Abstract
In both ecology and conservation, often a strong positive association is assumed between the diversity of plants as primary producers and that of animals, specifically primary consumers. Such a relationship has been observed at small spatial scales, and a begetting of diversity by diversity is expected under various scenarios of co-evolution and co-adaptation. But positive producer–consumer richness relationships may also arise from similar associations with past opportunities for diversification or contemporary environmental conditions, or from emerging properties of plant diversity such as vegetation complexity or productivity. Here we assess whether the producer–consumer richness relationship generalizes from plot to regional scale and provide a first global test of its strength for vascular plants and endothermic vertebrates. We find strong positive richness associations, but only limited congruence of the most diverse regions. The richness of both primary and higher-level consumers increases with plant richness at similar strength and rate. Environmental conditions emerge as much stronger predictors of consumer richness, and after accounting for environmental differences little variation is explained by plant diversity. We conclude that biotic interactions and strong local associations between plants and consumers only relatively weakly scale up to broad geographical scales and to functionally diverse taxa, for which environmental constraints on richness dominate.
Keywords: biodiversity, plants, vertebrates
1. Introduction
Understanding the mechanisms and predictors of the origin and maintenance of the geographical variation in species richness across taxa is a challenge central to ecology and conservation. Species interactions over evolutionary and ecological time scales suggest the potential for strong associations and high similarities in richness patterns across taxa. Such congruence may offer invaluable short cuts, ‘surrogates’, for conservation, but under global change discerning the strength of actually causal dependencies is crucial. While supported by select evidence (Prendergast et al. 1993; Dobson et al. 1997; Lamoreux et al. 2006) and already used in conservation planning (Myers et al. 2000), the generality and strength of patterns of congruence (Ceballos & Ehrlich 2006; Grenyer et al. 2006) are still debated and their causes only partly understood (Wolters et al. 2006). The idea that diversity at one trophic level (e.g. producer) begets diversity at others has strong prominence in ecology and is backed by intuitive causal links. Based on observed tight-knit associations between plants and specialized herbivorous insects, the putative increase of higher trophic-level richness with plant richness has formed one of the pillars of original thinking about ecological determinants of diversity (Hutchinson 1959).
But ecologists have early on appreciated the importance of energy availability and spatial heterogeneity for spatial patterns of diversity (Hutchinson 1959), effects that have since received ample support at broad scales (Brown 1981; Jetz & Rahbek 2002). This then raises the questions of the causes, and of both the absolute and relative strengths, of cross-trophic-level diversity relationships at broad scales. Here we assess patterns and strengths of these associations for plants and endotherm consumers (birds, mammals) worldwide.
Positive producer–consumer diversity correlations might arise in a variety of ways. Assuming that consumers compete for resources and exhibit at least some specialization, diverse resources are thought to provide opportunities for niche diversification and coexistence of diverse consumers (Hutchinson 1959; Chesson 2000; Novotny et al. 2006; Dyer et al. 2007). This ‘resource diversity hypothesis’ predicts a strong relationship between the diversity of primary producers and their consumers above and beyond other (e.g. environmental) correlates of richness, and a weaker link at higher trophic levels. Plant diversity may facilitate higher animal richness through emerging properties such as structural or habitat complexity instead of directly as a food resource. In the form of more complex vegetation structure (‘physiognomy’), increased plant richness may offer more niches for animals to coexist—this is the ‘vegetation structure hypothesis’ (MacArthur & MacArthur 1961; Cody 1985; Rotenberry 1985; Andrews & O'Brien 2000; Kissling et al. 2008). Niche space provided by structural heterogeneity is notoriously difficult to quantify over broad geographical scales, and tests to date have used relatively simple vegetation measures or indirect inference (Lee & Rotenberry 2005; Kissling et al. 2008).
Finally, the producer–consumer richness association may have no direct causality and may arise mainly from external factors with similar consequences for geographical patterns of diversification and co-occurrence of both groups (Hawkins & Porter 2003; Kissling et al. 2008). Chief among such external variables are general gradients in the potential for primary production and energy availability (e.g. by actual evapotranspiration, AET), which is widely accepted to affect number of individuals and, jointly or separately, number of species sustained (Hutchinson 1959; Brown 1981; Wright 1983; Mittelbach et al. 2001). But other variables include topographic and geographical habitat heterogeneity, temperature and more (Olff et al. 2002; Currie et al. 2004). These ‘energy’ and ‘environment’ hypotheses predict that, above all, geographical differences in consumer diversity are explained by environmental characteristics. Both producers and consumers may follow similar environmental gradients and therefore exhibit an apparent association with each other, but little variation in primary-consumer richness above and beyond environment would in fact be explained by producer richness. Critically, even more than plant richness, vegetation structure may show very strong collinearity with environmental variables such as energy availability (Holdridge 1947), hampering straightforward inference.
Given that higher-level consumers are at least one trophic link further removed from producers, contrasting their richness patterns may offer additional insights. While the purported positive effect of plant richness should at least partially cascade up to higher-level consumers, their more distant position should lead to weaker associations. Similar evolutionary histories and phylogenetic niche conservatism (which leads to similar ecological and environmental associations) would probably cause the geographical richness patterns of primary and higher-level vertebrate consumers to co-vary with each other. But is this within-vertebrate relationship affected by the diversity of (phylogenetically distant) producers? Do regions that are highly diverse in producers harbour a disproportionate number of primary producers? Here we examine how the proportional richness of primary vertebrate consumer changes along a global gradient of plant diversity.
The processes invoked by the listed hypotheses act at different spatial scales, characterized by different levels of species interactions (Holling 1992; Levin 1992). Therefore, any tests require an explicit appreciation of the scale of underlying mechanisms and the scale and grain size of analysis. Critically, Hutchinson formulated putative mechanisms of plant–herbivore richness associations based on observations of directly interacting taxa at local scale (Hutchinson 1959). At local scales and across relatively small sets of species, such associations have found ample observational and experimental support (Southwood et al. 1979; Siemann et al. 1998; Knops et al. 1999). Biotic interactions in local communities are expected to become weaker and more diffuse towards assemblages that are geographically (in terms of grain size) and ecologically (in terms of feeding associations) increasingly inclusive. Ecologically, the importance of resource diversity should therefore be highest for specialized food-plant–feeding-guild associations co-occurring over the geographical scale at which they interact. However, evolutionarily these local and specialized associations may be expected to promote consumer richness beyond single locales across regions worldwide (Price 2002; Novotny et al. 2006). Testing this assertion is the main aim of this study.
