chondroprogenitors (25). Based on the patterns of expression of COX-2 in fracture callus, we hypothesize that COX-2 functions as a critical periosteal signals targeting periosteal progenitors for efficient initiation of endochondral and intramembraneous healing.
To directly test the role of COX-2 in the initiation of periosteal healing, we utilize the bone graft transplantation model. We took the advantage of the live bone isograft transplantation model that allows a mix-and-match approach to examine the behavior of WT or COX-2-/- donor periosteal progenitors in a WT or COX-2 deficient host injury milieu during the early phase of healing. Our data demonstrates that COX-2 is essential for initiation of periosteal healing. COX-2 and its metabolites produced at the early inflammatory phase of healing constitute a crucial host injury milieu for activation and differentiation of donor periosteal progenitor cells. Transplanting WT graft into the COX-2-deficient injury milieu resulted in minimal periosteal bone formation and high incidence of fibrotic non-union. On the contrary, transplanting a COX-2-/- graft into WT host markedly suppressed fibrotic response and restored periosteal endochondral bone formation.
Materials and Methods
Experimental animals and materials
COX-2-/- mice were originally obtained from the breeding colony maintained at the University of North Carolina. They were further bred to 129/ola genetic background and intercrossed for about 30 generations. In all experiments, WT littermates were used as controls for COX-2-/- mice. All animal surgery procedures were approved by the University of Rochester Committee of Animal Resources (UCAR).
Bone graft transplantation
Live bone graft transplantation was performed as previously described (39). Briefly, ten-week-old COX-2-/- mice and their WT littermates were anesthetized by peritoneal injection of a mix of 10mg/ml Ketamine and 1mg/ml Xylazine. A 4 mm mid-diaphyseal segment was removed from the femur of the COX-2-/- mice or WT mice using an angled scissors. The graft was carefully dissected to remove the muscles without compromising the periosteum and immediately transplanted into COX-2-/- or WT mice with the same size defect (live bone graft transplantation). The bone graft was stabilized using a 25G metal pin placed through intramedullary marrow cavity. Four groups of bone grafting were performed: WT donor to WT host (WT-to-WT), COX-2-/- donor to COX-2-/- host (KO-to-KO), WT donor to COX-2-/- host (WT-to-KO), and COX-2-/- donor to WT host (KO-to-WT). The grafted femurs were processed for histological and microCT analyses at the end time points of the experiments. Eight animals were used in each group of transplantations (n=8).
Real Time PCR analyses for gene expression during bone graft healing
WT mice were anesthetized and a 4 mm mid-diaphyseal femoral segment was resected free of muscle by osteotomy. The bone segment were immediately placed into the defect in the same mice and stabilized by a 25G metal pin placed through intramedullary marrow cavity (autograft transplantation). Our previous publications have demonstrated an identical healing dynamics between live autograft healing and isograft healing (33, 39). For RNA analyses, mice were placed in groups of four (n=4) and sacrificed on day 0, 3, 5, 7, 10 and 14 following surgery. Autograft and the surrounding calluses were carefully dissected free of muscles and soft tissue and immediately snap frozen in a liquid nitrogen bath. Frozen tissue samples were homogenized using a liquid nitrogen-cooled mortar and pestle apparatus, and mRNA was purified via phase separation (TRIzol, Invitrogen, Carlsbad, CA). Exactly 0.5 μg of mRNA per callus was pooled and used in reverse transcription to make single-strand cDNA. Single-strand cDNA was synthesized using a commercial first strand cDNA synthesis kit (Invitrogen). qPCR reaction was performed using SyberGreen (ABgene, Rochester, NY) in a RotorGene real time PCR machine (Corbett Research, Carlsbad, CA). All genes were compared to a standard β-actin control. Data were assessed quantitatively using ANOVA comparing relative levels of transcript expression as a function of time. The following primers were used for the
| Genes | Primer-1 (Forward) | Primer-2 (Reverse) |
|---|---|---|
| Actin | 5′-CCC CAC TGA AGC CTA CAA AA-3 | 5′-GGG AGG CTC CTC CAT TC-3′ |
| OC | 5′ TGC TTG TGA CGA GCT ATC AG-3′ | 5′-GAG GAC AGG GAG GAT CAA GT-3′ |
| col-2 | 5′-CCA CAC CAA ATT CCT GTT CA-3′ | 5′-ACT GGT AAG TGG GGC AAG AC-3′ |
| col-X | 5′-CTT TGT GTG CCT TTC AAT CG-3′ | 5′-GTG AGG TAC AGC CTA CCA GTT TT-3′ |
| COX-2 | 5′-CAC AGC CTA CCA AAA CAG CCA-3′ | 5′-GCT CAG TTG AAC GCC TTT TGA-3′ |
assessment:
Histological and Histomorphometric analysis
Following sacrifice, the grafted femurs were harvested, fixed in 10% neutral buffered formalin, decalcified in 10% EDTA. Paraffin embedded sections were prepared and stained with H&E / alcian blue (38). Histomorphometric analyses were performed using Osteometrics™ software to determine the area of bone, cartilage and fibrotic tissue formation on the host and graft side. A hypothetical line was drawn in the middle of the distal or proximal junctions between graft and host bone to separate the callus into the graft side callus and the host side callus. Areas of bone, cartilage and fibrotic tissue were traced in computer program and percent area was used for analyses. At least three non-consecutive sections were used for histomorphometric analyses and a mean of three represents one sample. At least 8 samples were included in each group of transplantation. The mean from 8 samples was used in statistical analyses to determine the composition of the callus on host side and graft side.
