Abstract
The hypothesis that females of socially monogamous species obtain indirect benefits (good or compatible genes) from extra-pair mating behaviour has received enormous attention but much less generally accepted support. Here we ask whether selection for adult survival and fecundity or sexual selection contribute to indirect selection of the extra-pair mating behaviour in socially monogamous coal tits (Periparus ater). We tracked locally recruited individuals with known paternity status through their lives predicting that the extra-pair offspring (EPO) would outperform the within-pair offspring (WPO). No differences between the WPO and EPO recruits were detected in lifespan or age of first reproduction. However, the male WPO had a higher lifetime number of broods and higher lifetime number of social offspring compared with male EPO recruits, while no such differences were evident for female recruits. Male EPO recruits did not compensate for their lower social reproductive success by higher fertilization success within their social pair bonds. Thus, our results do not support the idea that enhanced adult survival, fecundity or within-pair fertilization success are manifestations of the genetic benefits of extra-pair matings. But we emphasize that a crucial fitness component, the extra-pair fertilization success of male recruits, has yet to be taken into account to fully appreciate the fitness consequences of extra-pair matings.
Keywords: adult survival selection, extra-pair paternity, lifetime reproductive success, local recruitment, longevity, Parus ater
1. Introduction
The adaptive significance of female extra-pair mating behaviour in socially monogamous bird species is one of the most contentious issues in evolutionary ecology (e.g. Griffith et al. 2002; Westneat & Stewart 2003; Arnqvist & Kirkpatrick 2005; Akçay & Roughgarden 2007; Griffith 2007). Why do females of so many species mate extra-pair so frequently? Any truly comprehensive understanding of avian mating systems and sexual selection in birds is impossible without providing a convincing answer to this long-standing question. Different hypotheses have been put forward in order to explain how a fitness benefit could outweigh the potential costs (e.g. Dixon et al. 1994) of female extra-pair mating behaviour. In particular, the idea that females mate extra-pair to obtain good or compatible genes that increase offspring genetic quality has received enormous attention but much less generally accepted empirical support (reviewed in Griffith et al. 2002; Westneat & Stewart 2003; Akçay & Roughgarden 2007).
Comparing fitness-related traits of the within-pair offspring (WPO) and the extra-pair offspring (EPO) is a strong test for the genetic benefits of extra-pair matings (e.g. Sheldon et al. 1997; Griffith et al. 2002). If genetic benefits select for extra-pair mating behaviour, EPO are predicted to have higher fitness than WPO and thus to outperform them in terms of viability, fecundity or sexual attractiveness. Some of the studies that compared half-sibling performance supported the good and/or compatible genes models of female extra-pair mating (e.g. Sheldon et al. 1997; Foerster et al. 2003; Fossøy et al. 2008), but others failed to do so (e.g. Lubjuhn et al. 1999a; Whittingham & Dunn 2001; Kleven & Lifjeld 2004). Furthermore, recent work suggests that environmental context dependence of paternal genetic effects adds another layer of complexity to this problem (Schmoll et al. 2005; Garvin et al. 2006). Thus, the evidence from this promising approach is mixed, and rather sceptical evaluations of the genetic benefit models of extra-pair mating tend to prevail in recent synthetic contributions (e.g. Westneat & Stewart 2003; Arnqvist & Kirkpatrick 2005; Akçay & Roughgarden 2007).
Failure to detect the predicted differences in half-sibling performance may result, for example, from insufficient statistical power due to small expected effect sizes (e.g. Møller & Alatalo 1999) or from environmental effects masking such differences (e.g. Schmoll et al. 2005). But it is also important to envision that to date no study has actually compared the lifetime reproductive performance or even fitness of WPO and EPO. In the majority of the studies, half siblings were compared at the nestling stage with respect to traits known or thought to be fitness relevant (e.g. fledgling size-corrected body mass, Sheldon et al. 1997; immunocompetence, Johnsen et al. 2000; heterozygosity, Foerster et al. 2003). Few studies were able to track the fate of individuals further and analysed fledgling survival (e.g. Suter et al. 2007) or local recruitment into the breeding population (which integrates the critical first winter survival and successful establishment as a breeder, e.g. Lubjuhn et al. 1999a). So far, in only one avian study system, was it directly investigated whether offspring—once recruited—differ in their (first-year) reproductive performance with respect to their paternity status (no such effects were detectable, Schmoll et al. 2003, 2005; but see Cohas et al. 2007 for an example in mammals). Yet, even in comparatively short-lived species similar to many passerines frequently investigated in this context, establishing oneself as a first-year breeder is only part of the exercise and the duration of the entire reproductive lifespan is potentially very important for variation in lifetime reproductive success. What happens beyond recruitment into the breeding population? It is easily conceivable that selection for adult survival and fecundity as well as sexual selection operates beyond recruitment and that they contribute to indirect selection of an extra-pair mating preference of females. To fully appreciate the fitness consequences of extra-pair matings, it is therefore of major importance to analyse the paternity-related fitness variation due to traits expressed beyond recruitment.
Building on previous work (Schmoll et al. 2003), we report here the data on long-term fitness consequences of extra-pair matings in the coal tit Periparus ater (formerly Parus ater), a socially monogamous passerine with a high frequency of extra-pair paternity as well as high local recruitment rates. We followed the fate of a total of 242 locally recruited individuals with known paternity status (WPO or EPO) from three cohorts to analyse how lifespan, lifetime reproductive performance and paternity loss (for male recruits) relate to paternity. Previous results from the study population showed that male extra-pair fertilization success is strongly and positively related to the male age (Schmoll et al. 2007). Assuming that females control extra-pair copulations, this result is compatible with an extra-pair mating preference of females for older males. Such a preference may be indirectly selected through genetic viability benefits according to an age-based indicator mechanism of genetic quality (reviewed in Brooks & Kemp 2001). If female coal tits mate extra-pair in order to obtain such viability benefits, EPO are predicted to have longer lifespan than their WPO half siblings and, accordingly, higher lifetime reproductive success.