Whether and how these ecological and coevolutionary connections scale up and, across landscapes and regions, translate into geographical patterns of co-variation in richness across broad taxa has to date eluded global scrutiny. At broad geographical scales, evolutionary signals of coevolution and biotic interactions may give way to taxon-specific patterns of dispersal and potential abiotic constraints on richness and the spatial turnover of species (Jetz & Rahbek 2002; Willis & Whittaker 2002; Hawkins & Porter 2003; Ruggiero & Kitzberger 2004; Currie et al. 2004; Buckley & Jetz 2007). While positive correlations between plant and vertebrate diversity have been demonstrated across large regions and countries, inference about the causes of this relationship is not straightforward (Andrews & O'Brien 2000; Hawkins & Porter 2003; Wolters et al. 2006; Qian et al. 2007). Confoundingly, the richness of vertebrate consumers and that of plants follows broadly similar climatic gradients: similar to birds and mammals, vascular plants are most diverse in warm, wet and topographically complex regions (Kreft & Jetz 2007). Therefore, quantifying the independent signal of cross-trophic biotic interactions in shaping gradients of consumer richness requires careful modelling of both abiotic and biotic components. Understanding the importance and scale dependence of biotic interactions for broad-scale patterns of diversity also has strong conservational relevance. Biotic constraints may limit species' responses, such as range shifts, in the face of the dramatic anthropogenic environmental change forecasted for this century (Carpenter et al. 2005; Jetz et al. 2007).
Here we aim to quantify the independent relationship between producer and consumer diversity at a global scale by separating their respective association from that with environment and space. Specifically, we analyse the richness of vascular plants (ferns, gymnosperms and angiosperms) and endotherm vertebrates (birds and mammals) across 639 geographical regions worldwide and ask: (i) how congruent producer and consumer richness are across all regions and, specifically, across plant diversity hotspots; (ii) whether producer diversity more strongly affects the diversity of primary rather than higher-level consumers; and (iii) how strong the independent effect of producer on consumer richness is after controlling for environmental and spatial associations.
2. Material and methods
(a) Plant and vertebrate data
A global-scale analysis of producer and vertebrate consumer diversity is limited by the data available for plants. While digital geographical range maps have been compiled for each mammal and bird species worldwide (Ceballos & Ehrlich 2006; Jetz et al. 2007), this is not the case for vascular plants. In a recent study, Kreft & Jetz (2007) used environment-richness relationships and geostatistical techniques to model the global pattern of vascular plant species richness. As the predicted gridded richness map is based on interpolations, for this study we use the original collection of floras and plant checklists used in that study, which includes the numbers of native vascular plant species (i.e. ferns, gymnosperms and angiosperms) for more than 1800 geographical units worldwide. Geographical units encompass natural (e.g. mountain ranges, deserts and biogeographic regions) as well as political entities (e.g. countries, states and provinces), and thus inevitably vary in size. This dataset is described in more detail elsewhere (Barthlott et al. 2005; Mutke & Barthlott 2005; Kreft & Jetz 2007) and full references of all sources are given in Kier et al. (2005). Because island-specific factors such as geographical isolation and immigration-extinction dynamics shape floras and faunas (Kreft et al. 2008), we excluded oceanic islands to facilitate inference. In order to minimize false presences of vertebrate species, we excluded geographical units below 10 000 km2 in size, the minimum size at which mammal and bird range map data are expected to be reliable (Hurlbert & Jetz 2007), which resulted in a final list of 639 geographical units. These units range from 10 000 to 575 430 km2 in size with a median of 52 481 km2. We acknowledge that the unavoidable variation in size and shape of our analysis units introduces potential scale and aggregation issues—the well-known modifiable areal unit problem in geographical sciences (Openshaw & Taylor 1981). But we expect this to have only a minor influence on our results. We performed a second set of analyses in which we excluded the larger half of spatial units specifically to tackle scale effects.
We used extent of occurrence maps of the geographical ranges of the world's 13 851 terrestrial endotherm vertebrate species, i.e. birds and mammals, to derive species lists for the floristic inventory units. We excluded species that predominantly feed in aquatic habitats (i.e. open ocean, lakes and rivers) during the breeding season to facilitate interpretation. Further, we used breeding ranges only, as accurate maps of avian winter distributions are not yet available and in terms of relative richness winter migrants play a relatively minor role. Altogether, maps for 8919 bird and 4932 mammal species entered the analysis (for sources and details see Ceballos & Ehrlich 2006; Jetz et al. 2007). Range maps were geographically overlaid with plant geographical units in a geographical information system, and the presence of every species with overlapping range was recorded. While erroneous inclusion of species whose ranges only narrowly overlap with a floristic unit may occur, this would only affect our results if such limited congruence was geographically or taxonomically highly non-random. Actual surveys of vertebrate species are unfortunately not available at broad spatial scales. For dietary information we used data from the primary and secondary literature (e.g. Nowak 1997; sources listed in Sekercioglu et al. 2004) and assigned species to two broad trophic levels: (i) primary consumers (i.e. predominantly feeding on plant material such as leaves, fruits, seeds, nectar, etc.; 3255 bird and 2573 mammal species) and (ii) secondary consumers (second trophic level and higher, feeding on invertebrates, vertebrates, etc.: 5664 bird and 2359 mammal species).
(b) Environmental data
Our choice of predictor variables was motivated by an attempt to minimize collinearity and to facilitate inference about established effects. We examined alternative measures for temperature and water availability, which did not yield stronger predictions or qualitatively different results (Kreft & Jetz 2007). Since the geographical units differ in size, we included the area sizes of the geographical units as a control variable. Area (km2) was calculated using the boundaries of the geographical units as published and as used for geographical data extraction. Mean values of climate, topography and landscape heterogeneity were extracted across all 639 geographical units. We used data for actual evapotranspiration (AET; mm yr−1) as a measure of potential net primary production (‘energy hypothesis’). As additional environmental predictors (‘environment hypothesis’), we used two climatic variables—mean annual temperature (Temp; °C), and mean annual number of days with more than 1 mm of precipitation (WetDays; d yr−1)—and two measures of landscape heterogeneity: as a measure of habitat diversity (HabDiv; n) we derived the number of ecosystems for each geographical unit and as a measure of topographic diversity (TopoDiv, m) we calculated the maximum elevational range within each geographical unit. All variables have been shown previously to be strong predictors of plant (Kreft & Jetz 2007) and/or vertebrate diversity (Jetz & Rahbek 2002; Ruggiero & Kitzberger 2004; Buckley & Jetz 2007). Data for AET were derived from Ahn & Tateishi (1994). The dataset has a spatial resolution of 30 min and is representative of the time period of 19.20–19.80. Data for Temp and WetDays (spatial resolution: 10 min) came from the dataset of the Climatic Research Unit for the time period 19.61–19.90 (New et al. 2002). HabDiv was derived as the number of ecosystems (Olson 1994) for each geographical unit (spatial resolution: 30 arc seconds). This dataset distinguishes 94 different ecosystem classes based on classified Advanced Very High Resolution Radiometer data for the period April 1992–March 1993. TopoDiv was based on the GTOPO30 dataset (USGS 1996) and calculated as the maximum elevational range for each geographical unit (spatial resolution: 30 arc seconds). All variables were extracted at their original resolution and aggregate measures calculated for each geographical unit. Dependent and independent variables were log10-transformed for analysis to achieve approximately normally distributed model residuals.
