Abstract
Thrombocytopenia affects up to 35% of all patients admitted to the neonatal intensive care unit (NICU). The causes of thrombocytopenia in neonates are very diverse, and include immune and non-immune disorders. Most cases of thrombocytopenia encountered in the NICU are non-immune, and these will constitute the focus of this review. Specifically, we will first discuss the biological differences between neonatal and adult megakaryocytopoiesis, which contribute to explain the vulnerability of neonates to develop thrombocytopenia. Next, we will review new diagnostic tools that have allowed for a better evaluation of platelet production in neonates, without having to obtain a bone marrow sample. Finally, we will summarize our current understanding of the mechanisms underlying the thrombocytopenia in several common neonatal conditions, such as chronic intrauterine hypoxia, sepsis and necrotizing enterocolitis (NEC), and viral infections. A better understanding of the mechanisms underlying these varieties of thrombocytopenia is critical to develop disease-specific treatment protocols, and to begin to entertain the possibility of using novel thrombopoietic growth factors to treat selected neonates with severe thrombocytopenia.
Platelet production in neonates
Platelet production, or thrombopoiesis, is a complex process that can be schematically represented as consisting of four main steps. The first step is the production of the thrombopoietic stimulus, which drives the generation of megakaryocytes and ultimately platelets. Although a number of cytokines (i.e. IL-3, IL-6, IL-11, GM-CSF) and chemokines (i.e. SDF and FGF-4) contribute to this process, thrombopoietin (Tpo) is now widely recognized as the most potent known stimulator of platelet production. Thrombopoietin mostly acts by promoting the proliferation of megakaryocyte progenitors (the cells that multiply and give rise to megakaryocytes), and the maturation of the megakaryocytes.1-3 Megakaryocyte maturation is a process characterized by a progressive increase in nuclear ploidy and cytoplasmic maturity that leads to the generation of large polyploid (8N-64N) megakaryocytes. Through a still poorly understood process, these mature megakaryocytes then generate and release new platelets into the circulation.4-6
Although, in general, the process of platelet production follows the same steps in neonates and adults, there are important developmental differences that need to be taken into consideration when evaluating neonates with platelet disorders (Table 1). Specifically, it is well known that plasma thrombopoietin concentrations are higher in normal neonates than in healthy adults,7,8 but neonates with thrombocytopenia have in general lower Tpo concentrations than adults with similar degrees and mechanisms of thrombocytopenia.9,10 Megakaryocyte progenitors of neonates have a higher proliferative potential than those of adults, and give rise to significantly larger megakaryocyte colonies when cultured in vitro.9,11,12 Furthermore, neonatal megakaryocyte progenitors are more sensitive to Tpo in vitro and in vivo than adult progenitors, and are present both in the bone marrow and the peripheral blood, while adult progenitors reside almost exclusively in the bone marrow. However, neonatal megakaryocytes are smaller and of lower ploidy than adult megakaryocytes, although they are otherwise mature cells.13,14 Since smaller megakaryocytes produce less platelets than larger megakaryocytes,15 it has been postulated that neonates maintain normal platelet counts on the basis of the increased proliferative potential of their progenitors.
Table 1.
Differences in megakaryocytopoiesis between neonates and adults.
| Adults | Neonates | |
|---|---|---|
| Tpo concentrations | Very high in hyporegenerative thrombocytopenia | Not as high in thrombocytopenic neonates (mostly SGA) as in thrombocytopenic adults |
| Megakaryocyte progenitors | Sparse in the blood Give rise to small colonies Less sensitive to Tpo |
Abundant in the blood Give rise to large colonies More sensitive to Tpo |
| Megakaryocytes | Large High ploidy levels |
Small Low ploidy levels |
| Effects of rTpo | Stimulates megakaryocyte polyploidization | Inhibits megakaryocyte polyploidization |
Adapted from: Sola-Visner MC. Thrombocytopenia in the NICU: New Insights into causative mechanisms and treatments. Haematologica reports 2006;2:65-69. Reprinted with permission.
Neonatal response to thrombocytopenia
An important question that had remained unanswered was whether and how these developmental differences affected the ability of neonates to respond to thrombocytopenia, particularly secondary to increased platelet consumption. Specifically, it was unknown if neonates could increase the number and/or size of their megakaryocytes, as adult patients with platelet consumptive disorders do in order to up-regulate platelet production.16 It was also unclear whether neonates could increase their megakaryocyte number, since their progenitors are proliferating at such a high rate at baseline. Finding the answers to these questions represented a significant challenge, mostly due to the limited availability of bone marrow specimens from living neonates, the rarity of megakaryocytes in the fetal marrow, and the lack of animal models of neonatal thrombocytopenia.
Our group recently applied immunohistochemistry and image analysis to systematically evaluate the number and size of megakaryocytes in the bone marrow of neonates and adults with and without thrombocytopenia. Findings from this study suggested that thrombocytopenic neonates could increase the number, but not the size, of their megakaryocytes.17 Adults with immune thrombocytopenic purpura, in contrast, exhibited significantly higher proportions of large megakaryocytes in their bone marrows than non-thrombocytopenic adults (Figure 1). We hypothesized that this relative developmental deficiency in the ability of neonates to increase their megakaryocyte size likely contributed to the predisposition of sick neonates to develop prolonged and severe thrombocytopenia. However, the causes of thrombocytopenia were highly diverse among the neonatal subjects, and it remained unclear whether the observed differences between neonates and adults reflected a true developmental limitation, or were rather explained by the different disease processes underlying neonatal and adult thrombocytopenia. Recent data from a murine model of fetal immune thrombocytopenia suggests that there is indeed a developmental limitation in the ability of neonates to increase their megakaryocyte size (Unpublished).
Figure 1.