Immunohistochemical staining for COX-2
Immunohistochemistry was performed as previously described (24, 37). Sections were deparaffinized using xylene and rehydrated using graded alcohols. The endogenous peroxidase was quenched using 3% hydrogen peroxide for 20 minutes. Non-specific binding epitopes were blocked using 1:20 normal goat serum. Slides were incubated at 4°C overnight with a 1:200 dilution of mouse primary COX-2 antibody (Cayman Chemical, Ann Arbor, MI). Sections were incubated with a 1:200 Goat Anti-Rabbit secondary antibody, rinsed again in PBS, and finally incubated with a 1:250 HRP Streptavidin for 30 minutes. Sections were counterstained with hematoxylin and rinsed.
MicroCT Imaging analyses
Femurs were harvested at indicated times and scanned using a Viva microCT system (Scanco Medical, Bassersdorf, Switzerland) at a voxel size of 10.5 μm to image bone or vasculature. New bone formation and vascular volume were measured as previously described (6, 7). Briefly, a lead chromate-based contrast agent, Microfil, was used to perfuse the mice via heart along with 4% paraformaldehyde. Samples were scanned before and after decalcification in order to examine bone and vessel formation in the same sample. Using proprietary software provided by Scanco, the periosteal external calluses were contoured and examined for bone volume and vascularization. To measure the vasculature, contour lines were drawn in the 2D slice images to exclude the cortical bone and the vessels in the surrounding tissues (39). New bone or vessel volume in a region of interest covering the entire length of bone graft and 1mm of the host bone at both distal and proximal bone graft junctions were used to evaluate graft healing.
Statistical analysis
Data are expressed as a mean value plus or minus the standard error of the mean. Statistical significance between experimental groups was determined using one-way ANOVA and a Tukey’s posthoc test. A P value <0.05 was considered statistically significant. Data analysis was performed using GraphPad Prism version 5.0 (GraphPad Software, San Diego, CA).
Results
COX-2-deficient injury milieu impairs periosteal progenitor cell-dependent initiation and progression of endochondral bone repair
Live bone isograft transplantation between WT and COX-2-/- (KO) mice was performed as described in the Materials and Methods. Histologic analyses demonstrated abundant new bone formation along the surface of the graft in WT to WT transplantations (Figure 1A). About 78% of the cortical junctions were bridged by either bone or cartilage 17 days after graft transplantation, similar to our previous observation using isografts from R26A mice (33, 39). Quantitative histomorphometric analyses were performed to evaluate the percent area of bone, cartilage, or fibrotic tissue on the donor graft as previously described (38, 39) (Figure 2A). WT-to-WT transplantation resulted in near completion of endochondral bone healing on day 17 post-surgery, as evidenced by markedly induction of periosteal bone formation with less than 10% of cartilage and fibrotic tissue remaining in the callus.
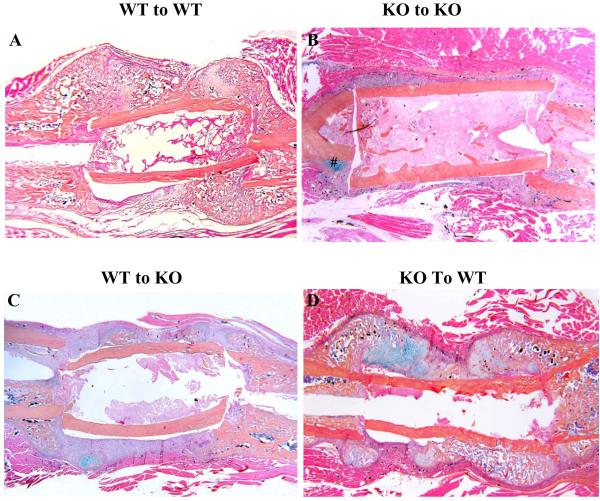
Figure 1. Initiation of donor bone graft healing is impaired in COX-2-/- host.
Femoral bone graft transplantations were performed between WT and COX-2-/- mice as described in the Materials and Methods. Samples were harvested on day 17 post-surgery. Representative alcian blue/hematoxylin sections of femoral bone graft transplantations between WT and COX-2-/- mice are shown: WT graft to WT host (A), KO graft to KO host (B), WT graft to KO host (C), KO graft to WT host (D). Of note is the nearly elimination of donor side bone and cartilage formation in KO-to-KO or WT-to-KO transplantations. When KO graft was transplanted into WT host, donor side bone and cartilage formation was restored.
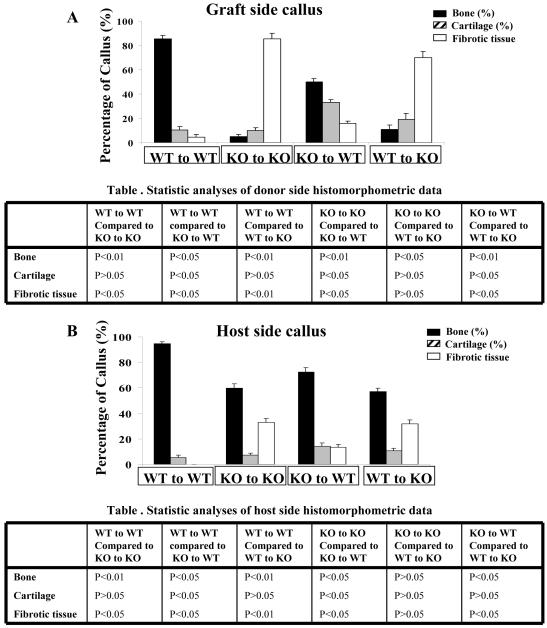
Figure 2. Histomorphometric analyses of bone graft healing in WT and COX-2-/- mice.
