Abstract
Tristetraprolin/zinc finger protein 36 (TTP/ZFP36) binds and destabilizes some proinflammatory cytokine mRNAs. TTP-deficient mice develop a profound inflammatory syndrome due to excessive production of proinflammatory cytokines. TTP gene expression is induced by various factors including insulin, cinnamon, and green tea extracts. Previous studies have shown that TTP is highly phosphorylated in vivo and multiple phosphorylation sites are identified in human TTP. This study evaluated the potential protein kinases that could phosphorylate recombinant TTP in vitro. Motif scanning suggested that TTP was a potential substrate for various kinases. SDS-PAGE showed that in vitro phosphorylation of TTP with p42 and p38 MAP kinases resulted in visible electrophoretic mobility shift of TTP to higher molecular masses. Autoradiography showed that TTP was phosphorylated in vitro by GSK3b, PKA, PKB, PKC, but not Cdc2, in addition to p42, p38, and JNK. These results demonstrate that TTP is a substrate for a number of protein kinases in vitro.
Keywords: Glycogen synthase kinase 3b, Inflammation, Phosphorylation, Protein kinase, Recombinant protein, Tristetraprolin, Zinc finger protein
1. INTRODUCTION
Tristetraprolin (TTP) is the best-understood member of a small family of tandem CCCH zinc finger proteins (ZFP). Similar tandem CCCH zinc finger sequences have been found in many species, ranging from human through yeasts and plants. TTP protein family consist of three known members in mammals (ZFP36 or TTP, ZFP36L1 or TIS11B, and ZFP36L2 or TIS11D) and the fourth member in mouse and rat but not in humans (ZFP36L3) (Blackshear 2002; Blackshear et al. 2005). Separate genes encode these four proteins, and their patterns of cell- and tissue-specific expression and agonist-stimulated expression are quite different. However, they share certain sequence characteristics: All four have highly conserved tandem zinc finger domains, in which each C8×C5×C3×H zinc finger is preceded by the sequence (R/K)YKTEL, and the two fingers are separated by 18 amino acids; and all are capable of binding AU-rich elements (ARE) within single stranded RNAs (Blackshear et al. 2003; Cao et al. 2003; Cao 2004; Carballo et al. 1998; Carballo et al. 2001; Phillips et al. 2004; Worthington et al. 2002) and promoting the deadenylation and subsequent destruction of those transcripts, both in transfection studies and in cell-free experiments (Lai et al. 1999; Lai et al. 2003). In intact animals, TTP deficiency causes a profound inflammatory syndrome with erosive arthritis, autoimmunity, and myeloid hyperplasia (Phillips et al. 2004; Taylor et al. 1996). This is apparently due almost entirely to excessive production of tumor necrosis factor α (TNF) and granulocyte-macrophage colony-stimulating factor, whose mRNAs are direct targets of TTP but are stabilized in cells from TTP knockout mice (Carballo et al. 1998; Carballo et al. 2000; Lai et al. 1999). For these reasons, TTP can be thought of as an anti-inflammatory protein and arthritis suppressor.
The cDNAs encoding TTP were originally cloned by three groups based on its very rapid and dramatic transcriptional induction in fibroblasts in response to insulin, phorbol esters, and serum (DuBois et al. 1990; Lai et al. 1990; Varnum et al. 1991). Recently, we have shown that TTP protein is a very low abundance cytosolic protein, whose level is also dramatically induced by lipopolysaccharide (LPS), fetal calf serum, cinnamon polyphenols, and green tea extract (Cao et al. 2004; Cao et al. 2007a; Cao et al. 2007b). The protein is very stable once induced, in contrast to its very labile mRNA (Cao et al. 2004). In addition, TTP in normal tissues and in stimulated cells exhibits a much larger molecular mass than the predicted size (Cao et al. 2004), probably due to extensive phosphorylation in intact cells and tissues. This conclusion is supported by the fact that dephosphorylation of TTP from transfected human 293 cells and LPS-stimulated mouse RAW 264.7 cells results in TTP close to the size of TTP expressed and purified from E. coli (Cao. 2004; Cao et al. 2004).
TTP phosphorylation has been studied by several laboratories. TTP could be phosphorylated in intact cells and in cell-free systems by p42 mitogen-activated protein kinase (MAPK) (ERK2) (Cao et al. 2003; Cao 2004; Taylor et al. 1995), p38 MAP kinase (Cao et al. 2003; Cao 2004; Carballo et al. 2001; Zhu et al. 2001), c-Jun N-terminal kinase (JNK) (Cao et al. 2003), and MAP kinase-activated protein kinase 2 (MAPKAP kinase 2 or MK2) (Chrestensen et al. 2004; Mahtani et al. 2001; Ming et al. 2001; Stoecklin et al. 2004). Mass spectrometry and site-directed mutagenesis have identified a number of phosphorylation sites in human and mouse TTP (Cao et al. 2006; Chrestensen et al. 2004; Taylor et al. 1995; Cao and Deterding 2007). However, much needs to be learned about the potential kinases that phosphorylate TTP. In this study, we extended our investigation on the identification of potential protein kinases targeting TTP using motif scanning, electrophoretic mobility shift assay, and in vitro phosphorylation assay. Our results demonstrate that TTP is a substrate for a number of protein kinases in vitro.