We found no evidence in support of the hypothesis that survival selection during the adult stage, fecundity selection or sexual selection indirectly select for the female extra-pair mating behaviour in our study population. But our analysis also highlights that a crucially important fitness component, the extra-pair fertilization success of the male offspring, is still missing from a comprehensive picture of the fitness consequences of extra-pair matings in natural populations.
2. Material and methods
(a) Study species, study population and general field methods
Coal tits are small, territorial, altricial, cavity-nesting passerine birds with biparental care (Glutz von Blotzheim & Bauer 1993). They are socially monogamous (Glutz von Blotzheim & Bauer 1993), but show comparatively high rates of extra-pair paternity (Lubjuhn et al. 1999b; Dietrich et al. 2004). From 2000 to 2007 we studied an established nest-box population of coal tits in a mixed coniferous forest near Lingen/Emsland (Lower Saxony, Germany, 52°27′ N, 7°15′ E). The 325 ha study area contained approximately 560 nest-boxes (only 470 nest-boxes in 2007), harbouring between 106 and 195 coal tit breeding pairs per year during the study period. Parentage analysis (see below) was conducted from 2000 to 2002 and the data on recapture and reproductive performance of locally recruited individuals were collected from 2001 to 2007. The percentage of broods with extra-pair paternity in the study population ranged from 66.3 to 67.0% in first brood periods and 83.6 to 91.3% in second brood periods with an overall proportion of the EPO ranging from 27.0 to 28.6% in first broods and 43.9 to 49.4% in second broods (for details see Dietrich et al. 2004). The study population is mainly non-migratory and natal dispersal (see Winkel 1981; Schmoll et al. 2005), and in particular breeding dispersal distances (Winkel 1981, and see below) are rather short. This leads to comparatively high local recapture rates that permit the estimation of the long-term fitness consequences of mating decisions.
During the breeding seasons (April–July), nest-boxes were monitored at least weekly to record breeding phenology (laying and hatching date), parameters of reproductive performance (e.g. brood size, hatching and recruitment success) and the identity of adult birds. Adults were captured while feeding 10–14-day-old nestlings and regarded as the social (i.e. putative) parents of the respective broods. Capturing effort was high and highly standardized over the study period so that only a very small fraction of the nest-box breeding population (estimated as less than 5%) could not be captured (including few very shy, capture-resistant individuals and individuals that had deserted their broods before capturing took place and also had not produced replacement clutches). Both adults and nestlings were banded with uniquely numbered metal rings of the Institute of Avian Research ‘Vogelwarte Helgoland’ (Wilhelmshaven, Germany). Blood samples (approx. 50 μl) were taken from the ulnar vein under license (no. 509f-42502-46), diluted in 250 μl APS buffer (Arctander 1988) and stored at −20°C until further use.
(b) Parentage analysis
We used multilocus DNA fingerprinting in order to exclude putative parents from genetic parentage. Details of the basic DNA fingerprinting procedures used for parentage exclusion have been described in detail elsewhere (Lubjuhn et al. 1999b; Dietrich 2001); hence the fundamental method is outlined only briefly. DNA was isolated according to a modified standard protocol (Lubjuhn & Sauer 1999) and digested with the restriction enzyme Hae III. After separation by horizontal agarose gel electrophoresis, gels were dried followed by in-gel hybridization using the 32P-labelled oligonucleotide (CA)8. The banding patterns were visualized by scanning with a phosphoimager (Storm 860, Amersham, Freiburg, Germany). Parentage exclusion gels always contained a brood's nestlings along with its social (i.e. putative) parents. Banding patterns were highly informative and analysed according to Westneat (1990) using the image-editing software Adobe Photoshop v. 5.5. The putative (i.e. social) fathers were excluded from genetic parentage if greater than or equal to two novel fragments (i.e. distinct fragments neither attributable to the social mother's nor to the social father's banding pattern) were present in the banding pattern of the focal nestling (Dietrich 2001). The probability of falsely assigning one putative parent to an offspring was as low as 1.1×10−5 (Dietrich 2001).
(c) Statistical analysis
In order to test for effects of paternity that become effective beyond local recruitment into the breeding population, we analysed patterns of presence or absence of breeding birds as well as the reproductive performance for recruits originating from three cohorts (2000, 2001 and 2002). Thus, we only included individuals for which (i) paternity status (WPO versus EPO) had been established at the nestling stage and (ii) that were recaptured at least once as a breeding bird in the study population. Overall, 9.2 per cent of the 3549 nestlings for which paternity status had been unequivocally determined were locally recruited. We excluded recruits from analysis that had either been raised in nests with experimentally manipulated brood sizes (enlarged or reduced) or that had provided parental care for broods with such experimentally manipulated brood sizes as adults because both kinds of manipulations may affect adult recapture probability in later years. This procedure resulted in a sample of 242 recruits (121 males and females each, 164 WPO and 78 EPO) originating from 168 successfully genotyped broods. Of these 242 recruits, 168 (81 males and 87 females, 100 WPO and 68 EPO) came from 116 broods with mixed paternity (MP; at least one WPO as well as one EPO present in the brood of origin). These broods are thereafter referred to as MP broods. In the text, we report and discuss results only for this latter sample, because the predicted differences between the WPO and EPO recruits should be most easily detectable in the MP broods sample despite its smaller size (WPO from broods with 100% within-pair paternity are expected to be of comparatively high quality and including them may blur the differences between the WPO and EPO descendants of those females that had actually mated extra-pair). Results based on the sample of all 168 genotyped broods are given in tabulated and graphical form in the electronic supplementary material.
