Abstract
Biological patterns are often constructed via a combination of mechanisms including self-organization, templates and recipes. Our understanding of self-organization is becoming increasingly clear, yet how multiple mechanisms work together and what selective advantage they confer over simpler mechanisms is poorly understood. Honeybee (Apis mellifera) combs exhibit a pattern of brood at the bottom, pollen in a band next to it and honey at the top. This study constructs an agent-based model, derived from experimental studies, to determine both how self-organization interacts with two templates and to elucidate a selective basis for the use of multiple mechanisms. The vertical pattern of honey and brood is shown to be dependent on a gravity-based template, while the pollen band is shown to form via the interaction of a queen-based template and self-organization. The study suggests that the selective basis for this complex mechanism may be that colonies have higher growth rates when multiple mechanisms are used as opposed to self-organization alone. As self-organization is used in many contexts in which the addition of supplemental mechanisms could be advantageous, this result may be of general significance to many biological systems.
Keywords: pattern formation, self-organization, templates, complexity, social insects, honeybees
1. Introduction
Social insects routinely construct spatiotemporal patterns on a scale far beyond their size (Wilson 1971; Michener 1974; Tschinkel 2004). Self-organization provides one explanation for how they accomplish this feat (Deneubourg & Goss 1989; Bonabeau et al. 1997; Camazine et al. 2001). Self-organization, as applied to insect societies, is a process in which workers perform simple acts without reference to global patterns. Mass action principles, such as the amplification of random disturbances, subsequently lead to large-scale patterns. Recently, it has been suggested that pattern formation mechanisms may be more complex and varied than previously thought (Boomsma & Franks 2006; Sumpter 2006). In particular, it is probable that multiple mechanisms work together both synergistically and independently. However, we do not yet have a good understanding of how self-organization works with alternative mechanisms, such as templates and recipes that reference global patterns. When using templates, for example, workers build over existing patterns in the environment (reviewed in Camazine et al. 2001). In the case of recipes, the global pattern is an intuitively obvious consequence of the underlying behaviour (Camazine et al. 2001).
In a ground-breaking study, Camazine (1991) proposed a self-organizing algorithm to explain the pattern of comb usage in honeybees (Apis mellifera). Honeybees rear brood at the bottom of their nests with pollen next to it and honey at the top and along the edges (figure 1a; Seeley & Morse 1976). Camazine argued that the pattern could be generated by a self-organizing algorithm of three simple rules: (i) the queen lays eggs in the centre of the comb, (ii) workers deposit pollen and nectar at random, and (iii) bees preferentially remove pollen and nectar from the brood nest relative to the honey storage area. Subsequent theoretical work seemed to support this view (Camazine et al. 1990; Jenkins et al. 1992), and several reviews (Bonabeau et al. 1997; Camazine et al. 2001; Theraulaz et al. 2003) have since heralded this as a classic bottom up demonstration of self-organization in the social insects.
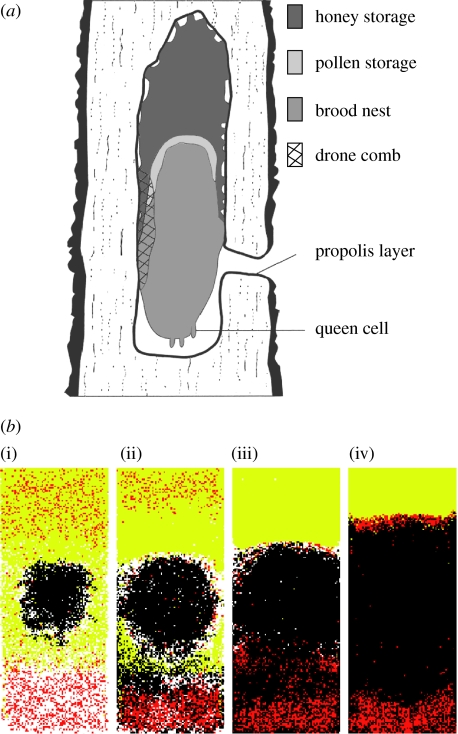
Figure 1.
