Abstract
While the number of studies providing evidence of actuarial senescence is increasing, and covers a wide range of taxa, the process of reproductive senescence remains poorly understood. In fact, quite high reproductive output until the last years of life has been reported in several vertebrate species, so that whether or not reproductive senescence is widespread remains unknown. We compared age-specific changes of reproductive parameters between two closely related species of long-lived seabirds: the small-sized snow petrel Pagodroma nivea, and the medium-sized southern fulmar Fulmarus glacialoides. Both are sympatric in Antarctica. We used an exceptional dataset collected over more than 40 years to assess age-specific variations of both breeding probability and breeding success. We found contrasted age-specific reproductive patterns between the two species. Reproductive senescence clearly occurred from 21 years of age onwards in the southern fulmar, in both breeding probability and success, whereas we did not report any decline in the breeding success of the snow petrel, although a very late decrease in the proportion of breeders occurred at 34 years. Such a contrasted age-specific reproductive pattern was rather unexpected. Differences in life history including size or migratory behaviour are the most likely candidates to account for the difference we reported in reproductive senescence between these sympatric seabird species.
Keywords: vertebrates, life history, senescence, breeding success, age, Antarctic seabirds
1. Introduction
The study of age-specific variation of life-history traits in vertebrates has become a popular topic (see for reviews on birds Bennett & Owens (2002) and on large mammalian herbivores Gaillard et al. (2003)). Of particular interest is senescence, defined as the decline in performance with age. The theory of senescence has been widely discussed (Partridge 1987; Kirkwood & Rose 1991; Ricklefs 1998; Partridge & Mangel 1999; Hughes et al. 2002), often from a theoretical perspective, because empirical observations in natura have often remained cryptic or disputed (Nisbet 2001). With the availability of both powerful statistical methods (e. g. mixed models providing the possibility to account for heterogeneity in quality among individuals Van de Pol & Verhulst 2006; Nussey et al. 2008) and long-term monitoring of known-aged individuals, such investigations have become possible. Moreover, age-related patterns in nature often do not follow a linear relationship with fitness traits (Weladji et al. 2006), which can make them difficult to describe accurately.
Owing to their generally extended lifespan (more then 60 years for albatrosses), seabirds are one of the predominant animal groups to fill the gap in knowledge of life-history patterns at old ages (Nisbet 2001; Reed et al. 2008). However, choosing seabirds as study species requires research programmes lasting for decades, in order to gather enough information on old birds, and due to the absence of external markers of age, birds have to be marked individually as nestlings, and recaptured throughout their lives.
Studies on ageing in wild seabirds have reported patterns of senescence, either linked to reproduction (Weimerskirch et al. 2005; Reed et al. 2008), to foraging abilities (Catry et al. 2006) or to survival (see Bennett & Owens (2002) for a review of case studies). On the other hand, some studies have reported an increase in reproductive performance with age (Mauck et al. 2004; Angelier et al. 2007). In the absence of comparative studies, the understanding of such contrasted age-specific variation remains scarce. To fill this gap, we provide here a first comparative study of the age-specific changes in reproductive output between two sympatric wild seabird species.
We studied populations of southern fulmar Fulmarus glacialoides and snow petrel Pagodroma nivea, which have been monitored using individual capture–mark–recapture methods since 1963. The two species are both very long-lived Antarctic seabirds, which only lay one egg per clutch and per year, and have high adult survival rates (Jenouvrier et al. 2003, 2005). Both species can thus be ranked close to the slow end of the slow–fast continuum of vertebrate life-history tactics (Gaillard 1989; Bielby et al. 2007), characterized by long generation times (Gaillard et al. 2005). We can therefore expect very weak senescence in both survival and reproduction in these species (Jones et al. 2008). However, since both species exhibit very high adult survival, the theory of life-history evolution suggests the existence of a trade-off, leading to a decreased reproductive output with increasing age. We therefore tested whether reproductive senescence occurred in these two closely related, sympatric species, and if so, at which age reproductive senescence begins.
2. Material and methods
(a) Study site and species
From 1963 onwards, annual ringing and recapture sessions of adult birds and chicks of both species took place on Ile des Pétrels, Pointe Géologie Archipelago (66°40′ S, 140°01′ E), Terre Adélie, Antarctica. The three colonies of snow petrels and the only colony of southern fulmars at Pointe Géologie were intensively surveyed each year. We pooled data from the three colonies of snow petrels because no difference occurred in breeding performance. More details on the monitoring are provided in Chastel et al. (1993) and Barbraud & Weimerskirch (2001).
The species considered here are very long lived, have a very high adult survival (93.4%±0.3 for the snow petrel (Chastel et al. 1993), 92.3%±0.6 for the southern fulmar (Jenouvrier et al. 2003)), lay only one egg, and both sexes contribute equally to parental care. However, the species differ in other life-history traits.
The snow petrel P. nivea (Forster) is the smallest species (approx. 400 g) of the fulmarine petrel group. Snow petrels breed in large numbers along the coast of Antarctica, where they forage in close association with the pack ice, feeding mostly on fish (Ainley et al. 1984; Ridoux & Offredo 1989). They are resident in Antarctic waters throughout the year. Snow petrels are characterized by a relatively low level of philopatry compared with other petrels (Chastel et al. 1993). In spite of this low philopatry, once a snow petrel has selected a breeding colony, it remains faithful to this place in the future and, therefore, if not observed between two breeding events, it can be confidently assumed that it did not breed elsewhere during this period (Jenouvrier et al. 2003).
Southern fulmars (700–1200 g) are cliff-nesting seabirds that forage in Antarctic waters in summer, but move up to sub-Antarctic waters in winter and prey mainly on euphausiids, fishes, crustaceans and squid (Ainley et al. 1984; Ridoux & Offredo 1989). Unlike snow petrels, southern fulmars are highly philopatric (Jenouvrier et al. 2003). As for the snow petrel, if a bird is not observed between two breeding attempts, it can be confidently assumed that it did not reproduce.