(i) Statistical modelling approach and general structure of the models
We used R v. 2.6.1 (R Development Core Team 2007) for statistical analyses, all tests were two-tailed and the null hypothesis was rejected at p<0.05. We fitted generalized linear models (GLMs) and generalized linear mixed models (GLMMs, R function lmer with Laplace approximation of the maximum likelihood as implemented in the R package lme4, Bates 2007) to our data. To obtain minimal adequate models, we removed fixed effect terms from a maximal model stepwise as long as this caused no significant decrease in model fit as assessed by likelihood ratio tests (Crawley 2005). Reported p values refer to the increase in deviance when respective terms are removed from the more complex models. All maximal models included paternity status (WPO versus EPO, thereafter referred to as paternity), cohort (2000, 2001 or 2002), brood period within which the recruits hatched (first versus second, thereafter referred to as hatch period) and sex (unless analyses were conducted separately for the sexes, see below) as categorical independent variables. Previous studies indicated that local recruitment into the study population is affected by paternity in a context-dependent way, in that EPO recruited better than their WPO half siblings when originating from broods late in the year (i.e. from second broods, see Schmoll et al. 2005). To test whether paternity effects vary with environmental conditions, maximal models included the two-way interactions of paternity with cohort and hatch period. Furthermore, we included the two-way interaction of paternity with sex when applicable.
To account for the non-independence of the data from recruits originating from the same brood and to control for random variation between the broods of origin, we always included a random effect of the brood of origin. Thus, we estimated the fixed effect of paternity status (and other independent variables of interest) while controlling for potentially confounding variation between broods that may be due to maternal genotype, nest environment, parental or territory quality. Broods of origin from which members of both half-sibling groups recruited have the greatest weight in these analyses, but—in contrast to classic pairwise comparisons—GLMMs can also make use of information for those broods from which only members of one half-sibling group recruited. The significance of random effects in these models was tested by removing them from the respective minimal adequate model and comparing the resulting increase in model deviance against a chi-square distribution. The proportion of variation explained by random effects was calculated as the adjusted coefficient of determination r2 (Nagelkerke 1991) of the minimal adequate GLMM minus the adjusted r2 of the corresponding GLM with an identical fixed effects structure, but lacking the focal random effect.
(ii) Recruit lifespan and latency to first reproduction
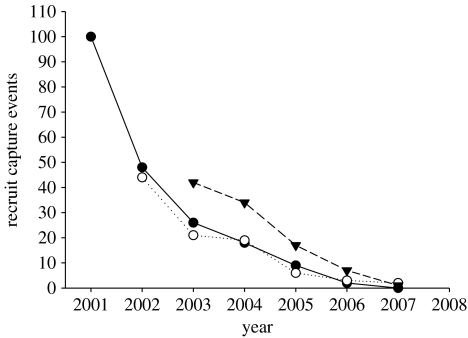
When analysing survival in natural populations, it is important to take into account that (i) the observed lifespan may be right censored because individuals may still be alive at the end of the study period and (ii) the capture probability of individuals is normally less than one so that observed periods of presence may underestimate true lifespan. We argue that censoring is not relevant for our datasets: by 2007, the number of capture events has dropped to nearly zero for recruits from all three focal cohorts (figure 1), and the predicted number of captures for a further sampling season amounted to 0.27, 0.41 and 1.26 individuals for the cohorts 2000, 2001 and 2002, respectively (predictions based on a minimal adequate GLM for the full sample with Poisson errors and log link and the significant predictor variables cohort, cohort age and squared cohort age, all p<0.01). Thus, we argue that sampling was performed for a sufficiently long time to reliably estimate the lifespan as well as the lifetime reproductive performance for members of these cohorts.
Figure 1.
Capture events over the recapture period (2001–2007) for locally recruited coal tits from different cohorts (n=242 recruits originating from all 168 successfully genotyped broods). Filled circles, 2000 cohort; open circles, 2001 cohort; triangles, 2002 cohort.
When analysing individual sequences of presence and absence in our samples over the recapture period (2001–2007), we furthermore found that absence followed by presence nearly always referred to special cases where individuals were not captured in the season(s) directly following their year of birth (implying a latency period to the first recorded reproduction), but were then continuously present from their first until their last recorded reproduction. Only 6 out of 168 individuals (3.6%) from MP broods and 10 out of 242 recruits (4.1%) from all genotyped broods were absent in between two presences (all these individuals were males). This implies that the capture probability of living individuals that had been captured at least once before is close to one. We thus measured recruit lifespan directly as the age when individuals were last captured breeding and separately analysed the latency to the first (recorded) reproduction to test for the differences between the WPO and EPO in this trait.
GLMMs with Poisson error structure and log-link function were fitted for both these dependent variables. Lifespan data were −1-transformed in order to meet Poisson assumptions (as only recruited individuals entered analyses, the minimum possible lifespan is 1 year, whereas a Poisson distribution has a non-zero probability of obtaining zero counts). In addition, pairwise Wilcoxon rank-sum tests of WPO versus EPO lifespan and latency to first reproduction were computed for 16 broods producing recruits of both types of maternal half siblings. If more than one individual per half-sibling type and brood had recruited, average values were used. Furthermore, multinomial log-linear models fitted via neural networks (R library nnet, Venables & Ripley 2002) were used to test for paternity effects on the entire distribution of lifespan (rather than just the mean lifespan).