2. MATERIALS AND METHODS
2.1. Chemicals and Reagents
The protein kinases used are glycogen synthase kinase b (GSK3β) (recombinant histidine-tagged rabbit protein from E. coli, Sigma, St. Louis, MO), protein kinase (PK)A (cAMP-dependent protein kinase, catalytic subunit from bovine heart, Calbiochem, La Jolla, CA), PKBα/Akt1 (histidine-tagged, activated human protein from S. frugiperda, Calbiochem), PKCμ (catalytic domain from rat brain, Calbiochem), Cdc2/CDK1/p34cdc2 (recombinant human Cdc2 kinase catalytic subunit from Sf9 insect cells, Sigma); p42/ERK2 MAPK (recombinant His-tagged rat protein purified from E. coli, Calbiochem,), p38 MAPK (GST-tagged mouse p38 MAP kinase purified from E. coli, Calbiochem), and JNK (calmodulin-binding peptide with 24 amino acid residues-tagged rat protein purified from E. coli, Calbiochem).
2.2. Protein Concentration Determination
Protein concentrations were determined with modifications using the Protein Assay Dye Reagent Concentrate (Bio-Rad) following NaOH treatment of the samples (Cao 2004). Bovine serum albumin from Bio-Rad was used as the protein standard.
2.3. SDS-PAGE and Immunoblotting
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting were performed (Cao et al. 2003). Briefly, proteins were separated by 10% SDS-PAGE and transferred onto nitrocellulose membranes. The membranes were blocked with 5% nonfat dry milk in TTBS buffer, and successively incubated in buffers containing the primary antibodies (1:10000 dilution) overnight and the secondary antibodies (1:10000 dilution) for 4 h. Proteins on the immunoblots were detected using SuperSignal West Pico Chemiluminescent Substrate (Pierce, Rockford, IL). The primary antibodies were anti-MBP-hTTP serum (Cao 2004) and anti-MBP-mTTP serum (Cao et al. 2004) raised against the recombinant full-length human or mouse TTP fused to E. coli maltose-binding protein. The secondary antibodies were affinity-purified goat anti-rabbit IgG (H+L) horseradish peroxidase conjugate with human IgG absorbed (GAR-HRP, Bio-Rad).
2.4. Recombinant TTP Expression and Purification
Plasmids pMBP-hTTP and pMBP-mTTP were used to express the full-length human TTP (hTTP, GenBank accession no. NP_003398) and mouse TTP (mTTP, GenBank accession no. NP_035886) as recombinant proteins fused to E. coli maltose binding protein (MBP) (Cao et al. 2003). Recombinant TTP was expressed and purified from E. coli (Cao et al. 2003). Briefly, plasmids were transformed into E. coli BL21(DE3) cells. TTP fusion proteins were induced with 0.4 mM isopropylthio-β-D-galactoside. Cells were homogenized in a buffer containing 20 mM Tris-HCl, pH 7.4, 200 mM NaCl, 10 mM β-mercaptoethanol, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 2 μM leupeptin, 2 μM pepstatin, 2 μg/ml aprotinin and with 2 mM zinc chloride.
MBP-TTP fusion proteins were purified by fast protein liquid chromatography (Amersham Pharmacia Biotech) from the 10000 g supernatant with amylose resin affinity chromatography in buffers with 1 mM zinc chloride (Cao et al. 2003). TTP fusion proteins were stored at −20 °C in 20% glycerol (v/v), 10 mM maltose, 20 mM Tris-HCl, pH 7.4, 200 mM NaCl, 10 mM β-mercaptoethanol), and 1 mM zinc chloride. MBP was affinity-purified from E. coli expressing plasmid pMAL2c (New England Biolabs) by the same amylose resin chromatography and used as a control in these experiments.
2.5. Protein Kinase Activity Assay in Vitro
The purified recombinant MBP-TTP or MBP alone was used for phosphorylation reactions with GSK3β, PKA, PKBα/Akt1, PKCμ, Cdc2, p42, p38, and JNK. The general procedures for the in vitro protein kinase assays were similar to those previously described (Cao et al. 2003). The phosphorylation reactions were initiated by the addition of labeled [γ32-P]-ATP, incubated at 30 °C for various times, and terminated by the addition of 1/5 volume of 5x SDS-PAGE sample buffer (Cao et al. 2003). The labeled protein was separated from free ATP by 10% SDS-PAGE. The gel was dried on Whatman paper for 60–90 min at 80 °C before being exposed to X-ray film and/or Phosphorimager (Molecular Dynamics, Sunnyvale, CA).