(b) Description, extraction and selection of the environmental variables
We used one local variable, the sea ice extent (SIE), and one large-scale variable, the southern oscillation index (SOI), to account for possible confounding effects of environmental conditions. Both variables are known to influence the focal species (Jenouvrier et al. 2003, 2005).
The SOI was obtained for the period 1973–2004 (see http://www.bom.gov.au/climate/current/soihtml.shtml).
The SIE, expressed in units of 1000 km2, was available from 1973 to 1990 only, for 1° latitude×10° longitude slices (see http://nsidc.org/data/g00917.html). Since Ile des Pétrels is situated at 66°40′ S, 140°01′ E, we extracted and averaged the SIE between the longitudes 130°–140° and 140°–150°. Since no SIE was available after 1990, we interpolated the second period from 1990 to 2004 using the sea ice concentration values (See http://ingrid.ldgo.columbia.edu/SOURCES/.IGOSS/.nmc/.Reyn_SmithOIv2/.monthly/.sea_ice/). These data covered a grid of 1°×1°, and a cell was considered as covered by ice when the concentration exceeded a specific value. We calculated the extent of ice (in units of 1000 km2) for longitudinal slices (130°–140° and 140°–150°). To correct for the difference in sampling between the two time periods (1973–1990 and 1990–2004), each measure was standardized with respect to the specific mean of the period. Finally, we used the mean SIE for April–June as it has been shown that this period critically influenced the breeding ecology of the focal species (Jenouvrier et al. 2005). Correlation tests (using Pearson's correlation coefficient) did not show any significant collinearity between the climatic variables.
(c) Data and statistical analysis
For both species, our analyses included all birds of known age (i.e. ringed as chicks and later recaptured as breeders), that reached sexual maturity and for which the breeding status was observed every year since fledging (snow petrel: n=112; southern fulmar: n=177). For the snow petrel, we only included individuals that reproduced more than once, to avoid considering transient birds. Age of maturity for each species was based on previous results (Chastel et al. 1993; Jenouvrier et al. 2003). We used the breeding success at fledging, defined as the probability of a chick fledging from the laid egg. Breeding probability was defined as the proportion of breeding birds in each age group, considering that they had reached maturity and that they were alive. For both species, the detection probability of an individual was close to one, because all nests are checked several times during each breeding season. We did not perform separate analyses for sexes, since the information was unavailable for the southern fulmar, and would have dramatically reduced the sample size for the snow petrel.
The two species (especially the snow petrel; Chastel et al. 1993) show a marked between-year variation in breeding success and breeding probability. They are prone to skip reproduction (breeding probability) during unfavourable environmental conditions. We therefore used the mean annual breeding success and breeding probability at the population level as a proxy for year quality by adding it as a covariate in our models in order to reduce the amount of variation not due to age effect.
We first created sets of candidate models and used the Akaike information criterion (AIC) to select the most parsimonious model (Burnham & Anderson 1998). We also computed Akaike weights (wi), which provide a measure of the relative likelihood of a given model to be the best among the models fitted.
We fitted linear, quadratic and logarithmic relationships on a logit scale to model the age-specific variation in reproductive traits (see table 1) by using generalized linear mixed models (package glmmML) in the software R, v. 2.6.2 (R Core Development Team 2005). Preliminary analyses showed that in all cases, mixed models described the data more appropriately than simple general linear models, confirming marked individual heterogeneities in reproductive traits. Such a procedure allowed us to account for the problem of pseudo-replication that occurs when using repeated measures of the same individuals (Hurlbert 1984). Note, however, that not accounting for individual variation (i.e. using GLM) led to much higher AIC but did not change the results, leading to only a small underestimation of slopes in the last life stage. Additionally, we fitted threshold models, including three stages: (i) the progressive access to reproduction from the age of maturity to a first threshold age τ1, (ii) a prime-age stage between τ1 and τ2 with a maximum reproductive output, and (iii) a senescent stage from the second threshold age τ2, from which the reproductive output decreases. For each combination of threshold values (e. g. τ1=12 years and τ2=23 years for a two-threshold model), a generalized linear mixed model was fitted (see table 1). The best threshold models were determined using AIC profiles. We further tested for interactions between year quality (measured by the mean annual breeding value) and senescence rate (see table 1) when senescence occurred, to assess a possible change of senescence patterns in response to variation in environmental conditions (as recently reported in red deer (Cervus elaphus) according to changes of density at birth, Nussey et al. 2007)
Table 1.
Summary of the 36 candidate models tested. (The model formula presents the full model for each trend fitted. All intermediate models were tested, see appendix (table S1 and S4 in the electronic supplementary material). Bs, breeding success; Bp, breeding probability; bsann, inter-annual variations in breeding success; bpann, inter-annual variations in breeding probability; SOI, southern oscillation index; SIEautumn, sea ice extent values for autumn; asterisk stands for an interaction.)