(iii) Recruit breeding dispersal
Differential breeding dispersal of WPO and EPO may affect adult recapture probability and could thus confound estimates of lifespan. We therefore tested whether any of our independent variables affected the lifetime short-range breeding dispersal, i.e. the vector of breeding dispersal distances within the study site measured over a recruit's lifetime. Breeding dispersal distances in the study population are very short (Winkel 1981, and see below), and many individuals or pairs reuse the same nest-box in a subsequent year. This results in a frequency distribution of dispersal distances with an excess of zero values. To account for this, GLMMs with gamma error structure and log-link function were fitted to dispersal distances +0.4 m (a small transformation constant had to be added in order to meet the assumption that gamma variables are strictly greater than zero). Furthermore, we included random effects of individual nested within the brood of origin in order to account for the non-independence of dispersal distances measured for the same recruit and for recruits originating from the same brood.
(iv) Recruit lifetime reproductive performance
The lifetime number of recorded broods (including first as well as second broods) and the lifetime number of hatchlings were used as dependent variables to analyse a recruit's lifetime reproductive performance. GLMMs with Poisson error structure and log-link function were fitted for both of these dependent variables. Number of broods was −1-transformed to meet Poisson assumptions (as only recruited individuals entered analyses, the minimum possible number of broods is one). In 2003, a balanced cross-foster experiment had been performed in some of the first broods, including broods for which recruits from the three focal cohorts provided parental care. Since nestlings were cross fostered at day 2 post-hatch, the number of fledglings cannot be used as a measure of reproductive performance in these broods. Hence, we used the lifetime number of hatchlings to quantify a recruit's lifetime reproductive performance. Reproductive performance was analysed separately for both sexes since the dependent variables can be compared meaningfully only within sexes. This is because the male genetic reproductive success cannot be estimated reliably from the number of hatchlings due to the high rates of extra-pair paternity (cf. Dietrich et al. 2004) in the study population. Thus, lifetime reproductive performance in these analyses refers to social (or apparent) reproductive success for male recruits but to genetic (or realized) reproductive success for female recruits (exclusion of genetic maternity was extremely rare in the study population, see Schmoll et al. 2008). Due to this splitting of the data, sample sizes for reproductive performance traits were too small for classic pairwise tests as a supplement to mixed model analyses.
(v) Paternity loss and within-pair fertilization success of male recruits
Data on paternity loss were available for a subsample, namely for male recruits of the 2000 cohort breeding in 2001 and 2002 and for recruits of the 2001 cohort breeding in 2002 (note that the data for recruits of the 2000 cohort breeding in 2001 were already reported in Schmoll et al. 2003). We use these data to assess whether male WPO recruits differ from male EPO recruits in terms of attractiveness or sperm competitiveness and thus in fertilization success within their social pair bonds. GLMMs with binomial error structure and logit-link function were fitted to model the proportion of EPO per brood using the number of successfully genotyped nestlings per brood as the binomial denominator. GLMMs with Poisson error structure and log-link function were fitted to model the number of WPO sired (reflecting fertilization success within the social pair bond). In addition to the independent variables used in other analyses (see above), this analysis included also year and brood period (first versus second) as fixed categorical variables. Furthermore, we included random effects of individual nested within the brood of origin in order to account for the non-independence of the data measured for the same recruit and for recruits originating from the same brood.
3. Results
(a) Recruit lifespan
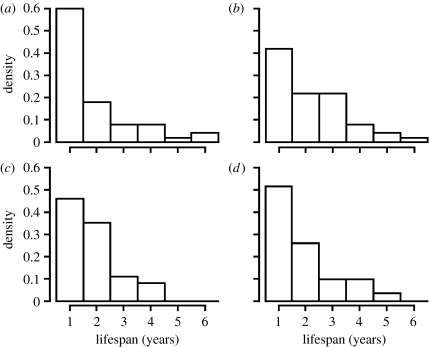
Lifespan ranged from 1 to 6 years with a median of 1.5 years. Frequency distributions of lifespan in relation to paternity and sex are given in figure 2 (see also figure S1 in the electronic supplementary material). Neither paternity (Χ12=0.51, p=0.47) nor any other independent variable nor their two-way interactions with paternity predicted recruit lifespan (all other p>0.05, see table S1 in the electronic supplementary material for full details of analysis of deviance). There was significant random variation in lifespan between recruits' broods of origin (Χ12=15.97, p<0.001), which explained 11.4 per cent of the total variation in lifespan in the minimal adequate model. A pairwise test of WPO versus EPO lifespan within those broods producing recruits of both half-sibling types revealed no difference (Wilcoxon rank-sum test: W=14.0, p=0.67, n=16 pairwise comparisons involving 40 recruits) and thus supported the results of the mixed model analysis. Inspection of the data suggested that WPO and EPO recruits may differ in the shape of their lifespan distributions (in particular, female WPO compared with female EPO, figure 2). However, multinomial log-linear models indicated that neither paternity nor sex or their two-way interaction had significant effects on the distribution of recruits across lifespan classes (all Χ52<6.25, all p>0.25).
Figure 2.
Relative frequency distributions of recruit lifespan in relation to paternity and sex for 168 recruits originating from 116 broods with MP (WPO, within-pair offspring; EPO, extra-pair offspring; n=50 female WPO, 50 male WPO, 37 female EPO and 31 male EPO). (a) Female WPO, (b) male WPO, (c) female EPO and (d) male EPO.
(b) Lifetime breeding dispersal
Breeding dispersal distances ranged from 0 to 510 m with a median of 25 m and a modal value of 0 m. Lifetime short-range breeding dispersal was not affected either by paternity (Χ12=0.01, p=0.91) or by any other predictor variable or their two-way interactions with paternity (all other p>0.20; see table S2 in the electronic supplementary material for details).