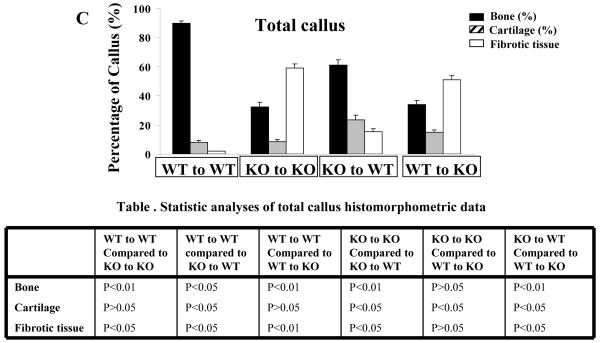
Femoral bone graft transplantations were performed between WT and COX-2-/- mice. Samples were harvested on day 17 post-surgery. Histomorphometric analyses of callus were performed on histologic sections prepared from four groups of transplantations as described in the Materials and Methods. Each group consists at least 8 transplants. The mean percent area of bone (solid bar), cartilage (hatched bar) and fibrotic tissue (empty bar) on the graft side (A), host side (B) and combined host and donor sites (C) are shown. A summary of statistical analyses is included in a separate table below each graph.
In contrast to WT-to-WT transplantation, KO-to-KO transplantation (autograft or isograft) led to fibrotic nonunion at 96% of the cortical junctions (Figure 1B). Minimal periosteal bone formation was observed along the surface of the graft, indicating the nearly elimination of donor periosteal progenitor cell-initiated chondrogenesis and osteogenesis. Although bone formation was found on the host bone, only 4% of the cortical junctions reached union by day 17 primarily due to the lack of bone formation on the KO donor bone graft. Histomorphometric analyses showed that KO-to-KO transplantation resulted in 96% reduction of periosteal bone formation along the surface of the graft (n=8, p<0.05). And over 90% of the callus was occupied by fibrotic tissues (Figure 2A).
When WT donor graft was transplanted into a KO host, similar reduction of bone formation on donor graft surface was observed (Figure 1C). Although WT donor graft displayed a small increase of bone formation in the KO host compared to KO to KO transplantation, about 90% of the cortical junctions remained non-united 17 days following grafting. Large amount of fibrotic tissues were found at the cortical junctions and along bone graft surface, similar to KO-to-KO transplantation. Histomorphometric analyses (Figure 2A) demonstrated that WT-to-KO transplantation resulted in about 2-fold increase of bone formation on the graft compared to KO-to-KO transplantations (n=8, p<0.05). Despite the increase in bone and cartilage formation, fibrotic tissue was predominant in the callus and about 80% of the callus was occupied by fibrotic tissues (p<0.01).
In contrast to WT-to-KO transplantation, transplantation of KO graft into WT host resulted in marked recovery of periosteal bone and cartilage formation on donor graft. About 64% of cortical bone junctions were bridged by bone or cartilaginous tissue on day 17, along with marked reduction of fibrotic tissue on bone graft (Figure 1D). Histomorphometric analyses showed that KO-to-WT transplantation led to 10-fold increase of bone formation and 3-fold increase of cartilage formation compared to KO-to-KO transplantation (n=8, p<0.01), indicating a rescue of KO donor progenitor cells in a WT host (Figure 3A). In KO-to-WT transplantation, we noticed that the percent of cartilage on KO graft remained 3 fold higher than WT graft (p<0.05), suggesting increased chondrogenesis but decreased conversion of cartilage to bone in the KO-to-WT transplantations.
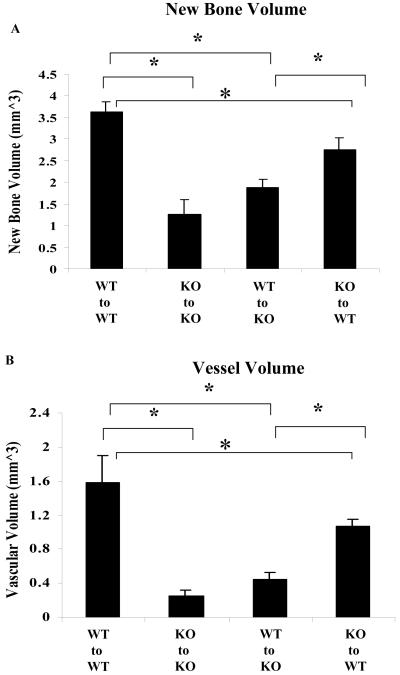
Figure 3. Quantitative analyses of bone and vasculature during graft healing.
Femoral bone graft transplantations were performed between WT and COX-2-/- mice as described in the Materials and Methods. Samples were harvested on day 17. Quantitative microCT analyses of new bone (A) and vasculature (B) on donor bone graft are shown. Data shown are shown as mean ± SEM, * (P<0.05, n=8).
