Abstract
The ‘challenge hypothesis’ posits that testosterone facilitates reproductive effort (investment in male–male competition and mate-seeking) at the expense of parenting effort (investment in offspring and mates). Multiple studies, primarily in North America, have shown that men in committed relationships, fathers, or both maintain lower levels of testosterone than unpaired men. Data from non-western populations, however, show inconsistent results. We hypothesized that much of this cross-cultural variation can be attributed to differential investment in mating versus parenting effort, even among married fathers. Here, we directly test this idea by comparing two neighbouring Tanzanian groups that exhibit divergent styles of paternal involvement: Hadza foragers and Datoga pastoralists. We predicted that high levels of paternal care by Hadza fathers would be associated with decreased testosterone in comparison with non-fathers, and that no such difference between fathers and non-fathers would be evident in Datoga men, who provide minimal direct paternal care. Twenty-seven Hadza men and 80 Datoga men between the ages of 17 and 60 provided morning and afternoon saliva samples from which testosterone was assayed. Measurements in both populations confirmed these predictions, adding further support to the hypothesis that paternal care is associated with decreased testosterone production in men.
Keywords: fatherhood, parenting effort, challenge hypothesis, mating effort, Hadza, Datoga
1. Introduction
Humans are unique among primates in forming long-term pair bonds in the context of multi-male, multi-female social groups, despite men and women typically spending most of their waking hours in separate locations (Rodseth et al. 1991). Humans are also exceptional among mammals for their level of direct parental investment, which in foraging societies can extend for almost two decades (Kaplan 1997; Kaplan et al. 2000). This rare combination of traits links female reproductive success to male investment, while presenting both sexes with continual opportunities for extra-pair copulation. Consequently, human males face a fundamental trade-off between allocating resources towards mating effort (investment in male–male competition and mate attraction) and parenting effort (investment in offspring and mates), with the optimal pattern of allocation dependent on local social and ecological constraints (Trivers 1972; Lancaster & Kaplan 1992; Marlowe 2003a).
The endocrine system plays an important role in modulating life-history strategies, such as reproductive effort, by coordinating morphological, physiological and behavioural responses to environmental factors, such as energy availability and social context (Wingfield et al. 2000; Ellison 2003). In a range of vertebrates, the steroid hormone testosterone has been shown to play a critical role in mediating the trade-off between mating effort and parenting effort. The evidence for this effect is particularly clear in birds, which show dramatic interspecific and individual differences in temporal patterns of testosterone secretion, explicable by variation in two key variables: intensity of male mating competition and degree of paternal care (Wingfield et al. 1990, 2000; Beletsky et al. 1995). Data from more than 60 avian species are consistent with the ‘challenge hypothesis’ (Wingfield et al. 1990; Hirschenhauser & Oliveira 2006), which proposes that testosterone levels increase when males must respond to threats from conspecifics—particularly during territory formation and mate guarding—and decrease during periods when males must provide care to offspring (Wingfield et al. 2000). Experimental manipulations of male birds have confirmed that high levels of testosterone suppress parental behaviour in favour of male–male competition (Hegner & Wingfield 1987; De Ridder et al. 2000; Peters et al. 2002). In a number of mammals that exhibit paternal care, male testosterone levels also decrease from the period of gestation to lactation (Brown et al. 1995; Roberts et al. 1996; Reburn & Wynne-Edwards 1999; Nunes et al. 2000).
Although numerous studies have investigated the role that testosterone plays in facilitating aggression and status-seeking behaviour in men (reviewed in Archer 2006), until recently, few had addressed the hypothesis that pair bonding and paternal care are associated with low levels of testosterone. In the 1990s, two studies of military personnel found that married men exhibited slightly lower testosterone levels than unmarried men, though the difference was modest (Booth & Dabbs 1993; Mazur & Michalek 1998). Subsequently, Storey et al. (2000) reported that in 34 couples taking childbirth classes, men exhibited chronic declines in testosterone production over the course of the pregnancy. They also exhibited acute decreases in circulating testosterone in response to visual, tactile, olfactory and auditory stimuli associated with paternal care (i.e. when listening to a tape of a crying baby, and holding a doll that had been wrapped in a blanket worn by a real baby). A related study by Berg & Wynne-Edwards (2001) showed that, in comparison with control men, a sample of fathers in prenatal classes had decreased testosterone in the weeks surrounding parturition. More recently, a series of cross-sectional studies have variously demonstrated that either men in committed, long-term relationships, fathers, or both have lower levels of testosterone than unpaired men (Gray et al. 2002, 2004a,b; Burnham et al. 2003; McIntyre et al. 2006).
Most of the studies showing an effect of marriage and fatherhood on men's testosterone have examined North American populations living under conditions of relative energy abundance. Data from less affluent, non-western populations show inconsistent results. One study in Beijing revealed significantly lower testosterone levels in married fathers than in non-fathers, but no significant difference between married and unmarried men without children (Gray et al. 2006). No significant difference was apparent in testosterone levels between married and unmarried men in Dominica (Flinn et al. 1998), nor among Kenyan men on the island of Lamu (Gray 2003). In the latter population, polygynously married men actually exhibited higher levels of testosterone than monogamously married men. Among Ariaal pastoralists in northern Kenya, the transition from life as a bachelor and warrior to monogamous marriage was associated with lower testosterone levels (Gray et al. 2007).
Cross-cultural variation in testosterone responses to marriage and fatherhood could potentially result from differential patterns of investment in mating and parenting effort, even among married fathers (Muller & Wrangham 2001). For example, McIntyre et al. (2006) showed that within an American undergraduate population, pair-bonded men who maintained a strong interest in sexual activity with women other than their primary partner exhibited higher testosterone levels than those who favoured fidelity. If North American men typically invest more in marital bonds and paternal care than men in, for example, polygynous societies, then this might account for the more predictable association between reduced testosterone and fatherhood in these populations (Gray 2003; Gray et al. 2007).