(c) Statistical analysis
We first looked at single-predictor relationships between environmental predictor variables and species richness of vascular plants, and each group of vertebrates and dietary level (birds, mammals, both combined, primary consumers, higher-level consumers). To control for spatial non-independence and associated Type I errors, we performed spatial linear models (SLM; simultaneous autoregressive model, error type, weighted neighbourhood structure; for details see Kreft & Jetz 2007) in addition to non-spatial generalized linear models (GLM). In a second step, we performed multivariate statistical models (both GLM and SLM) incorporating climate and landscape variables. In addition to our GLM and SLM approach, we conducted structural equation modelling (SEM). SEM allows specific tests of different multivariate hypotheses by assigning directional relationships and by providing a straightforward approach for multi-collinearity among predictor variables (Grace 2006). Furthermore, it allows separating the effects of predictor on response variables into direct and indirect effects. Our SEMs were designed to test the hypothesis that plant richness directly drives vertebrate richness and to disentangle the specific respective roles of climate, heterogeneity and producer richness. All statistical analyses (except for SEM) were performed with the software R and the package spdep for spatial analyses. SEM was performed with the software AMOS v. 5.0 (Arbuckle 2003). We note that hierarchical Bayesian models, which were outside the scope of this study, represent a powerful alternative way of addressing the intricate dependencies often observed in macroecological data (Ellison 2004).
3. Results and discussion
(a) Plants as richness and hotspot surrogates for vertebrates
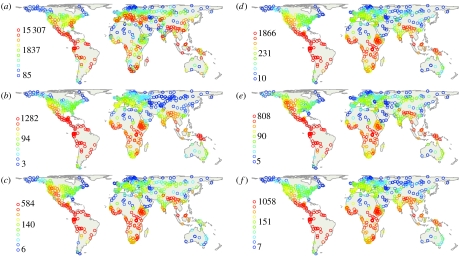
We find that plants, birds, mammals, and birds and mammals combined (endotherm vertebrates) all show geographically uneven patterns of richness (figure 1a–f). On the whole, vertebrate diversity is positively associated with plant richness (figure 2, left panels)—an association that is similarly strong for birds, mammals and both combined. The observed diversity for birds and mammals increases with increasing plant richness (Plants) in a decelerating fashion (slopes <1 in log–log space, table 1). Independently, increases in richness are much steeper in birds (N=8919 species in the analysis; Birds∝Plants0.71) than they are in mammals (N=4932 species; Mammals∝Plants0.43). While several of the regions rich or depauperate in species are identical across all groups, strong discrepancies exist. Regions of particularly high plant diversity unmatched by vertebrates include the European Mediterranean, Madagascar and the South African Cape floristic region (electronic supplementary material, fig. 1a). Conversely, certain vertebrate richness peaks such as those in the Ethiopian highlands (Birds) and Central Africa (Mammals) are not as outstanding in plants. Only 31 and 38 per cent of the 5 per cent most plant species-rich locations (N=32) are also among the top 5 per cent diverse mammal and bird locations, respectively; this congruence is somewhat higher for all endotherm vertebrates (44%). The observed congruence in the tropical Andes, the East African Mountains, or parts of Indo-Malaysia should simplify conservation planning, but dramatically more area is needed to account for all diversity centres, even just for groups in this study. The existence of so important taxon-specific centres such as the South African Cape flora region (for plants) or Central Africa (for vertebrates) raises a question about the use of these taxa as surrogates for overall biodiversity patterns.
Figure 1.
Global patterns of species richness of 639 geographical units analysed in this study. (a) Plants, (b) birds, (c) mammals, (d) endotherm vertebrates, (e) primary consumers and (f) higher-level consumers. Endotherm vertebrates comprised mammals and birds, and are separated in primary and secondary (and higher-level) consumers. Quantile classification. Maps are projected using Behrmann projection.
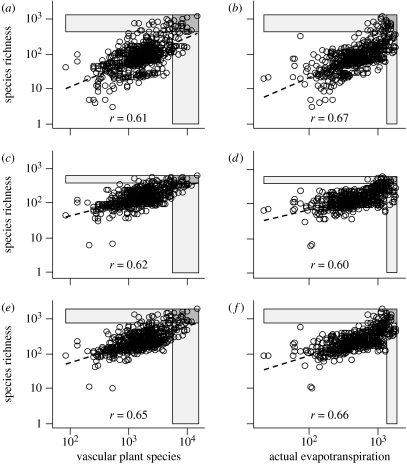
Figure 2.
Relationships between vascular plant species richness, actual evapotranspiration (AET, mm/a) and the species richness of (a,b) birds, (c,d) mammals and (e, f) endotherm vertebrates (i.e. mammals+birds). r-values are Pearson correlations of log-transformed data and dashed lines indicate linear fits. Plant richness and AET are collinear (r=0.64, p>0.001). Grey boxes mark upper 95% percentiles and dark grey boxes the overlap between the two.
Table 1.