| model formula | biological meaning | number |
|---|---|---|
| Bs (or Bp)∼1 | no effect of age on reproduction | 1 |
| Bs (or Bp)∼age | linear effect of age on reproduction | 2 |
| Bs (or Bp)∼age+age2+bsann (or bpann)+SOI+SIEautumn | quadratic effect of age on reproduction | 3–10 |
| Bs (or Bp)∼log(age)+bsann (or bpann)+SOI+SIEautumn | logarithmic effect of age on reproduction | 11–18 |
| Bs (or Bp)∼bsann (or bpann) | mean annual breeding output only explains variations of reproduction | 19 |
| Bs (or Bp)∼T1+T2+ bsann (or bpann)+SOI+SIEautumn | existence of one threshold age (6<τ1<34) | 20 to 27 |
| Bs (or Bp)∼T1+T2+T3+ bsann (or bpann)+SOI+SIEautumn | existence of two threshold ages (6<τ1<20, 21<τ2<34) | 28–35 |
| Bs (or Bp)∼T1+T2+T3+ bsann (or bpann)+bsann (or bpann)*T3 | existence of two threshold ages and an interaction between year quality and age | 36 |
3. Results
(a) Age-specific breeding success
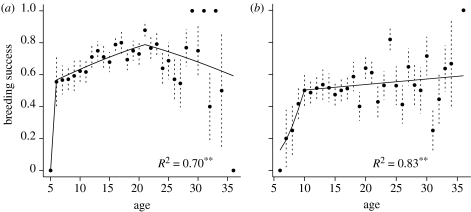
The breeding success of the southern fulmar was best fitted by a two-threshold model including the effects of year-to-year variations in breeding success and SOI (Model 33, table 1). The first threshold age was 6 years, and between 6 and 21 years, the reproductive success of birds increased from 55 per cent at 6 years to 75 per cent at 21 years, at an annual rate of 0.07 on a logit scale (±0.02, table 2). From 21 years of age onwards, the breeding success of birds decreased with age at an annual rate of 0.07 (slope of −0.07±0.04, table 2). Towards the end of their life, southern fulmars have approximately the same breeding success as they had at 6 years of age. This model also included a positive effect of the SOI on breeding success (slope of 0.23±0.09 on a logit scale, table 2) and accounted for 70 per cent of the variation observed in breeding success among individual fulmars (wi=0.46; figure 1a; appendix, table 6 for threshold selection, and table S1 in the electronic supplementary material for details of model selection). A model including an interaction term between senescence rate and annual breeding success did not improve the fit (see appendix, model 36, table S1 in the electronic supplementary material), indicating that senescence of breeding success was not influenced by environmental conditions.
Table 2.
Effect of age on the breeding success—parameter estimates from the best model—SOUTHERN FULMAR—estimates of each parameter are presented with their standard error (s.e.). (bsann: inter-annual variations in breeding success, SOI, southern oscillation index).
| two thresholds | ||
|---|---|---|
| term | estimate | s.e. |
| (intercept) | −55.772 | 70.526 |
| slope before the first threshold age | 8.822 | 11.754 |
| slope between the first and the second threshold ages | 0.072 | 0.022 |
| slope after the second age threshold | −0.068 | 0.044 |
| bsann | 4.630 | 0.744 |
| SOI | 0.234 | 0.093 |
Figure 1.
Breeding success in relation to age, starting at age of first breeding. (a) The southern fulmar and (b) the snow petrel. The average observed value for each age is plotted with dotted standard error bars, with predictions from the threshold model. Thresholds are at 6 years and 21 years for the southern fulmar, and at 10 years for the snow petrel. Pearson's correlation coefficients between the prediction of the best model and the averaged observed value are indicated below the curve. **p<0.01.
Table 6.
Breeding probability—threshold model selection—SOUTHERN FULMAR. (AIC values from the models corresponding to the first (columns) and second (rows) threshold age are shown. The lowest AIC value is given in italic.)
| 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 21 | 977.5115 | 983.342 | 987.203 | 989.818 | 991.8056 | 993.0454 | 993.2465 | 994.0215 | 995.5467 | 996.815 | 997.036 | 998.0204 | 999.9394 | 1000.888 | 1001.649 |
| 22 | 978.5372 | 984.268 | 987.954 | 990.4034 | 992.32 | 993.4628 | 993.5284 | 994.2164 | 995.7027 | 996.9365 | 997.0619 | 997.9885 | 999.904 | 1000.747 | 1001.191 |
| 23 | 979.6701 | 985.2917 | 988.7886 | 991.0576 | 992.8896 | 993.9207 | 993.8375 | 994.424 | 995.8542 | 997.0364 | 997.0589 | 997.921 | 999.8055 | 1000.547 | 1000.819 |
| 24 | 981.1244 | 986.6188 | 989.8846 | 991.924 | 993.6393 | 994.517 | 994.2335 | 994.6726 | 996.007 | 997.0982 | 996.9584 | 997.7026 | 999.524 | 1000.118 | 1000.185 |
| 25 | 981.955 | 987.3627 | 990.4737 | 992.3611 | 993.999 | 994.7812 | 994.3833 | 994.744 | 996.0303 | 997.0806 | 996.8891 | 997.611 | 999.4153 | 1000.005 | 1000.126 |
| 26 | 982.6218 | 987.949 | 990.9244 | 992.6807 | 994.2521 | 994.9553 | 994.469 | 994.7733 | 996.0275 | 997.0546 | 996.8472 | 997.5778 | 999.384 | 1000.011 | 1000.238 |
| 27 | 982.6157 | 987.9129 | 990.8427 | 992.5703 | 994.1366 | 994.8435 | 994.3805 | 994.7243 | 996.0201 | 997.0979 | 997.005 | 997.8527 | 999.7137 | 1000.494 | 1000.973 |
| 28 | 981.6836 | 986.9987 | 989.9667 | 991.7594 | 993.3772 | 994.163 | 993.8191 | 994.2827 | 995.6783 | 996.8586 | 996.9688 | 997.9938 | 999.9212 | 1000.890 | 1001.643 |
| 29 | 980.206 | 985.5454 | 988.5608 | 990.4216 | 992.088 | 992.9443 | 992.6996 | 993.2573 | 994.728 | 995.9817 | 996.2377 | 997.3794 | 999.3355 | 1000.410 | 1001.305 |
| 30 | 980.1788 | 985.4833 | 988.4404 | 990.2446 | 991.8832 | 992.7063 | 992.4237 | 992.9589 | 994.4203 | 995.6688 | 995.9227 | 997.0711 | 999.0329 | 1000.117 | 1001.028 |
| 31 | 979.999 | 985.2826 | 988.2048 | 989.9698 | 991.5869 | 992.3863 | 992.0764 | 992.5947 | 994.0482 | 995.291 | 995.5426 | 996.6952 | 998.6602 | 999.75 | 1000.670 |
| 32 | 981.6989 | 986.8965 | 989.6652 | 991.2631 | 992.7835 | 993.4646 | 993.0127 | 993.4212 | 994.8006 | 995.9794 | 996.126 | 997.211 | 999.1652 | 1000.208 | 1001.073 |
| 33 | 980.844 | 986.0489 | 988.8236 | 990.423 | 991.9443 | 992.6277 | 992.1806 | 992.595 | 993.981 | 995.1668 | 995.3314 | 996.4319 | 998.3914 | 999.4472 | 1000.331 |
| 34 | 981.9437 | 987.026 | 989.6746 | 991.1377 | 992.5757 | 993.1571 | 992.6025 | 992.9308 | 994.2653 | 995.3908 | 995.4805 | 996.5204 | 998.4728 | 999.4835 | 1000.322 |
The best model (model 21, table 1) for snow petrels was a one-threshold model, including annual breeding success and showing the expected increase of breeding success throughout the early ages (threshold at 10 years), at a rate of 0.64 (±0.27, table 3) on a logit scale. After having reached a breeding success of 50 per cent at 10 years, the birds maintain high reproductive success until the oldest ages, since the slope (0.03±0.02, table 3) indicates a trend of increasing success with increasing age. This model fitted the data very well, accounting for approximately 83 per cent of the observed variation in breeding success among individuals (wi=0.25). No influence of either the SOI or the SIE could be detected, as the models incorporating those variables had very low wi (figure 1b; appendix, table 7 for threshold selection, and table S2 in the electronic supplementary material for details of model selection).