(a) Idealized drawing of the characteristic pattern on the surface of wild honeybee colonies (after Seeley & Morse 1976). (b) Pattern formation via self-organization and two template effects during a period of high nectar intake ((i) day 1, (ii) day 4, (iii) day 7 and (iv) day 14). Honey cells are yellow, pollen cells are red and brood cells are black. Initially, pollen is scattered throughout the nest, but once open brood is present pollen localizes within the bottom of the nest. Nectar shows an immediate bias towards being unloaded at the top of the nest due to the upward movement of nectar receivers. By day 7, the complete pattern is almost present with the exception of the pollen band, which forms by day 14.
Although Camazine's model provides a good starting point for understanding honeybee comb usage, it is far from complete. Briefly, it focuses on one feature of the pattern (the pollen band), which can be explained with a simple self-organization algorithm, while neglecting several other features that cannot. Camazine described the pattern as concentric (not requiring a directional component), whereas the pattern is actually strongly vertical with the honey always being above and never below the brood (Seeley & Morse 1976). Camazine (1991) also concluded that workers unload pollen and nectar at random. However, we now know that pollen foragers prefer to unload pollen near open brood (Dreller & Tarpy 2000), and nectar receivers have a bias towards unloading at the top of the nest (Seeley 1989; Johnson & Baker 2007).
The purpose of the present study is to re-examine pattern formation on honeybee combs in the light of these new findings. The goal was to construct an agent-based model of a honeybee colony that produces the characteristic pattern and elucidates the roles played by nectar receivers, pollen foragers and nurse bees in its construction. The upward movement of nectar receivers as they search for a place to unload is hypothesized to be a mechanism for generating the vertical pattern of honey and brood. As receivers use gravity as a reference for what direction they are moving in, this would be a gravity-based template. Pollen foragers unloading near open brood is also a simple template mechanism that could be important for pattern formation. Because the queen is responsible for the compact distribution of the brood, I refer to this as a queen-based template. Camazine showed that nurses differentially remove pollen and nectar from near the brood, leading to the formation of a pollen band via self-organization. I explore how the addition of two templates affects this process. A further goal of this study is to suggest an adaptive benefit of not relying on self-organization alone for pattern formation. I examined this by comparing the growth rates (a key fitness component) of colonies using self-organization only versus those using a mechanism incorporating self-organization and two templates.
This work builds on previous work on the interplay between self-organization and templates. Franks & Deneubourg (1997) explored a mechanism similar to the queen-based template when they showed that ants use the cluster of brood as a template for building a wall around the nest via self-organization. Recently, Jost et al. (2007) explored how corpse clustering in ants, a self-organizing process, is affected by wind currents, an environmental template. The present study will expand on these studies by exploring a more complex process (self-organization and two templates) and by exploring the selective basis of a multimodal pattern formation mechanism. This evolutionary perspective was missing from previous studies and should be a valuable addition to the literature on self-organization as this field moves from demonstrating basic principles in abstract settings to elucidating complex biological mechanisms and their evolutionary bases.
2. The model
(a) The nest
An agent-based model of a honeybee colony was constructed using the NetLogo programming language (Wilensky 1999). The nest surface consisted of 14 025 cells. This number of cells corresponds in size to a two-frame observation hive (typical population size=4000). Many experimental studies have used this nest size, thereby simplifying model parametrization (reviewed in Seeley 1995). The shape of the nest is based upon what is typically found in nature: long and narrow, a result of most nests being in tree cavities (Seeley & Morse 1976). Cells could contain only one material at a time: pollen; honey; or a developing bee. A cell could hold 40 loads of nectar, but only 17 loads of pollen (Schmickl & Crailsheim 2007). This differs from previous models, which assumed that all the cells in the nest were the same size. However, bees routinely lengthen honey cells (particularly at the top of the nest) to a much greater depth than cells in the brood zone (Seeley & Morse 1976). Larvae grew at an exponential rate, were capped at 9 days and emerged at 21 days (reviewed in Winston 1987).
(b) The bees
Four types of bee were modelled: a queen; pollen foragers; nectar receivers; and nurses. Each bee acted independently according to a set of instructions specific to its class. One iteration of the model corresponded to 1 min of time. Queens acted out their behavioural sequence each minute, foragers and receivers every 20 min, and nurses every 30 min. This staggered pattern was necessary because the full model required a long computation time even with these simplifications. Pollen and nectar foraging occurred for 12 hours per day, while the rest of the bees in the model were active 24 hours. The electronic supplementary material shows simulations in which the day length was varied.