Relationships between abiotic predictor variables, plant and vertebrate consumer species richness. (AET: actual evapotranspiration. Env refers to models including main effects of the independent variables Area (size of the geographical unit; km2), Temp (mean annual temperature; °C), WetDays (mean annual number of days with precipitation greater than 1 mm; d yr−1), TopoDiv (topographic heterogeneity measured as maximum elevational range; m) and HabDiv (number of different ecosystems; n). All independent and dependent variables were log-transformed. Slopes (b), tests for slopes differing from zero (t) and according p-values are reported. Akaike information criterion (AIC) values and likelihood-based r2-values are provided as measures of fit. r2-values for SLMs refer to the non-spatial component of the model fit. Absolute Moran's I of residuals are greater than 0.60 and significant for all GLMs, and less than 0.07 and not significant for all SLMs. Significance coding: *p≤0.05; ***p≤0.001.)
| GLM | SLM | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| b | t | AIC | r2 | b | t | AIC | r2 | ||
| Birds | Area | 0.04 | 1.16 | 624.2 | 0.00 | 0.12 | 8.58*** | −505.8 | 0.10 |
| AET | 0.88 | 22.73*** | 246.1 | 0.45 | 0.54 | 15.24*** | −633.5 | 0.27 | |
| Plants | 0.71 | 19.56*** | 325.0 | 0.38 | 0.32 | 14.49*** | −618.0 | 0.25 | |
| AET+Plants | 176.4 | 0.51 | −725.4 | 0.37 | |||||
| Env | −125.4 | 0.70 | −861.4 | 0.90 | |||||
| Env+Plants | −127.2 | 0.70 | −868.5 | 0.91 | |||||
| Mammals | Area | 0.12 | 5.52*** | −42.8 | 0.05 | 0.13 | 11.25*** | −763.7 | 0.16 |
| AET | 0.47 | 18.74*** | −293.5 | 0.36 | 0.35 | 11.11*** | −760.2 | 0.16 | |
| Plants | 0.43 | 19.95*** | −323.1 | 0.38 | 0.28 | 14.61*** | −833.1 | 0.25 | |
| AET+Plants | −396.2 | 0.45 | −870.8 | 0.29 | |||||
| Env | −486.6 | 0.53 | −1013.0 | 0.79 | |||||
| Env+Plants | −503.2 | 0.54 | −1024.0 | 0.80 | |||||
| Endotherms | Area | 0.09 | 3.38*** | 191.8 | 0.02 | 0.12 | 10.83*** | −766.5 | 0.15 |
| AET | 0.62 | 22.33*** | −166.4 | 0.44 | 0.41 | 13.52*** | −818.6 | 0.22 | |
| Plants | 0.54 | 21.60*** | −147.9 | 0.42 | 0.29 | 15.50*** | −863.5 | 0.27 | |
| AET+Plants | −273.2 | 0.53 | −934.6 | 0.35 | |||||
| Env | −460.2 | 0.65 | −1111.5 | 0.87 | |||||
| Env+Plants | −472.8 | 0.66 | −1122.7 | 0.88 | |||||
| Primary | Area | 0.13 | 4.90*** | 200.0 | 0.04 | 0.15 | 12.75*** | −739.2 | 0.20 |
| AET | 0.59 | 19.83*** | −83.5 | 0.38 | 0.40 | 12.19*** | −728.2 | 0.19 | |
| Plants | 0.54 | 20.76*** | −106.6 | 0.40 | 0.31 | 16.13*** | −814.3 | 0.29 | |
| AET+Plants | −192.5 | 0.48 | −863.3 | 0.34 | |||||
| Env | −314.9 | 0.58 | −1042.1 | 0.87 | |||||
| Env+Plants | −326.9 | 0.58 | −1053.6 | 0.87 | |||||
| Higher-level | Area | 0.05 | 2.06* | 195.0 | 0.01 | 0.10 | 9.38*** | −801.8 | 0.12 |
| AET | 0.62 | 22.57*** | −176.3 | 0.45 | 0.41 | 14.12*** | −891.9 | 0.24 | |
| Plants | 0.53 | 20.81*** | −132.3 | 0.41 | 0.26 | 14.47*** | −900.8 | 0.25 | |
| AET+Plants | −267.8 | 0.52 | −987.1 | 0.34 | |||||
| Env | −541.7 | 0.69 | −1143.2 | 0.88 | |||||
| Env+Plants | −553.0 | 0.70 | −1153.5 | 0.88 | |||||
(b) Producer–consumer associations across trophic levels
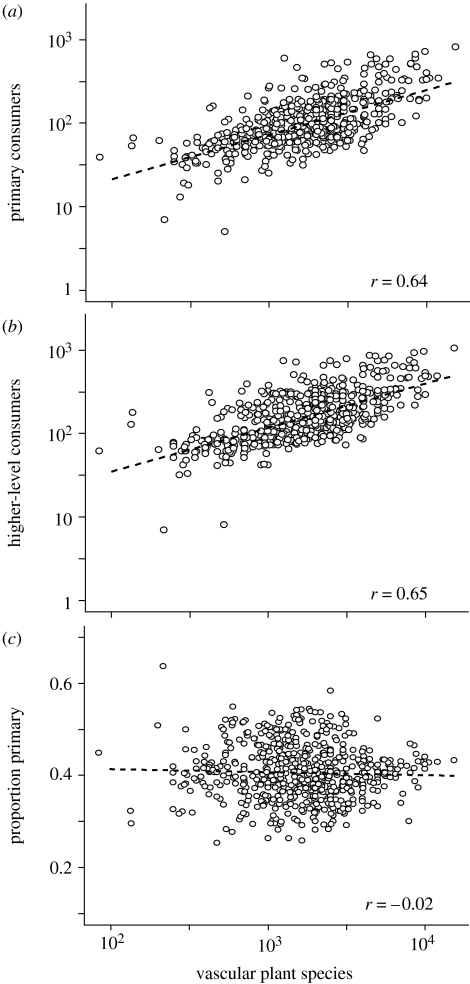
Given their direct dependence on plants, primary consumers may be expected to show stronger associations with plant richness than higher-level consumers as these are at least one trophic link further removed (Hutchinson 1959). Of all 13 851 bird and mammal species, 5828 (i.e. 42%) are primary and 8023 (58%) are higher-level consumers. We find that in both trophic-level categories, consumer diversity exhibits an increase with plant richness that is remarkably similar in shape (figure 1, table 1): Primary∝Plants0.54, Higher-level∝Plants0.53. Contrary to our initial prediction, the richness relationship is somewhat stronger for higher-level than primary consumers. This might imply that richness relationships are driven more generally by the higher resource availability (Naeem et al. 1995; Tilman et al. 1996; Siemann et al. 1998) and more complex vegetation structure (MacArthur & MacArthur 1961; Andrews & O'Brien 2000; Lee & Rotenberry 2005; Kissling et al. 2008) that high plant richness entails rather than specific functional connections. We find surprising invariance in primary–higher-level consumer richness ratios along the global plant richness gradient (figure 3), highlighting how much tighter associations are within these two clades than between them and plants. Across all locations, primary consumers constitute 40.6 per cent (s.e. 0.2%) of all endotherm vertebrate species, and across the over two-order magnitude variation in plant richness, this ratio does not show any significant trend (Proportion Primary∝Plants−0.00, GLM on log-transformed data, t=−0.52, n.s.). The ratio between primary and higher-level consumers is not directly comparable with predator–prey ratios known from foodwebs (Cohen 1977), as many higher-level vertebrate consumers in fact prey on invertebrates. Several hypotheses have been discussed to explain roughly invariant predator–non-predator ratios (Warren & Gaston 1992). Further study into the macroecology of this pattern in vertebrates is required, but as a working hypothesis we attribute much of the observed association to the broadly similar environmental adaptations and evolutionary histories among these relatively closely related species.