Histomorphometric analyses were also performed to examine the host side bone and cartilage formation as described in the Materials and Methods. Smaller but similar differences in bone and cartilage formation were found on the host side indicating that the donor graft had a smaller influence on the host-side bone formation (Figure 2B). This observation is consistent with our previous finding that shows a localized donor cell-dependent bone/cartilage formation overlying donor bone graft (39). Notably, the fibrotic tissues on the host side remained higher in the COX-2-/- mice, suggesting that COX-2 deficient host injury milieu significant impaired cortical bone union.
If combining histomorphometric data from host and graft (Figure 2C), we were able to compare the composition of total callus among four groups of transplantations. Remarkably, we found that composition of the total callus was similar to that of the graft side callus as shown in Figure 2A, indicating that graft side callus can be used as a good indicator for evaluation of cortical bone graft healing.
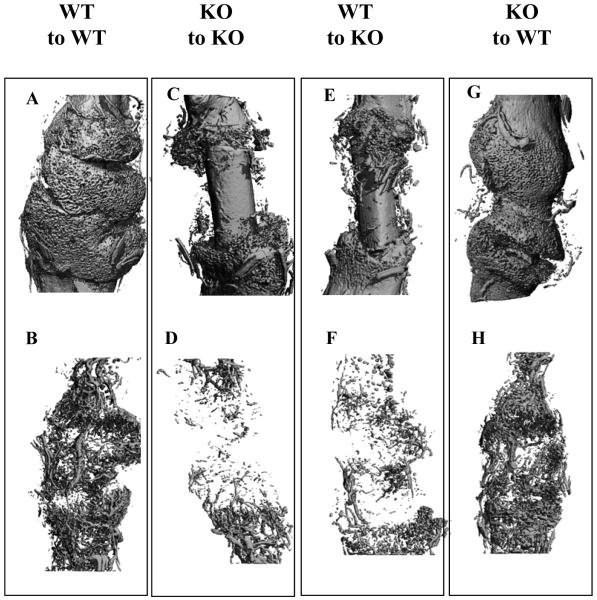
MicroCT 3D reconstructions of grafted femurs further confirmed the histologic findings (Figure 3 and 4). Quantitative analyses of total bone and vascular volume at the cortical junction showed that new bone was reduced by about 3-fold in KO-to-KO transplantations compared to WT-to-WT transplantation (n=8, p<0.01), whereas the total vessel volume was reduced by 5-fold in the same group (Figure 5A, B) (n=8, p<0.01). Transplantation of WT grafts into KO hosts increased bone formation by about 30% when compared to KO-to-KO transplantation (n=8, p>0.05). On the other hand, transplantation of KO graft into WT host restored 76% of new bone and 78% of new vessel formation, respectively (Figure 3 A and B) (p<0.01, n=8). The representative microCT images from four groups of transplantations showed marked differences among WT and KO mice. Transplantation of WT graft to KO host resulted in smaller improvement in bone and vessel formation. In contrast, transplantation of KO graft to WT host resulted in rescue of donor dependent bone and vessel formation.
Figure 4. Representative MicroCT imaging of bone graft transplantation in WT and KO mice.
Femoral bone graft transplantations were performed between WT and COX-2-/- mice as described in the Materials and Methods. Samples are harvested on day 17. MicroCT images of bone (A, C, E, G) and vasculature (B, D, F, H) of grafted femurs at day 17 post-surgery are shown (A-H). Four different transplantations were carried out: WT graft to WT host (A, B), KO graft to KO host (C, D), WT graft to KO host (E, F), KO graft to WT host (G, H).
Figure 5. Induction of COX-2 during bone autograft healing.
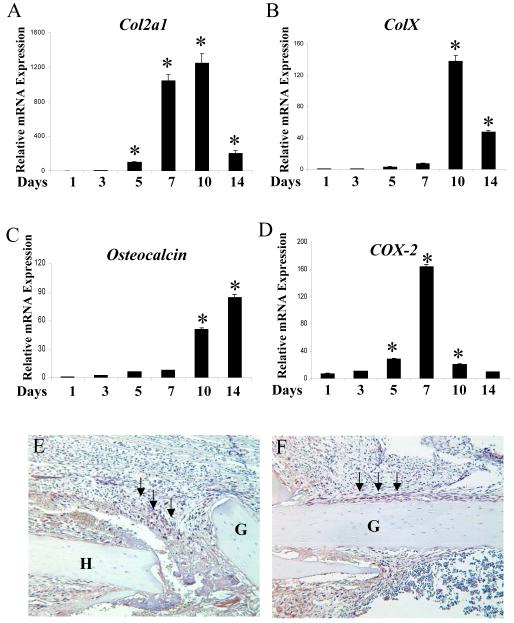
To determine the timing of COX-2 expression, total RNA was harvested from the donor bone graft at day 0, 3, 5, 7, 10, 14 following surgery in WT mice as described in the Materials and Methods. Real-time RT-PCR analyses were performed to examine the expression of Col2a1 (A), ColX (B), Osteocalcin (C) and COX-2 (D) gene expression during donor graft healing. COX-2 expression is significantly induced at day 5, 7 and 10, correlating with Col2a1 expression (*, n=4, p<0.05). To determine the location of COX-2 during early phase of healing, immunostaining for COX-2 was performed in samples harvested on day 3 (E) and day 5 (F). Strong staining of COX-2 was localized in infiltrating inflammatory cells at the cortical bone junction (arrows in E) as well as in periosteal progenitor cells on donor graft surface (arrows in F). G indicates donor graft. H indicates host bone.