Queen behaviour was modelled following Camazine (1991). Queens lay up to one egg per minute in cells within four cells of another brood cell. Queens began by filling up most of the cells in the centre of the nest before searching at random throughout the nest. The queen avoided areas of solid nectar by slightly increasing her speed in a random direction whenever she took more than 20 consecutive steps on nectar cells. This allowed the queen to search the honey zone without spending an inordinate amount of time in a region of the nest where no eggs can be laid. The qualitative behaviour of the model is the same with and without this behaviour, but the pattern forms more quickly when the queen has this bias. As queens are typically found in the brood nest of colonies (Winston 1987), this is a reasonable assumption.
Pollen foragers began at the entrance of the nest and conducted a random walk in search of open brood (Dreller & Tarpy 2000). Once they located open brood, they began a random search for a cell in which to unload (empty or containing pollen). If they were unable to find an open brood cell after an extensive search, they unloaded at random into an empty or pollen cell. Overall pollen collection rates were modelled via a negative feedback process that captures the basic dynamics of the natural system (Fewell & Winston 1992; Seeley 1995; Fewell & Bertram 1999; Rotjan et al. 2002). If more than 10 per cent of the cells in the nest contained pollen, then only 10 per cent of the pollen foragers foraged (specialist pollen foragers). When less than 10 per cent of the cells in the nest contained pollen, all the pollen foragers foraged. The maximum pollen foraging rate was set to 80 per cent of the nectar foraging rate (Seeley 1985). Thus, the rate of pollen foraging fluctuated over the course of the day with most of it occurring in the morning when pollen stores were at their lowest. Overall, colonies stockpiled enough pollen to get through approximately 3 rainy days.
Nectar foragers were not modelled since they unload their nectar to receiver bees shortly after entering the nest (reviewed in Seeley 1995). Nectar receivers began on the dance floor where they filled their crops with nectar. They then walked up a variable distance before searching for either an empty or nectar cell in which to unload. If they were unsuccessful in their first attempt, they repeated this behaviour until they reached the top of the nest at which point they began a random search for a suitable cell. This behaviour was modelled after the observations of Seeley (1989) who observed bees walking up and out of the brood nest before searching for cells in which to unload. Rates of nectar collection for all simulations presented in figures were set to 30 bees min−1. Thus, 600 nectar receivers unloaded honey every 20 min. In a previous study, using colonies of the same size as in the model, this rate of foraging during a nectar flow was recorded (Johnson 2005). Modelling pattern formation during a period of high nectar influx saved computation time, as it takes the pattern less time to form when more honey is coming into the nest. Simulations with lower rates were performed and, as long as the colony is bringing in enough to not starve, the rate of collection did not qualitatively affect the nature of pattern formation (see the electronic supplementary material).
Nurse bees serve as a protein source for larva and all the other temporal castes of adult bees (Crailsheim 1991, 1992). Nurses affect pattern formation by removing pollen and nectar from cells near the brood (Camazine 1991). Because nurses only affect pattern formation via feeding, only this behaviour was modelled. This was accomplished by having hungry nurses conduct random searches for either pollen or nectar starting from a random place within the brood nest. The colony-level amount of pollen and honey eaten was a function of the number of brood cells (factoring in age effects in larvae) and the food needs of adult bees (inside and outside workers) in the colony (after Crailsheim 1991, 1992; Schmickl & Crailsheim 2007). One larva required approximately 145 mg of pollen and 160 mg of nectar during its development, while adult bees (in total) were fed approximately 16.5 per cent of the total fed to larva. This value for adult food need is consistent with the conclusions of Crailsheim (1991, 1992), but as this value is impossible to quantify exactly, the effect of strongly varying this parameter was explored (see the electronic supplementary material).
(c) Rain
Some simulations explored pattern formation during periods of rainy weather, which has important consequences for honeybee foraging (reviewed in Seeley 1985). Rain had two effects on the behaviour of the bees. First, foragers did not forage on rainy days (Seeley 1985) and, on those days when rain led to the loss of pollen stores, the brood were cannibalized (Schmickl & Crailsheim 2001). The pattern of rain was implemented in two ways. To facilitate comparisons between runs of the model with different combinations of mechanisms, rain occurred on days 9–11 for results shown in the main text. The electronic supplementary material shows the results of simulations for which rain occurred stochastically throughout the first two weeks of pattern formation.
A complete list of parameters, their values, and the references on which they were based is included in the electronic supplementary material, table 1 along with the model's complete computer code. The model was run 30 times for each question explored in §3.