The purpose of this study is to directly test the relationship between paternal care and testosterone in men by comparing two neighbouring Tanzanian groups that exhibit divergent patterns of paternal involvement: Hadza foragers and Datoga pastoralists. These populations provide an ideal test case because they live in close proximity around Lake Eyasi in northern Tanzania, but their cultural norms of parenting reflect a broader ethnographic pattern in which foragers often maintain close father–infant bonds, while pastoralists tend to show lower levels of direct paternal care (Marlowe 2000; figure 1).
Figure 1.
Father–infant proximity by subsistence mode. Data are from the standard cross-cultural sample (SCCS; n=139; Murdock & White 1980). The scale from the SCCS is as follows: 1, no close proximity; 2, rare instances of close proximity; 3, occasional or irregular close proximity; 4, frequent close proximity; 5, regular, close relationship or companionship.
Specifically, among the Hadza, most marriages are monogamous (with approx. 4% of men having two wives at any given time) and most couples (approx. 68%) co-reside in a camp with the wife's mother (Marlowe 1999a, 2003b; Blurton Jones et al. 2005). Hadza men exhibit high rates of direct paternal care, including carrying, holding, cleaning, feeding and pacifying infants, with biological children receiving significantly more care than stepchildren (Marlowe 1999a,b, 2005). In focal follows, Marlowe (2005) observed Hadza men holding their infants 5.6 per cent of the time in daylight and evening hours. When fathers with children under 3 years of age were present in the camp, they spent more than 20 per cent of their time interacting with those children (2005). Fathers also slept in close proximity to offspring at a shared hearth, and were thus in contact with children from approximately 21.00 to 07.00 (2005).
A very different pattern of paternal care is evident among the Eyasi Datoga, patrilineal pastoralists with a strong ‘warrior tradition’ (Klima 1970). Approximately 40 per cent of Datoga families are polygynous, living in widely spaced individual homesteads consisting of a thorn-bush fence in the shape of a figure of eight, half of which contains living huts and the other half a corral for herds of cattle, sheep and goats (Klima 1970; Borgerhoff Mulder 1992; Sellen 1999a,b). Datoga men spend much of their day away from their homesteads, herding cattle, visiting other men and travelling to markets (Klima 1970; Sellen 1999a,b). When at their homesteads, men take their meals in a separate men's hut, and sleep in a separate room from their wives and children (Klima 1970). Although men sometimes interact with older children, particularly those who have started to help tend herd animals, direct interaction with infants is minimal, and men express a strong belief that caring for infants is ‘women's work’ (‘kazi ya wanawake’ was the Kiswahili phrase employed by multiple informants). Before weaning, mother and infant are considered to be ‘one body’, just as they were prior to parturition (Blystad & Rekdal 2003).
Accordingly, the challenge hypothesis makes two predictions about fathers' testosterone levels in these populations. First, Hadza men caring for young children are expected to maintain lower levels of testosterone than men not engaging in paternal care. Second, Datoga men with young children in their homesteads should show no difference in testosterone from men who do not have offspring.
A third hypothesis is suggested by Storey et al.'s (2000) data demonstrating an acute suppressive effect of infant cues on paternal testosterone production. Because Hadza men's direct paternal care (infant holding, feeding and caregiving) peaks around nine months after parturition, and decreases steadily thereafter as offspring mature (Marlowe 2005), Hadza fathers with relatively young children are exposed to infant stimuli at elevated rates throughout the day. Consequently, such fathers are expected to exhibit a more pronounced diurnal decline in testosterone than fathers of older children (e.g. Gray et al. 2002). Accordingly, Hadza, but not Datoga, fathers are predicted to show a correlation between the age of the youngest child and the relative decrease in testosterone levels from morning to evening.
2. Material and methods
In most western populations, free testosterone levels peak during young adulthood and decline steadily thereafter with age (Vermeulen et al. 1999). By contrast, in many non-western populations, men exhibit comparatively lower testosterone levels in early adulthood, and thereafter show little or no reduction (Ellison et al. 2002). In this study, we restricted our sample to men between the ages of 17 and 60 in order to eliminate variation in testosterone resulting solely from disparate maturation rates in younger men, or the effects of ill health in older men, that might be mistaken for an age effect (e.g. Muller et al. 2003).
Eighty Datoga participants between the ages of 18 and 59 were recruited by word of mouth in and around the villages of Mangola and Matala, along Lake Eyasi, during August 2003. Interviews were conducted either in Kiswahili, by R.B. and M.N.M., or in Kidatoga, with the assistance of an experienced Datoga field assistant. Questions focused on men's ages, and their marital and reproductive histories. Thirty-two men in the sample had no children and, of those, 25 had no wife. Of the 48 men who had children, all were married (12 polygynously), with the age of the youngest child ranging from less than one month to 11 years (mean=2.24 years). Most young men knew their date of birth; for older men, this was estimated with reference to major political and social events (e.g. Tanzanian Independence 1961), and in relation to known ages of other men. Anthropometric data included measures of height and weight, together with body fat estimates from a bioelectrical impedance scale (Tanita BF522). BMI was calculated as weight (kg)/height2 (m2).
Twenty-seven Hadza participants between the ages of 17 and 51 were recruited in the Sipunga area, east of Lake Eyasi, during January 2004. Collection of saliva samples was coordinated by R.B. F.W.M. collected anthropometric data and conducted interviews (in Kiswahili) with adult Hadza to gather data on marital status and number, ages and residential status of children. Fifteen of the Hadza participants either had no children (n=6) or were not involved in caring for presumed children because they had separated from the mother (n=9). None of the 15 was residing with a stepchild. These men were classified as ‘non-fathers’. Ten Hadza men had presumed biological children (ages ranging from less than one month to 7 years; mean=3.2 years) that they were actively nurturing, provisioning and sharing a hearth with (‘fathers’). Two men had no children, but were expecting with their pregnant spouses. These two men were excluded from analyses comparing fathers and non-fathers. However, including them in the former group as ‘expectant fathers’ (as per Storey et al. 2000; Berg & Wynne-Edwards 2001) had no effect on any of the father/non-father comparisons (see below). Hadza ages were known with greater precision than those of the Datoga, because births have been recorded for several decades as part of a long-term demographic study (Blurton Jones et al. 1992; Marlowe in press).