Table 3.
Effect of age on the breeding success—parameter estimates from the best model—SNOW PETREL—estimates of each parameter are presented with their standard error (s.e.). (bsann: inter-annual variations in breeding success).
| one threshold | ||
|---|---|---|
| term | estimate | s.e. |
| (intercept) | −9.253 | 2.714 |
| slope before the threshold | 0.642 | 0.273 |
| slope after the threshold | 0.027 | 0.017 |
| bsann | 5.227 | 0.554 |
Table 7.
Breeding probability—threshold model selection—SNOW PETREL. (AIC values from the models corresponding to the first threshold age (rows) are shown. The lowest AIC value is given in italics.)
| age at the first threshold | AIC |
|---|---|
| 7 | 892.3198 |
| 8 | 890.8771 |
| 9 | 889.458 |
| 10 | 888.7441 |
| 11 | 889.273 |
| 12 | 889.578 |
| 13 | 890.095 |
| 14 | 890.8103 |
| 15 | 891.3826 |
| 16 | 891.5965 |
| 17 | 891.7053 |
| 18 | 891.6908 |
| 19 | 891.8913 |
| 20 | 891.5893 |
| 21 | 891.6745 |
| 22 | 891.991 |
| 23 | 892.0299 |
| 24 | 891.8657 |
| 25 | 892.361 |
| 26 | 892.7444 |
| 27 | 892.8373 |
| 28 | 893.0806 |
| 29 | 893.254 |
| 30 | 893.3174 |
| 31 | 892.5926 |
| 32 | 891.5535 |
| 33 | 891.376 |
(b) Age-specific breeding probability
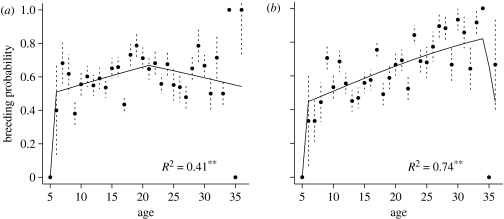
For southern fulmars, the model selected to describe breeding probability (model 29, table 1) followed the same pattern as for breeding success, except that the effects of SOI were not included. Breeding probability increased to 50 per cent at 6 years, and continued to increase (slope of 0.06±0.02 on a logit scale, table 4) until 21 years of age, when it reached a peak, with 68 per cent of birds breeding. From 21 years of age onwards, breeding probability decreased at a high rate (slope of −0.10±0.04 on a logit scale, table 4). The selected model (wi=0.41) accounted for 41 per cent of the variation in breeding probability observed among individual fulmars (figure 2a; appendix, table 8 for threshold selection, and table S3 in the electronic supplementary material for details of model selection). Again the model including an interaction term between senescence rate and annual breeding probability did not improve the fit (see appendix, model 36, table S3 in the electronic supplementary material).
Table 4.
Effect of age on the breeding probability—parameter estimates from the best model—SOUTHERN FULMAR—estimates of each parameter are presented with their standard error (s.e.). (bpann: inter-annual variations in breeding probability).
| two thresholds | ||
|---|---|---|
| term | estimate | s.e. |
| (intercept) | −68.527 | 302.655 |
| slope before the first threshold age | 10.908 | 50.443 |
| slope between the first and the second threshold ages | 0.058 | 0.018 |
| slope after the second age threshold | −0.096 | 0.035 |
| bpann | 5.486 | 0.409 |
Figure 2.
Breeding probability in relation to age, starting at age of first breeding. (a) The southern fulmar and (b) the snow petrel. The average observed value for each age is plotted with standard error bars, with predictions from the threshold model. Thresholds are at 6 years and 21 years for the southern fulmar, and at 6 years and 34 years for the snow petrel. Pearson's correlation coefficients between the prediction of the best model and the averaged observed value are indicated below the curve. *p<0.05. **p<0.01.
Table 8.
Breeding probability—threshold model selection—SOUTHERN FULMAR. (AIC values from the models corresponding to the first (columns) and second (rows) threshold age are shown. The lowest AIC value is given in italic.)