3. Results
Figure 1b shows the course of pattern formation for the full model including self-organization, and the two template effects. Initially, there is a disorganized phase when pollen is unloaded throughout the nest and honey is present both above and below the brood. By day 4, the queen has laid a compact central brood area and most of the honey is above the brood. Most of the pollen, however, is at the bottom of the nest and a pollen band has not formed between the brood and honey. This is because pollen foragers, on average, are more likely to have their first encounter with open brood close to the nest entrance than at the top of the nest. By day 7, the brood zone occupies most of the nest (common for small colonies) and is below the honey zone with a small empty zone in the middle. This space between the brood and honey contains pollen, but the pattern is not as strong as that described by Seeley & Morse (1976). This is because the bees in the model have a large stockpile of pollen at the bottom of the nest and are only foraging for more at a low rate.
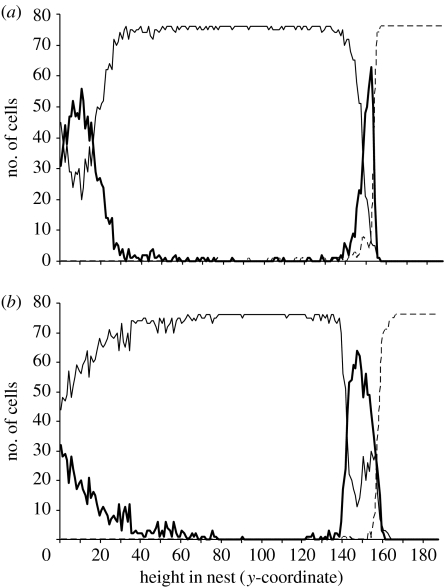
The pattern drawn by Seeley & Morse (1976), based on wild colonies, is idealized and pollen does occur throughout the brood zone (T. D. Seeley 2008, personal communication). However, a thick band of pollen between the brood and honey is quite distinctive in wild colonies and requires an explanation (the model, as discussed thus far, leads to a thin band of pollen with most of the pollen in the bottom of the nest). Given that colonies stockpile pollen to get them through rainy spells when they cannot forage (reviewed in Seeley 1985), I hypothesized that rain may be the key to solving the problem. Specifically, I hypothesized that because colonies often eat much of their pollen stores on rainy days, the queen might fill in the newly empty cells with eggs so that when foraging recommences, the only empty cells would be between the brood and the honey. To test this, simulations were run in which it rained on days 9–11 and in which it rained with a constant 25 per cent probability per day for the first 13 days (the simulations were halted after 14 days). Both patterns of rain led to the formation of a thicker pollen band relative to simulations minus rain (fixed rain, 818.5 cells ±241.4 s.d.; random rain, 668.4 cells ±209.7 s.d.; no rain, 391.0 cells ±91.8 s.d.; ANOVA, F2,87=33.76, p<0.0001; see the electronic supplementary material for more information). Random rain and the fixed rain pattern did not significantly differ (Tukey's test), though the bands in the fixed rain pattern trended towards being higher. A comparison of the size of the pollen band in simulations with and without a 3-day rainy spell is shown in figure 2.
Figure 2.
The role played by rainy days in the formation of the pollen band between the brood and honey. The results of two simulations (with and without 3 days of rain) are shown. Shown is the number of brood (thin solid line), honey (dashed line) and pollen (thick solid line) cells at different heights within the nest. The two representative simulations were chosen because their pollen bands were equal to the average of 30 simulations. (a) In the absence of rain, a relatively weak pollen band forms between the brood and honey, but most of the pollen is at the bottom of the nest. (b) During rainy spells, the bees eat through most of their pollen stores and the queen fills in many of the newly empty cells with brood. This leads to the only empty cells being between the brood and honey, where pollen foragers unload when foraging recommences. Thus, a larger pollen band forms in the presence of rain (fixed rain, 818.5 cells ±241.4 s.d.; random rain, 668.4 cells ±209.7 s.d.; no rain, 391.0 cells ±91.8 s.d.; ANOVA, F2,87=33.76, p<0.0001).