Figure 3.
Relationships between vascular plant species richness and the richness of (a) primary and (b) higher-level vertebrate consumers. The ratio of primary to higher-level consumer richness of endotherm vertebrates in relation to plant richness is shown in (c). r-values are Pearson correlations of log-transformed data and hatched lines indicate linear fits.
(c) Disentangling environment and species interactions
Geographical patterns of terrestrial vertebrate richness also vary strongly along environmental gradients such as water-temperature variables or net primary productivity (Jetz & Rahbek 2002; Hawkins & Porter 2003; Currie et al. 2004). Here we use AET, which encapsulates water and temperature dynamics and is a good proxy for the potential for primary production and energy availability. Endotherm vertebrate richness shows a consistent increase towards high-AET regions that at first glance appears comparable in shape and strength to that towards high plant richness (figure 2, right panels). The congruence of top 5 per cent endotherm richness and AET ‘hotspots’ is comparable to that found for plants (figure 2; electronic supplementary material, fig. 1b; birds: 34%, mammals: 25%, endotherms: 38%). AET is stronger than plant richness in predicting bird richness gradients, but is weaker in explaining mammal richness in both spatial and non-SLM. In comparison, the variation in study area size has relatively little effect on plant or vertebrate richness (GLM r2=0.01 to 0.05; table 1), but gains importance when the spatial structure in the data is accounted for (SLM r2=0.10 to 0.20). Given the favourable conditions for growth inherent in high AET, plant richness and AET are themselves strongly positively associated (GLM r2=0.41, p<0.001; Kreft & Jetz 2007), but complement each other as predictors of vertebrate richness (table 1): in all cases both variables combined yield a slightly stronger model than each individually.
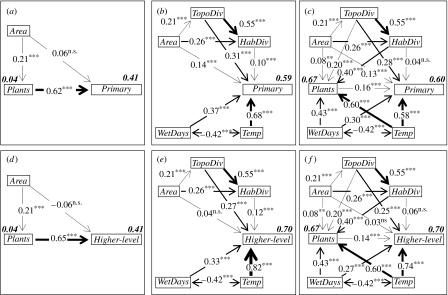
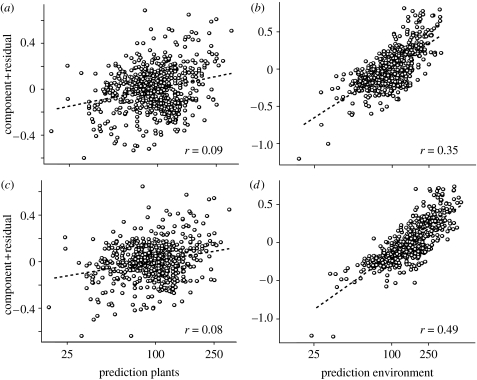
In addition to AET, a multitude of environmental (including topographic) attributes have been found to affect spatial patterns of vertebrate diversity (Jetz & Rahbek 2002; Hawkins et al. 2003; Ruggiero & Kitzberger 2004; Buckley & Jetz 2007; Davies et al. 2007). To document and separately identify the potential importance of plant richness in representing biotic determinants of vertebrate richness above and beyond recognized abiotic factors, we use SEM. This represents a powerful statistical approach to disentangle direct from indirect effects in multivariate hypotheses and to explicitly test different competing functional relationships (Grace 2006). We extend the range of abiotic predictors to include topographic heterogeneity (TopoDiv) and habitat heterogeneity (HabDiv). To address primary abiotic variables, we fit average annual temperature (Temp) and annual wet days (WetDays) instead of AET, with which both are collinear (r=0.75; Kreft & Jetz 2007). While plant richness itself provides a strong prediction (figure 4, left), the five environmental variables (Env; table 1) explain primary, higher-level and combined endotherm richness dramatically better (figure 4, middle). WetDays, Temp and TopoDiv in particular have strong positive effects on vertebrate richness, yielding path coefficients (in the case of temperature) and coefficients of determination that are much higher than those observed for plant richness. When all environmental effects on vertebrate richness are accounted for (figure 4, right), there is very little variation for plants, richness left to explain (figure 4). In turn, select abiotic variables such as HabDiv noticeably decrease in their importance when plant richness is controlled for (change in beta from 0.13 to 0.07 for endotherm vertebrates). However, together the abiotic (‘environmental’) predictors are much stronger determinants of vertebrate richness variation above and beyond that explained by plant richness than vice versa (in both GLM and models addressing spatial autocorrelation; table 1). At the grain size of our analysis, the independent signal of plant richness compared with that of environment is negligible (table 1; figure 5). The same qualitative results hold true for mammals, birds and both combined (electronic supplementary material fig. 2) and for an analysis that excluded the larger half of spatial units which may be affected by biogeographic turnover and other scale effects (median size of units analysed=23 343 km2; electronic supplementary material, table 1).
Figure 4.
(a–f) Structural equation models of the relationships between plant richness, environment, and primary and higher-level consumer vertebrate richness. (a,d) Direct effects of plant richness, (b,e) environmental effects on vertebrate richness and (c,f) combined environmental/richness model. The numbers at bidirectional relationships represent correlations among predictors. Standardized path coefficients are shown for all unidirectional relationships. These illustrate the effects in units of standard deviation or, in other words, the correlations that represent the variation associated with a relationship. The width of the arrows indicates the strength of the effects. Numbers in bold italics next to dependent variables indicate squared multiple correlations.
Figure 5.
Component-residual plots of the independent effects of plant richness and environment on the richness of primary and higher level vertebrate consumers. (a,c) vertebrate richness predicted by plant richness controlled for environment (five variables, for details see table 1 and figure 4). (a) Primary consumers; (c) higher-level consumers. (b,d) Vertebrate richness predicted by environment (same five variables) controlled for plant richness. (b) Primary consumers; (d) higher-level consumers. These are component-residual plots based on multi-predictor GLM models. r-values are Pearson correlations of the plotted relationships.