(c) Latency to first reproduction
Out of 168 recruits from MP broods 19.0 per cent were not captured in the season directly following their year of birth but only in later years (data for all genotyped broods: 23.1% out of 242 recruits). Thus, while paternity did not affect lifespan (see above), it might still affect the age at first (recorded) reproduction. Male recruits had a significantly longer latency than females in terms of the number of years before first recorded breeding (Χ12=7.35, p=0.007). Furthermore, a marginally significant cohort effect was also detected (Χ22=6.01, p=0.049). Yet, neither paternity (Χ12=2.31, p=0.13) nor any other independent variable nor their two-way interaction with paternity was a significant predictor of the latency to first reproduction (all other effects p>0.1; see table S3 in the electronic supplementary material for details). In the minimal adequate model, there was a tendency for random variation between recruits' broods of origin (Χ12=3.17, p=0.07), which explained 2.9 per cent of the total variation in latency. A pairwise test of WPO versus EPO latency within those broods producing recruits of both half-sibling types revealed no difference (Wilcoxon rank-sum test: W=19.0, p=0.38, n=16 pairwise comparisons involving 40 recruits).
When further exploring the detected sex difference in latency to first reproduction, we found that in 78 per cent of the total of 45 cases in which only one of the two attending adults of a breeding pair were captured, this was the female partner (binomial p<0.001 for a deviation from a probability of 0.5 based on a period of 6 years from 2001 to 2006 with a high and highly standardized capturing effort).
(d) Lifetime reproductive performance
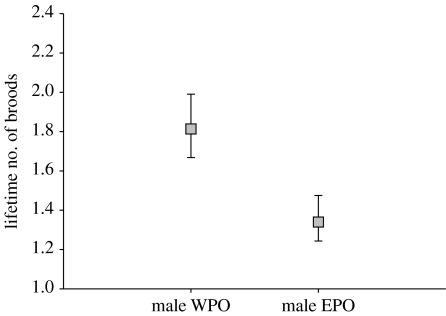
The lifetime number of recorded broods ranged from one to eight with a median of one for females and from one to five with a median of one for males. Relative frequency distributions of the lifetime number of broods in relation to paternity and sex are given in figure S2 in the electronic supplementary material (see also figure S3 in the electronic supplementary material). There was an overall positive correlation between the lifespan and the lifetime number of broods recorded (Spearman's rank correlation: rs=0.79, p<0.001, sexes combined). For female recruits, neither paternity (Χ12=0.003, p=0.96) nor any other independent variable or their two-way interactions with paternity predicted the lifetime number of broods (all other p>0.05; see table S4a,b in the electronic supplementary material for details). However, male EPO recruits produced significantly less broods over their lifetime than male WPO recruits (Χ12=6.07, p=0.014; figure 3; see also figure S4 in the electronic supplementary material). This effect was not caused by a differential propensity of male WPO and EPO recruits to engage in second broods as there was no significant difference in the lifetime number of second broods (main effect of paternity: Χ12=0.86, p=0.35). None of the other fixed effect terms reached significance (all p>0.10; table S4c, see also table S4d in the electronic supplementary material). However, there was significant random variation in the number of broods between the female as well as male recruits' broods of origin (Χ12=42.1, p<0.001 for females and Χ12=7.11, p=0.008 for males), explaining 43.3 and 9.7 per cent, respectively, of the total variation in number of broods in the minimal adequate models.
Figure 3.
GLMM estimates (±s.e.) for the lifetime number of broods recorded for male WPO and EPO recruits (n=81 recruits originating from 62 broods with MP).
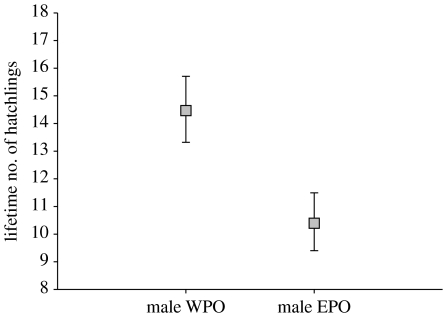
The lifetime number of hatchlings ranged from 2 to 72 with a median of 9 for females and from 2 to 40 with a median of 9 for males. Relative frequency distributions of the lifetime number of hatchlings in relation to paternity and sex are given in figure S5 in the electronic supplementary material (see also figure S6 in the electronic supplementary material). There was an overall positive correlation between the lifetime number of broods recorded and the lifetime number of hatchlings produced (Spearman's rank correlation: rs=0.90, p<0.001, sexes combined). For female recruits, neither paternity (Χ12=0.38, p=0.54) nor any other independent variable nor their two-way interactions with paternity predicted the lifetime number of hatchlings (all other p>0.10; see table S5a,b in the electronic supplementary material for details). However, male EPO recruits produced significantly less hatchlings over their lifetime than male WPO recruits (Χ12=7.50, p=0.006; figure 4; see also figure S7 in the electronic supplementary material). None of the other terms reached significance (all p>0.05; see table S5c,d in the electronic supplementary material for details). When including the lifetime number of broods as a covariate in the minimal adequate model for the lifetime number of hatchlings, the main effect of paternity turned insignificant (Χ12=0.80, p=0.37). This indicates that the paternity-related difference in lifetime number of hatchling of males largely result from the paternity-related difference in the lifetime number of broods.
Figure 4.
GLMM estimates (±s.e.) for the lifetime number of hatchlings produced by male WPO and EPO recruits (n=81 recruits originating from 62 broods with MP).
(e) Paternity loss and within-pair fertilization success of male recruits
Paternity loss in broods of male recruits ranged from 0 to 100% (note that data on paternity loss for male recruits were only available for the breeding seasons 2001 and 2002, and thus for broods of recruits from the cohorts 2000 and 2001). There was no difference in the mean proportion of EPO in broods of male WPO versus EPO recruits (n=57 male recruits caring for 74 broods: Χ12=0.24, p=0.62; see table S6a,b in the electronic supplementary material for details). Within-pair fertilization success of male recruits ranged from 0 to 10 genetic offspring per brood. There was no difference in the mean number of WPO in broods of male WPO versus EPO recruits (Χ12=0.12, p=0.73; see table S7a,b in the electronic supplementary material for details).