COX-2 expression during bone autograft healing
The defective healing in COX-2-/- host indicates that COX-2 is required at the initiation stage of healing. To further determine the timing and localization of COX-2 expression during graft healing, we performed both real time RT-PCR analyses and immunohistochemical staining using samples prepared from autograft transplantation. RNA analyses showed that Col2a1 gene expression was induced on day 5 and peaked on day 7 and 10. ColX gene followed and peaked at day 10. By day 14, expressions of both Col2a1 and ColX were markedly reduced whereas Osteocalcin gene expression was marked induced, indicating the conversion of cartilage to bone (Figure 5A, B, C). COX-2 mRNA expression was markedly induced during early chondrogenic phase of healing at day 5 and 7. The expression declined during the phase of chondrocyte maturation and bone formation (Figure 5D), remarkably similar to our previous finding in fracture callus (25). Since RNA analyses only included the tissues on the donor grafts but not host tissue, we therefore performed further immunohistochemical staining to determine the localization of COX-2 protein in the surrounding soft tissue, particularly at the early stage of healing. As presented in Figure 5E and F, COX-2 specific staining was found as early as day 3 in autografting tissue sections. Strong COX-2 staining was found in infiltrating cells at the cortical bone junction surrounding periosteum, particularly in cells around the hematoma (arrows in Figure 5E). In addition to infiltrating cells, we also found strong expression of COX-2 in periosteal progenitor cells at day 5 (arrows in Figure 5F), corroborating with the results from the RNA analyses.
Discussion
The goal of this current study is to examine the role of COX-2 in the initiation of cortical bone healing. We took the advantage of a segmental bone graft transplantation model, which allows a mix-and-match approach to examine the phenotype of WT or COX-2-/- donor periosteal progenitors in a WT or COX-2 deficient host injury environment during the early phase of healing. Our data provides strong evidence to show that COX-2, produced by infiltrating inflammatory cells and early periosteal progenitors, functions as an essential modulator in host injury milieu directing the efficient initiation of cortical bone healing.
COX-2 is critically implicated in fracture healing (30, 38). In COX-2-/- mice, fracture healing is delayed due to impaired mesenchyme differentiation and delayed transition of cartilage to bone. High incidence of non-union is found in these mice due to impaired chondrogenesis and osteogenesis. Compared to fracture healing, segmental bone graft transplantation represents a more challenging endochondral bone repair model, in which a large critical defect must be repaired. As expected, when graft transplantation was performed in the KO mice, a more severe phenotype was observed. Histologic analyses showed that donor progenitor cell-initiated bone formation was nearly abolished in KO-to-KO transplantation. As a result, more than 90% of the cortical bone junctions failed to reach histological union in COX-2 knockout host as compared to only 22% in WT-to-WT transplantation. These results underscore the importance of COX-2 in cortical bone healing and provide strong evidence to show that COX-2 plays a key role during the initiation phase of cortical bone healing.
To further determine the host vs. donor contribution to the healing, we performed graft transplantation between WT and KO mice. This approach allows mutant progenitor cells to be placed in a WT host or vise versa. Interestingly, our data demonstrated that when a live WT graft with intact periosteum was transplanted into COX-2-/- mice, donor periosteal progenitor cell initiated chondrogenesis and bone formation were abolished. On the contrary, when a COX-2-/- graft was placed into WT mice, donor periosteum initiated bone formation and angiogenesis were markedly enhanced. These data strongly suggest that host plays a predominant role in activation and initiation of periosteal endochondral and intramembraneous bone formation. COX-2, likely functions through its metabolites such as prostaglandins, is required in early host injury milieu for effective initiation of chondrogenesis and osteoblastogenesis.
The early blockade of progenitor cell differentiation in the COX-2-/- host suggests that COX-2 produced during inflammatory phase of healing by host infiltrating cells and progenitors plays a key role. RT-PCR analyses showed that COX-2 was induced as early as day 5 and peaked on day 7, paralleled with Col2a1 expression. Using immunohistochemical staining for COX-2, we were able to localize COX-2 protein in infiltrating inflammatory cells at the cortical bone junction as early as day 3. More intensified staining was found in the cells surrounding the hematoma. In addition to sporadic staining in soft tissue callus, we also found intense staining of COX-2 in chondroprogenitor cells at day 5, which correlated with the induction of COX-2 mRNA expression. These data corroborate with our previous finding in fracture healing model, in which COX-2 was found in infiltrating cells as well as in chondroprogenitors and proliferating chondrocytes in the early fracture callus (25). The localization of COX-2 in early infiltrating cells and mesenchymal progenitors during inflammatory phase of healing show that COX-2 plays a key role in modulating early inflammatory response and coordinating the activation and initiation of progenitor cell-dependent endochondral and intramembraneous bone formation.