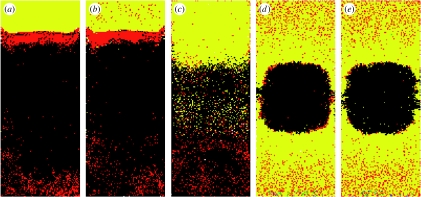
Figure 3 explores the role played by each of the models' mechanisms: the gravity-based template; the queen-based template; and self-organization. All simulations had the same pattern of rain (on days 9–11). By day 14, the pattern had reached its final form for each case. Figure 3a shows the full model for comparison. Figure 3b shows the behaviour of the model with random unloading by pollen foragers as opposed to the template effect of unloading near brood. The pattern is incomplete because the pollen is scattered throughout the honey zone. Figure 3c shows the behaviour of the model with random removal of the honey and pollen (minus the self-organizing process). As Camazine showed, when there is no differential removal, the pollen band does not form and the brood and honey zones are not distinct. Figure 3d shows the behaviour of the model with random unloading of honey as opposed to upward movement by nectar receivers (the gravity-based template). In this case, the pattern is concentric as there is no directional bias in nectar deposition. Figure 3e shows how the model behaves with random unloading of pollen and nectar and with differential removal of pollen and nectar from the brood nest (Camazine's original model). The resulting pattern is missing both the vertical component of the pattern (honey above brood) and the pollen band. This is in contrast to Camazine's treatment, which showed a pollen band. The difference is caused by the size of the pollen stores in the present model. Camazine's model allowed for almost no stores, while the present study allows for 3 days' worth, a more realistic situation. With pollen stores, the differential removal of pollen and honey from around the brood nest alone is insufficient to create a pollen band. A similar process is at play in figure 3b. Here, there is differential removal of pollen from near the brood; however, the amount of pollen being stored in the nest is such that both the template and self-organizing effects are necessary for the pollen to become completely localized near the brood zone.
Figure 3.
The role played by each of the mechanisms underlying pattern formation. SO, self-organization; T1, gravity-based template; T2, queen-based template. Each picture shows the pattern at 14 days. The full model (with rain) is shown for comparison in (a) (SO+T1+T2). (b) Without the queen-based template, the pattern is incomplete in that the pollen is scattered throughout the honey zone (SO+T1). (c) Without the self-organizing mechanism not only does the pollen band not form, but also the brood and honey zones are not distinct (T1+T2). (d) Without the gravity-based template, the pattern remains concentric as opposed to vertical (SO+T2). (e) The original self-organization model of Camazine does not lead to pattern formation under realistic parameter settings (SO; see text for an explanation).
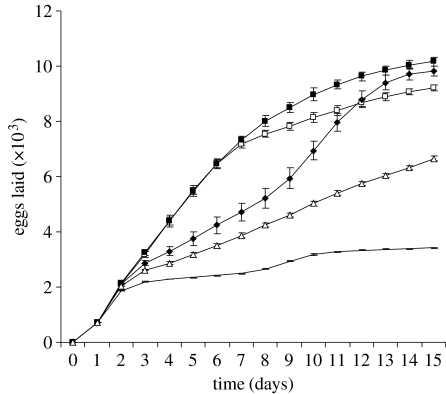
The second goal of the study was to suggest an adaptive benefit for a complex pattern formation mechanism. Figure 4 shows the growth rate (in eggs laid) of simulations with different patterns of nectar unloading. Nectar unloading is the key to the optimal use of space within the nest because nectar is the most heavily collected resource. Random unloading (Camazine's original model) led to the slowest colony growth rate. Although the queen began by laying at her maximal rate, this rate quickly decreased because much of the nest filled with a light layer of honey. This resulted in few empty cells for the queen to lay in. Although preferential removal of honey and pollen from the brood zone caused the brood nest to expand, it did so slowly. When bees have an upward bias in their direction of unloading of honey, however, the rate of egg laying remains at the maximum for much longer. This is because the amount of nectar being consumed from the brood zone matches (for some parameter values) that which is placed there with the remainder going into the honey zone. Furthermore, as the brood zone forms, the upward movement of bees searching for places to unload leads to an increase in the amount of nectar placed in the honey zone via positive feedback. This is because bees are increasingly unlikely to find empty cells in the brood zone as the queen lays there. Consequently, the brood zone grows in the downward direction away from where the honey is stored.
Figure 4.