In order to control for diurnal variation in testosterone levels (Van Cauter 1990), we collected both morning and afternoon samples from each subject during specified hours. For Datoga subjects, one morning sample was collected between 07.34 and 08.18, and one evening sample between 17.45 and 19.03. Two Datoga men contributed matched morning and evening samples on two separate days, and for these individuals the average of each pair was used in all analyses. Nine Datoga men failed to return for evening sample collection, so only their morning samples were available for analyses. Hadza subjects contributed matched morning (07.10–08.35) and evening (17.00–18.00) samples on one to three different days (median=3), and median values for each man were used in all analyses.
All participants avoided eating, drinking, chewing or smoking for 30 min, and rinsed their mouths with a small amount of clean water, prior to sample collection. Trident sugarless gum was provided to stimulate saliva production, and sodium azide was subsequently added to sample tubes to inhibit bacterial growth. Samples were maintained at ambient temperature for four to six weeks before being transported to Harvard University, where they were stored frozen at −20°C until April 2004, when they were assayed for testosterone. Lipson & Ellison (1989) have previously validated all sample collection and storage procedures. Informed consent was obtained from all participants, and the Human Subjects Committee at Harvard University approved all research protocols.
Testosterone assays were performed by M.N.M. in the Reproductive Ecology Laboratory at Harvard University, using a modified application of the I125 double antibody kit from Diagnostic Systems Laboratories (Webster, TX). Sample and standard reactions were run in duplicate. Substrate (400 μl) was pipetted into borosilicate tubes containing either 200 μl of sample and 200 μl of buffered saline or, for the standard reactions, a standard preparation run at six concentrations from 2 to 375 pg ml−1. Antiserum (20 μl) and tracer (50 μl) were added to sample and standard tubes. Reactions incubated overnight at 4°C, after which precipitating reagent (500 μl) was added, tubes were centrifuged for 45 min and aspirated. The assays were sensitive to 14 pmol l−1 T, and the interassay coefficient of variation was 7.9 per cent.
All comparisons between independent groups employed the Mann–Whitney U-test, and dependent groups the Wilcoxon signed-rank test. All correlations report Spearman's rank correlation coefficient (ρ). All statistical tests are two tailed. Unless otherwise indicated, means are reported ±s.e.
3. Results
The two study populations were comparable in terms of age and anthropometry (table 1). Datoga men are generally taller than Hadza men, and this resulted in significant differences between the groups in both height (nD=80, nH=27, Z=−6.077, p<0.001) and weight (nD=80, nH=27, Z=−4.770, p<0.001). However, both populations experience suboptimal access to energy, and consequently maintain minimal levels of body fat and low BMI (see also Sellen 1999a,b). No significant difference was apparent between the groups in either of these measures (BMI: nD=80, nH=27, Z=−0.269, p=0.788; body fat %: nD=79, nH=27, Z=−0.715, p=0.475) or in age (age: nD=80, nH=27, Z=−1.17, p=0.242).
Table 1.
Comparison of anthropometric measures and salivary testosterone (T) levels in the Hadza and the Datoga.
| variable | Hadza mean±s.d. | Datoga mean±s.d. | p-value |
|---|---|---|---|
| age (years) | 33.4±10.1 | 31.0±10.9 | 0.242 |
| height (cm) | 158.4±7.6 | 170.6±6.9 | <0.001 |
| weight (kg) | 49.5±5.9 | 57.2±7.3 | <0.001 |
| BMI (kg m−2) | 19.7±1.9 | 19.6±1.8 | 0.788 |
| body fat (%) | 10.3±3.8 | 10.9±4.3 | 0.475 |
| morning T (pmol l−1) | 150±66 | 171±105 | 0.691 |
| evening T (pmol l−1) | 129±62 | 140±86 | 0.769 |
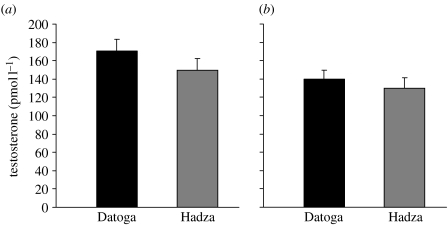
Within each population, men's morning and evening salivary testosterone levels were positively correlated (Datoga: Spearman's ρ=0.322, p=0.006, n=71; Hadza: Spearman's ρ=0.373, p=0.055, n=27), and average testosterone levels were higher in the morning than in the evening (Datoga: Z=−2.423, p=0.015, n=71; Hadza: Z=−1.727, p=0.084, n=27; table 1); however, in the smaller Hadza group, these tests fell just short of significance. There were no significant differences between the two populations in either morning or evening measures of salivary testosterone (morning: Z=−0.398, p=0.691, nD=80, nH=27; evening: Z=−0.294, p=0.769, nD=71, nH=27; figure 2).
Figure 2.
No significant differences were apparent between the two populations in either (a) morning (p=0.691) or (b) evening (p=0.769) measures of salivary testosterone.
Among the Hadza, mean age did not differ significantly between fathers and non-fathers (fathers: 38±2.9 years, n=10; non-fathers: 31±2.7 years, n=15; Z=−1.5, p=0.14). Among the Datoga, mean age was significantly higher for fathers (fathers: 35.5±1.4 years, n=48; non-fathers: 24±1.6 years, n=30; Z=−5.126, p<0.001). However, there was no relationship between salivary testosterone and age in either population (Datoga morning: Spearman's ρ=−0.175, p=0.120, n=80; Datoga evening: Spearman's ρ=−0.094, p=0.434, n=71; Hadza morning: Spearman's ρ=−0.154, p=0.442, n=27; Hadza evening: Spearman's ρ=−0.182, p=0.363, n=27).
