Abstract
Social context has been shown to have a profound influence on brain activation in a wide range of vertebrate species. Best studied in songbirds, when males sing undirected song, the level of neural activity and expression of immediate early genes (IEGs) in several song nuclei is dramatically higher or lower than when they sing directed song to other birds, particularly females. This differential social context-dependent activation is independent of auditory input and is not simply dependent on the motor act of singing. These findings suggested that the critical sensory modality driving social context-dependent differences in the brain could be visual cues. Here, we tested this hypothesis by examining IEG activation in song nuclei in hemispheres to which visual input was normal or blocked. We found that covering one eye blocked visually induced IEG expression throughout both contralateral visual pathways of the brain, and reduced activation of the contralateral ventral tegmental area, a non-visual midbrain motivation-related area affected by social context. However, blocking visual input had no effect on the social context-dependent activation of the contralateral song nuclei during female-directed singing. Our findings suggest that individual sensory modalities are not direct driving forces for the social context differences in song nuclei during singing. Rather, these social context differences in brain activation appear to depend more on the general sense that another individual is present.
Keywords: egr-1, ZENK, directed singing, social behaviour, vocal nuclei, courtship
1. Introduction
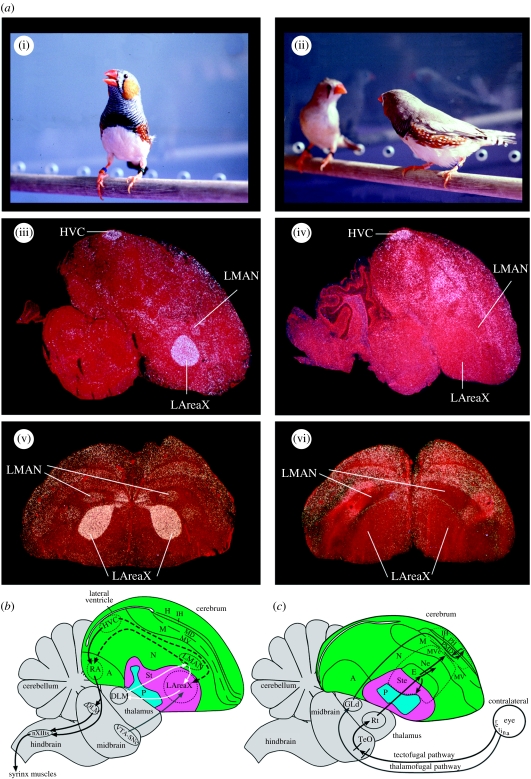
Interactions with conspecifics, either positive or negative, are critical in many ways for the survival of individuals and species. Within these interactions, the precise social context in which animals interact can strongly modulate the level of activity in specific brain areas in a wide range of vertebrates, from fishes (Burmeister et al. 2005), frogs (Yang & Wilczynski 2007) and birds (Jarvis et al. 1998; Hessler & Doupe 1999; Vignal et al. 2005), to primates (Fujii et al. 2007), including humans (Sassa et al. 2007; Van den Bos et al. 2007). Social context-dependent brain modulation is especially strong in relation to communicative behaviour, where brain activity during the production of specific vocalizations is dependent on the presence of a communicative target individual. Such modulation has been well characterized in the songbird brain during singing (Jarvis et al. 1998; Hessler & Doupe 1999), and recently found in human language production and processing brain areas during speaking (Sassa et al. 2007). In both systems, the level of brain activation during vocalization differs by orders of magnitude depending on who is listening. When male zebra finches sing undirected song, the level of neural activity and expression of the immediate early gene (IEG) egr-1 (also known as zif268, NGF1-A, krox24 and avian ZENK) is high throughout the vocal motor pathway (figure 1a(i)(iii); black solid arrows, figure 1b) and the vocal pallial-basal-ganglia-thalamic loop (figure 1a(i)(iii)(v); white arrows, figure 1b). However, when they sing directed song, activity and/or egr-1 levels are low in the robust nucleus of the arcopallium (RA) of the vocal motor pathway and in the lateral portion of the vocal pallial-basal-ganglia loop (figure 1a(ii)(iv)(vi)) (Jarvis et al. 1998; Hessler & Doupe 1999). The opposite result is found for the FoxP2 gene, a gene necessary for normal speech production in humans and song learning in songbirds (Lai et al. 2001; Haesler et al. 2004, 2007), which shows decreased expression in AreaX of the vocal pallial-basal-ganglia-thalamic loop during undirected singing but no change during directed singing (Teramitsu & White 2006). Directed singing is used during courtship to females, and undirected singing appears to have other communicative functions (Dunn & Zann 1996), including for practice or vocal exploration (Jarvis et al. 1998; Olveczky et al. 2005; Kao & Brainard 2006). However, the songs produced are very similar, with only subtle differences in tempo (directed sung slightly faster) and variability (undirected more variable) (Sossinka & Bohner 1980; Kao & Brainard 2006).