Growth rate (in eggs laid) of colonies when workers varied in their bias towards unloading at the top of the nest. No upward bias (random unloading) led to the slowest growth rate. When bees had a slight bias towards moving up (12.5% of the nest height between inspections), the growth rate was also low due to too much honey being deposited where the queen prefers to lay eggs. When bees had a stronger upward bias, however, the rate of egg laying remained at the maximum for much longer. Thus, the extent to which the bees' unloading decisions allow them to make efficient use of their space strongly affects colony growth rate. Open squares, 50%; filled squares, 37.50%; diamonds, 25%; triangles, 12.50%; solid line, random.
4. Discussion
Recent reviews have called for more sophisticated pattern formation models that incorporate more detailed worker behaviour (Boomsma & Franks 2006; Sumpter 2006). The present study shows that pattern formation on honeybee combs is dependent on self-organization and at least two templates. These processes are the result of the behaviour of four different groups of bees: the queen; the nectar receivers; the pollen foragers; and the nurses. The vertical pattern of honey on top and brood at the bottom arises from a gravity-based template effect, whereas the pollen band is the result of the combined effects of a queen-based template and a self-organization process. Colonies using this complex pattern formation mechanism had higher growth rates than colonies using self-organization alone. This was due to the inefficient placement of nectar in the self-organization model slowing the egg laying rate of queens. Without a bias in the direction of nectar unloading, honey quickly blankets the whole nest, which prevents the queen from laying at her optimal rate. Given that the main selection pressure for small colonies is to grow as quickly as possible (Seeley 1985), this is clearly an untenable situation. Selection may thus add other processes onto self-organizing algorithms whenever useful information is present in the environment and it is within the cognitive ability of the workers to make use of it.
This study builds on previous work showing multiple processes at play in the construction of social insect nests (reviewed in Theraulaz et al. (2003) and Jost et al. (2007)). Franks et al. (1992) and Franks & Deneubourg (1997), working with Temnothorax sp., found that the workers use the brood as a template around which to construct a nest wall. The ants then use a self-organization algorithm to ensure that the wall is compact. Termites (Macrotermes sp.) likewise use the queen as a template in constructing royal chambers (Bonabeau et al. 1998). In the present study, the brood, which is laid in a compact cluster, acts as a template for the deposition of pollen. Given the importance of the queen and brood to colony fitness, and the similarity of all of these mechanisms, it is possible that the queen plays a central role in generating templates in many social insects.
In a paper focused on determining when biological systems should use self-organization, Seeley (2002) concluded that they should do so when other means are beyond their ability. This is because alternative mechanisms are often superior in terms of speed and efficiency. Of course, some alternatives to self-organization require information not at the disposal of the individual. Consequently, such processes (central organization, in particular) may be more difficult for selection to implement. However, this does not apply to templates or recipes that are no more difficult for insects to apply than are self-organization algorithms. All three processes depend on the performance of many simple context-dependent behaviours. The only difference has to do with whether these behaviours make reference to global patterns. Thus, the commonly cited fact that social insect behaviours are relatively simple is not evidence in favour of self-organization over alternative pattern formation mechanisms.
In applying the concept of self-organization to biology, it is useful to keep in mind Turing's (1952) paper in which he proposed the reaction–diffusion model and essentially founded the subject. Turing was interested in determining how a biological system could generate a pattern from an initially homogenous distribution of potential reactants. In other words, given a situation in which no information is available (no template), how can purely physical processes generate patterns from homogeneity? His answer relies on the fact that random perturbations can be amplified in such a way as to create many different patterns. This was an ingenious discovery and obliviously of great importance (reviewed in Nicolis & Prigogine 1989 and Camazine et al. 2001). However, early applications of this theory to biology (and to the social insects in particular) lost sight of the domain of pure self-organization models (Bonabeau et al. 1997). Although worker behaviour can be analogous to molecular interactions in particular contexts, social insects are not limited to interacting with their nearest neighbours. They process information in sophisticated manners while moving throughout their environment (Johnson 2008). Cells also process information about their environment and are connected to one another in complex circuits. In short, biological systems can do better than self-organization in many contexts. In the present case, because self-organization alone leads to the poor use of space within the nest, it is not surprising that selection has acted on variation in worker behaviour to construct a more efficient algorithm. With respect to pattern formation on combs, this involves the use of at least two templates. This argument, of course, does not lessen the importance of self-organization to biological systems. In the present case, the self-organizing effect is as important as any of the other mechanisms. Furthermore, because there are many cases in which templates are either unavailable or their use would be beyond the information processing ability of workers, self-organization is often the only option. Hence, self-organization is probably the key mechanism for many cases of pattern formation. In cases where templates are available, however, this study suggests that selection may often favour mechanisms that make use of multiple processes.