Consistent with data from a range of non-western populations (Bribiescas 2001; Ellison 2003), both Hadza and Datoga men maintained low levels of testosterone in comparison with North American men. Morning testosterone levels averaged 151 and 170 pmol l−1 in the Hadza and the Datoga, respectively. The same testosterone assay conducted in the same laboratory revealed average morning levels of testosterone in American men from 250 to more than 400 pmol l−1, depending on the population sampled (Burnham et al. 2003; McIntyre et al. 2003).
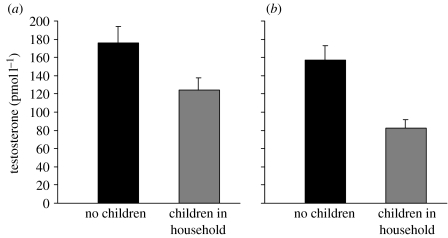
Among the Datoga, fathers with children under 11 in their homesteads showed no significant difference from non-fathers in either morning (fathers: 166±15.5 pmol l−1, n=48; non-fathers: 176±18.5 pmol l−1, n=32; Z=−0.629, p=0.53) or evening (fathers: 141±12.9 pmol l−1, n=41; non-fathers: 138±16.7 pmol l−1, n=30; Z=−0.116, p=0.907) measures of testosterone (figure 3). Mean testosterone levels in polygynously married Datoga men were lower than those of monogamously married men, but this difference was not significant in either morning (polygynous: 142±32 pmol l−1, n=12; monogamous: 174±18 pmol l−1, n=36; Z=−1.19, p=0.234) or evening samples (polygynous: 93±28 pmol l−1, n=9; monogamous: 170±39 pmol l−1, n=32; Z=−1.8, p=0.068).
Figure 3.
Datoga fathers with children in their homesteads showed no significant difference from non-fathers in either (a) morning (p=0.530) or (b) evening (p=0.907) measures of salivary testosterone.
Among the Hadza, by contrast, fathers currently caring for children exhibited significantly lower levels of testosterone than men not caring for children in both morning (fathers: 124±13.6 pmol l−1, n=10; non-fathers: 176±17.7 pmol l−1, n=15; Z=−02.164, p=0.03) and evening samples (figure 4; fathers: 83±8.83 pmol l−1, n=10; non-fathers: 157±16.3 pmol l−1, n=15; Z=−2.691, p=0.007). Including two expectant Hadza men in the ‘father’ group (as per Storey et al. 2000 and Berg & Wynne-Edwards 2001) had no significant effect on the Hadza father/non-father comparisons (morning with expectant fathers: Z=−2.44, p=0.015; evening with expectant fathers: Z=−2.416, p=0.016; nf=12, nnf=15).
Figure 4.
Hadza fathers currently caring for children exhibited 30 per cent lower levels of testosterone in (a) the morning (p=0.031) and 47 per cent lower levels of testosterone in (b) the evening (p=0.007) than men not caring for children.
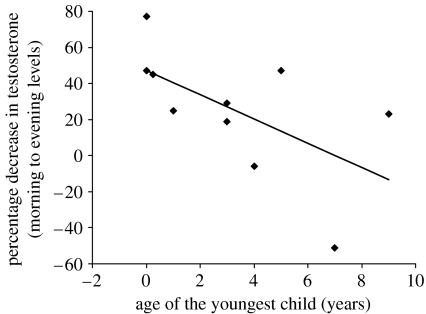
Among the 10 Hadza fathers currently caring for offspring, the age of the youngest child was negatively and significantly correlated with the median percentage decrease in salivary testosterone from morning to evening (Spearman's ρ=−0.659, p=0.024, n=10; figure 5). This was not the case for Datoga fathers (Spearman's ρ=0.064, p=0.704, n=38).
Figure 5.
Hadza fathers caring for younger offspring showed a larger decrease in salivary testosterone from morning to evening than fathers with older children (Spearman's ρ=−0.659, p=0.024).
4. Discussion
Previous studies of non-western populations have revealed inconsistent associations between men's testosterone levels and paternal or marital status. We hypothesized that, consistent with the challenge hypothesis, much of this variation can be attributed to differential investment in mating versus parenting effort, even among married fathers. Our cross-cultural data support this idea by showing that, among the Hadza, a group exhibiting high levels of paternal involvement, men caring for offspring maintain lower levels of testosterone than men who are not engaged in such care. By contrast, Datoga fathers, who exhibit low levels of paternal involvement, maintained levels of testosterone similar to those of non-fathers. To our knowledge, this is the first demonstration that increased levels of parental care are directly associated with low testosterone in fathers.
The Hadza data are particularly interesting because they represent the first examination of testosterone and reproductive effort in a foraging population, where direct paternal care is known to be higher than in many socioecological contexts, including agricultural and industrial societies (Hewlett 1991; Hewlett et al. 2000). Among foragers such as the Hadza, when they are not out of camp foraging, men are often near their children, sometimes babysitting weanlings while mothers forage. Because men sleep together with their wives and children, they have direct physical contact with younger children throughout the night. This sort of intimacy is the likely context within which human paternal investment evolved. The increased rates of polygyny and diminished paternal involvement of pastoralists such as the Datoga are probably driven by wealth inequalities among men in the form of cattle ownership that would have been absent prior to the domestication of herd animals.
The role of fathers in Hadza childrearing is often overlooked, owing to the focus on grandmothering in this group (Hawkes et al. 1997). However, in Marlowe's (2005) data, genetic fathers held their children and interacted with their children twice as much as maternal grandmothers. The intensity of care by genetic fathers may be responsible for the relatively robust effects reported here, with morning testosterone in fathers 30 per cent lower and evening levels almost 50 per cent lower than non-fathers. By contrast, in several North American studies, testosterone differences between fathers and non-fathers were less prominent, and in some cases—particularly among morning samples—non-significant (Berg & Wynne-Edwards 2001; Gray et al. 2002, 2004a,b).