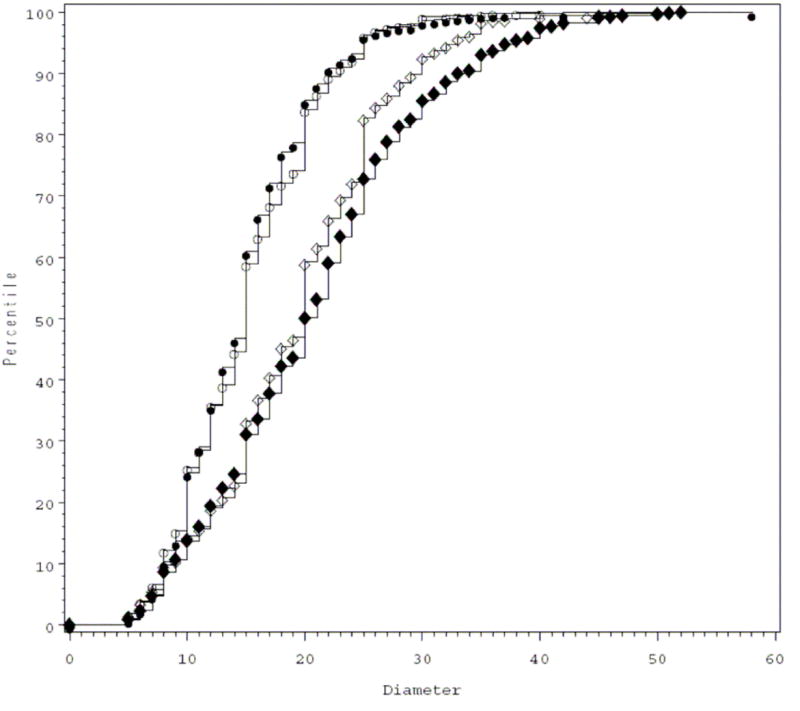
Cumulative distribution plots of megakaryocyte diameters in the bone marrow of thrombocytopenic neonates (dark circles), non-thrombocytopenic neonates (clear circles), thrombocytopenic adults (dark diamonds), and non-thrombocytopenic adults (clear diamonds). The megakaryocyte diameters are displayed on the X-axis, and their cumulative distribution on the Y-axis. Both adult curves are shifted to the right compared to the neonatal curves, indicating the predominance of larger megakaryocytes in these samples. Furthermore, the curve for the thrombocytopenic adults is also shifted to the right compared to that for the non-thrombocytopenic adults, indicating a higher percentage of large megakaryocytes. In contrast, the curves of thrombocytopenic and non-thrombocytopenic neonates are overlapping, indicating no change in the megakaryocyte size distribution in this cohort of thrombocytopenic neonates compared to controls. (From Sola-Visner MC, Christensen RD, Hutson AD, Rimsza LM. Megakaryocyte size and concentration in the bone marrow of thrombocytopenic and nonthrombocytopenic neonates. Pediatr Res. 2007;61:479-484. Repinted with permission).
The reasons underlying the lack of increase in megakaryocyte size in neonates are unclear. Previous transplant studies by our group evaluating the phenotype of neonatal megakaryocytes in an adult microenvironment demonstrated that the developmental differences between neonatal and adult megakaryocytes are due both to cell-intrinsic and to microenvironmental factors.18,19 Among the cell-intrinsic differences, we recently reported that the response of human megakaryocytes to Tpo is different at various stages of development. Specifically, under identical culture conditions, Tpo potently stimulated the maturation and polyploidization of adult megakaryocytes, while it inhibited the nuclear maturation of neonatal megakaryocytes (Figure 2).20 It is therefore tempting to hypothesize that the early rise in Tpo levels that usually accompanies thrombocytopenia (induced by the acute drop in platelet count) at least partially explains the differences in megakaryocyte size between thrombocytopenic neonates and adults.
Figure 2.
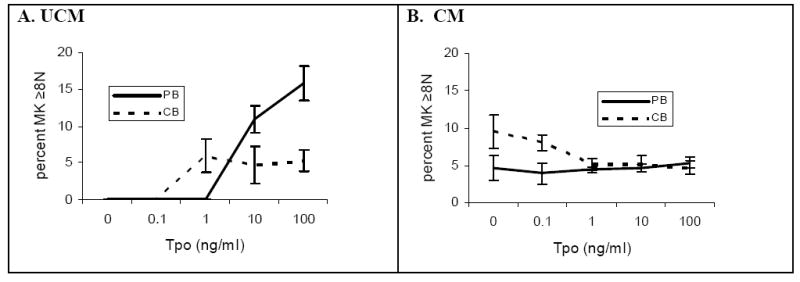
The percentage of megakaryocytes with ploidy levels ≥ 8N in peripheral blood (PB)- and cord blood (CB)-derived megakaryocyte cultures differed depending on media source and rTpo concentration. PB- and CB-CD34+ cells were cultured for 14 days in serum free unconditioned media (A) and in adult bone marrow stromal conditioned media (B), with varying rTpo concentrations. PB-derived megakaryocytes (solid lines) cultured in unconditioned media (A) exhibited a rTpo dose-dependent increase in ploidy levels, an effect inhibited by the presence of conditioned media (B). CB-derived megakaryocytes (dashed lines) reached highest ploidy levels when cultured in conditioned media with no rTpo, and effect that was reversed by rTpo concentrations ≥1 ng/ml (B). Data shown represent the means and standard error of the mean (SEM) of four separate experiments. (From Pastos KM, Slayton WB, Rimsza LM, et al. Differential effects of recombinant thrombopoietin and bone marrow stromal-conditioned media on neonatal versus adult megakaryocytes. Blood 2006;108:3360-3362. Reprinted with permission).
In regard to the fetal vs. adult hematopoietic microenvironment, developmental differences may exist in the expression levels of factors stimulating or suppressing megakaryocytopoiesis (i.e. IL-6, IL-11, VEGF, PF4), or of chemokines that have been proven critical for megakaryocyte maturation, such as SDF and/or FGF-4. Such differences in the microenvironment could also explain the variability in megakaryocyte size and concentration observed in different organs (i.e. bone marrow, spleen and liver), although this remains to be determined.
New tests to evaluate platelet production in neonates
Since bone marrow studies are technically difficult in neonates (particularly in those born prematurely), significant efforts have been aimed at developing blood tests to evaluate platelet production that are suitable for neonates. Among those tests, plasma or serum Tpo concentrations, circulating Mk progenitors, reticulated platelet percentages (RP%), and glycocalicin concentrations have been used by different investigators, and have shown promising results. Tpo concentrations are a good measure of the thrombopoietic stimulus. Because serum Tpo levels are a reflection of both the level of Tpo production and the availability of Tpo receptor (on progenitor cells, megakaryocytes, and platelets), elevated Tpo levels in the presence of thrombocytopenia usually suggest inflammatory conditions leading to upregulated gene expression (i.e. during infections), or hyporegenerative thrombocytopenias characterized by a decreased megakaryocyte mass (such as aplastic anemia or chemotherapy-induced thrombocytopenia). Normal to only minimally elevated Tpo concentrations have been reported in patients with ITP21 and in patients with thrombocytopenia secondary to liver failure22 (Table 2), in whom lack of Tpo production by the failing liver is thought to contribute to the thrombocytopenia. In regard to neonates, several investigators have published Tpo concentrations in healthy preterm and term neonates as well as in neonates with thrombocytopenia secondary to different etiologies.23 Overall, Tpo levels can be helpful in the mechanistic evaluation of thrombocytopenia, but due to the complex regulation of plasma Tpo levels, they are difficult to interpret in the absence of other test(s) of thrombopoiesis. Tpo concentrations are also not routinely available in the clinical setting.