Acknowledgments
The experiments reported here comply with the current laws of the USA.
I thank James Nieh, David Holway and three anonymous referees for carefully reading the manuscript. This work was supported by a National Science Foundation Postdoctoral Fellowship.
Supplementary Material
Additional Methods, Figures, and Computer Code
References
- Bonabeau E., Theraulaz G., Deneubourg J.L., Aron S., Camazine S. Self-organization in social insects. Trends Ecol. Evol. 1997;12:188–193. doi: 10.1016/s0169-5347(97)01048-3. doi:10.1016/S0169-5347(97)01048-3 [DOI] [PubMed] [Google Scholar]
- Bonabeau E., Theraulaz G., Deneubourg J.L., Franks N.R., Rafelsberger O., Joly J.L., Blanco S. A model for the emergence of pillars, walls and royal chambers in termite nests. Phil. Trans. R. Soc. B. 1998;353:1561–1576. doi:10.1098/rstb.1998.0310 [Google Scholar]
- Boomsma J.J., Franks N.R. Social insects: from selfish genes to self organisation and beyond. Trends Ecol. Evol. 2006;21:303–308. doi: 10.1016/j.tree.2006.04.001. doi:10.1016/j.tree.2006.04.001 [DOI] [PubMed] [Google Scholar]
- Camazine S. Self-organization pattern formation on the combs of honey bee colonies. Behav. Ecol. Sociobiol. 1991;28:61–76. doi:10.1007/BF00172140 [Google Scholar]
- Camazine S., Sneyd J., Jenkins M.J., Murray J.D. A mathematical model of self organized pattern formation on the combs of honey bee colonies. J. Theor. Biol. 1990;147:553–571. doi:10.1016/S0022-5193(05)80264-4 [Google Scholar]
- Camazine S., Deneubourg J., Franks N.R., Sneyd J., Theraulaz G., Bonabeau E. Princeton University Press; Princeton, NJ: 2001. Self-organization in biological systems. [Google Scholar]
- Crailsheim K. Interadult feeding of jelly in the honeybee (Apis mellifera) colonies. J. Comp. Physiol. B: Biochem. Syst. Environ. Physiol. 1991;161:55–60. doi:10.1007/BF00258746 [Google Scholar]
- Crailsheim K. The flow of jelly within a honeybee colony. J. Comp. Physiol. B: Biochem. Syst. Environ. Physiol. 1992;162:681–689. doi:10.1007/BF00301617 [Google Scholar]
- Deneubourg J.L., Goss S. Collective patterns and decision making. Ethol. Ecol. Evol. 1989;1:295–311. [Google Scholar]
- Dreller C., Tarpy D.R. Perception of the pollen need by foragers in a honeybee colony. Anim. Behav. 2000;59:91–96. doi: 10.1006/anbe.1999.1303. doi:10.1006/anbe.1999.1303 [DOI] [PubMed] [Google Scholar]
- Fewell J.H., Bertram S.M. Division of labor in a dynamic environment: response by honeybees (Apis mellifera) to graded changes in colony pollen stores. Behav. Ecol. Sociobiol. 1999;46:171–179. doi:10.1007/s002650050607 [Google Scholar]
- Fewell J.H., Winston M.L. Colony state and regulation of pollen foraging in the honey bee, Apis mellifera L. Behav. Ecol. Sociobiol. 1992;30:387–393. doi:10.1007/BF00176173 [Google Scholar]
- Franks N.R., Deneubourg J.L. Self-organizing nest construction in ants: individual worker behaviour and the nest's dynamics. Anim. Behav. 1997;54:779–796. doi: 10.1006/anbe.1996.0496. doi:10.1006/anbe.1996.0496 [DOI] [PubMed] [Google Scholar]
- Franks N.R., Wilby A., Silverman B.W., Tofts C. Self organizing nest construction in ants: sophisticated building by blind bulldozing. Anim. Behav. 1992;44:357–375. doi:10.1016/0003-3472(92)90041-7 [Google Scholar]
- Jenkins M.J., Sneyd J., Camazine S., Murray J.D. On a simplified model for pattern formation in honey bee colonies. J. Math. Biol. 1992;30:281–306. doi:10.1007/BF00176152 [Google Scholar]
- Johnson B.R. Limited flexibility in the temporal caste system of the honey bee. Behav. Ecol. Sociobiol. 2005;58:219–226. doi:10.1007/s00265-005-0949-z [Google Scholar]