4. Discussion
There was a substantial variation in the age when individual breeding birds were last captured (figure 2), suggesting a considerable potential for selection on adult survival to affect variation in fitness. Yet, in contrast to our prediction, WPO and EPO recruits differed neither in the mean lifespan nor in the frequency distribution of lifespan. In addition, none of our other independent variables or their interactions with paternity affected this fitness component. Thus, our data do not support the hypothesis that females benefit from extra-pair matings by obtaining ‘good longevity genes’ and that selection on adult survival contributes to indirect selection for female extra-pair mating behaviour. Differential breeding dispersal with respect to paternity may affect recapture probabilities of WPO and EPO and could thus confound estimates of recruit lifespan. However, breeding dispersal distances in our population showed a median of only 25 m and a modal value of 0 m indicating the frequent reuse of the same territory or even nest-box across years. Furthermore, paternity did not influence the short-range breeding dispersal of recruits. While long-range dispersal can never be ruled out completely as a confounding factor for survival estimates in wild populations, we argue that paternity effects on breeding dispersal did not bias our lifespan estimates.
Male recruits showed a significantly longer latency to their first recorded reproduction than females. This may result from two different but not mutually exclusive mechanisms. First, the pattern suggests that more males than females delay their first reproduction or that they have to use (probably suboptimal) natural cavities for the first breeding attempt(s), e.g. due to intraspecific competition for high-quality nesting sites. Second, in the great majority of those cases where we could catch only one of the two partners of a breeding pair, this was the female. This indicates that males were more difficult to capture than females and/or that they are more likely to have deserted a brood at the time of capturing (nestling day 10). Given that males in this population have, on average, a much lower certainty of genetic parentage than females, sex differences in parental investment (e.g. in vigilance or risk taking during feeding and nest defence behaviour) may indeed be expected. In fact, if the differential capture probability mainly resulted from younger males being less likely to be captured than their female partners, this could also confound estimates of males' age at first reproduction (see above). Importantly, however, we found no significant differences when comparing the latency with the first recorded reproduction of WPO and EPO recruits. Thus, there is no evidence to suggest that paternity status affects the age of first reproduction as a major life-history trait.
Lifetime reproductive success may differ for individuals with similar lifespan and we thus analysed—separately for the sexes—the lifetime reproductive performance of recruits. Contrary to our prediction, male WPO recruits had a higher lifetime number of hatchlings than male EPO recruits (figure 4). This effect was mainly due to a higher number of broods raised (figure 3), although neither lifespan, latency to first reproduction nor the number of attended second broods differed significantly between the (male) WPO and EPO recruits. However, inspection of the sign of the parameter estimates in these analyses for males revealed that the male WPO were on average slightly younger when they were first captured, slightly older when they were last captured, and that they were also captured slightly more often attending second broods (data not shown). In combination, these non-significant effects resulted in a significantly higher lifetime number of broods and hatchlings for male WPO recruits, while there were no such differences for female recruits.
Are male WPO better off than EPO recruits after all? Given that the high frequency of extra-pair paternity in the study population precludes reliable inference of male genetic reproductive success from male social success, it is difficult to draw firm conclusions based on the social reproductive success of males alone. For example, male EPO recruits could be more attractive as within-pair and/or extra-pair mating partners pre copula or they could be more competitive fertilizers under a regime of intense sperm competition post copula. Such a genetic attractiveness benefit (cf. Kokko et al. 2002) of extra-pair matings might (over-) compensate for the lower social reproductive success of male EPO recruits. In fact, their lower apparent success could even represent a consequence of high investment in sexual attractiveness, e.g. in terms of extra-pair mating effort (e.g. Hunt et al. 2004 for an example of trading sexual attractiveness against other fitness components). We were not able to measure the lifetime total fertilization success of males as a function of their paternity status, because genetic data over the lifetime of focal cohorts were not available. However, when analysing paternity loss and within-pair fertilization success (as a measure of the within-pair sexual attractiveness) in the subsample for which these data were available, we found no differences between the male WPO and EPO recruits (in line with previously reported results, cf. Schmoll et al. 2003). Thus, it seems unlikely that male EPO can compensate for their lower social reproductive success through higher within-pair fertilization success. However, for a truly comprehensive picture, a crucial and still missing part is whether male EPO can outperform male WPO recruits in gaining extra-pair fertilizations in broods attended by other males. This could make all the difference given that extra-pair fertilizations have been shown to be a very important component of variance in male total fertilization success in other socially monogamous species (Kleven et al. 2006; Webster et al. 2007). We therefore suggest that future studies should pay special attention to male extra-pair fertilization success and consider it as a major task to quantify the total (i.e. within-pair and extra-pair) lifetime fertilization success of males in relation to paternity status.
Previous results from the study population suggested a context-dependent genetic benefit of extra-pair matings as EPO recruited better than their WPO half siblings when originating from second broods (see Schmoll et al. 2005). Modelling approaches may be useful for integrating this earlier finding on juvenile survival and recruitment with the adult fitness components analysed in the present study to obtain an overall assessment of the adaptive significance of extra-pair matings in the study population.
We conclude that our results do not support the hypothesis that survival selection in the adult stage, fecundity selection or sexual selection contributed to indirect selection for female extra-pair mating behaviour in the coal tit study population. However, we would like to stress that a crucial fitness component, namely male extra-pair mating success, has not yet been taken into account by any study. We therefore call to analyse not only lifetime reproductive success, but in particular male lifetime extra-pair and total fertilization success in relation to paternity in this and other socially monogamous species to fully appreciate the fitness consequences of extra-pair matings before rejecting genetic benefit models of extra-pair mating (e.g. Arnqvist & Kirkpatrick 2005; Akçay & Roughgarden 2007).