A number of studies have reported that evening testosterone levels in humans and chimpanzees show stronger correlates with behavioural measures than do morning samples (Worthman & Konner 1987; Berg & Wynne-Edwards 2001; Gray et al. 2002; Muller & Wrangham 2004). This pattern may be widespread because morning testosterone levels reflect physiology during sleep, whereas evening samples are influenced by the cumulative outcomes of diurnal social interactions. In the present study, we found significant differences between fathers and non-fathers in both morning and evening samples, but, in evening samples, the magnitude of the disparity was greater and the p-value lower.
Evidence for a suppressive effect of interactions with offspring on testosterone comes from the correlation between the age of the youngest child and the relative diurnal decline in testosterone observed in Hadza fathers. Although the sample size is small, the data support the idea that, within populations, the level of direct paternal involvement with offspring affects short-term testosterone production, and that this phenomenon is not limited to a brief period following parturition (Storey et al. 2000).
Owing to the cross-sectional and correlational nature of our data, however, we cannot entirely rule out the possibility that men with high testosterone levels are less likely to care for young children. Nine of the 15 Hadza men in our ‘non-father’ category had biological children, but were not providing for them, and had terminated relations with the mother. High testosterone levels in these men may have increased the probability of separation from the mother, and one might expect that significant variation exists among men in the degree to which they are responsive to the suppressive effects of infant stimuli. Similar variation might also exist among Datoga men, but would not be detectable given their generally low levels of interaction with children. Longitudinal data are needed to address this critical issue, and these should be available from the Hadza in the near future.
Why should testosterone levels be reduced in men caring for young children? As with male birds, it seems likely that testosterone facilitates reproductive effort in the form of male–male competition and mate-seeking behaviour, both of which interfere with effective paternal care. Experimental studies in humans have shown that testosterone enhances responsiveness to social challenges (van Honk et al. 1999, 2001; Benderlioglu et al. 2004; Hermans et al. 2008), which can result in a lower latency to reactive aggression in high testosterone men (Kouri et al. 1995). A low threshold for provocation could prove costly in the context of childcare, not only because it might involve men in aggressive interactions with other men, but also because it could potentially lead to child abuse, such as infant battering. Although we are not aware of any studies specifically linking androgens to the physical abuse of children, high testosterone in men has been implicated in spousal abuse (Soler et al. 2000).
Mate-seeking behaviour is also likely to conflict with paternal investment. Increased opportunity for extra-pair matings has been shown to decrease paternal care in birds (Magrath & Elgar 1997), and Marlowe (1999b) documented a similar effect among Hadza fathers, who spend less time caring for and interacting with their children when staying in camps with a larger number of fecund women. Experimental data have shown that men's testosterone levels increase in response to interactions with potential mating partners (Roney et al. 2003, 2007), and this is a plausible mechanism for calibrating men's mating effort to local socioecological conditions. The role of testosterone in promoting libido (Isidori et al. 2005) and the specific association between testosterone and extra-pair mating interest (McIntyre et al. 2006) are consistent with this model. The fact that men's testosterone levels appear to be suppressed in response to infant cues is a strong indicator of the importance of pair bonding and paternal care in human evolutionary history, despite a multi-male, multi-female, fission–fusion social system that promotes opportunities for extra-pair mating.
Acknowledgments
We thank the Tanzanian Commission for Science and Technology for permission to conduct research, Prof. Audax Mabulla for logistical aid, Susan Lipson for laboratory assistance, Sherry Nelson for assistance in the field, Melissa Emery Thompson for helpful comments on the manuscript and E. O. Wilson for support from the Arthur Green Fund. Additional financial support came from an L. S. B. Leakey Foundation grant to M.N.M. and P.T.E., and a US National Science Foundation grant (no. 0242455) to F.W.M.
References
- Archer J. Testosterone and human aggression: an evaluation of the challenge hypothesis. Neurosci. Biobehav. Rev. 2006;30:319–345. doi: 10.1016/j.neubiorev.2004.12.007. doi:10.1016/j.neubiorev.2004.12.007 [DOI] [PubMed] [Google Scholar]
- Beletsky L.D., Gori D.F., Freeman S., Wingfield J.C. Testosterone and polygyny in birds. In: Power D.M., editor. Current ornithology. vol. 12. Plenum Press; New York, NY: 1995. pp. 1–42. [Google Scholar]