The GSK3β kinase assay was performed for various times at 30 °C in 20 μl containing ~10 pmol protein, 1 unit enzyme, and 10 μM ATP (200 pmol, ~1 μCi) in GSK3β buffer (25 mM Tris-HCl, pH 7.4, 10 mM MgCl2, 1 mM DTT, 0.1 mM EGTA, and 0.1 mM Na3VO4). The PKA kinase assay was performed for various times at 30 °C in 20 μl containing ~10 pmol protein, 1 μl enzyme, and 10 μM ATP (200 pmol, ~1 μCi) in PKA buffer (40 mM MES, pH 6.0, 1 mM EGTA, 10 mM MgCl2). The PKBα (Akt1) kinase assay was performed for various times at 30 °C in 20 μl containing ~10 pmol protein, 12 unit (1 μl) enzyme, and 10 μM ATP (200 pmol, ~1.5 μCi) in PKBα buffer (20 mM Tris-HCl, pH 7.5, 25 mM β-glycerol phosphate, 5 mM EGTA, 1 mM sodium orthovanadate, 1 mM DTT, 20 mM MgCl2). The PKCμ kinase assay was performed for various times at 30 °C in 20 μl containing ~10 pmol protein, 0.1 μl enzyme, and 10 μM ATP (200 pmol, ~2.5 μCi) in PKCμ buffer (40 mM Hepes, pH 7.4, 1 mM EGTA, 10 mM MgCl2, 1 mM CaCl2). The time course of GSK3β phosphorylation was done for 0–80 min at 30 °C in 150 μl containing ~100 pmol protein, 7.5 unit enzyme, and 10 μM ATP (1500 pmol, ~8 μCi) in GSK3β buffer and the phosphorylation product was analyzed at each time point using 10 μl reaction mixture (~6.5 pmol protein, 0.5 unit enzyme, 100 pmol ATP). The kinetics of GSK3β phosphorylation were done for 15 min at 30 °C in 10 μl containing 0–20 pmol protein, 0.35 unit enzyme, and 10 μM ATP (100 pmol, ~1 μCi) in GSK3β buffer. P42, p38, and JNK phosphorylation assays were previously described (Cao et al. 2003). The Cdc2 kinase assay was performed for various times at 30 °C in 20 μl containing ~10 pmol protein, 0.1 μl enzyme, and 100 μM ATP (200 pmol, ~2.5 μCi) in a buffer (50 mM Tris-HCl, pH 7.5, 1 mM EGTA, 10 mM MgCl2, 2 mM DTT).
3. RESULTS
3.1. Prediction of Putative Protein Kinases for the Phosphorylation of TTP
Human TTP protein sequence was imported into a motif scanning program (http://scansite.mit.edu) (Obenauer et al. 2003; Yaffe et al. 2001) for predicting potential phosphorylation sites and their putative protein kinases. Motif scanning results suggested that human TTP was a potential substrate for a variety of protein kinases (Table 1), including: 1) ERK1 [The consensus sequence motif is Px1–4(S/T)P] (Pearson et al. 2001) sites at S41 [PWSLSP in hTTP], S88 [PELSP in hTTP], S218 [PSSSP in hTTP], and S228 [PLSP in hTTP]; 2) p38 MAPK [The consensus sequence motif is Px(S/T)P] (Hawkins et al. 2000) sites at S93 [PTSP in hTTP] and T238 [PGTP in hTTP]; 3) a Cdc2 [The consensus sequence motif is x(S/T)Px(R/K)] (Blom et al. 2004) site at T238 [GTPLAR in hTTP]; 4) a GSK3 [The consensus sequence motif is (S/T)xxx(S/T)] (Frame and Cohen 2001) site at S218 [SPSSS in hTTP]; 5) PKA [The consensus sequence motif is Rx1–2S/Tx] (Blom et al. 2004) sites at S197 [RTSP in hTTP] and T257 [RATP in hTTP]; 6) a PKCμ [The consensus sequence motif is LVxxxS] (Nishikawa et al. 1997) site at S66 [LVEGRS in hTTP]; and 7) a PKC [The consensus sequence motif is x(S/T)x(R/K)] (Blom et al. 2004) site at S252 [PSCR in hTTP]. Some of the predicted phosphorylation sites are identified by mass spectrometry (Table 1) (Cao et al. 2006).
Table 1.