| 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 21 | 1771.379 | 1777.174 | 1788.309 | 1793.409 | 1793.352 | 1793.514 | 1794.074 | 1794.323 | 1794.515 | 1794.436 | 1794.493 | 1794.634 | 1793.677 | 1791.614 | 1790.848 |
| 22 | 1772.353 | 1777.917 | 1789.178 | 1794.538 | 1794.370 | 1794.461 | 1795.083 | 1795.357 | 1795.600 | 1795.349 | 1795.336 | 1795.574 | 1793.660 | 1791.219 | 1790.594 |
| 23 | 1773.583 | 1778.867 | 1790.250 | 1795.864 | 1795.545 | 1795.520 | 1796.157 | 1796.394 | 1796.606 | 1796.102 | 1795.906 | 1796.051 | 1793.288 | 1790.419 | 1789.642 |
| 24 | 1774.306 | 1779.393 | 1790.847 | 1796.614 | 1796.178 | 1796.058 | 1796.68 | 1796.866 | 1797.025 | 1796.347 | 1796.022 | 1796.075 | 1793.036 | 1790.271 | 1789.768 |
| 25 | 1775.161 | 1779.990 | 1791.520 | 1797.45 | 1796.854 | 1796.6 | 1797.177 | 1797.272 | 1797.331 | 1796.410 | 1795.884 | 1795.773 | 1792.256 | 1789.259 | 1788.666 |
| 26 | 1775.580 | 1780.207 | 1791.785 | 1797.824 | 1797.098 | 1796.731 | 1797.261 | 1797.275 | 1797.246 | 1796.15 | 1795.483 | 1795.261 | 1791.540 | 1788.555 | 1788.055 |
| 27 | 1775.670 | 1780.174 | 1791.777 | 1797.873 | 1797.068 | 1796.634 | 1797.133 | 1797.100 | 1797.025 | 1795.852 | 1795.137 | 1794.887 | 1791.225 | 1788.464 | 1788.19 |
| 28 | 1775.67 | 1780.188 | 1791.786 | 1797.876 | 1797.081 | 1796.665 | 1797.175 | 1797.164 | 1797.117 | 1796.023 | 1795.400 | 1795.246 | 1791.981 | 1789.710 | 1789.807 |
| 29 | 1775.668 | 1780.202 | 1791.796 | 1797.878 | 1797.095 | 1796.694 | 1797.214 | 1797.221 | 1797.198 | 1796.165 | 1795.609 | 1795.523 | 1792.536 | 1790.588 | 1790.901 |
| 30 | 1775.633 | 1780.118 | 1791.72 | 1797.823 | 1797.007 | 1796.582 | 1797.091 | 1797.083 | 1797.047 | 1795.998 | 1795.437 | 1795.354 | 1792.429 | 1790.589 | 1790.976 |
| 31 | 1775.411 | 1779.850 | 1791.460 | 1797.583 | 1796.734 | 1796.283 | 1796.781 | 1796.757 | 1796.705 | 1795.632 | 1795.059 | 1794.969 | 1792.064 | 1790.280 | 1790.708 |
| 32 | 1775.333 | 1779.788 | 1791.395 | 1797.511 | 1796.675 | 1796.238 | 1796.744 | 1796.735 | 1796.701 | 1795.669 | 1795.139 | 1795.09 | 1792.339 | 1790.715 | 1791.233 |
| 33 | 1774.982 | 1779.432 | 1791.041 | 1797.161 | 1796.32 | 1795.882 | 1796.389 | 1796.380 | 1796.349 | 1795.324 | 1794.803 | 1794.764 | 1792.062 | 1790.496 | 1791.046 |
| 34 | 1775.311 | 1779.802 | 1791.405 | 1797.507 | 1796.697 | 1796.287 | 1796.808 | 1796.822 | 1796.816 | 1795.844 | 1795.372 | 1795.375 | 1792.812 | 1791.369 | 1791.981 |
| 35 | 1774.062 | 1778.502 | 1790.109 | 1796.240 | 1795.41 | 1794.976 | 1795.491 | 1795.517 | 1795.479 | 1794.488 | 1794.024 | 1794.017 | 1791.427 | 1789.969 | 1790.573 |
For snow petrels, the model including two thresholds and the year-to-year variations of breeding decision (model 29, table 1) best described individual variation in observed breeding probabilities. Breeding probabilities increased from 0 at 5 years of age to 45 per cent at 6 years, the first threshold age, then increased at an annual rate of 0.02 (±0.01 on a logit scale, table 5), but over an extended period (between 6 and 34 years of age), by the end of which, approximately 80 per cent of birds were breeding. After this, breeding probability dropped abruptly (slope of −1.28±0.57 on a logit scale, table 5). This model accounted for 74 per cent of the observed variations in breeding probability of individual petrels (wi=0.33; figure 2b; appendix, table 9 for threshold selection, and table S4 in the electronic supplementary material for details of model selection).
Table 5.
Effect of age on the breeding probability—parameter estimates from the best model—SNOW PETREL—estimates of each parameter are presented with their standard error (s.e.). (bpann: inter-annual variations in breeding probability).
| two thresholds | ||
|---|---|---|
| term | estimate | s.e. |
| (intercept) | −36.339 | 68.620 |
| slope before the first threshold age | 5.630 | 11.437 |
| slope between the first and the second threshold ages | 0.021 | 0.013 |
| slope after the second age threshold | −1.281 | 0.568 |
| bpann | 4.714 | 0.435 |
Table 9.
Breeding probability—threshold model selection—SNOW PETREL. (AIC values from the models corresponding to the first (columns) and second (rows) threshold age are shown. The lowest AIC value is given in italic.)