Table 2.
Mechanisms of thrombocytopenia based on different tests of thrombopoiesis.
| Tpo | CMPs | RP% | RP Count | Interpretation |
|---|---|---|---|---|
| NL | ↓ | ↓ | ↓↓ | Decreased platelet production secondary to low Tpo:
|
| ↑↑↑ | ↓ | ↓ | ↓↓ | Decreased platelet production secondary to decreased marrow megakaryocytes:
|
| ↑ | ↑ | ↓ | ↓↓ | Ineffective platelet production:
|
| ↑↑ | ↑ | ↑ | ↓ to NL | Thrombocytopenia due to increased platelet consumption with inflammation (Tpo up-regulation):
|
| NL to slightly ↑ | ↑ | ↑ | ↓ to NL | Thrombocytopenia due to immune-mediated platelet consumption:
|
NL=Normal; CAMT=Congenital Amegakaryocytic Thrombocytopenia; HIV=Human Immunodeficiency Virus; ITP=Immune Thrombocytopenic Purpura; NEC=Necrotizing Enterocolitis
Adapted from: Brown et al., Effects of sepsis on neonatal thrombopoiesis. Pediatric Research 2008. Reprinted with permission
In neonates, megakaryocyte progenitors (the precursors for megakaryocytes) are present both in the blood and in the bone marrow. Several investigators have capitalized on this observation, and have used the concentration of circulating progenitors as an indirect marker of marrow megakaryocytopoiesis,24-26 although the correlation between blood progenitors and marrow progenitors has never been clearly established in newborn infants. Nevertheless, the quantification of circulating megakaryocyte progenitors has provided significant insight into the mechanisms underlying several varieties of neonatal thrombocytopenia (see below), and has enhanced our understanding of normal and abnormal neonatal thrombopoiesis. It is unlikely, however, that this relatively labor-intensive test (which requires culturing the megakaryocyte progenitors for 10 days and then quantifying the megakaryocyte colonies formed) will ever become available for routine clinical use.
Perhaps the most promising test of platelet production to emerge in the last decade has been the reticulated platelet (RP) percentage. RPs are newly released platelets (<24 hours old) that contain residual RNA, which allows their detection and quantification in the blood by flow cytometry. In adults and children, the reticulated platelet percentage (RP%) has been evaluated as a way of classifying thrombocytopenia into hyporegerative and consumptive varieties, similar to the way the reticulocyte count is used to evaluate anemia.27,28 Thus, a low RP% suggests diminished platelet production, while an elevated RP% indicates increased platelet production. Unfortunately, RP% values reported for healthy adults and children have varied significantly among publications, mostly because of the lack of consistent methodology. Four prior studies measured RP% in non-thrombocytopenic term and preterm neonates, and (similar to adult studies) the methods used and the RP% values varied significantly.29-32 In one of these publications, our group used a modified whole blood method to determine RP% in preterm neonates admitted to the NICU over the first 28 days of life. We found that the RP% in neonates increased over the first 2-5 days of life and then decreased to a stable level of 3.2±1.1%. In addition, RP% were higher in neonates than adults, and (in the first day of life) they were inversely related to gestational age.32
Recently, a test similar to the RP% was developed for clinical use. This RP% equivalent, termed the “immature platelet fraction (IPF)” can be measured by a standard hematology analyzer (XE-2100, Sysmex, Kobe, Japan) as part of a routine complete blood count.33,34 Similar to the RP%, IPF values were shown to be elevated in thrombocytopenic conditions associated with increased platelet destruction (i.e. ITP), and decreased in thrombocytopenias due to decreased platelet production (i.e. aplastic anemia, chemotherapy-induced thrombocytopenia).35,36 Only one study has evaluated IPF values in thrombocytopenic and non-thrombocytopenic neonates.37 In this study, as in our previous RP% study, normal neonatal IPF values were higher than those previously reported in adults, likely reflecting an increased level of platelet turnover in neonates (necessary to maintain a normal platelet count during a period of rapid growth). Most importantly, however, findings from this study suggested that the IPF could be used (at least in certain varieties of thrombocytopenia) to predict recovery of the platelet count within the next 24 hours. Thus, the IPF has the potential of becoming an important consideration when deciding whether to give or withhold a platelet transfusion in a thrombocytopenic neonate.
Glycocalicin concentrations, measured by ELISA, have also been used in a number of research studies as a marker of platelet turnover. Glycocalicin is a soluble proteolytic fragment of the α-subunit of glycoprotein Ib (GPIb), which is normally expressed on mature megakaryocytes and platelets. Its levels are increased in patients with increased platelet destruction, and decreased in patients with defective platelet formation.38-40 This test is currently not available for clinical use.
Although none of these tests have yet been adequately validated with concomitant bone marrow or platelet kinetic studies in neonates, recent reports in adults and children have suggested that the use of several tests in combination can help differentiate between disorders of increased platelet destruction and those of decreased production (Table 2).38-42 Particularly in neonates with severe and unexplained thrombocytopenia, this type of evaluation is likely to offer useful information leading to the diagnosis, although at this point it is limited to research settings. For the clinicians, it is anticipated that the IPF (as it becomes more widely available) will become an integral part of the evaluation of thrombocytopenia, similarly to the reticulocyte count in anemia. Bone marrow studies still provide information that cannot be obtained through any indirect measures of platelet production (such as marrow cellularity and megakaryocyte morphology), and should be performed in selected patients.43
Mechanisms of thrombocytopenia in specific neonatal conditions
Chronic intrauterine hypoxia
Chronic intrauterine hypoxia is the most frequent cause of early-onset thrombocytopenia (within the first 72 hours of life) in preterm neonates.44 Chronic intrauterine hypoxia is commonly seen in maternal conditions associated with placental insufficiency, such as pregnancy-induced hypertension and diabetes,45 and is usually manifested in the fetus by intrauterine growth restriction and hematological abnormalities. The thrombocytopenia associated with this condition is almost always mild to moderate, with platelet counts between 50 and 100 × 109/L. In fact, if the thrombocytopenia is severe (<50 × 109/L), other causes of severe early onset thrombocytopenia (such as immune mediated thrombocytopenia, infections, genetic disorders) should be considered. Since the thrombocytopenia associated with chronic intrauterine hypoxia and intrauterine growth restriction is usually resolved by days of life 7-10, persistence of the thrombocytopenia beyond this period should also trigger an evaluation for other causes.