Acknowledgments
Blood samples from birds were taken under licence no. 509f-42502-46
We thank Sabrina Bleidissel, Verena Dietrich-Bischoff, Maria Orland and Christiane Wallnisch for their help in the laboratory; Julia Delingat, Julia Eggert, Jorg Welcker and especially Verena Dietrich-Bischoff, Volker Janzon, Verena Mund, Darius Stiels and Anja Quellmalz for their support in the field; and Karin and Herbert Körner for their hospitality during the field work. Bart Kempenaers and two anonymous reviewers provided valuable comments on an earlier version of this manuscript. This project was supported by the Deutsche Forschungsgemeinschaft (Lu 572/2). Frank Schurr acknowledges support from the European Union through Marie Curie Transfer of Knowledge Project FEMMES (MTKD-CT-2006-042261).
Footnotes
We wish to dedicate this contribution to the memory of Doris Winkel who unexpectedly passed away in June 2007 and who contributed so much to this work.
Supplementary Material
Additional figures
Analysis of deviance tables
References
- Akçay E., Roughgarden J. Extra-pair paternity in birds: review of the genetic benefits. Evol. Ecol. Res. 2007;9:855–868. [Google Scholar]
- Arctander P. Comparative studies on avian DNA restriction fragment length polymorphism analysis: convenient procedures based on blood samples from live birds. J. Ornithol. 1988;129:205–216. doi:10.1007/BF01647289 [Google Scholar]
- Arnqvist G., Kirkpatrick M. The evolution of infidelity in socially monogamous passerines: the strength of direct and indirect selection on extrapair copulation behavior in females. Am. Nat. 2005;165:S26–S37. doi: 10.1086/429350. doi:10.1086/429350 [DOI] [PubMed] [Google Scholar]
- Bates, D. 2007 lme4: linear mixed-effects models using S4 classes. R package, version 0.99875-9.
- Brooks R., Kemp D.J. Can older males deliver the good genes? Trends Ecol. Evol. 2001;16:308–313. doi: 10.1016/s0169-5347(01)02147-4. doi:10.1016/S0169-5347(01)02147-4 [DOI] [PubMed] [Google Scholar]
- Cohas A., Bonenfant C., Gaillard J.M., Allaine D. Are extra-pair young better than within-pair young? A comparison of survival and dominance in alpine marmot. J. Anim. Ecol. 2007;76:771–781. doi: 10.1111/j.1365-2656.2007.01253.x. doi:10.1111/j.1365-2656.2007.01253.x [DOI] [PubMed] [Google Scholar]
- Crawley M.J. Wiley; Chichester, UK: 2005. Statistics—an introduction using R. [Google Scholar]
- Dietrich, V. 2001 Zum Auftreten alternativer Fortpflanzungsstrategien in einer Lingener Population der Tannenmeise (Parus ater). Diploma thesis, TU Braunschweig, Braunschweig, Germany.
- Dietrich V., Schmoll T., Winkel W., Epplen J.T., Lubjuhn T. Pair identity—an important factor concerning variation in extra-pair paternity in the coal tit (Parus ater) Behaviour. 2004;141:817–835. doi:10.1163/1568539042265644 [Google Scholar]
- Dixon A., Ross D., O'Malley S.L.C., Burke T. Paternal investment inversely related to degree of extra-pair paternity in the reed bunting. Nature. 1994;371:698–700. doi:10.1038/371698a0 [Google Scholar]
- Foerster K., Delhey K., Johnsen A., Lifjeld J.T., Kempenaers B. Females increase offspring heterozygosity and fitness through extra-pair matings. Nature. 2003;425:714–717. doi: 10.1038/nature01969. doi:10.1038/nature01969 [DOI] [PubMed] [Google Scholar]
- Fossøy F., Johnsen A., Lifjeld J.T. Multiple genetic benefits of female promiscuity in a socially monogamous passerine. Evolution. 2008;62:145–156. doi: 10.1111/j.1558-5646.2007.00284.x. doi:10.1111/j.1558-5646.2007.00284.x [DOI] [PubMed] [Google Scholar]
- Garvin J.C., Abroe B., Pedersen M.C., Dunn P.O., Whittingham L.A. Immune response of nestling warblers varies with extra-pair paternity and temperature. Mol. Ecol. 2006;15:3833–3840. doi: 10.1111/j.1365-294X.2006.03042.x. doi:10.1111/j.1365-294X.2006.03042.x [DOI] [PubMed] [Google Scholar]
- Glutz von Blotzheim U.N., Bauer K.M. Aula; Wiesbaden, Germany: 1993. Handbuch der Vögel Mitteleuropas, 13/I, Passeriformes (part 4) [Google Scholar]
- Griffith S.C. The evolution of infidelity in socially monogamous passerines: neglected components of direct and indirect selection. Am. Nat. 2007;169:274–281. doi: 10.1086/510601. doi:10.1086/510601 [DOI] [PubMed] [Google Scholar]
- Griffith S.C., Owens I.P.F., Thuman K.A. Extra pair paternity in birds: a review of interspecific variation and adaptive function. Mol. Ecol. 2002;11:2195–2212. doi: 10.1046/j.1365-294x.2002.01613.x. doi:10.1046/j.1365-294X.2002.01613.x [DOI] [PubMed] [Google Scholar]
- Hunt J., Brooks R., Jennions M.D., Smith M.J., Bentsen C.L., Bussiere L.F. High-quality male field crickets invest heavily in sexual display but die young. Nature. 2004;432:1024–1027. doi: 10.1038/nature03084. doi:10.1038/nature03084 [DOI] [PubMed] [Google Scholar]