TTP is predicted to be phosphorylated at multiple sites by several protein kinases
| Predicted protein kinase | Predicted phosphorylaton site position | Predicted peptide sequence with phosphorylaton site in bold letter | Phosphorylation sites observed by mass spectrometric analysis (Cao et al. 2006) |
|---|---|---|---|
| ERK1 | 41 | SSGPWSLSPSDSSPS | S41 |
| 88 | PRLGPELSPSPTSPT | S88 | |
| 214 | SLSSSSFSPSSSPPP | ||
| 218 | SSFSPSSSPPPPGDL | S218 | |
| 228 | PPGDLPLSPSAFSAA | S228 | |
|
| |||
| P38 | 93 | ELSPSPTSPTATSTT | S93 |
| 238 | AFSAAPGTPLARRDP | T238 | |
|
| |||
| GSK3 | 35 | SSPGWGSSGPWSLSP | |
| 39 | WGSSGPWSLSPSDSS | ||
| 52 | SSPSGVTSRLPGRST | ||
| 214 | SLSSSSFSPSSSPPP | ||
| 218 | SSFSPSSSPPPPGDL | S218 | |
|
| |||
| PKA | 257 | CPSCRRATPISVWGP | T257 |
| 197 | LPSGRRTSPPPPGLA | S197 | |
|
| |||
| PKB/Akt1 | 60 | RLPGRSTSLVEGRSC | |
| 113 | TELCRTFSESGRCRY | ||
|
| |||
| PKC-a/b/g | 252 | PTPVCCPSCRRATPI | S252 |
| -epsilon | 111 | YKTELCRTFSESGRC | T111 |
| -mu | 66 | TSLVEGRSCGWVPPP | S66 |
| -zeta | 144 | NRHPKYKTELCHKFY | |
|
| |||
| Cdc2/Cdk5 | 238 | AFSAAPGTPLARRDP | T238 |
| 100 | SPTATSTTPSRYKTE | ||
3.2. Scanning Protein Kinases by Electrophoretic Mobility Shift Assay
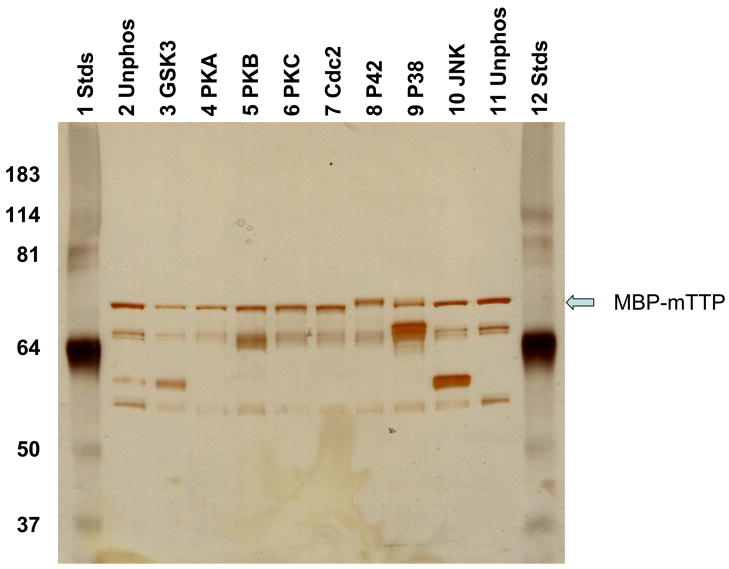
Non-fusion TTP is difficult to be purified from expressed E. coli due to insolubility of the protein (Cao et al. 2003). Therefore, recombinant MBP-TTP was selected for our current experiments. MBP-mTTP was incubated with various protein kinases. The results showed that only p42 and p38 MAPK reactions resulted in visible shift of the protein on SDS-PAGE (Fig. 1). The MBP-TTP bands were positively identified by immunoblotting using antibodies against recombinant MBP-TTP proteins (data not shown). No apparent mobility shift of TTP was detected by in vitro phosphorylation with GAS3b, PKA, PKB, PKC, Cdc2, or JNK kinases (Fig. 1).
Fig. 1.
Effects of in vitro phosphorylation on the electrophoretic mobility of TTP. MBP-mTTP was phosphorylated in vitro. The reaction mixtures were separated by SDS-PAGE. The proteins were visualized by staining the gel with silver reagent.
3.3. Phosphorylation of Recombinant TTP by Glycogen Synthase Kinase-3β in Vitro
The increased size of MBP-mTTP on SDS-PAGE suggest that the induced TTP was phosphorylated by p42 and p38 MAPK, but the mobility shift assay is not always definitive and other kinases might phosphorylate TTP but not cause visible changes in gel mobility. We therefore confirmed the test by use of an in vitro phosphorylation assay.
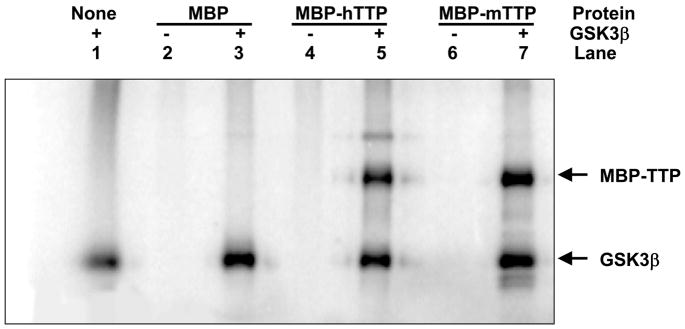
We first investigated if TTP is a substrate for GSK3β in vitro. MBP-TTP (both MBP-hTTP and MBP-mTTP) purified from E. coli by amylose resin affinity columns was used to investigate the phosphorylation of TTP. MBP-TTP but not MBP was phosphorylated by GSK3β (Fig. 2, lanes 5 and 7), while MBP-TTP was not auto-phosphorylated (lanes 4 and 6). There was, however, apparent auto-phosphorylation of GSK3β protein (lanes 1, 3, 5 and 7). These results suggest that TTP is specifically phosphorylated by GSKβ in vitro.