(d) Conclusions
Taken together, our results confirm a strong positive correlation between producer and vertebrate consumer diversity across regions worldwide, but, for the scale of analysis, offer only little support for a direct causal link as suggested by the ‘resource diversity’ hypothesis. Lack of a direct ecological signal of biotic interactions is not surprising, given the large spatial extent of regions and the ecological breadth of consumers included. More expected was a potential coevolutionary signal of resource diversity in shaping broad-scale geographical patterns. But our findings suggest that the cross-regional spatial congruence of plant and endotherm vertebrate richness emerges more strongly from similar responses to environmental gradients, and particularly support the energy hypothesis of both producers and consumers following the same gradients of kinetic energy and water availability (Jetz & Rahbek 2002; Hawkins et al. 2003; Currie et al. 2004; Buckley & Jetz 2007; Kreft & Jetz 2007). This highlights the limited direct mechanistic support for the use in conservation of plants as cross-regional surrogates for vertebrate richness and vice versa. For the scale of the analysis, we were unable to quantify the structural complexity of vegetation as a potential functional link between producer and consumer diversity (vegetation structure hypothesis; MacArthur & MacArthur 1961; Rotenberry 1985; Andrews & O'Brien 2000; Kissling et al. 2008). We expect vegetation structure to co-vary strongly with environmental variables, particularly AET, and it may well play a dominant role behind the strong environmental correlations for consumer richness that we find in our analysis.
There is abundant evidence for biotic interactions causing co-variation between plant and consumer richness at local scales (Southwood et al. 1979; Siemann et al. 1998; Knops et al. 1999) and, at least for functionally tightly linked producer–consumer groups (e.g. between arboreal mammals and woody-plant richness; Andrews & O'Brien 2000), at broader scales and coarser spatial resolution (Kissling et al. 2007). If a multitude of such associations combined similarly across the many functional guilds that make up vertebrate consumers and are spatially scaled up, we would expect a strong plant–consumer richness relationship above and beyond environmental variation. The putative increase of diversity and complexity of vegetation structure with plant richness and its positive effect on niche space (MacArthur & MacArthur 1961; Pearson 1975) should equally lead to noticeable positive correlations between plant and vertebrate diversity. After accounting for environmental variation, we found a relatively weak association, which indicates that neither mechanism plays a dominant role in explaining the cross-regional variation in richness of diverse vertebrate taxa. However, this does not discount the importance of coevolutionary and co-adaptive mechanisms in the diversification of endotherm vertebrates. Coevolution between trophic levels undoubtedly played an important role for the original diversification of consumers (Price 2002; Novotny et al. 2006) at these geographical scales but might be masked by, for example, taxon-specific key-radiations, patterns of dispersal and spatial turn-over. Further, much of the signal of past or contemporary plant–vertebrate interactions and habitat structure may be collinear with contemporary environmental gradients—a long-appreciated conundrum in broad-scale diversity analyses (Endler 1982).
Nevertheless, we conclude that at broad geographical scales environmental constraints on richness dominate. Mechanisms that have been rightly recognized as pivotal at one scale of investigation (Hutchinson 1959) may prove to be of only secondary importance for understanding patterns at another. It is this scale-dependent context of mechanisms that may have stirred many debates about the determinants of richness in the past decades. However, the weak spatial scaling up we find for cross-taxon biotic effects does not imply that their relative unimportance at broad scales would scale back down to local communities. Species interactions, playing out locally and among select functional associations, may limit the coarse-grain distribution of species even when environmental conditions may indicate strong suitability—a remaining challenge for understanding the impacts of global change on biodiversity.
Acknowledgements
We thank Wilhelm Barthlott and Gerold Kier (Nees Institute, University of Bonn, Germany) for granting us access to the plant richness dataset, and Cagan Sekercioglu for sharing avian diet data. We are grateful to Daniel Kissling, Tien Ming Lee, Frank La Sorte and four anonymous referees for helpful comments on the manuscript. W.J. acknowledges the support by National Science Foundation grant BCS-0648733. During the preparation of this work, H.K.'s work was supported by a scholarship from the German National Academic Foundation and by a Feodor-Lynen postdoctoral fellowship from the Alexander von Humboldt Foundation. G.C.'s work was supported by DGAPA of the Universidad Nacional Auto´noma de Me´xico. W.J., H.K. and J.M. designed this work. J.M., W.J. and G.C. contributed data. H.K. and W.J. processed and analysed the data. W.J. wrote the initial draft of the paper. All authors discussed the results, and commented on and contributed to subsequent drafts. The authors declare no competing financial interest.
Supplementary Material
(a) 5%-hotspot congruence between vascular plants (P), birds (B), and mammals (M); and (b) between actual evapotranspiration (AET) and the two vertebrate groups. Grey dots mark upper 95%-quantile regions where no congruence exists between the respective group and plant or AET hotspots, respectively. Maps are projected using Behrmann projection.
(a,d,g) Direct effects of plant richness, (b,e,h) environmental effects on vertebrate richness and (c,f,i) combined environmental / richness model. For other details see Figure 4.
References
- Ahn C.-H., Tateishi R. Development of a global 30-minute grid potential evapotranspiration data set. J. Jpn. Soc. Photogr. Remote Sensing. 1994;33:12–21. [Google Scholar]
- Andrews P., O'Brien E.M. Climate, vegetation, and predictable gradients in mammal species richness in southern Africa. J. Zool. 2000;251:205–231. doi:10.1111/j.1469-7998.2000.tb00605.x [Google Scholar]
- Arbuckle, J. L. 2003 AMOS 5.0. Chicago, IL: SmallWaters.