The pathophysiology of the thrombocytopenia in fetuses and neonates exposed to chronic intrauterine hypoxia is not completely understood, but several studies have implicated decreased platelet production as the main mechanism. Murray and Roberts observed that preterm neonates with early-onset thrombocytopenia (associated with chronic intrauterine hypoxia in most cases) had decreased concentrations of circulating megakaryocyte progenitors compared to their non-thrombocytopenic counterparts, and that the number of progenitors increased during the period of platelet recovery (Figure 3).25 Similarly, our group reported decreased numbers of megakaryocytes in the bone marrow of three thrombocytopenic preterm neonates born from pregnancies complicated by placental insufficiency.10 Interestingly, both groups of investigators reported that plasma Tpo concentrations in these neonates were normal to minimally elevated, suggesting that an inadequate up-regulation of Tpo production could contribute to the thrombocytopenia.10,46 The reasons for this are unknown.
Figure 3.
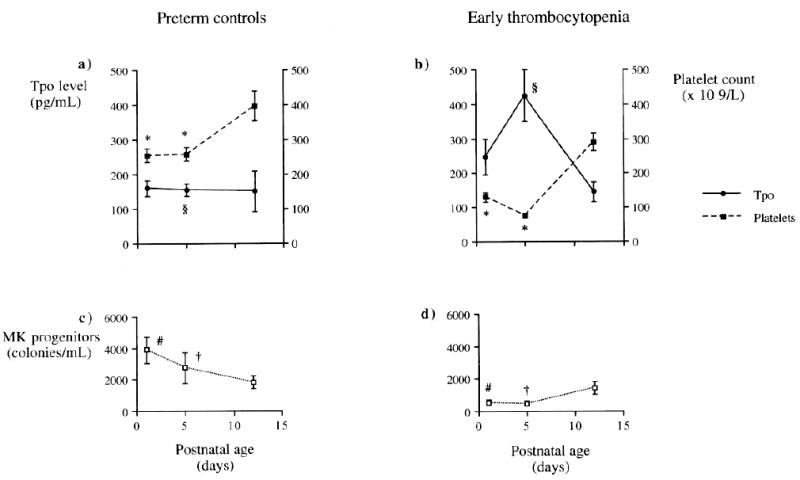
Changes in thrombopoietin (Tpo) level, platelet count, and megakaryocyte (MK) progenitor numbers during the first 12 d of life in control preterm babies and preterm babies with early onset thrombocytopenia. Results expressed as mean ± SEM. Despite platelet counts being significantly lower in thrombocytopenic babies at d 1 and d 4/5 compared with control babies (*p < 0.001), Tpo levels were only significantly higher at d 4/5 (§p < 0.001) (a and b). As with platelet counts, MK progenitors were also significantly lower at d 1 (#p < 0.001) and d 4/5 (†p < 0.05) in thrombocytopenic babies compared with control babies (c and d). There was no significant difference among platelet count, Tpo level, or MK progenitors between study and control groups by d 12. (From Watts TL, Murray NA, Roberts IA. Thrombopoietin has a primary role in the regulation of platelet production in preterm babies. Pediatr Res. 1999;46:28-32. Reprinted with permission).
The effects of chronic hypoxia on thrombopoiesis in vivo have been studied in several animal models. These confirmed the pathogenic association between chronic hypoxia and thrombocytopenia, and showed reduced megakaryocyte differentiation of hematopoietic precursors and decreased Mk numbers in adult and newborn animals exposed to hypoxia.47,48 Since chronic intrauterine hypoxia also causes polycythemia due to hypoxic upregulation of erythropoietin,49 it was hypothesized that increased erythropoiesis might contribute to the thrombocytopenia by “stealing” progenitor cells from the megakaryocytic to the erythroid lineage.50 Against this hypothesis, however, thrombocytopenia has not been observed in anemic preterm infants treated with recombinant erythropoietin for up to several weeks.51,52 Furthermore, erythropoietin displays synergistic effects with Tpo, likely due to their high degree of structural homology.53
Saxonhouse et al. investigated the effects of chronic hypoxia on the clonogenic potential of preterm, term and adult megakaryocyte progenitors exposed to different oxygen tensions (1, 5, or 20 % oxygen for 10-12 days). These experiments demonstrated that hypoxia had no direct deleterious effect on megakaryocyte progenitors. However, if megakaryocyte progenitor cells obtained from the cord blood of preterm neonates were co-cultured with non-progenitor (CD34 negative) bone marrow cells, exposure to hypoxia resulted in decreased megakaryocyte colony formation.54 These findings suggested that the hematopoietic microenvironment plays a significant role in mediating chronic hypoxia-induced suppression of megakaryocytopoiesis, and that megakaryocyte progenitors from preterm neonates are more vulnerable than progenitors from term neonates or adults.
Sepsis and necrotizing enterocolitis
Sepsis and necrotizing enterocolitis (NEC) are among the most common causes of severe thrombocytopenia in the neonatal intensive care unit (NICU). Despite their prevalence, the mechanisms underlying the thrombocytopenia in these conditions had not been clearly elucidated. Over the last decade, several groups evaluated thrombopoietin concentrations in neonates with infection. These studies consistently observed that septic neonates had elevated plasma Tpo concentrations, compared to their healthy counterparts.55 However, it was unclear whether these elevated Tpo levels were the result of increased Tpo production, or rather reflected decreased megakaryocytes and therefore sepsis-mediated suppression of megakaryocytopoiesis.