- Benderlioglu Z., Sciulli P.W., Nelson R.J. Fluctuating asymmetry predicts human reactive aggression. Am. J. Hum. Biol. 2004;16:458–469. doi: 10.1002/ajhb.20047. doi:10.1002/ajhb.20047 [DOI] [PubMed] [Google Scholar]
- Berg S.J., Wynne-Edwards K.E. Changes in testosterone, cortisol, and estradiol levels in men becoming fathers. Mayo Clin. Proc. 2001;76:582–592. doi: 10.4065/76.6.582. [DOI] [PubMed] [Google Scholar]
- Blurton Jones N.C., Smith L.C., O'Connell J.F., Hawkes K., Kamuzora C.L. Demography of the Hadza, an increasing and high density population of savanna foragers. Am. J. Phys. Anthropol. 1992;89:159–181. doi: 10.1002/ajpa.1330890204. doi:10.1002/ajpa.1330890204 [DOI] [PubMed] [Google Scholar]
- Blurton Jones N.G., Hawkes K., O'Connell J.F. Older Hadza men and women as helpers: residence data. In: Hewlett B.S., Lamb M.E., editors. Hunter–gatherer childhoods: evolutionary, developmental and cultural perspectives. Transaction; New Brunswick, NJ: 2005. pp. 214–236. [Google Scholar]
- Blystad A., Rekdal O.B. Datoga. In: Ember C.R., Ember M., editors. Encyclopedia of medical anthropology: health and illness in the world's cultures. vol. 2. Kluwer Academic/Plenum Publishers; New York, NY: 2003. pp. 629–638. [Google Scholar]
- Booth A., Dabbs J. Testosterone and men's marriages. Soc. Forces. 1993;72:463–477. doi:10.2307/2579857 [Google Scholar]
- Borgerhoff Mulder M. Demography of pastoralists: preliminary data on the Datoga of Tanzania. Hum. Ecol. 1992;20:383–405. doi: 10.1007/BF00890427. doi:10.1007/BF00890427 [DOI] [PubMed] [Google Scholar]
- Bribiescas R.G. Reproductive ecology and life history of the human male. Yearb. Phys. Anthropol. 2001;44:148–176. doi: 10.1002/ajpa.10025.abs. doi:10.1002/ajpa.10025 [DOI] [PubMed] [Google Scholar]
- Brown R.E., Murdoch T., Murphy P.R., Moger W.H. Hormonal responses of male gerbils to stimuli from their mate and pups. Horm. Behav. 1995;29:474–491. doi: 10.1006/hbeh.1995.1275. doi:10.1006/hbeh.1995.1275 [DOI] [PubMed] [Google Scholar]
- Burnham T.C., Flynn Chapman J., Gray P.B., McIntyre M., Lipson S.F., Ellison P.T. Men in committed, romantic relationships have lower testosterone. Horm. Behav. 2003;44:119–122. doi: 10.1016/s0018-506x(03)00125-9. doi:10.1016/S0018-506X(03)00125-9 [DOI] [PubMed] [Google Scholar]
- De Ridder E., Pinxten R., Eens M. Experimental evidence of a testosterone-induced shift from paternal to mating behaviour in a facultatively polygynous songbird. Behav. Ecol. Sociobiol. 2000;49:24–30. doi:10.1007/s002650000266 [Google Scholar]
- Ellison P.T. Energetics and reproductive effort. Am. J. Hum. Biol. 2003;15:342–351. doi: 10.1002/ajhb.10152. doi:10.1002/ajhb.10152 [DOI] [PubMed] [Google Scholar]
- Ellison P.T., Bribiescas R.G., Bentley G.R., Campbell B.C., Lipson S.F., Panter-Brick C., Hill K. Population variation in age-related decline in male salivary testosterone. Hum. Reprod. 2002;17:3251–3253. doi: 10.1093/humrep/17.12.3251. doi:10.1093/humrep/17.12.3251 [DOI] [PubMed] [Google Scholar]
- Flinn M.V., Baewald C., Decker S., England B. Evolutionary functions of neuroendocrine response to social environment. Behav. Brain Sci. 1998;21:372–374. doi:10.1017/S0140525X98361221 [Google Scholar]
- Gray P.B. Marriage, parenting, and testosterone variation among Kenyan Swahili men. Am. J. Phys. Anthropol. 2003;122:279–286. doi: 10.1002/ajpa.10293. doi:10.1002/ajpa.10293 [DOI] [PubMed] [Google Scholar]
- Gray P.B., Kahlenberg S.M., Barrett E.S., Lipson S.F., Ellison P.T. Marriage and fatherhood are associated with lower testosterone in males. Evol. Hum. Behav. 2002;23:193–201. doi:10.1016/S1090-5138(01)00101-5 [Google Scholar]
- Gray P.B., Campbell B.C., Marlowe F.W., Lipson S.F., Ellison P.T. Social variables predict between-subject but not day-to-day variation in the testosterone of US men. Psychoneuroendocrinology. 2004a;29:1153–1162. doi: 10.1016/j.psyneuen.2004.01.008. doi:10.1016/j.psyneuen.2004.01.008 [DOI] [PubMed] [Google Scholar]
- Gray P.B., Flynn Chapman J., Burnham T.C., McIntyre M.H., Lipson S.F., Ellison P.T. Human male pair bonding and testosterone. Hum. Nat. 2004b;15:119–131. doi: 10.1007/s12110-004-1016-6. doi:10.1007/s12110-004-1016-6 [DOI] [PubMed] [Google Scholar]
- Gray P.B., Yang C.-F. J., Pope H.G. Fathers have lower salivary testosterone levels than unmarried men and married non-fathers in Beijing, China. Proc. R. Soc. B. 2006;273:333–339. doi: 10.1098/rspb.2005.3311. doi:10.1098/rspb.2005.3311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray P.B., Ellison P.T., Campbell B.C. Testosterone and marriage among Ariaal men of northern Kenya. Curr. Anthropol. 2007;48:750–755. doi:10.1086/522061 [Google Scholar]
- Hawkes K., O'Connell J.F., Blurton Jones N.G. Hadza women's time allocation, offspring provisioning, and the evolution of long postmenopausal life spans. Curr. Anthropol. 1997;38:551–577. doi:10.1086/204646 [Google Scholar]
- Hegner R.E., Wingfield J.C. Effects of experimental manipulation of testosterone levels on parental investment and breeding success in male house sparrows. Auk. 1987;104:462–469. [Google Scholar]