- Barthlott W., Mutke J., Rafiqpoor M.D., Kier G., Kreft H. Global centres of vascular plant diversity. Nova Acta Leopoldina. 2005;92:61–83. [Google Scholar]
- Brown J.H. 2 Decades of homage to Santa-Rosalia—toward a general-theory of diversity. Am. Zool. 1981;21:877–888. [Google Scholar]
- Buckley L.B., Jetz W. Environmental and historical constraints on global patterns of amphibian richness. Proc. R. Soc. B. 2007;274:1167–1173. doi: 10.1098/rspb.2006.0436. doi:10.1098/rspb.2006.0436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter S.R., Pingali P.L., Bennett E.M., Zurek M.B. Island Press; Washington, DC: 2005. Ecosystems and human well-being: scenarios. [Google Scholar]
- Ceballos G., Ehrlich P.R. Global mammal distributions, biodiversity hotspots, and conservation. Proc. Natl. Acad. Sci. 2006;103:19 374–19 379. doi: 10.1073/pnas.0609334103. doi:10.1073/pnas.0609334103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesson P. Mechanisms of maintenance of species diversity. Annu. Rev. Ecol. Syst. 2000;31:343–366. doi:10.1146/annurev.ecolsys.31.1.343 [Google Scholar]
- Cody M.L. Academic Press; Orlando, FL: 1985. Habitat selection in birds. [Google Scholar]
- Cohen J.E. Ratio of prey to predators in community food webs. Nature. 1977;270:165–166. doi:10.1038/270165a0 [Google Scholar]
- Currie D.J., et al. Predictions and tests of climate-based hypotheses of broad-scale variations in taxonomic richness. Ecol. Lett. 2004;7:1121–1134. doi:10.1111/j.1461-0248.2004.00671.x [Google Scholar]
- Davies R.G., et al. Topography, energy and the global distribution of bird species richness. Proc. R. Soc. B. 2007;274:1189–1197. doi: 10.1098/rspb.2006.0061. doi:10.1098/rspb.2006.0061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson A.P., Rodriguez J.P., Roberts W.M., Wilcove D.S. Geographic distribution of endangered species in the United States. Science. 1997;275:550–553. doi: 10.1126/science.275.5299.550. doi:10.1126/science.275.5299.550 [DOI] [PubMed] [Google Scholar]
- Dyer L.A., et al. Host specificity of Lepidoptera in tropical and temperate forests. Nature. 2007;448:696–699. doi: 10.1038/nature05884. doi:10.1038/nature05884 [DOI] [PubMed] [Google Scholar]
- Ellison A.M. Bayesian inference in ecology. Ecol. Lett. 2004;7:509–520. doi:10.1111/j.1461-0248.2004.00603.x [Google Scholar]
- Endler J.A. Problems in distinguishing historical from ecological factors in biogeography. Am. Zool. 1982;22:441–452. doi:10.1093/icb/22.2.441 [Google Scholar]
- Grace J.B. Cambridge University Press; Cambridge, UK: 2006. Structural equation modelling and natural systems. [Google Scholar]
- Grenyer R., et al. Global distribution and conservation of rare and threatened vertebrates. Nature. 2006;444:93–96. doi: 10.1038/nature05237. doi:10.1038/nature05237 [DOI] [PubMed] [Google Scholar]
- Hawkins B.A., Porter E.E. Does herbivore diversity depend on plant diversity? The case of California butterflies. Am. Nat. 2003;161:40–49. doi: 10.1086/345479. doi:10.1086/345479 [DOI] [PubMed] [Google Scholar]
- Hawkins B.A., et al. Energy, water, and broad-scale geographic patterns of species richness. Ecology. 2003;84:3105–3117. doi:10.1890/03-8006 [Google Scholar]
- Holdridge L.R. Determination of world plant formations from simple climatic data. Science. 1947;105:367–368. doi: 10.1126/science.105.2727.367. doi:10.1126/science.105.2727.367 [DOI] [PubMed] [Google Scholar]
- Holling C.S. Cross-scale morphology, geometry and dynamics of ecosystems. Ecol. Monogr. 1992;62:447–502. doi:10.2307/2937313 [Google Scholar]
- Hurlbert A.H., Jetz W. Species richness, hotspots and the scale dependence of range maps in ecology and conservation. PNAS. 2007;104:13384–13389. doi: 10.1073/pnas.0704469104. doi:10.1073/pnas.0704469104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson G.E. Homage to Santa Rosalia or why are there so many kinds of animals? Am. Nat. 1959;93:145–159. doi:10.1086/282070 [Google Scholar]
- Jetz W., Rahbek C. Geographic range size and determinants of avian species richness. Science. 2002;297:1548–1551. doi: 10.1126/science.1072779. doi:10.1126/science.1072779 [DOI] [PubMed] [Google Scholar]
- Jetz W., Wilcove D.S., Dobson A.P. Projected impacts of climate and land-use change on the global diversity of birds. PLoS Biol. 2007;5:1211–1219. doi: 10.1371/journal.pbio.0050157. doi:10.1371/journal.pbio.0050157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kier G., Mutke J., Dinerstein E., Ricketts T.H., Kuper W., Kreft H., Barthlott W. Global patterns of plant diversity and floristic knowledge. J. Biogeogr. 2005;32:1107–1116. doi:10.1111/j.1365-2699.2005.01272.x [Google Scholar]
- Kissling W., Rahbek C., Böhning-Gaese K. Food plant diversity as broad-scale determinant of avian frugivore richness. Proc. R. Soc. B. 2007;274:799–808. doi: 10.1098/rspb.2006.0311. doi:10.1098/rspb.2006.0311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissling, W. D., Field, R. & Böhning-Gaese, K. 2008 Spatial patterns of woody plant and bird diversity: functional relationships or environmental effects? Global Ecol. Biogeogr.17, 327–339. (doi:10.1111/j.1466-8238.2007.00379.x)
- Knops J.M.H., et al. Effects of plant species richness on invasion dynamics, disease outbreaks, insect abundances and diversity. Ecol. Lett. 1999;2:286–293. doi: 10.1046/j.1461-0248.1999.00083.x. doi:10.1046/j.1461-0248.1999.00083.x [DOI] [PubMed] [Google Scholar]
- Kreft H., Jetz W. Global patterns and determinants of vascular plant diversity. Proc. Natl. Acad. Sci. 2007;104:5925–5930. doi: 10.1073/pnas.0608361104. doi:10.1073/pnas.0608361104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreft H., Jetz W., Mutke J., Kier G., Barthlott W. Global diversity of island floras from a macroecological perspective. Ecol. Lett. 2008;11:116–127. doi: 10.1111/j.1461-0248.2007.01129.x. [DOI] [PubMed] [Google Scholar]
- Lamoreux J.F., Morrison J.C., Ricketts T.H., Olson D.M., Dinerstein E., McKnight M.W., Shugart H.H. Global tests of biodiversity concordance and the importance of endemism. Nature. 2006;440:212–214. doi: 10.1038/nature04291. doi:10.1038/nature04291 [DOI] [PubMed] [Google Scholar]