- Johnsen A., Andersen V., Sunding C., Lifjeld J.T. Female bluethroats enhance offspring immunocompetence through extra-pair copulations. Nature. 2000;406:296–299. doi: 10.1038/35018556. doi:10.1038/35018556 [DOI] [PubMed] [Google Scholar]
- Kleven O., Lifjeld J.T. Extra-pair paternity and offspring immunocompetence in the reed bunting (Emberiza schoeniclus) Anim. Behav. 2004;68:283–289. doi:10.1016/j.anbehav.2003.11.016 [Google Scholar]
- Kleven O., Jacobsen F., Izadnegahdar R., Robertson R.J., Lifjeld J.T. Male tail streamer length predicts fertilization success in the North American barn swallow (Hirundo rustica erythrogaster) Behav. Ecol. Sociobiol. 2006;59:412–418. doi:10.1007/s00265-005-0065-0 [Google Scholar]
- Kokko H., Brooks R., McNamara J.M., Houston A.I. The sexual selection continuum. Proc. R. Soc. B. 2002;269:1331–1340. doi: 10.1098/rspb.2002.2020. doi:10.1098/rspb.2002.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubjuhn T., Sauer K.P. DNA fingerprinting and profiling in behavioural ecology. In: Epplen J.T., Lubjuhn T., editors. DNA profiling and DNA fingerprinting. Birkhäuser; Basel, Switzerland: 1999. pp. 39–52. [Google Scholar]
- Lubjuhn T., Strohbach S., Brün J., Gerken T., Epplen J.T. Extra-pair paternity in great tits (Parus major)—a long term study. Behaviour. 1999a;136:1157–1172. doi:10.1163/156853999501810 [Google Scholar]
- Lubjuhn T., Gerken T., Brün J., Epplen J.T. High frequency of extra-pair paternity in the coal tit. J. Avian Biol. 1999b;30:229–233. doi:10.2307/3677134 [Google Scholar]
- Møller A.P., Alatalo R.V. Good-genes effects in sexual selection. Proc. R. Soc. B. 1999;266:85–91. doi:10.1098/rspb.1999.0607 [Google Scholar]
- Nagelkerke N.J.D. A note on a general definition of the coefficient of determination. Biometrika. 1991;78:691–692. doi:10.1093/biomet/78.3.691 [Google Scholar]
- R Development Core Team. R Foundation for Statistical Computing; Vienna, Austria: 2007. R: a language and environment for statistical computing.http://www.R-project.org [Google Scholar]
- Schmoll T., Dietrich V., Winkel W., Epplen J.T., Lubjuhn T. Long-term fitness consequences of female extra-pair matings in a socially monogamous passerine. Proc. R. Soc. B. 2003;270:259–264. doi: 10.1098/rspb.2002.2216. doi:10.1098/rspb.2002.2216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmoll T., Dietrich V., Winkel W., Epplen J.T., Schurr F., Lubjuhn T. Paternal genetic effects on offspring fitness are context dependent within the extrapair mating system of a socially monogamous passerine. Evolution. 2005;59:645–657. doi:10.1111/j.0014-3820.2005.H.01023.x [PubMed] [Google Scholar]
- Schmoll T., Mund V., Dietrich-Bischoff V., Winkel W., Lubjuhn T. Male age predicts extrapair and total fertilization success in the socially monogamous coal tit. Behav. Ecol. 2007;18:1073–1081. doi:10.1093/beheco/arm082 [Google Scholar]
- Schmoll T., Winkel W., Lubjuhn T. Molekulargenetischer Nachweis gemischter Mutterschaften in Bruten der Tannenmeise Parus ater. Vogelwarte. 2008;48:223–227. [Google Scholar]
- Sheldon B.C., Merilä J., Qvarnström A., Gustafsson L., Ellegren H. Paternal genetic contribution to offspring condition predicted by size of male secondary sexual character. Proc. R. Soc. B. 1997;264:297–302. doi:10.1098/rspb.1997.0042 [Google Scholar]
- Suter S.M., Keiser M., Feignoux R., Meyer D.R. Reed bunting females increase fitness through extra-pair mating with genetically dissimilar males. Proc. R. Soc. B. 2007;274:2865–2871. doi: 10.1098/rspb.2007.0799. doi:10.1098/rspb.2007.0799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venables W.N., Ripley B.D. 4th edn. Springer; New York, NY: 2002. Modern applied statistics with S-Plus. [Google Scholar]
- Webster M.S., Tarvin K.A., Tuttle E.M., Pruett-Jones S. Promiscuity drives sexual selection in a socially monogamous bird. Evolution. 2007;61:2205–2211. doi: 10.1111/j.1558-5646.2007.00208.x. doi:10.1111/j.1558-5646.2007.00208.x [DOI] [PubMed] [Google Scholar]
- Westneat D.F. Genetic parentage in the indigo bunting: a study using DNA fingerprinting. Behav. Ecol. Sociobiol. 1990;27:67–76. doi:10.1007/BF00183315 [Google Scholar]
- Westneat D.F., Stewart I.R.K. Extra-pair paternity in birds: causes, correlates, and conflict. Annu. Rev. Ecol. Evol. Syst. 2003;34:365–396. doi:10.1146/annurev.ecolsys.34.011802.132439 [Google Scholar]
- Whittingham L.A., Dunn P.O. Survival of extrapair and within-pair young in tree swallows. Behav. Ecol. 2001;12:496–500. doi:10.1093/beheco/12.4.496 [Google Scholar]
- Winkel W. Zum Ortstreue-Verhalten von Kohl-, Blau- und Tannenmeisen (Parus major, P. caeruleus und P. ater) in einem 325 ha großen Untersuchungsgebiet. Vogelwelt. 1981;102:81–106. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional figures
Analysis of deviance tables