| 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 21 | 1338.170 | 1339.13 | 1337.555 | 1337.323 | 1340.65 | 1341.784 | 1342.233 | 1341.156 | 1340.598 | 1340.905 | 1341.303 | 1341.749 | 1340.618 | 1340.507 | 1341.193 |
| 22 | 1338.413 | 1339.387 | 1337.927 | 1337.778 | 1341.016 | 1342.081 | 1342.343 | 1341.162 | 1340.589 | 1340.894 | 1341.287 | 1341.726 | 1340.726 | 1340.778 | 1341.434 |
| 23 | 1339.022 | 1340.028 | 1338.774 | 1338.763 | 1341.834 | 1342.755 | 1342.596 | 1341.033 | 1340.263 | 1340.494 | 1340.829 | 1341.227 | 1339.771 | 1339.537 | 1339.853 |
| 24 | 1339.172 | 1340.186 | 1339.013 | 1339.055 | 1342.052 | 1342.907 | 1342.601 | 1340.946 | 1340.154 | 1340.379 | 1340.709 | 1341.099 | 1339.740 | 1339.614 | 1340.013 |
| 25 | 1339.285 | 1340.306 | 1339.215 | 1339.310 | 1342.226 | 1343.012 | 1342.539 | 1340.762 | 1339.921 | 1340.127 | 1340.44 | 1340.813 | 1339.451 | 1339.345 | 1339.744 |
| 26 | 1339.261 | 1340.305 | 1339.308 | 1339.461 | 1342.281 | 1342.977 | 1342.289 | 1340.331 | 1339.401 | 1339.566 | 1339.843 | 1340.181 | 1338.715 | 1338.562 | 1338.911 |
| 27 | 1339.029 | 1340.081 | 1339.163 | 1339.364 | 1342.097 | 1342.711 | 1341.836 | 1339.722 | 1338.714 | 1338.842 | 1339.087 | 1339.394 | 1337.854 | 1337.670 | 1337.989 |
| 28 | 1338.764 | 1339.848 | 1338.962 | 1339.184 | 1341.880 | 1342.460 | 1341.527 | 1339.394 | 1338.394 | 1338.531 | 1338.785 | 1339.102 | 1337.663 | 1337.552 | 1337.921 |
| 29 | 1338.696 | 1339.778 | 1338.887 | 1339.108 | 1341.807 | 1342.397 | 1341.515 | 1339.476 | 1338.555 | 1338.734 | 1339.028 | 1339.381 | 1338.156 | 1338.170 | 1338.615 |
| 30 | 1338.105 | 1339.168 | 1338.314 | 1338.559 | 1341.213 | 1341.761 | 1340.797 | 1338.704 | 1337.765 | 1337.938 | 1338.226 | 1338.576 | 1337.368 | 1337.395 | 1337.842 |
| 31 | 1337.865 | 1338.952 | 1338.085 | 1338.325 | 1340.993 | 1341.559 | 1340.661 | 1338.669 | 1337.805 | 1338.015 | 1338.335 | 1338.711 | 1337.670 | 1337.776 | 1338.256 |
| 32 | 1338.181 | 1339.259 | 1338.346 | 1338.561 | 1341.279 | 1341.901 | 1341.163 | 1339.362 | 1338.622 | 1338.887 | 1339.248 | 1339.653 | 1338.835 | 1339.028 | 1339.526 |
| 33 | 1337.154 | 1338.201 | 1337.296 | 1337.518 | 1340.226 | 1340.841 | 1340.095 | 1338.304 | 1337.575 | 1337.844 | 1338.208 | 1338.614 | 1337.827 | 1338.028 | 1338.522 |
| 34 | 1336.985 | 1338.043 | 1337.124 | 1337.339 | 1340.061 | 1340.690 | 1339.987 | 1338.243 | 1337.545 | 1337.825 | 1338.197 | 1338.607 | 1337.869 | 1338.085 | 1338.577 |
| 35 | 1338.911 | 1339.963 | 1338.973 | 1339.145 | 1341.941 | 1342.644 | 1342.135 | 1340.595 | 1340.014 | 1340.334 | 1340.729 | 1341.145 | 1340.579 | 1340.836 | 1341.308 |
4. Discussion
Our main goal was to examine whether senescence of reproductive traits can be detected in two populations of long-lived seabirds, using a remarkably long dataset and accounting for individual differences in quality that can prevent the detection of senescence (Cam et al. 2002). We found that a marked contrast occurred in age-specific changes of reproduction between the two sympatric long-lived bird species. The southern fulmar, with an annual adult survival of 0.923 (Jenouvrier et al. 2003), showed clear evidence of senescence in both breeding probability and breeding success from 21 years of age onwards, for a maximum longevity of more than 45 years, whereas the snow petrel, with an annual adult survival of 0.934 (Chastel et al. 1993), did not show any sign of senescence in breeding success, and breeding probability did not decrease before 34 years of age for a maximum longevity of more than 46 years.
(a) Southern fulmar: evidence of senescence
Of the two species studied, only the southern fulmar showed clear evidence of reproductive senescence. The decrease was clear for both breeding success and breeding probability. Reproductive senescence has already been shown in numerous studies carried out on a wide range of vertebrates (Bennett & Owens 2002 on birds, Gaillard et al. 2003 on large mammals and Reznick et al. 2002 on fishes), including seabirds (Weimerskirch et al. 2005 on wandering albatross, Diomedea exulaus, Reed et al. 2008 on common guillemot, Uria aalge). The pattern of age-specific breeding success found in the fulmar is very similar to that of wandering albatrosses (Weimerskirch et al. 2005), i.e. a progressive decline when only half the maximum longevity is reached. Since the study on guillemots did not include age-specific breeding success, but time before death (Reed et al. 2008), the pattern observed is not directly comparable, although this study showed an abrupt decline 3 years before death and a progressive decline 10 years before death over a study period of 23 years, a pattern similar to that reported here for fulmars. Reproductive senescence in fulmar did not seem to be influenced by variation in the year quality. This might reflect a true independence between environmental conditions and senescence patterns. Alternatively, fluctuating year quality throughout the reproductive life of individuals in this long-lived bird could have masked any effect of environmental variation on senescence in our analysis.
(b) Snow petrel: no or very late indication for senescence
Interestingly, we did not find any support for senescence in the breeding success of snow petrels. On the contrary, the tendency was even towards a slight continuous increase. Another study, using a measure of prolactin levels throughout life (Angelier et al. 2007), reported the absence of a decrease in a reproductive trait with age in snow petrels. It shows that older breeders had higher prolactin levels than younger ones, which is associated with a lower probability of neglecting the egg. Our results therefore go in the same direction. However, we found an indication that breeding probability may decline at old ages in this species. Owing to this late decline (i.e. when two-third or more of the maximum longevity has been reached), we can suspect that it represents only the trajectory of very few birds, or the possibility that after a certain age, the birds remain at sea, avoiding this way the costs of a breeding event during poor years. Thus, in old age, birds could breed successfully only when conditions are favourable, and otherwise skip a breeding attempt. Since intermittent breeding is common in petrels and albatrosses, it is important to consider also the probability of breeding as a measure of breeding performance, when studying these species. By looking at only breeding success, we would be unable to distinguish between those birds that are alive but not willing to reproduce, and birds that have died.