- Lee P.-Y., Rotenberry J.T. Relationships between bird species and tree species assemblages in forested habitats of eastern North America. J. Biogeogr. 2005;32:1139–1150. doi:10.1111/j.1365-2699.2005.01254.x [Google Scholar]
- Levin S.A. The problem of pattern and scale in ecology. Ecology. 1992;73:1943–1967. doi:10.2307/1941447 [Google Scholar]
- MacArthur R.H., MacArthur J.W. On bird species diversity. Ecology. 1961;42:594–598. doi:10.2307/1932254 [Google Scholar]
- Mittelbach G.G., Steiner C.F., Scheiner S.M., Gross K.L., Reynolds H.L., Waide R.B., Willig M.R., Dodson S.I., Gough L. What is the observed relationship between species richness and productivity? Ecology. 2001;82:2381–2396. doi:10.2307/2679922 [Google Scholar]
- Mutke J., Barthlott W. Patterns of vascular plant diversity at continental to global scales. Biologiske Skrifter. 2005;55:521–538. [Google Scholar]
- Myers N., Mittermeier R.A., Mittermeier C.G., da Fonseca G.A.B., Kent J. Biodiversity hotspots for conservation priorities. Nature. 2000;403:853–858. doi: 10.1038/35002501. doi:10.1038/35002501 [DOI] [PubMed] [Google Scholar]
- Naeem S., Thompson L.J., Lawler S.P., Lawton J.H., Woodfin R.M. Empirical evidence that declining species diversity may alter the performance of terrestrial ecosystems. Phil. Trans. R. Soc. B. 1995;347:249–262. doi:10.1098/rstb.1995.0025 [Google Scholar]
- New M., Lister D., Hulme M., Makin I. A high-resolution data set of surface climate over global land areas. Climate Res. 2002;21:1–25. doi:10.3354/cr021001 [Google Scholar]
- Novotny V., Drozd P., Miller S.E., Kulfan M., Janda M., Basset Y., Weiblen G.D. Why are there so many species of herbivorous insects in tropical rainforests? Science. 2006;313:1115–1118. doi: 10.1126/science.1129237. doi:10.1126/science.1129237 [DOI] [PubMed] [Google Scholar]
- Nowak R.M. The Johns Hopkins University Press; Baltimore, MD: 1997. Walker's mammals of the world. [Google Scholar]
- Olff H., Ritchie M.E., Prins H.H.T. Global environmental controls of diversity in large herbivores. Nature. 2002;415:901–904. doi: 10.1038/415901a. doi:10.1038/415901a [DOI] [PubMed] [Google Scholar]
- Olson J.S. USGS EROS Data Center; Sioux Falls, SD: 1994. Global ecosystem framework-definitions: USGS EROS Data Center internal report, p. 37. [Google Scholar]
- Openshaw S., Taylor P.J. The modifiable areal unit problem. In: Wrigley N., Bennett R., editors. Quantitative geography: a British view. Routledge and Kegan Paul; London, UK: 1981. pp. 60–69. [Google Scholar]
- Pearson D.L. Relation of foliage complexity to ecological diversity of 3 Amazonian bird communities. Condor. 1975;77:453–466. doi:10.2307/1366092 [Google Scholar]
- Prendergast J.R., Quinn R.M., Lawton J.H., Eversham B.C., Gibbons D.W. Rare species, the coincidence of diversity hotspots and conservation strategies. Nature. 1993;365:335–337. doi:10.1038/365335a0 [Google Scholar]
- Price P.W. Species interactions and the evolution of biodiversity. In: Herrera C.M., Pellmyr O., editors. Plant-animal interactions—an evolutionary approach. Blackwell; Oxford, UK: 2002. pp. 3–25. [Google Scholar]
- Qian H., White P.S., Song J.-S. Effects of regional vs. ecological factors on plant species richness: an intercontinental analysis. Ecology. 2007;88:1440–1453. doi: 10.1890/06-0916. doi:10.1890/06-0916 [DOI] [PubMed] [Google Scholar]
- Rotenberry J.T. The role of habitat in avian community composition: physiognomy or floristics? Oecologia. 1985;67:213–217. doi: 10.1007/BF00384286. doi:10.1007/BF00384286 [DOI] [PubMed] [Google Scholar]
- Ruggiero A., Kitzberger T. Environmental correlates of mammal species richness in South America: effects of spatial structure, taxonomy and geographic range. Ecography. 2004;27:401–417. doi:10.1111/j.0906-7590.2004.03801.x [Google Scholar]
- Sekercioglu C.H., Daily G.C., Ehrlich P.R. Ecosystem consequences of bird declines. Proc. Natl. Acad. Sci. USA. 2004;101:18 042–18 047. doi: 10.1073/pnas.0408049101. doi:10.1073/pnas.0408049101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemann E., Tilman D., Haarstad J., Ritchie M. Experimental tests of the dependence of arthropod diversity on plant diversity. Am. Nat. 1998;152:738–750. doi: 10.1086/286204. doi:10.1086/286204 [DOI] [PubMed] [Google Scholar]
- Southwood T.R.E., Brown V.K., Reader P.M. Relationships of plant and insect diversities in succession. Biol. J. Linn. Soc. 1979;12:327–348. doi:10.1111/j.1095-8312.1979.tb00063.x [Google Scholar]
- Tilman D., Wedin D., Knops J. Productivity and sustainability influenced by biodiversity in grassland ecosystems. Nature. 1996;379:718–720. doi:10.1038/379718a0 [Google Scholar]
- USGS 1996 GTOPO30/global digital elevation model, http://edc.usgs.gov/products/elevation/gtopo30/gtopo30.html. Sioux Falls, SD: US Geological Survey.
- Warren P.H., Gaston K.J. Predator–prey ratios—a special case of a general pattern. Phil. Trans. R. Soc. B. 1992;338:113–130. doi:10.1098/rstb.1992.0135 [Google Scholar]
- Willis K., Whittaker R.J. Species diversity—scale matters. Science. 2002;295:1245–1248. doi: 10.1126/science.1067335. doi:10.1126/science.1067335 [DOI] [PubMed] [Google Scholar]
- Wolters V., Bengtsson J., Zaitsev A.S. Relationship among the species richness of different taxa. Ecology. 2006;87:1886–1895. doi: 10.1890/0012-9658(2006)87[1886:ratsro]2.0.co;2. doi:10.1890/0012-9658(2006)87[1886:RATSRO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Wright D.H. Species energy theory—an extension of species-area theory. Oikos. 1983;41:496–596. doi:10.2307/3544109 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(a) 5%-hotspot congruence between vascular plants (P), birds (B), and mammals (M); and (b) between actual evapotranspiration (AET) and the two vertebrate groups. Grey dots mark upper 95%-quantile regions where no congruence exists between the respective group and plant or AET hotspots, respectively. Maps are projected using Behrmann projection.
(a,d,g) Direct effects of plant richness, (b,e,h) environmental effects on vertebrate richness and (c,f,i) combined environmental / richness model. For other details see Figure 4.