Fig. 2.
Phosphorylation of TTP by GSK3β in vitro. MBP-mTTP and MBP-hTTP (1 μg) were purified by amylose resin column and used as a substrate for GSK3β (1 unit). The GSK3β kinase assay was performed for 30 min at 30 °C in 20 μl containing ~10 pmol protein, 1 unit enzyme, and 10 μM ATP (200 pmol, ~1 μCi). The labeled protein was separated from free ATP by 10% SDS-PAGE. The gel was dried and exposed to X-ray film.
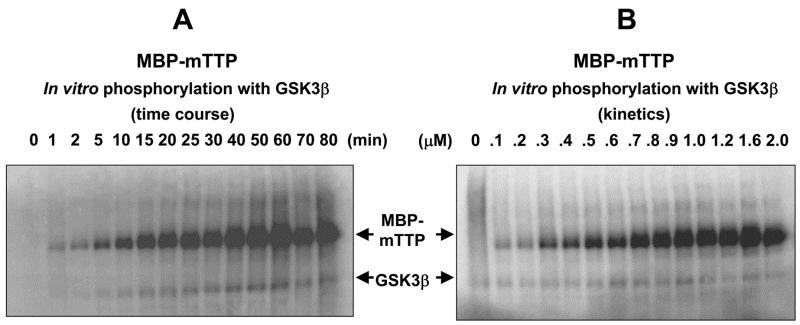
When 1 μM MBP-mTTP was used as a substrate, half-maximal phosphorylation was achieved within 30 min and phosphorylation reached maximal at about 60 min at 30 °C with GSK3β (Fig. 3A). Increasing concentrations of MBP-mTTP showed half-maximal phosphorylation at approximately 0.8 μM in the linear portion of the time course analysis at 15 min (Fig. 3B).
Fig. 3.
Time-course and kinetics of phosphorylation of TTP by GSK3β in vitro. (A) Time course of the phosphorylation reactions. The reactions were performed at 30 °C for various times using 1 pmol MBP-mTTP as the substrate in 150 μl (10 μl per time point). (B) Substrate concentration dependence of the phosphorylation reactions. The reactions were performed at 30 °C for 15 min using various amounts of MBP-mTTP in 20 μl. The labeled protein was separated from free ATP by 10% SDS-PAGE. The gel was dried and exposed to Phosphorimager.
3.4. Phosphorylation of Recombinant TTP by Protein Kinases A, B, and C in Vitro
Motif scanning (http://scansite.mit.edu) also predicted that TTP was potentially phosphorylated by PKA and PKCμ (Table 1). MBP-mTTP could be phosphorylated in vitro by PKA (Fig. 4A), PKBα (Fig. 4B), and PKCμ (Fig. 4C), while MBP was not phosphorylated by these same protein kinases (data not shown).
Fig. 4.
Phosphorylation of TTP by PKA, PKBα (Akt1), and PKCμ in vitro. MBP-mTTP (1 μg) was purified by amylose resin column and used as a substrate for the protein kinase assays in 20 μl for 30 min at 30 °C. The labeled protein was separated from free ATP by 10% SDS-PAGE. The gel was dried and exposed to X-ray film. (A) The PKA kinase assay contained ~10 pmol protein, 1 μl enzyme, and 10 μM ATP. (B) The PKBα (Akt1) kinase assay contained ~10 pmol protein, 12 unit (1 μl) enzyme, and 10 μM ATP. (C) The PKCμ kinase assay contained ~10 pmol protein, 0.1 μl enzyme, and 10 μM ATP.
4. DISCUSSION
It has been known for many years that TTP is highly phosphorylated in intact cells, and that this modification can affect the electrophoretic mobility of the protein (Blackshear 2002). Mass spectrometry and site-directed mutagenesis have identified a number of phosphorylation sites in human TTP, including S66, S88, T92, S169, S186, S197, S218, S228, S276, and S296, and 29 other potential phosphorylation sites (Cao et al. 2006; Cao and Deterding 2007). To better understand TTP phosphorylation reactions, we investigated the protein kinases that may phosphorylate TTP using motif scanning, electrophoretic mobility shift assay, and in vitro phosphorylation assay. Our results demonstrate that TTP is a substrate for various protein kinases in vitro.