Senescence is widespread among seabirds, but our results showing the absence of reproductive senescence in snow petrel are in line with those from a study on Leach's storm petrel Oceanodroma leucorhoa (Mauck et al. 2004). In this species, hatching success, defined as the presence or absence of a chick after one egg was laid did not decline with increasing age, but remained constant until old age, after an initial sharp increase.
(c) Possible explanation for contrasted patterns of reproductive senescence between two closely related species
Why are these two closely related species so different in the way breeding success and decision change with age? Two important differences exist between them. First, snow petrels are smaller than southern fulmars, and it is noticeable that the only other seabird species showing a similar pattern to that of the snow petrel is a small-sized storm petrel (Mauck et al. 2004). Although both species have similar life histories (high survival rates, low number of eggs laid, large amount of parental care, etc.), snow petrels are longer lived than fulmars and skip reproduction more frequently (Jenouvrier et al. 2005), leading them to have a longer generation time than fulmars. Jones et al. (2008) have recently shown that the magnitude of senescence is tightly linked with generation time, with slower species having later and weaker senescence. Note that although Jones et al. (2008) included the fulmar population analysed here in their comparative work, differences in reproductive measures considered and different analytical procedures preclude any comparison between the two studies. The between-species difference in reproductive senescence we report here could illustrate such a link between senescence and speed of the life-history cycle.
The other major difference between the two species is the migratory behaviour of the southern fulmar during winter, whereas the snow petrel is sedentary and remains closely associated with the pack ice during the whole year. Møller & De Lope (1999) found that the migratory performance of barn swallows, Hirundo rustica, decreased with age (delay in spring arrival on the breeding grounds), and Catry et al. (2006) have shown that reproductive senescence of another very long-lived seabird, the grey-headed albatross Diomedea chrysostoma, could be linked to reduced foraging performance at old age. Since the migratory behaviour of southern fulmars is closely associated with the search for food, a decline in migratory abilities also means a decline in foraging success. They might therefore be more strongly affected by ageing processes as compared with snow petrels.
Future studies should investigate these hypotheses to determine whether reproductive senescence is associated with a decrease in migratory ability, and whether the longest lived species show slower ageing pattern, together with possible causes for this, by comparing senescence patterns between more than two species.
Acknowledgments
The field study was approved by the ethics committee of the French Polar Institute.
We thank the wintering fieldworkers involved in the long-term monitoring of both species on Terre Adélie, Antarctica, and Dominique Besson for her help in data management. We also thank Christophe Bonenfant and Fitsum Abadi Gebreselassie for their useful comments, and Myles Menz for improving the English. We are grateful to Owen Jones, Dan Nussey, and an anonymous referee for constructive comments on a previous version of this paper. The long-term study was funded by IPEV, program 109 to Henri Weimerskirch, and supported by the program GICC2.
Appendix A.
Supplementary Material
Tables 1 to 5
References
- Ainley, D. G., O'Connor, E. G. & Boekelheide, R. J. 1984 The marine ecology of birds in the Ross Sea, Antarctica Ornithological Monographs N°32. Washington, DC: Amercian Ornithologists' Union.
- Angelier F., Moe B., Weimerskirch H., Chastel O. Age-specific reproductive success in a long-lived bird: do older parents resist better? J. Anim. Ecol. 2007;76:1181–1191. doi: 10.1111/j.1365-2656.2007.01295.x. doi:10.1111/j.1365-2656.2007.01295.x [DOI] [PubMed] [Google Scholar]
- Barbraud C., Weimerskirch H. Contrasting effects of the extent of sea-ice on the breeding performance of an Antarctic top predator, the snow petrel Pagodroma nivea. J. Avian Biol. 2001;32:297–302. doi:10.1111/j.0908-8857.2001.320402.x [Google Scholar]
- Bennett P.M., Owens I.P.F. Oxford University Press; Oxford, UK: 2002. Evolutionary ecology of birds: life histories, mating systems, and extinction. [Google Scholar]
- Bielby J., Mace G.M., Bininda-Emonds O.R.P., Cardillo M., Gittleman J.L., Jones K.E., Orme C.D.L., Purvis A. The fast–slow continuum in mammalian life history: an empirical reevaluation. Am. Nat. 2007;169:748–757. doi: 10.1086/516847. doi:10.1086/516847 [DOI] [PubMed] [Google Scholar]
- Burnham K.P., Anderson D.R. Springer; New York, NY: 1998. Model selection and inference. A practical information–theoretic approach. [Google Scholar]
- Cam E., Link W.A., Cooch E.G., Monnat J.Y., Danchin E. Individual covariation in life-history traits: seeing the trees despite the forest. Am. Nat. 2002;159:96–105. doi: 10.1086/324126. doi:10.1086/324126 [DOI] [PubMed] [Google Scholar]
- Catry P., Phillips R.A., Phalan B., Croxall J.P. Senescence effects in an extremely long-lived bird: the grey-headed albatross Thalassarche chrysostoma. Proc. R. Soc. B. 2006;273:1625–1630. doi: 10.1098/rspb.2006.3482. doi:10.1098/rspb.2006.3482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chastel O., Weimerskirch H., Jouventin P. High annual variability in reproductive success and survival of an Antarctic seabird, the snow petrel Pagodroma nivea. Oecologia. 1993;94:278–285. doi: 10.1007/BF00341328. doi:10.1007/BF00341328 [DOI] [PubMed] [Google Scholar]