To answer this question, we recently applied a panel of tests to evaluate platelet production in 20 neonates with sepsis and/or NEC.56 This panel included plasma Tpo concentrations, circulating megakaryocyte progenitors, RP% and RP counts. Findings from this study demonstrated that, in neonates with sepsis and/or NEC, Tpo concentrations and circulating megakaryocyte progenitors were elevated, suggesting an up-regulation of thrombopoiesis mediated by Tpo. The RP% was also increased in neonates with thrombocytopenia. Overall, observations from this study demonstrated that thrombopoiesis is up-regulated in neonatal sepsis and/or NEC, although the degree of up-regulation observed in our study was relatively modest. Interestingly, the neonates with Gram-negative sepsis did not have the highest degree of up-regulation, despite more significant levels of thrombocytopenia and more severe illness. This observation suggested that the magnitude of the thrombopoietic response can be down-regulated during severe illness, and can reach a state of “relative hypoproliferation”, previously defined as a less than two-fold increase in megakaryocyte mass in response to thrombocytopenia.16 The mechanisms responsible for this down-regulated response are unknown, but we hypothesized that it could be mediated by high levels of platelet factor 4 being released from activated platelets during severe sepsis. Platelet factor 4 has been recently found to be a potent inhibitor of megakaryocyte proliferation, thus negatively regulating megakaryocytopoiesis.57
Congenital viral infections: HIV, CMV, and enterovirus
Neonates suffering from infections with viral pathogens such as human immunodeficiency virus (HIV), cytomegalovirus (CMV), or enterovirus frequently exhibit thrombocytopenia. The mechanisms underlying the decreased platelet counts under these conditions differ and are incompletely understood.
Thrombocytopenia is a well-studied complication of HIV-infection in children and adults.58 However, information regarding HIV-associated thrombocytopenia in the neonatal population is sparse. Roux et al. showed that 47% out of 34 HIV-exposed neonates were thrombocytopenic, which led the authors to suggest that thrombocytopenia could serve as a maker for HIV-exposure in neonates.59
The mechanisms responsible for the thrombocytopenia in HIV infected subjects are complex, and likely involve a combination of immune-mediated thrombocytopenia,58,60 sequestration, and suppression of platelet production. The most comprehensive study to date focusing on the mechanisms underlying HIV-related thrombocytopenia in adult patients was carried out by Cole et al. These investigators observed that subjects with HIV-associated thrombocytopenia had splenic platelet sequestration, but also had evidence of decreased platelet production despite a substantial increase in their megakaryocyte mass.61 This dissociation between the platelet producing substrate (i.e. the megakaryocytes) and the actual level of platelet production is known as “ineffective thrombopoiesis”, and was identified as the main mechanism accounting for the thrombocytopenia in these patients. Direct HIV infection of the megakaryocytes and their progenitors, which express CD4 and CXCR4, was hypothesized to be the cause for their impaired platelet release.62,63
The mechanisms contributing to neonatal HIV-related thrombocytopenia have extensively been investigated in only a single neonate. Tighe et al. applied a panel of tests consisting of plasma Tpo concentrations, circulating megakaryocyte progenitors, and RP% to evaluate a neonate with severe HIV infection and thrombocytopenia (Table 2). This study confirmed ineffective platelet production as the main mechanism of the thrombocytopenia, which resolved within 10 days of starting treatment with Zydovudine.64
In contrast to the rather uncommon congenital HIV-infection, fetal CMV infection is frequent, affecting 1% of all life births in the United States, 10% of which are symptomatic.65 The rate of thrombocytopenia among those infants ranges from 50% to 77%.66,67 Despite the high clinical relevance of CMV-induced thrombocytopenia, the pathophysiology of this condition has only been poorly evaluated in neonates. Affected infants have been reported to exhibit massive splenomegaly and to often require repeated transfusions, likely because of platelet sequestration in the spleen.68,69 Furthermore, CMV might affect megakaryocytopoiesis by directly infecting bone marrow stromal cells.70 However, it is not known whether CMV infection interferes with platelet release from the megakaryocytes and causes ineffective platelet production in a manner similar to HIV.
The mechanisms underlying the thrombocytopenia associated with other viral infections (e.g. enterovirus infection) are only speculative, but seem to involve hepatosplenomegaly causing increased platelet sequestration, as well as increased platelet destruction associated with disseminated intravascular coagulation.71,72
Conclusion
Thrombocytopenia is a common problem among sick neonates admitted to the NICU. Despite its prevalence, the responsible pathophysiologic mechanisms remain unclear in the majority of cases. Over the last decade, a mounting body of evidence has suggested that megakaryocytes and megakaryocyte progenitors from neonates are biologically substantially different from those of adults, and that these differences contribute to explain the predisposition of neonates to develop thrombocytopenia. Our ability to evaluate individual neonates with thrombocytopenia has also been enhanced by the availability of new tests to evaluate platelet production. Among these tests, the immature platelet fraction (IPF) is already clinically available in several institutions, and will likely become an integral part of the evaluation of thrombocytopenia in any age group. With this and other tools in hand, we have gained a better understanding of the mechanisms that underlie several common and some less common types of neonatal thrombocytopenia. This improved understanding is critical to begin to explore the potential of judiciously using thrombopoietic growth factors to treat certain groups of thrombocytopenic neonates.