In this report, we have shown by motif scanning program that human TTP is potentially phosphorylated at multiple sites by a variety of protein kinases, including: 1) ERK1; 2) p38 MAPK; 3) Cdc2; 4) GSK3; 5) PKA; 6) PKCμ; and 7) PKC. Some of the predicted phosphorylation sites are experimentally identified by mass spectrometry (Table 1) (Cao et al. 2006). Electrophoretic mobility shift assay showed that p42 and p38 MAP kinases caused slower mobility of recombinant TTP on SDS-PAGE after in vitro phosphorylation reactions than those untreated TTP. Previous studies have demonstrated that decreases in the electrophoretic mobility of proteins on SDS-PAGE can serve as an indicator of stoichiometric phosphorylation in some proteins (Rangel-Aldao et al. 1979; Rodriguez et al. 2003). Similar to these reports, in vitro phosphorylation of TTP using protein kinases results in a decrease in mobility on SDS-PAGE (Cao 2004). On the other hand, dephosphorylation of TTP from mammalian cells significantly increases its electrophoretic mobility on SDS-PAGE (Cao 2004; Cao et al. 2004; Carballo et al. 2001; Taylor et al. 1995). These results support the notion that p42 and p38 MAP kinases stoichiometrically phosphorylate recombinant TTP in vitro (Cao et al. 2003; Cao 2004).
Recombinant TTP was further shown to be phosphorylated by GSK3β, PKA, PKBα (Akt1), and PKCμ under in vitro conditions. Recombinant MBP-TTP was used in this study because non-fusion TTP protein is difficult to be purified from expressed E. coli due to insolubility and MBP fusion increases the solubility of its fusion partners (Cao et al. 2003; Kapust and Waugh 1999). Because MBP forms separate domains from its fusion partner, MBP may not interfere with its fusion partner’s conformation (Liu et al. 2001). Therefore, recombinant MBP could be an appropriate negative control for the phosphorylation reactions of recombinant MBP-TTP. Under this assumption and since MBP alone was not phosphorylated by the protein kinases we tested, we conclude that TTP is phosphorylated by GSK3β, PKA, PKBα (Akt1), and PKCμ. These results extend previous observations that recombinant TTP is phosphorylated in vivo and in vitro by a number of protein kinases, including p42 MAP kinase, p38 MAP kinase, JNK, and MK2 (Chrestensen et al. 2004; Mahtani et al. 2001; Ming et al. 2001; Stoecklin et al. 2004). Since GSK3β and PKBα (Akt1) act downstream in the insulin signal transduction pathway (Frame and Cohen 2001), our results suggest that TTP phosphorylation may be similarly affected by insulin induced protein kinases.
It is noted that recombinant MBP-TTP without phosphorylation and those treated with several protein kinases appeared as sharp bands on SDS-PAGE after silver staining (Fig. 1). However, those MBP-TTP molecules treated with GSK3β, PKA, PKBα, and PKCμunder in vitro phosphorylation conditions resulted in much broader and more diffuse bands on autoradiograms (Figs. 2–4). The reason for this discrepancy is probably due to the much more sensitive detection method with autoradiography than that with silver staining.
A major task for future studies is to understand the phosphorylation reactions on TTP function(s). A number of investigations have studied the effects of phosphorylation on TTP function(s). MK2 phosphorylation on TTP stability and function is well documented in recent publications. MK2 was shown to be essential for the stabilization of TTP mRNA. MK2 phosphorylation leads to increased TTP protein stability but reduced ARE affinity (Hitti et al. 2006). Mouse TTP is phosphorylated by MK2 at S52 (corresponding to S60 in human TTP) and S178 (corresponding to S186 in human TTP) in vivo and in vitro (Chrestensen et al. 2004). The regulation of both subcellular localization and protein stability of mouse TTP is dependent on MK2 and on the integrity of S52 and S178 (Brook et al. 2006). Phosphorylation of mouse TTP at S178 increases the relative ratio of TTP protein in the cytoplasm (Johnson et al. 2002). Recent experiments have shown that mutation of S52 to A52 in mouse TTP weakly reduces the assembly of TTP-14-3-3, whereas mutation of S178 to A178 and of S52/178 to A52/178 substantially reduces the association of mouse TTP with 14-3-3 (Sun et al. 2007). Finally, global dephosphorylation of mouse TTP by calf intestine alkaline phosphatase (CIAP) prevents TTP from binding to 14-3-3 proteins (Johnson et al. 2002). However, little information is available about the impact of phosphorylation by other protein kinases on TTP functions.
The relationship between TTP phosphorylation and its mRNA binding activity requires more studies in the future. It is well known that phosphorylation of TTP decreases its mRNA binding activity. TTP expressed in human 293 cells and then dephosphorylated by CIAP is able to bind more tightly to an ARE probe than native, phosphorylated TTP (Carballo et al. 2001). TTP purified from overexpressed E. coli exhibits approximately 2-fold greater affinity for the TNF mRNA ARE than the protein purified from transfected human 293 cells (Cao 2004). However, mRNA binding activity of TTP is not apparently affected by individual phosphorylation with p42/ERK2, p38, or JNK MAP kinases (Cao et al. 2003), or by MK2 (Worthington et al. 2002) under in vitro conditions. Understanding the importance of TTP phosphorylation on its function(s) will require further studies to confirm the types of protein kinases and their TTP targeting sites in vivo.