The high percentage of fibrotic tissue and high incidence of fibrotic nonunion in COX-2-/- mice indicate a potential role of COX-2 in modulating fibrotic tissue formation and inflammatory response during early stage of bone healing. Experiments transplanting Rosa-LacZ mice into COX-2-/- mice showed that the fibrotic tissues surrounding donor bone graft was derived from the host (data not shown) suggesting that COX-2 produced from the surrounding inflammatory tissues in the host is required for suppression of fibrotic tissue formation during healing. PGE2 has been known to suppress the growth of fibroblasts (4). COX-2-/- mice develop fibrosis in multiple organs (5, 23). In COX-2-/- mice, fibrotic response following lung injury is increased accompanied by an enhanced and persistent inflammatory response to injury (17). The studies using COX-2-/- mice further show that COX-2 derived prostaglandins promote early microphage infiltration but also restrain the inflammatory response at the late stage thereby are involved in the resolution of exacerbated inflammation (5, 13, 20, 22). Additionally, one recent study show that COX-2 is involved in modulating early inflammatory response during muscle healing by counteracts TGF-β induced fibrotic response (29). Based on these findings, we deduce that COX-2 plays an intricate role modulating the inflammatory response during the initiation of bone healing. On one hand, COX-2/PGE2 stimulates bone progenitor cell proliferation and differentiation. On the other hand, COX-2/PGE2 suppresses fibrotic response associated with injury and healing. Lack of COX-2 in the host disrupts the intricate balance in host injury milieu, resulting in detrimental effects on activation and differentiation of donor periosteal stem/progenitor cells. Restoration of balanced host injury milieu re-establishes the initiation of donor graft healing and repair.
Aside from deficiency in the initiation of graft healing, careful histomorphometric studies by quantifying percent bone and cartilage formation in graft transplantation experiments also revealed that COX-2-/- donor graft displayed a delayed mineralization and neovascularization in a wild type host, similar to what we have observed in the COX-2-/- fracture callus (30, 38). Interestingly, the delayed cartilage ossification was incompletely restored in wild type host (KO-to-WT transplantation), as evidenced by higher percentage of cartilage tissue on the bone graft and relatively lower vessel volume when compared to WT-to-WT transplantation. This data argue that COX-2 and its metabolites play a role in endochondral ossification and neovascularization in donor chondrocytes, presumably through an autocrine mechanism of prostaglandins. The effect of COX-2 on cartilage tissue turnover may involve a cell autonomous mechanism that cannot be completely corrected by WT host environment. Further studies are needed to address the mechanisms involved in cartilage turnover and neovascularization.
Induction of COX-2 is the hallmark of inflammatory response following tissue injury (36). Anti-inflammatory drugs NSAIDs suppress production of prostaglandins via suppression of COX-2. NSAIDs have been shown to delay or impair skeletal healing in human and animal models (2, 12, 14, 16). Several recent studies further show that COX-2 inhibitor when administered at the inflammatory phase significantly perturbs fracture healing in rats (11, 31). Previous study from our laboratory, in which we used a similar allograft transplantation model in mice, have demonstrated that the use of NSAIDs, including inhibitor of COX-2 at the early stage of healing leads to increased incidence of nonunions (27). Using COX-2-/- mice, our current study provides further evidence for a critical role of COX-2 in the early inflammatory phase of healing. Taken together, our data argue against the use of high dose of NSAIDs or COX-2 inhibitors during the initiation stage of skeletal repair and reconstruction.
In summary, we have demonstrated that COX-2 in injury milieu is critical for initiation of donor bone graft healing. In the absence of COX-2, the activation, proliferation and differentiation of donor periosteal progenitor cells are markedly impaired, leading to high incidence of fibrotic nonunion in bone graft transplantation. This study suggests that suppression of COX-2 at the initiation stage of healing could lead to negative effects on bone healing and repair.
Acknowledgements
This study is supported by grants from the Musculoskeletal Transplant Foundation, the National Institute of Health (AR051469, AR46545, P50AR054041). We thank Krista Canary and Laura Yanoso for their assistance with histological work and microCT analyses and Kimberly Napoli for her assistance in preparing this manuscript.
References