Acknowledgments
This work was partially supported by NIH grant HL69990 (MSV)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Debili N, Wendling F, Katz A, et al. The Mpl-ligand or thrombopoietin or megakaryocyte growth and differentiative factor has both direct proliferative and differentiative activities on human megakaryocyte progenitors. Blood. 1995;86:2516–25. [PubMed] [Google Scholar]
- 2.Kaushansky K. The molecular mechanisms that control thrombopoiesis. J Clin Invest. 2005;115:3339–47. doi: 10.1172/JCI26674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaushansky K. Historical review: megakaryopoiesis and thrombopoiesis. Blood. 2008;111:981–6. doi: 10.1182/blood-2007-05-088500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Junt T, Schulze H, Chen Z, et al. Dynamic visualization of thrombopoiesis within bone marrow. Science. 2007;317:1767–70. doi: 10.1126/science.1146304. [DOI] [PubMed] [Google Scholar]
- 5.Patel SR, Hartwig JH, Italiano JE., Jr The biogenesis of platelets from megakaryocyte proplatelets. J Clin Invest. 2005;115:3348–54. doi: 10.1172/JCI26891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richardson JL, Shivdasani RA, Boers C, et al. Mechanisms of organelle transport and capture along proplatelets during platelet production. Blood. 2005;106:4066–75. doi: 10.1182/blood-2005-06-2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sola MC, Juul SE, Meng YG, et al. Thrombopoietin (Tpo) in the fetus and neonate: Tpo concentrations in preterm and term neonates, and organ distribution of Tpo and its receptor (c-mpl) during human fetal development. Early Hum Dev. 1999;53:239–50. doi: 10.1016/s0378-3782(98)00077-2. [DOI] [PubMed] [Google Scholar]
- 8.Dame C. Developmental biology of thrombopoietin in the human fetus and neonate. Acta Paediatr Suppl. 2002;91:54–65. doi: 10.1111/j.1651-2227.2002.tb02900.x. [DOI] [PubMed] [Google Scholar]
- 9.Murray NA, Watts TL, Roberts IA. Endogenous thrombopoietin levels and effect of recombinant human thrombopoietin on megakaryocyte precursors in term and preterm babies. Pediatr Res. 1998;43:148–51. doi: 10.1203/00006450-199801000-00023. [DOI] [PubMed] [Google Scholar]
- 10.Sola MC, Calhoun DA, Hutson AD, Christensen RD. Plasma thrombopoietin concentrations in thrombocytopenic and non-thrombocytopenic patients in a neonatal intensive care unit. Br J Haematol. 1999;104:90–2. doi: 10.1046/j.1365-2141.1999.01154.x. [DOI] [PubMed] [Google Scholar]
- 11.Nishihira H, Toyoda Y, Miyazaki H, et al. Growth of macroscopic human megakaryocyte colonies from cord blood in culture with recombinant human thrombopoietin (c-mpl ligand) and the effects of gestational age on frequency of colonies. Br J Haematol. 1996;92:23–8. doi: 10.1046/j.1365-2141.1996.00287.x. [DOI] [PubMed] [Google Scholar]
- 12.Sola MC, Du Y, Hutson AD, Christensen RD. Dose-response relationship of megakaryocyte progenitors from the bone marrow of thrombocytopenic and non-thrombocytopenic neonates to recombinant thrombopoietin. Br J Haematol. 2000;110:449–53. doi: 10.1046/j.1365-2141.2000.02163.x. [DOI] [PubMed] [Google Scholar]
- 13.Olson TA, Levine RF, Mazur EM, et al. Megakaryocytes and megakaryocyte progenitors in human cord blood. Am J Pediatr Hematol Oncol. 1992;14:241–7. doi: 10.1097/00043426-199208000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Levine RF, Olson TA, Shoff PK, et al. Mature micromegakaryocytes: an unusual developmental pattern in term infants. Br J Haematol. 1996;94:391–9. doi: 10.1046/j.1365-2141.1996.00666.x. [DOI] [PubMed] [Google Scholar]
- 15.Mattia G, Vulcano F, Milazzo L, et al. Different ploidy levels of megakaryocytes generated from peripheral or cord blood CD34+ cells are correlated with different levels of platelet release. Blood. 2002;99:888–97. doi: 10.1182/blood.v99.3.888. [DOI] [PubMed] [Google Scholar]
- 16.Harker LA, Finch CA. Thrombokinetics in man. J Clin Invest. 1969;48:963–74. doi: 10.1172/JCI106077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sola-Visner MC, Christensen RD, Hutson AD, Rimsza LM. Megakaryocyte size and concentration in the bone marrow of thrombocytopenic and nonthrombocytopenic neonates. Pediatr Res. 2007;61:479–84. doi: 10.1203/pdr.0b013e3180332c18. [DOI] [PubMed] [Google Scholar]
- 18.Slayton WB, Wainman DA, Li XM, et al. Developmental differences in megakaryocyte maturation are determined by the microenvironment. Stem Cells. 2005;23:1400–8. doi: 10.1634/stemcells.2004-0373. [DOI] [PubMed] [Google Scholar]
- 19.Ignatz M, Sola-Visner M, Rimsza LM, et al. Umbilical cord blood produces small megakaryocytes after transplantation. Biol Blood Marrow Transplant. 2007;13:145–50. doi: 10.1016/j.bbmt.2006.10.032. [DOI] [PubMed] [Google Scholar]
- 20.Pastos KM, Slayton WB, Rimsza LM, et al. Differential effects of recombinant thrombopoietin and bone marrow stromal-conditioned media on neonatal versus adult megakaryocytes. Blood. 2006;108:3360–2. doi: 10.1182/blood-2006-04-018036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Emmons RV, Reid DM, Cohen RL, et al. Human thrombopoietin levels are high when thrombocytopenia is due to megakaryocyte deficiency and low when due to increased platelet destruction. Blood. 1996;87:4068–71. [PubMed] [Google Scholar]
- 22.Peck-Radosavljevic M, Wichlas M, Zacherl J, et al. Thrombopoietin induces rapid resolution of thrombocytopenia after orthotopic liver transplantation through increased platelet production. Blood. 2000;95:795–801. [PubMed] [Google Scholar]
- 23.Dame C. Thrombopoietin in thrombocytopenias of childhood. Semin Thromb Hemost. 2001;27:215–28. doi: 10.1055/s-2001-15251. [DOI] [PubMed] [Google Scholar]