Acknowledgments
This work was supported in part by USDA-ARS Human Nutrition Research Program and NIH-NIEHS Intramural Research Program. A preliminary report of this study was presented at the Experimental Biology 2006 in San Francisco, California, on April 1–5, 2006. We greatly appreciate Dr. Perry J. Blackshear (NIH/NIEHS) for his generous support. We also thank Dr. Joseph F. Urban Jr. (USDA/ARS) for his helpful comments on the manuscript.
Abbreviations
- TTP
tristetraprolin
- hTTP
human TTP
- mTTP
mouse TTP
- ARE
AU-rich element
- Cdc2
cyclin-dependent kinase 2
- CIAP
calf intestine alkaline phosphatase
- GSK3b
glycogen synthase kinase 3B
- JNK
c-Jun-N-terminal kinase
- LPS
lipopolysaccharide
- MAPK
mitogen-activated protein kinase
- MBP
maltose binding protein
- MK2
MAP kinase-activated protein kinase 2
- PK
protein kinase
- SDS-PAGE
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- TNF
tumor necrosis factor alpha
- ZFP
zinc finger protein
- ZFP36L
ZFP36-like
References
- Blackshear PJ. Biochem Soc Trans. 2002;30:945–952. doi: 10.1042/bst0300945. [DOI] [PubMed] [Google Scholar]
- Blackshear PJ, Lai WS, Kennington EA, Brewer G, Wilson GM, Guan X, Zhou P. J Biol Chem. 2003;278:19947–19955. doi: 10.1074/jbc.M301290200. [DOI] [PubMed] [Google Scholar]
- Blackshear PJ, Phillips RS, Ghosh S, Ramos SB, Richfield EK, Lai WS. Biol Reprod. 2005;73:297–307. doi: 10.1095/biolreprod.105.040527. [DOI] [PubMed] [Google Scholar]
- Blom N, Sicheritz-Ponten T, Gupta R, Gammeltoft S, Brunak S. Proteomics. 2004;4:1633–1649. doi: 10.1002/pmic.200300771. [DOI] [PubMed] [Google Scholar]
- Brook M, Tchen CR, Santalucia T, McIlrath J, Arthur JS, Saklatvala J, Clark AR. Mol Cell Biol. 2006;26:2408–2418. doi: 10.1128/MCB.26.6.2408-2418.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H. Biochemistry. 2004;43:13724–13738. doi: 10.1021/bi049014y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Deterding LJ, Blackshear PJ. Expert Rev Proteomics. 2007;4:711–726. doi: 10.1586/14789450.4.6.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Deterding LJ, Venable JD, Kennington EA, Yates JR, III, Tomer KB, Blackshear PJ. Biochem J. 2006;394:285–297. doi: 10.1042/BJ20051316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Dzineku F, Blackshear PJ. Arch Biochem Biophys. 2003;412:106–120. doi: 10.1016/s0003-9861(03)00012-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Kelly MA, Kari F, Dawson HD, Urban JF, Jr, Coves S, Roussel AM, Anderson RA. J Inflamm (Lond) 2007a;4:1–12. doi: 10.1186/1476-9255-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Polansky MM, Anderson RA. Arch Biochem Biophys. 2007b;459:214–222. doi: 10.1016/j.abb.2006.12.034. [DOI] [PubMed] [Google Scholar]
- Cao H, Tuttle JS, Blackshear PJ. J Biol Chem. 2004;279:21489–21499. doi: 10.1074/jbc.M400900200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carballo E, Cao H, Lai WS, Kennington EA, Campbell D, Blackshear PJ. J Biol Chem. 2001;276:42580–42587. doi: 10.1074/jbc.M104953200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carballo E, Lai WS, Blackshear PJ. Science. 1998;281:1001–1005. doi: 10.1126/science.281.5379.1001. [DOI] [PubMed] [Google Scholar]
- Carballo E, Lai WS, Blackshear PJ. Blood. 2000;95:1891–1899. [PubMed] [Google Scholar]
- Chrestensen CA, Schroeder MJ, Shabanowitz J, Hunt DF, Pelo JW, Worthington MT, Sturgill TW. J Biol Chem. 2004;279:10176–10184. doi: 10.1074/jbc.M310486200. [DOI] [PubMed] [Google Scholar]
- DuBois RN, McLane MW, Ryder K, Lau LF, Nathans D. J Biol Chem. 1990;265:19185–19191. [PubMed] [Google Scholar]
- Frame S, Cohen P. Biochem J. 2001;359:1–16. doi: 10.1042/0264-6021:3590001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins J, Zheng S, Frantz B, LoGrasso P. Arch Biochem Biophys. 2000;382:310–313. doi: 10.1006/abbi.2000.2005. [DOI] [PubMed] [Google Scholar]