- Johnson B.R. Global information sampling in the honey bee. Naturwissenschaften. 2008;95:523–530. doi: 10.1007/s00114-008-0354-3. doi:10.1007/s00114-008-0354-3 [DOI] [PubMed] [Google Scholar]
- Johnson B.R., Baker N. Adaptive spatial biases in nectar deposition in the nests of honey bees. Insectes Soc. 2007;54:351–355. doi:10.1007/s00040-007-0953-6 [Google Scholar]
- Jost C., Verret J., Casellas E., Gautrais J., Challet M., Lluc J., Blanco S., Clifton M.J., Theraulaz G. The interplay between a self-organized process and an environmental template: corpse clustering under the influence of air currents in ants. J. R. Soc. Interface. 2007;4:107–116. doi: 10.1098/rsif.2006.0156. doi:10.1098/rsif.2006.0156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michener C.D. Harvard University Press; Cambridge, MA: 1974. The social behaviour of the bees. [Google Scholar]
- Nicolis G., Prigogine I. W. H. Freeman Press; New York, NY: 1989. Exploring complexity. [Google Scholar]
- Rotjan R.D., Calderone N.W., Seeley T.D. How a honey bee colony mustered additional labour for the task of pollen foraging. Apidologie. 2002;33:367–373. doi:10.1051/apido:2002026 [Google Scholar]
- Schmickl T., Crailsheim K. Cannibalism and early capping: strategy of honey bee colonies in times of experimental pollen shortages. J. Comp. Physiol. A. 2001;187:541–547. doi: 10.1007/s003590100226. doi:10.1007/s003590100226 [DOI] [PubMed] [Google Scholar]
- Schmickl T., Crailsheim K. HoPoMo: a model of honeybee intracolonial population dynamics and resource management. Ecol. Modell. 2007;204:219–245. doi:10.1016/j.ecolmodel.2007.01.001 [Google Scholar]
- Seeley T.D. Harvard University Press; Cambridge, MA: 1985. Honey bee ecology. [Google Scholar]
- Seeley T.D. Social foraging in honey bee: how nectar foragers assess their colony nutritional status. Behav. Ecol. Sociobiol. 1989;24:181–199. doi:10.1007/BF00292101 [Google Scholar]
- Seeley T.D. Harvard University Press; Cambridge, MA: 1995. The wisdom of the hive: the social physiology of honey bee colonies. [Google Scholar]
- Seeley T.D. When is self-organization used in biological systems? Biol. Bull. 2002;202:314–318. doi: 10.2307/1543484. doi:10.2307/1543484 [DOI] [PubMed] [Google Scholar]
- Seeley T.D., Morse R.A. The nest of the honey bee (Apis mellifera L.) Insectes Soc. 1976;23:495–512. doi:10.1007/BF02223477 [Google Scholar]
- Sumpter D.J.T. The principles of collective animal behaviour. Phil. Trans. R. Soc. B. 2006;361:5–22. doi: 10.1098/rstb.2005.1733. doi:10.1098/rstb.2005.1733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theraulaz G., Gautrais J., Camazine S., Deneubourg J.L. The formation of spatial patterns in social insects: from simple behaviours to complex structures. Phil. Trans. R. Soc. A. 2003;361:1263–1282. doi: 10.1098/rsta.2003.1198. doi:10.1098/rsta.2003.1198 [DOI] [PubMed] [Google Scholar]
- Tschinkel W.R. The nest architecture of the Florida harvester ant, Pogonomyrmex badius. J. Insect Sci. 2004;4:19. doi: 10.1093/jis/4.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turing A.M. The chemical basis of morphogenesis. Phil. Trans. R. Soc. B. 1952;237:37–72. doi:10.1098/rstb.1952.0012 [Google Scholar]
- Wilensky, U. 1999 NetLogo Centre for connected learning and computer-based modelling, Northwestern University, Evanston, IL. See http://ccl.northwestern.edu/netlogo/
- Wilson E.O. Harvard University Press; Cambridge, MA: 1971. The insect societies. [Google Scholar]
- Winston M.L. Harvard University Press; Cambridge, MA: 1987. The biology of the honey bee. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Methods, Figures, and Computer Code