- 24.Murray NA, Roberts IA. Circulating megakaryocytes and their progenitors (BFU-MK and CFU-MK) in term and pre-term neonates. Br J Haematol. 1995;89:41–6. doi: 10.1111/j.1365-2141.1995.tb08913.x. [DOI] [PubMed] [Google Scholar]
- 25.Murray NA, Roberts IA. Circulating megakaryocytes and their progenitors in early thrombocytopenia in preterm neonates. Pediatr Res. 1996;40:112–9. doi: 10.1203/00006450-199607000-00020. [DOI] [PubMed] [Google Scholar]
- 26.Saxonhouse MA, Christensen RD, Walker DM, et al. The concentration of circulating megakaryocyte progenitors in preterm neonates is a function of post-conceptional age. Early Hum Dev. 2004;78:119–24. doi: 10.1016/j.earlhumdev.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 27.Ault KA. Flow cytometric measurement of platelet function and reticulated platelets. Ann N Y Acad Sci. 1993;677:293–308. doi: 10.1111/j.1749-6632.1993.tb38785.x. [DOI] [PubMed] [Google Scholar]
- 28.Ault KA, Rinder HM, Mitchell J, et al. The significance of platelets with increased RNA content (reticulated platelets). A measure of the rate of thrombopoiesis. Am J Clin Pathol. 1992;98:637–46. doi: 10.1093/ajcp/98.6.637. [DOI] [PubMed] [Google Scholar]
- 29.Joseph MA, Adams D, Maragos J, Saving KL. Flow cytometry of neonatal platelet RNA. J Pediatr Hematol Oncol. 1996;18:277–81. doi: 10.1097/00043426-199608000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Peterec SM, Brennan SA, Rinder HM, et al. Reticulated platelet values in normal and thrombocytopenic neonates. J Pediatr. 1996;129:269–74. doi: 10.1016/s0022-3476(96)70253-6. [DOI] [PubMed] [Google Scholar]
- 31.Jilma-Stohlawetz P, Homoncik M, Jilma B, et al. High levels of reticulated platelets and thrombopoietin characterize fetal thrombopoiesis. Br J Haematol. 2001;112:466–8. doi: 10.1046/j.1365-2141.2001.02524.x. [DOI] [PubMed] [Google Scholar]
- 32.Saxonhouse MA, Sola MC, Pastos KM, et al. Reticulated platelet percentages in term and preterm neonates. J Pediatr Hematol Oncol. 2004;26:797–802. [PubMed] [Google Scholar]
- 33.Briggs C, Hart D, Kunka S, et al. Immature platelet fraction measurement: a future guide to platelet transfusion requirement after haematopoietic stem cell transplantation. Transfus Med. 2006;16:101–9. doi: 10.1111/j.1365-3148.2006.00654.x. [DOI] [PubMed] [Google Scholar]
- 34.Briggs C, Kunka S, Hart D, et al. Assessment of an immature platelet fraction (IPF) in peripheral thrombocytopenia. Br J Haematol. 2004;126:93–9. doi: 10.1111/j.1365-2141.2004.04987.x. [DOI] [PubMed] [Google Scholar]
- 35.Abe Y, Wada H, Tomatsu H, et al. A simple technique to determine thrombopoiesis level using immature platelet fraction (IPF) Thromb Res. 2006;118:463–9. doi: 10.1016/j.thromres.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 36.Saigo K, Sakota Y, Masuda Y, et al. Automatic detection of immature platelets for decision making regarding platelet transfusion indications for pediatric patients. Transfus Apher Sci. 2008;38:127–32. doi: 10.1016/j.transci.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 37.Cremer M, Paetzold J, Schmalisch G, et al. Immature platelet fraction as novel laboratory parameter predicting the course of neonatal thrombocytopenia. Br J Haematol. doi: 10.1111/j.1365-2141.2008.07485.x. in press. [DOI] [PubMed] [Google Scholar]
- 38.Robinson M, Machin S, Mackie I, Harrison P. Comparison of glycocalicin, thrombopoietin and reticulated platelet measurement as markers of platelet turnover in HIV+ samples. Platelets. 2001;12:108–13. doi: 10.1080/09537100020047093. [DOI] [PubMed] [Google Scholar]
- 39.van den Oudenrijn S, Bruin M, Folman CC, et al. Three parameters, plasma thrombopoietin levels, plasma glycocalicin levels and megakaryocyte culture, distinguish between different causes of congenital thrombocytopenia. Br J Haematol. 2002;117:390–8. doi: 10.1046/j.1365-2141.2002.03455.x. [DOI] [PubMed] [Google Scholar]
- 40.Fabris F, Cordiano I, Steffan A, et al. Indirect study of thrombopoiesis (TPO, reticulated platelets, glycocalicin) in patients with hereditary macrothrombocytopenia. Eur J Haematol. 2000;64:151–6. doi: 10.1034/j.1600-0609.2000.90072.x. [DOI] [PubMed] [Google Scholar]
- 41.Koike Y, Yoneyama A, Shirai J, et al. Evaluation of thrombopoiesis in thrombocytopenic disorders by simultaneous measurement of reticulated platelets of whole blood and serum thrombopoietin concentrations. Thromb Haemost. 1998;79:1106–10. [PubMed] [Google Scholar]
- 42.Kurata Y, Hayashi S, Kiyoi T, et al. Diagnostic value of tests for reticulated platelets, plasma glycocalicin, and thrombopoietin levels for discriminating between hyperdestructive and hypoplastic thrombocytopenia. Am J Clin Pathol. 2001;115:656–64. doi: 10.1309/RAW2-0LQW-8YTX-941V. [DOI] [PubMed] [Google Scholar]
- 43.Sola MC, Rimsza LM, Christensen RD. A bone marrow biopsy technique suitable for use in neonates. Br J Haematol. 1999;107:458–60. doi: 10.1046/j.1365-2141.1999.01712.x. [DOI] [PubMed] [Google Scholar]
- 44.Murray NA. Evaluation and treatment of thrombocytopenia in the neonatal intensive care unit. Acta Paediatr Suppl. 2002;91:74–81. doi: 10.1111/j.1651-2227.2002.tb02908.x. [DOI] [PubMed] [Google Scholar]
- 45.Roberts IA, Murray NA. Thrombocytopenia in the newborn. Curr Opin Pediatr. 2003;15:17–23. doi: 10.1097/00008480-200302000-00004. [DOI] [PubMed] [Google Scholar]
- 46.Watts TL, Murray NA, Roberts IA. Thrombopoietin has a primary role in the regulation of platelet production in preterm babies. Pediatr Res. 1999;46:28–32. doi: 10.1203/00006450-199907000-00005. [DOI] [PubMed] [Google Scholar]
- 47.Meberg A. Transitory thrombocytopenia in newborn mice after intrauterine hypoxia. Pediatr Res. 1980;14:1071–3. doi: 10.1203/00006450-198009000-00010. [DOI] [PubMed] [Google Scholar]