- Hitti E, Iakovleva T, Brook M, Deppenmeier S, Gruber AD, Radzioch D, Clark AR, Blackshear PJ, Kotlyarov A, Gaestel M. Mol Cell Biol. 2006;26:2399–2407. doi: 10.1128/MCB.26.6.2399-2407.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA, Stehn JR, Yaffe MB, Blackwell TK. J Biol Chem. 2002;277:18029–18036. doi: 10.1074/jbc.M110465200. [DOI] [PubMed] [Google Scholar]
- Kapust RB, Waugh DS. Protein Sci. 1999;8:1668–1674. doi: 10.1110/ps.8.8.1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai WS, Carballo E, Strum JR, Kennington EA, Phillips RS, Blackshear PJ. Mol Cell Biol. 1999;19:4311–4323. doi: 10.1128/mcb.19.6.4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai WS, Kennington EA, Blackshear PJ. Mol Cell Biol. 2003;23:3798–3812. doi: 10.1128/MCB.23.11.3798-3812.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai WS, Stumpo DJ, Blackshear PJ. J Biol Chem. 1990;265:16556–16563. [PubMed] [Google Scholar]
- Liu Y, Manna A, Li R, Martin WE, Murphy RC, Cheung AL, Zhang G. Proc Natl Acad Sci U S A. 2001;98:6877–82. doi: 10.1073/pnas.121013398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahtani KR, Brook M, Dean JL, Sully G, Saklatvala J, Clark AR. Mol Cell Biol. 2001;21:6461–6469. doi: 10.1128/MCB.21.9.6461-6469.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming XF, Stoecklin G, Lu M, Looser R, Moroni C. Mol Cell Biol. 2001;21:5778–5789. doi: 10.1128/MCB.21.17.5778-5789.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa K, Toker A, Johannes FJ, Songyang Z, Cantley L. J Biol Chem. 1997;272:952–960. doi: 10.1074/jbc.272.2.952. [DOI] [PubMed] [Google Scholar]
- Obenauer JC, Cantley LC, Yaffe MB. Nucl Acids Res. 2003;31:3635–3641. doi: 10.1093/nar/gkg584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, Cobb MH. Endocr Rev. 2001;22:153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- Phillips K, Kedersha N, Shen L, Blackshear PJ, Anderson P. Proc Natl Acad Sci U S A. 2004;101:2011–2016. doi: 10.1073/pnas.0400148101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel-Aldao R, Kupiec JW, Rosen OM. J Biol Chem. 1979;254:2499–508. [PubMed] [Google Scholar]
- Rodriguez P, Bhogal MS, Colyer J. J Biol Chem. 2003;278:38593–600. doi: 10.1074/jbc.C301180200. [DOI] [PubMed] [Google Scholar]
- Stoecklin G, Stubbs T, Kedersha N, Wax S, Rigby WF, Blackwell TK, Anderson P. EMBO J. 2004;23:1313–1324. doi: 10.1038/sj.emboj.7600163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Stoecklin G, Van WS, Hinkovska-Galcheva V, Guo RF, Anderson P, Shanley TP. J Biol Chem. 2007;282:3766–3777. doi: 10.1074/jbc.M607347200. [DOI] [PubMed] [Google Scholar]
- Taylor GA, Carballo E, Lee DM, Lai WS, Thompson MJ, Patel DD, Schenkman DI, Gilkeson GS, Broxmeyer HE, Haynes BF, Blackshear PJ. Immunity. 1996;4:445–454. doi: 10.1016/s1074-7613(00)80411-2. [DOI] [PubMed] [Google Scholar]
- Taylor GA, Thompson MJ, Lai WS, Blackshear PJ. J Biol Chem. 1995;270:13341–13347. doi: 10.1074/jbc.270.22.13341. [DOI] [PubMed] [Google Scholar]
- Varnum BC, Ma QF, Chi TH, Fletcher B, Herschman HR. Mol Cell Biol. 1991;11:1754–1758. doi: 10.1128/mcb.11.3.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthington MT, Pelo JW, Sachedina MA, Applegate JL, Arseneau KO, Pizarro TT. J Biol Chem. 2002;277:48558–48564. doi: 10.1074/jbc.M206505200. [DOI] [PubMed] [Google Scholar]
- Yaffe MB, Leparc GG, Lai J, Obata T, Volinia S, Cantley LC. Nat Biotechnol. 2001;19:348–53. doi: 10.1038/86737. [DOI] [PubMed] [Google Scholar]
- Zhu W, Brauchle MA, Di Padova F, Gram H, New L, Ono K, Downey JS, Han J. Am J Physiol Lung Cell Mol Physiol. 2001;281:499–508. doi: 10.1152/ajplung.2001.281.2.L499. [DOI] [PubMed] [Google Scholar]