- Hermans E.J., Ramsey N.F., Van Honk J. Exogenous testosterone enhances responsiveness to social threat in the neural circuitry of social aggression in humans. Biol. Psychiatry. 2008;63:263–270. doi: 10.1016/j.biopsych.2007.05.013. doi:10.1016/j.biopsych.2007.05.013 [DOI] [PubMed] [Google Scholar]
- Hewlett B.S. University of Michigan Press; Ann Arbor, MI: 1991. Intimate fathers. [Google Scholar]
- Hewlett B.S., Lamb M.E., Leyendecker B., Scholmerich A. Parental investment strategies among Aka foragers, Ngandu farmers, and Euro-American urban-industrialists. In: Cronk L., Chagnon N., Irons W., editors. Adaptation and human behavior. Aldine de Gruyter; New York, NY: 2000. pp. 155–178. [Google Scholar]
- Hirschenhauser K., Oliveira R.F. Social modulation of androgens in male vertebrates: meta-analyses of the challenge hypothesis. Anim. Behav. 2006;71:265–277. doi:10.1016/j.anbehav.2005.04.014 [Google Scholar]
- Isidori A.M., Giannetta E., Gianfrilli D., Greco E.A., Bonifacio V., Aversa A., Isidori A., Fabbri A., Lenzi A. Effects of testosterone on sexual function in men: results of a meta-analysis. Clin. Endocrinol. 2005;63:381–394. doi: 10.1111/j.1365-2265.2005.02350.x. doi:10.1111/j.1365-2265.2005.02350.x [DOI] [PubMed] [Google Scholar]
- Kaplan H. The evolution of the human life course. In: Wachter K.W., Finch C.E., editors. Between Zeus and the Salmon: the biodemography of longevity. National Academy Press; Washington, DC: 1997. pp. 175–211. [PubMed] [Google Scholar]
- Kaplan H., Hill K., Lancaster J., Hurtado A.M. A theory of human life history evolution: diet, intelligence, and longevity. Evol. Anthropol. 2000;9:156–185. doi:10.1002/1520-6505(2000)9:4<156::AID-EVAN5>3.0.CO;2-7 [Google Scholar]
- Klima G. Waveland Press; Prospect Heights, IL: 1970. The Barabaig: East African cattle-herders. [Google Scholar]
- Kouri E.M., Lukas S.E., Pope H.G., Oliva P.S. Increased aggressive responding in male volunteers following the administration of gradually increasing doses of testosterone cypionate. Drug Alcohol Depend. 1995;40:73–79. doi: 10.1016/0376-8716(95)01192-7. doi:10.1016/0376-8716(95)01192-7 [DOI] [PubMed] [Google Scholar]
- Lancaster J.B., Kaplan H. Human mating and family formation strategies: the effects of variability among males in quality and the allocation of mating effort and parental investment. In: Nishida T., McGrew W.C., Marler P., Pickford M., de Waal F.B.M., editors. Topics in primatology. vol. 1. University of Tokyo Press; Tokyo, Japan: 1992. pp. 21–33. [Google Scholar]
- Lipson S.F., Ellison P.T. Development of protocols for the application of salivary steroid analyses to field conditions. Am. J. Hum. Biol. 1989;1:249–255. doi: 10.1002/ajhb.1310010304. doi:10.1002/ajhb.1310010304 [DOI] [PubMed] [Google Scholar]
- Magrath M.J.L., Elgar M.A. Paternal care declines with increased opportunity for extra-pair matings in fairy martins. Proc. R. Soc. B. 1997;264:1731–1736. doi:10.1098/rspb.1997.0240 [Google Scholar]
- Marlowe F. Male care and mating effort among the Hadza. Behav. Ecol. Sociobiol. 1999a;46:57–64. doi:10.1007/s002650050592 [Google Scholar]
- Marlowe F. Showoffs or providers? The parenting effort of Hadza men. Evol. Hum. Behav. 1999b;20:391–404. doi:10.1016/S1090-5138(99)00021-5 [Google Scholar]
- Marlowe F. Paternal investment and the human mating system. Behav. Process. 2000;51:45–61. doi: 10.1016/s0376-6357(00)00118-2. doi:10.1016/S0376-6357(00)00118-2 [DOI] [PubMed] [Google Scholar]
- Marlowe F.W. The mating system of foragers in the standard cross-cultural sample. Cross-Cult. Res. 2003a;37:282–306. doi:10.1177/1069397103254008 [Google Scholar]
- Marlowe F. A critical period for provisioning by Hadza men: implications for pair bonding. Evol. Hum. Behav. 2003b;24:217–229. doi:10.1016/S1090-5138(03)00014-X [Google Scholar]
- Marlowe F.W. Who tends Hadza children? In: Hewlett B.S., Lamb M.E., editors. Hunter–gatherer childhoods: evolutionary, developmental and cultural perspectives. Transaction; New Brunswick, NJ: 2005. pp. 177–190. [Google Scholar]
- Marlowe, F. W. In press The Hadza: hunter–gatherers of Tanzania Berkeley, CA: University of California Press.
- Mazur A., Michalek J. Marriage, divorce, and male testosterone. Soc. Forces. 1998;77:315–330. doi:10.2307/3006019 [Google Scholar]
- McIntyre M.H., Lipson S.F., Ellison P.T. Effects of developmental and adult androgens on male abdominal adiposity. Am. J. Hum. Biol. 2003;15:662–666. doi: 10.1002/ajhb.10201. doi:10.1002/ajhb.10201 [DOI] [PubMed] [Google Scholar]
- McIntyre M., Gangestad S.W., Gray P.B., Flynn Chapman J., Burnham T.C., O'Rourke M.T., Thornhill R. Romantic involvement often reduces men's testosterone levels—but not always: the moderating role of extrapair sexual interest. J. Pers. Soc. Psychol. 2006;91:642–651. doi: 10.1037/0022-3514.91.4.642. doi:10.1037/0022-3514.91.4.642 [DOI] [PubMed] [Google Scholar]
- Muller M.N., Wrangham R.W. The reproductive ecology of male hominoids. In: Ellison P.T., editor. Reproductive ecology and human evolution. Aldine; New York, NY: 2001. pp. 397–427. [Google Scholar]
- Muller M.N., Wrangham R.W. Dominance, aggression and testosterone in wild chimpanzees: a test of the challenge hypothesis. Anim. Behav. 2004;67:113–123. doi:10.1016/j.anbehav.2003.03.013 [Google Scholar]