- 48.McDonald TP, Cottrell MB, Steward SA, et al. Comparison of platelet production in two strains of mice with different modal megakaryocyte DNA ploidies after exposure to hypoxia. Exp Hematol. 1992;20:51–6. [PubMed] [Google Scholar]
- 49.Fandrey J. Oxygen-dependent and tissue-specific regulation of erythropoietin gene expression. American Journal of Physiology. 2004;286:R977–88. doi: 10.1152/ajpregu.00577.2003. [DOI] [PubMed] [Google Scholar]
- 50.McDonald TP, Clift RE, Cottrell MB. Large, chronic doses of erythropoietin cause thrombocytopenia in mice. Blood. 1992;80:352–8. [PubMed] [Google Scholar]
- 51.Ohls RK, Ehrenkranz RA, Wright LL, et al. Effects of early erythropoietin therapy on the transfusion requirements of preterm infants below 1250 grams birth weight: a multicenter, randomized, controlled trial. Pediatrics. 2001;108:934–42. doi: 10.1542/peds.108.4.934. [DOI] [PubMed] [Google Scholar]
- 52.Maier RF, Obladen M, Muller-Hansen I, et al. Early treatment with erythropoietin beta ameliorates anemia and reduces transfusion requirements in infants with birth weights below 1000 g. J Pediatr. 2002;141:8–15. doi: 10.1067/mpd.2002.124309. [DOI] [PubMed] [Google Scholar]
- 53.Papayannopoulou T, Brice M, Farrer D, Kaushansky K. Insights into the cellular mechanisms of erythropoietin-thrombopoietin synergy. Exp Hematol. 1996;24:660–9. [PubMed] [Google Scholar]
- 54.Saxonhouse MA, Rimsza LM, Stevens G, et al. Effects of hypoxia on megakaryocyte progenitors obtained from the umbilical cord blood of term and preterm neonates. Biol Neonate. 2006;89:104–8. doi: 10.1159/000088561. [DOI] [PubMed] [Google Scholar]
- 55.Colarizi P, Fiorucci P, Caradonna A, et al. Circulating thrombopoietin levels in neonates with infection. Acta Paediatr. 1999;88:332–7. doi: 10.1080/08035259950170132. [DOI] [PubMed] [Google Scholar]
- 56.Brown RE, Rimsza LM, Pastos K, et al. Effects of Sepsis on Neonatal Thrombopoiesis. Pediatr Res. 2008 doi: 10.1203/PDR.0b013e318181ad49. [DOI] [PubMed] [Google Scholar]
- 57.Lambert MP, Rauova L, Bailey M, et al. Platelet factor 4 is a negative autocrine in vivo regulator of megakaryopoiesis: clinical and therapeutic implications. Blood. 2007;110:1153–60. doi: 10.1182/blood-2007-01-067116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Scaradavou A. HIV-related thrombocytopenia. Blood Rev. 2002;16:73–6. doi: 10.1054/blre.2001.0188. [DOI] [PubMed] [Google Scholar]
- 59.Roux W, Pieper C, Cotton M. Thrombocytopoenia as marker for HIV exposure in the neonate. J Trop Pediatr. 2001;47:208–10. doi: 10.1093/tropej/47.4.208. [DOI] [PubMed] [Google Scholar]
- 60.Nardi M, Tomlinson S, Greco MA, Karpatkin S. Complement-independent, peroxide-induced antibody lysis of platelets in HIV-1-related immune thrombocytopenia. Cell. 2001;106:551–61. doi: 10.1016/s0092-8674(01)00477-9. [DOI] [PubMed] [Google Scholar]
- 61.Cole JL, Marzec UM, Gunthel CJ, et al. Ineffective platelet production in thrombocytopenic human immunodeficiency virus-infected patients. Blood. 1998;91:3239–46. [PubMed] [Google Scholar]
- 62.Sato T, Sekine H, Kakuda H, et al. HIV infection of megakaryocytic cell lines. Leuk Lymphoma. 2000;36:397–404. doi: 10.3109/10428190009148861. [DOI] [PubMed] [Google Scholar]
- 63.Rozmyslowicz T, Majka M, Kijowski J, et al. Platelet- and megakaryocyte-derived microparticles transfer CXCR4 receptor to CXCR4-null cells and make them susceptible to infection by X4-HIV. Aids. 2003;17:33–42. doi: 10.1097/00002030-200301030-00006. [DOI] [PubMed] [Google Scholar]
- 64.Tighe P, Rimsza LM, Christensen RD, et al. Severe thrombocytopenia in a neonate with congenital HIV infection. J Pediatr. 2005;146:408–13. doi: 10.1016/j.jpeds.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 65.Fowler KB, Stagno S, Pass RF. Maternal age and congenital cytomegalovirus infection: screening of two diverse newborn populations, 1980-1990. J Infect Dis. 1993;168:552–6. doi: 10.1093/infdis/168.3.552. [DOI] [PubMed] [Google Scholar]
- 66.Boppana SB, Pass RF, Britt WJ, et al. Symptomatic congenital cytomegalovirus infection: neonatal morbidity and mortality. Pediatr Infect Dis J. 1992;11:93–9. doi: 10.1097/00006454-199202000-00007. [DOI] [PubMed] [Google Scholar]
- 67.Kylat RI, Kelly EN, Ford-Jones EL. Clinical findings and adverse outcome in neonates with symptomatic congenital cytomegalovirus (SCCMV) infection. Eur J Pediatr. 2006;165:773–8. doi: 10.1007/s00431-006-0172-6. [DOI] [PubMed] [Google Scholar]
- 68.Aslam M, Anderson JL, Guglietti D, Cardwell D. CMV-induced neonatal thrombocytopenia: a case report and review of the literature. Am J Perinatol. 2007;24:429–34. doi: 10.1055/s-2007-984409. [DOI] [PubMed] [Google Scholar]
- 69.Muller A, Eis-Hubinger AM, Brandhorst G, et al. Oral valganciclovir for symptomatic congenital cytomegalovirus infection in an extremely low birth weight infant. J Perinatol. 2008;28:74–6. doi: 10.1038/sj.jp.7211854. [DOI] [PubMed] [Google Scholar]
- 70.Steinberg HN, Anderson J, Jr, Lim B, Chatis PA. Cytomegalovirus infection of the BS-1 human stroma cell line: effect on murine hemopoiesis. Virology. 1993;196:427–32. doi: 10.1006/viro.1993.1498. [DOI] [PubMed] [Google Scholar]
- 71.Abzug MJ, Levin MJ, Rotbart HA. Profile of enterovirus disease in the first two weeks of life. Pediatr Infect Dis J. 1993;12:820–4. doi: 10.1097/00006454-199310000-00005. [DOI] [PubMed] [Google Scholar]
- 72.Cheng LL, Ng PC, Chan PK, et al. Probable intrafamilial transmission of coxsackievirus b3 with vertical transmission, severe early-onset neonatal hepatitis, and prolonged viral RNA shedding. Pediatrics. 2006;118:e929–33. doi: 10.1542/peds.2006-0554. [DOI] [PubMed] [Google Scholar]