- [1].Allen MR, Hock JM, Burr DB. Periosteum: biology, regulation, and response to osteoporosis therapies. Bone. 2004;35:1003–12. doi: 10.1016/j.bone.2004.07.014. [DOI] [PubMed] [Google Scholar]
- [2].Altman RD, Latta LL, Keer R, Renfree K, Hornicek FJ, Banovac K. Effect of nonsteroidal antiinflammatory drugs on fracture healing: a laboratory study in rats. J Orthop Trauma. 1995;9:392–400. doi: 10.1097/00005131-199505000-00006. [DOI] [PubMed] [Google Scholar]
- [3].Barnes GL, Kostenuik PJ, Gerstenfeld LC, Einhorn TA. Growth factor regulation of fracture repair. J Bone Miner Res. 1999;14:1805–15. doi: 10.1359/jbmr.1999.14.11.1805. [DOI] [PubMed] [Google Scholar]
- [4].Clark JG, Kostal KM, Marino BA. Modulation of collagen production following bleomycin-induced pulmonary fibrosis in hamsters. Presence of a factor in lung that increases fibroblast prostaglandin E2 and cAMP and suppresses fibroblast proliferation and collagen production. J Biol Chem. 1982;257:8098–105. [PubMed] [Google Scholar]
- [5].Dinchuk JE, Car BD, Focht RJ, Johnston JJ, Jaffee BD, Covington MB, Contel NR, Eng VM, Collins RJ, Czerniak PM, et al. Renal abnormalities and an altered inflammatory response in mice lacking cyclooxygenase II. Nature. 1995;378:406–9. doi: 10.1038/378406a0. [DOI] [PubMed] [Google Scholar]
- [6].Duvall CL, Robert Taylor W, Weiss D, Guldberg RE. Quantitative microcomputed tomography analysis of collateral vessel development after ischemic injury. Am J Physiol Heart Circ Physiol. 2004;287:H302–10. doi: 10.1152/ajpheart.00928.2003. [DOI] [PubMed] [Google Scholar]
- [7].Duvall CL, Taylor WR, Weiss D, Wojtowicz AM, Guldberg RE. Impaired angiogenesis, early callus formation, and late stage remodeling in fracture healing of osteopontin-deficient mice. J Bone Miner Res. 2007;22:286–97. doi: 10.1359/jbmr.061103. [DOI] [PubMed] [Google Scholar]
- [8].Frost HM. The biology of fracture healing. An overview for clinicians. Part I. Clin Orthop Relat Res. 1989;248:283–93. [PubMed] [Google Scholar]
- [9].Frost HM. The biology of fracture healing. An overview for clinicians. Part II. Clin Orthop Relat Res. 1989;248:294–309. [PubMed] [Google Scholar]
- [10].Gerstenfeld LC, Cullinane DM, Barnes GL, Graves DT, Einhorn TA. Fracture healing as a post-natal developmental process: molecular, spatial, and temporal aspects of its regulation. J Cell Biochem. 2003;88:873–84. doi: 10.1002/jcb.10435. [DOI] [PubMed] [Google Scholar]
- [11].Gerstenfeld LC, Thiede M, Seibert K, Mielke C, Phippard D, Svagr B, Cullinane D, Einhorn TA. Differential inhibition of fracture healing by non-selective and cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs. J Orthop Res. 2003;21:670–5. doi: 10.1016/S0736-0266(03)00003-2. [DOI] [PubMed] [Google Scholar]
- [12].Giannoudis PV, MacDonald DA, Matthews SJ, Smith RM, Furlong AJ, De Boer P. Nonunion of the femoral diaphysis. The influence of reaming and non-steroidal anti-inflammatory drugs. J Bone Joint Surg Br. 2000;82:655–8. doi: 10.1302/0301-620x.82b5.9899. [DOI] [PubMed] [Google Scholar]
- [13].Gilroy DW, Colville-Nash PR, Willis D, Chivers J, Paul-Clark MJ, Willoughby DA. Inducible cyclooxygenase may have anti-inflammatory properties. Nat Med. 1999;5:698–701. doi: 10.1038/9550. [DOI] [PubMed] [Google Scholar]
- [14].Goodman SB. Use of COX-2 specific inhibitors in operative and nonoperative management of patients with arthritis. Orthopedics. 2000;23:S765–8. doi: 10.3928/0147-7447-20000702-07. [DOI] [PubMed] [Google Scholar]
- [15].Herschman HR. Prostaglandin synthase 2. Biochem. Biophys. Acta. 1996;1229:125–140. doi: 10.1016/0005-2760(95)00194-8. [DOI] [PubMed] [Google Scholar]
- [16].Ho ML, Chang JK, Chuang LY, Hsu HK, Wang GJ. Effects of nonsteroidal anti-inflammatory drugs and prostaglandins on osteoblastic functions. Biochem Pharmacol. 1999;58:983–90. doi: 10.1016/s0006-2952(99)00186-0. [DOI] [PubMed] [Google Scholar]
- [17].Hodges RJ, Jenkins RG, Wheeler-Jones CP, Copeman DM, Bottoms SE, Bellingan GJ, Nanthakumar CB, Laurent GJ, Hart SL, Foster ML, McAnulty RJ. Severity of lung injury in cyclooxygenase-2-deficient mice is dependent on reduced prostaglandin E(2) production. Am J Pathol. 2004;165:1663–76. doi: 10.1016/S0002-9440(10)63423-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ito H, Koefoed M, Tiyapatanaputi P, Gromov K, Goater JJ, Carmouche J, Zhang X, Rubery PT, Rabinowitz J, Samulski RJ, Nakamura T, Soballe K, O’Keefe RJ, Boyce BF, Schwarz EM. Remodeling of cortical bone allografts mediated by adherent rAAV-RANKL and VEGF gene therapy. Nat Med. 2005;11:291–7. doi: 10.1038/nm1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kapoor M, Shaw O, Appleton I. Possible anti-inflammatory role of COX-2-derived prostaglandins: implications for inflammation research. Curr Opin Investig Drugs. 2005;6:461–6. [PubMed] [Google Scholar]