- Muller M., den Tonkelaar I., Thijssen J.H.H., Grobbee D.E., van der Schouw Y.T. Endogenous sex hormones in men aged 40–80 years. Eur. J. Endocrinol. 2003;149:583–589. doi: 10.1530/eje.0.1490583. doi:10.1530/eje.0.1490583 [DOI] [PubMed] [Google Scholar]
- Murdock G.P., White D.R. Standard cross-cultural sample. In: Barry H., Schlegel A., editors. Cross cultural samples and codes. University of Pittsburgh Press; Pittsburgh, PA: 1980. pp. 3–43. [Google Scholar]
- Nunes S., Fite J.E., French J.A. Variation in steroid hormones associated with infant care behaviour and experience in male marmosets (Callithrix kuhlii) Anim. Behav. 2000;60:857–865. doi: 10.1006/anbe.2000.1524. doi:10.1006/anbe.2000.1524 [DOI] [PubMed] [Google Scholar]
- Peters A., Cockburn A., Cunningham R. Testosterone treatment suppresses paternal care in superb fairy-wrens, Malurus cyaneus, despite their concurrent investment in courtship. Behav. Ecol. Sociobiol. 2002;51:538–547. doi:10.1007/s00265-002-0472-4 [Google Scholar]
- Reburn C.J., Wynne-Edwards K.E. Hormonal changes in males of a naturally biparental and a uniparental mammal. Horm. Behav. 1999;35:163–176. doi: 10.1006/hbeh.1998.1509. doi:10.1006/hbeh.1998.1509 [DOI] [PubMed] [Google Scholar]
- Roberts R.L., Zullo A., Gustafson E.A., Carter C.S. Perinatal steroid treatments alter alloparental and affiliative behavior in prairie voles. Horm. Behav. 1996;30:576–582. doi: 10.1006/hbeh.1996.0060. doi:10.1006/hbeh.1996.0060 [DOI] [PubMed] [Google Scholar]
- Rodseth L., Wrangham R.W., Harrigan A.M., Smuts B.B. The human community as a primate society. Curr. Anthropol. 1991;32:221–254. doi:10.1086/203952 [Google Scholar]
- Roney J.R., Mahler S.V., Maestripieri D. Behavioral and hormonal responses of men to brief interactions with women. Evol. Hum. Behav. 2003;24:365–375. doi:10.1016/S1090-5138(03)00053-9 [Google Scholar]
- Roney J.R., Lukaszewski A.W., Simmons Z.L. Rapid endocrine responses of young men to social interactions with young women. Horm. Behav. 2007;52:326–333. doi: 10.1016/j.yhbeh.2007.05.008. doi:10.1016/j.yhbeh.2007.05.008 [DOI] [PubMed] [Google Scholar]
- Sellen D.W. Growth patterns among seminomadic pastoralists (Datoga) of Tanzania. Am. J. Phys. Anthropol. 1999a;109:187–209. doi: 10.1002/(SICI)1096-8644(199906)109:2<187::AID-AJPA5>3.0.CO;2-P. doi:10.1002/(SICI)1096-8644(199906)109:2<187::AID-AJPA5>3.0.CO;2-P [DOI] [PubMed] [Google Scholar]
- Sellen D.W. Polygyny and child growth in a traditional pastoral society: the case of the Datoga of Tanzania. Hum. Nat. 1999b;10:329–371. doi: 10.1007/s12110-999-1007-8. doi:10.1007/s12110-999-1007-8 [DOI] [PubMed] [Google Scholar]
- Soler H., Vinayak P., Quadagno D. Biosocial aspects of domestic violence. Psychoneuroendocrinology. 2000;25:721–739. doi: 10.1016/s0306-4530(00)00022-6. doi:10.1016/S0306-4530(00)00022-6 [DOI] [PubMed] [Google Scholar]
- Storey A.E., Walsh C.J., Quinton R.L., Wynne-Edwards K.E. Hormonal correlates of paternal responsiveness in new and expectant fathers. Evol. Hum. Behav. 2000;21:79–95. doi: 10.1016/s1090-5138(99)00042-2. doi:10.1016/S1090-5138(99)00042-2 [DOI] [PubMed] [Google Scholar]
- Trivers R.L. Parental investment and sexual selection. In: Campbell B., editor. Sexual selection and the descent of man, 1871–1971. Aldine; Chicago, IL: 1972. pp. 136–179. [Google Scholar]
- Van Cauter E. Diurnal and ultradian rhythms in human endocrine function: a minireview. Horm. Res. 1990;34:45–53. doi: 10.1159/000181794. [DOI] [PubMed] [Google Scholar]
- van Honk J., Tuiten A., Verbaten R., Hout M.v.d., Koppeshaar H., Thijssen J., Haan E.d. Correlations among salivary testosterone, mood, and selective attention to threat in humans. Horm. Behav. 1999;36:17–24. doi: 10.1006/hbeh.1999.1521. doi:10.1006/hbeh.1999.1521 [DOI] [PubMed] [Google Scholar]
- van Honk J., Tuiten A., Hermans E., Putman P., Koppeschaar H., Thijssen J., Verbaten R., van Doornen L. A single administration of testosterone induces cardiac accelerative responses to angry faces in healthy young women. Behav. Neurosci. 2001;115:238–242. doi: 10.1037/0735-7044.115.1.238. doi:10.1037/0735-7044.115.1.238 [DOI] [PubMed] [Google Scholar]
- Vermeulen A., Goemaere S., Kaufman J.M. Testosterone, body composition and aging. J. Endocrinol. Invest. 1999;22:110–116. [PubMed] [Google Scholar]
- Wingfield J.C., Hegner R.E., Dufty A.M., Ball G.F. The ‘challenge hypothesis’: theoretical implications for patterns of testosterone secretion, mating systems, and breeding strategies. Am. Nat. 1990;136:829–846. doi:10.1086/285134 [Google Scholar]
- Wingfield J.C., Jacobs J.D., Tramontin A.D., Perfito N., Meddle S., Maney D.L., Soma K. Toward an ecological basis of hormone–behavior interactions in reproduction of birds. In: Wallen K., Schneider J.E., editors. Reproduction in context. MIT Press; Cambridge, MA: 2000. pp. 85–128. [Google Scholar]
- Worthman C.M., Konner M.J. Testosterone levels change with subsistence hunting effort in !Kung San men. Psychoneuroendocrinology. 1987;12:449–458. doi: 10.1016/0306-4530(87)90079-5. doi:10.1016/0306-4530(87)90079-5 [DOI] [PubMed] [Google Scholar]