- Gaillard J.-M. An analysis of demographic tactics in birds and mammals. Oikos. 1989;56:59–76. doi:10.2307/3566088 [Google Scholar]
- Gaillard J.-M., Loison A., Festa-Bianchet M., Yoccoz N.G., Solberg E. Ecological correlates of life span in populations of large herbivorous mammals. Popul. Dev. Rev. 2003;29:39–56. [Google Scholar]
- Gaillard J.-M., Yoccoz N.G., Lebreton J.-D., Bonenfant C., Devillard S., Loison A., Pontier D., Allaine D. Generation time: a reliable metric to measure life-history variation among mammalian populations. Am. Nat. 2005;166:124–128. doi: 10.1086/430330. doi:10.1086/430330 [DOI] [PubMed] [Google Scholar]
- Hughes K.A., Alipaz J.A., Drnevich J.M., Reynolds R.M. A test of evolutionary theories of aging. Proc. Natl Acad. Sci. USA. 2002;99:14 286–14 291. doi: 10.1073/pnas.222326199. doi:10.1073/pnas.222326199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlbert S.H. Pseudoreplication and the design of ecological field experiments. Ecol. Monogr. 1984;54:187–211. doi:10.2307/1942661 [Google Scholar]
- Jenouvrier S., Barbraud C., Weimerskirch H. Effects of climate variability on the temporal population dynamics of southern fulmars. J. Anim. Ecol. 2003;72:576–587. doi: 10.1046/j.1365-2656.2003.00727.x. doi:10.1046/j.1365-2656.2003.00727.x [DOI] [PubMed] [Google Scholar]
- Jenouvrier S., Barbraud C., Weimerskirch H. Long-term contrasted responses to climate of two Antarctic seabird species. Ecology. 2005;86:2889–2903. doi:10.1890/05-0514 [Google Scholar]
- Jones O.R., et al. Senescence rates are determined by ranking on the fast-slow life-history continuum. Ecol. Lett. 2008;11:664–673. doi: 10.1111/j.1461-0248.2008.01187.x. doi:10.1111/j.1461-0248.2008.01187.x [DOI] [PubMed] [Google Scholar]
- Kirkwood T.B.L., Rose M.R. Evolution of senescence: late survival sacrificed for reproduction. Phil. Trans. R. Soc. B. 1991;332:15–24. doi: 10.1098/rstb.1991.0028. doi:10.1098/rstb.1991.0028 [DOI] [PubMed] [Google Scholar]
- Mauck R.A., Huntington C.E., Grubb T.C., Jr Age-specific reproductive success: evidence for the selection hypothesis. Evolution. 2004;58:880–885. doi: 10.1111/j.0014-3820.2004.tb00419.x. doi:10.1554/03-147 [DOI] [PubMed] [Google Scholar]
- Møller A.P., De Lope F. Senescence in a short-lived migratory bird: age-dependant morphology, migration, reproduction and parasitism. J. Anim. Ecol. 1999;68:163–171. doi:10.1046/j.1365-2656.1999.00274.x [Google Scholar]
- Nisbet I.C.T. Detecting and measuring senescence in wild birds: experience with long-lived seabirds. Exp. Gerontol. 2001;36:833–843. doi: 10.1016/s0531-5565(00)00244-8. doi:10.1016/S0531-5565(00)00244-8 [DOI] [PubMed] [Google Scholar]
- Nussey D., Kruuk L.E.B., Morris A., Clutton-Brock T.H. Environmental conditions in early life influence ageing rates in a wild population of red deer. Curr. Biol. 2007;17:R1000–R1001. doi: 10.1016/j.cub.2007.10.005. doi:10.1016/j.cub.2007.10.005 [DOI] [PubMed] [Google Scholar]
- Nussey D., Coulson J.C., Festa-Bianchet M., Gaillard J.-M. Measuring senescence in wild animal populations: towards a longitudinal approach. Funct. Ecol. 2008;22:393–406. doi:10.1111/j.1365-2435.2008.01408.x [Google Scholar]
- Partridge L. Is accelerated senescence a cost of reproduction? Funct. Ecol. 1987;1:317–320. doi:10.2307/2389786 [Google Scholar]
- Partridge L., Mangel M. Messages from mortality: the evolution of death rates in the old. Trends Ecol. Evol. 1999;14:438–442. doi: 10.1016/s0169-5347(99)01646-8. doi:10.1016/S0169-5347(99)01646-8 [DOI] [PubMed] [Google Scholar]
- Reed T.E., Kruuk L.E.B., Wanless S., Frederiksen M., Cunningham E.J.A., Harris M.P. Reproductive senescence in a long-lived seabird: rates of decline in late-life performance are associated with varying costs of early reproduction. Am. Nat. 2008;171:E89–E101. doi: 10.1086/524957. doi:10.1086/524957 [DOI] [PubMed] [Google Scholar]
- Reznick D., Ghalambor C., Nunney L. The evolution of senescence in fish. Mech. Ageing Dev. 2002;123:773–789. doi: 10.1016/s0047-6374(01)00423-7. doi:10.1016/S0047-6374(01)00423-7 [DOI] [PubMed] [Google Scholar]
- Ricklefs R.E. Evolutionary theories of ageing: confirmation of a fundamental prediction, with implications for the genetic basis and evolution of life span. Am. Nat. 1998;152:24–44. doi: 10.1086/286147. doi:10.1086/286147 [DOI] [PubMed] [Google Scholar]
- Ridoux V., Offredo C. The diets of five summer breeding seabirds in Adelie Land, Antarctica. Polar Biol. 1989;9:137–145. doi:10.1007/BF00297168 [Google Scholar]
- Van de Pol M., Verhulst S. Age-dependent traits: a new statistical model to separate within- and between-individual effects. Am. Nat. 2006;167:766–773. doi: 10.1086/503331. doi:10.1086/503331 [DOI] [PubMed] [Google Scholar]
- Weimerskirch H., Lallemand J., Martin J. Population sex ratio variation in a monogamous long-lived bird, the wandering albatross. J. Anim. Ecol. 2005;74:285–291. doi:10.1111/j.1365-2656.2005.00922.x [Google Scholar]
- Weladji R.B., Gaillard J.-M., Yoccoz N.G., Holand O., Mysterud A., Loison A., Nieminen M., Stenseth N.C. Good reindeers mothers live longer and become better in raising offspring. Proc. R. Soc. B. 2006;273:1239–1244. doi: 10.1098/rspb.2005.3393. doi:10.1098/rspb.2005.3393 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables 1 to 5