- [20].Langenbach R, Loftin C, Lee C, Tiano H. Cyclooxygenase knockout mice: models for elucidating isoform-specific functions. Biochem Pharmacol. 1999;58:1237–46. doi: 10.1016/s0006-2952(99)00158-6. [DOI] [PubMed] [Google Scholar]
- [21].Le AX, Miclau T, Hu D, Helms JA. Molecular aspects of healing in stabilized and non-stabilized fractures. J Orthop Res. 2001;19:78–84. doi: 10.1016/S0736-0266(00)00006-1. [DOI] [PubMed] [Google Scholar]
- [22].Mizuno H, Sakamoto C, Matsuda K, Wada K, Uchida T, Noguchi H, Akamatsu T, Kasuga M. Induction of cyclooxygenase 2 in gastric mucosal lesions and its inhibition by the specific antagonist delays healing in mice. Gastroenterology. 1997;112:387–97. doi: 10.1053/gast.1997.v112.pm9024292. [DOI] [PubMed] [Google Scholar]
- [23].Morham SG, Langenbach R, Loftin CD, Tiano HF, Vouloumanos N, Jennette JC, Mahler JF, Kluckman KD, Ledford A, Lee CA, et al. Prostaglandin synthase 2 gene disruption causes severe renal pathology in the mouse. Cell. 1995;83:473–82. doi: 10.1016/0092-8674(95)90125-6. [DOI] [PubMed] [Google Scholar]
- [24].Mungo DV, Zhang X, O’Keefe RJ, Rosier RN, Puzas JE, Schwarz EM. COX-1 and COX-2 expression in osteoid osteomas. J Orthop Res. 2002;20:159–62. doi: 10.1016/S0736-0266(01)00065-1. [DOI] [PubMed] [Google Scholar]
- [25].Naik A, Xie C, Zuscik M, Kingsley P, Schwarz E, Awad H, Guldberg R, Drissi H, Boyce B, Zhang X, O’keefe R. Impaired Cox2/EP4 signaling leads to delayed fracture healing in aged mice. Journal of Bone & Mineral Research. 2008 doi: 10.1359/jbmr.081002. in Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].O’Driscoll SW, Fitzsimmons JS. The role of periosteum in cartilage repair. Clin Orthop Relat Res. 2001;391(Suppl):S190–207. doi: 10.1097/00003086-200110001-00019. [DOI] [PubMed] [Google Scholar]
- [27].O’Keefe RJ, Tiyapatanaputi P, Xie C, Li TF, Clark C, Zuscik MJ, Chen D, Drissi H, Schwarz E, Zhang X. COX-2 has a critical role during incorporation of structural bone allografts. Ann N Y Acad Sci. 2006;1068:532–42. doi: 10.1196/annals.1346.012. [DOI] [PubMed] [Google Scholar]
- [28].Orwoll ES. Toward an expanded understanding of the role of the periosteum in skeletal health. J Bone Miner Res. 2003;18:949–54. doi: 10.1359/jbmr.2003.18.6.949. [DOI] [PubMed] [Google Scholar]
- [29].Shen W, Li Y, Zhu J, Schwendener R, Huard J. Interaction between macrophages, TGF-beta1, and the COX-2 pathway during the inflammatory phase of skeletal muscle healing after injury. J Cell Physiol. 2008;214:405–12. doi: 10.1002/jcp.21212. [DOI] [PubMed] [Google Scholar]
- [30].Simon AM, Manigrasso MB, O’Connor JP. Cyclo-oxygenase 2 function is essential for bone fracture healing. J Bone Miner Res. 2002;17:963–76. doi: 10.1359/jbmr.2002.17.6.963. [DOI] [PubMed] [Google Scholar]
- [31].Simon AM, O’Connor JP. Dose and time-dependent effects of cyclooxygenase-2 inhibition on fracture-healing. J Bone Joint Surg Am. 2007;89:500–11. doi: 10.2106/JBJS.F.00127. [DOI] [PubMed] [Google Scholar]
- [32].Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, and molecular biology. Ann. Rev. Biochem. 2000;69:145–82. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- [33].Tiyapatanaputi P, Rubery PT, Carmouche J, Schwarz EM, O’Keefe R,J, Zhang X. A novel murine segmental femoral graft model. J Orthop Res. 2004;22:1254–60. doi: 10.1016/j.orthres.2004.03.017. [DOI] [PubMed] [Google Scholar]
- [34].Wang D, Mann JR, DuBois RN. The role of prostaglandins and other eicosanoids in the gastrointestinal tract. Gastroenterology. 2005;128:1445–61. doi: 10.1053/j.gastro.2004.09.080. [DOI] [PubMed] [Google Scholar]
- [35].Wlodarski KH. Normal and heterotopic periosteum. Clin Orthop Relat Res. 1989;241:265–77. [PubMed] [Google Scholar]
- [36].Wu KK. Control of cyclooxygenase-2 transcriptional activation by pro-inflammatory mediators. Prostaglandins Leukot Essent Fatty Acids. 2005;72:89–93. doi: 10.1016/j.plefa.2004.11.001. [DOI] [PubMed] [Google Scholar]
- [37].Zhang X, Morham SG, Langenback R, Young DA, Xing L, Boyce BF, Puzas JE, Rosier RN, O’Keefe RJ, Schwarz EM. Evidence for a Direct Role of COX-2 in Implant Wear Debris Induced Osteolysis. J. Bon. Min. Res. 2001;16:660–669. doi: 10.1359/jbmr.2001.16.4.660. [DOI] [PubMed] [Google Scholar]
- [38].Zhang X, Schwarz EM, Young DA, Puzas JE, Rosier RN, O’Keefe RJ. Cyclooxygenase-2 regulates mesenchymal cell differentiation into the osteoblast lineage and is critically involved in bone repair. J Clin Invest. 2002;109:1405–15. doi: 10.1172/JCI15681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Zhang X, Xie C, Lin AS, Ito H, Awad H, Lieberman JR, Rubery PT, Schwarz EM, O’Keefe RJ, Guldberg RE. Periosteal progenitor cell fate in segmental cortical bone graft transplantations: implications for functional tissue engineering. J Bone Miner Res. 2005;20:2124–37. doi: 10.1359/JBMR.050806. [DOI] [PMC free article] [PubMed] [Google Scholar]