Figure 1.
Social context-dependent brain activation differences in the zebra finch and schematics of songbird brain areas involved in singing and vision. (a) Social context difference in the zebra finch brain. (i) Male singing undirected song, (ii) male (zebra-striped chest) singing directed song to a female, (iii) example sagittal section (cresyl violet stained) showing induced egr-1 mRNA (white) in the HVC, LMAN and LAreaX song nuclei after 30 min of undirected singing, (iv) example sagittal section showing the directed singing-driven expression pattern and (v, vi) example frontal sections from other animals showing dramatic differences in LMAN and LAreaX in the same social contexts. (a(i)–(iv)) Modified from Jarvis et al. (1998) and (a(v)(vi)) modified from Hara et al. (2007). (b) Song system: black solid arrows, vocal motor pathway; white arrows, vocal pallial-basal-ganglia-thalamic loop; dashed black arrows, connections between the two vocal pathways; grey arrow, ventral tegmental area dopaminergic projection to LAreaX; weaker projections exist to HVC and RA (not shown). Connectivity of vocal pathways is as summarized in Jarvis (2004); not all connections are shown and only the lateral part of the vocal-basal-ganglia loop is shown. (c) Visual pathways, located lateral to (b): grey arrows, thalamofugal pathway; black arrows, tectofugal pathway; dashed lines, boundary of visual areas as revealed in this study. Connectivity of visual pathways is as summarized in Shimizu & Bowers (1999) and Krutzfeldt & Wild (2004). Green, pallium; pink, striatum; turquoise, pallidum. See abbreviation list for anatomical terms and §2d for further definitions.
This social context-dependent modulation of brain activation does not depend on the males' hearing their own voice during singing or hearing the calls of the females in response to their singing, as it still occurs in deaf birds when they sing (Jarvis et al. 1998; Hessler & Doupe 1999). In addition, directed singing is associated with increased neural activity levels and egr-1 expression in brainstem motivation-related areas, namely the ventral tegmental area (VTA; Yanagihara & Hessler 2006; Hara et al. 2007). In mammals, activity of VTA neurons is driven by visual input and it receives visual input from the superior colliculus (Comoli et al. 2003; McHaffie et al. 2006), the homologue of the avian optic tectum. In both mammals and birds, the VTA has been implicated in reward and social context-dependent sexual behaviour (Maney et al. 2003; Riters et al. 2004; Young & Wang 2004; Aron et al. 2005; Esch & Stefano 2005; Heimovics & Riters 2005), and provides a strong dopaminergic input to the striatum, including to the lateral AreaX (LAreaX) of the vocal pallial-basal-ganglia loop (figure 1b; Lewis et al. 1981). The above findings led to the hypothesis that social context-dependent activation of vocal communication brain systems could depend on the visual stimulus reaching the VTA or some other brain areas and then those brain areas modulate the social context activation of the song system.
Here, we tested this hypothesis by blocking visual input into one hemisphere while male zebra finches sang directed song to females. There are two visual pathways in birds and mammals: the thalamofugal pathway (grey arrows, figure 1c) and the tectofugal pathway (black arrows, figure 1c; Karten 1991; Shimizu & Bowers 1999). In birds with laterally placed eyes, such as the zebra finch, the visual pathways are nearly completely crossed at the optic chiasm, such that visual input from one eye projects primarily to both visual pathways of the contralateral hemisphere (Weidner et al. 1985). We found that although visual input was necessary for the visually induced IEG activation of the visual pathways of the contralateral hemisphere and for the activation in part of the VTA, it was not apparently required for the social context-dependent activation of the song system. Our findings suggest that the social context-dependent activation of the brain is not driven by direct primary sensory or motor processes, but rather by an indirect perhaps association process indicating that another individual is present.
2. Material and methods
(a) Animals
We used 19 adult male zebra finches (more than 90 days old) that were bred in our aviaries at RIKEN and at the Duke University Medical Center. All experiments were performed according to the RIKEN BSI guidelines and were approved by the RIKEN Animal Experiments Committee and the Duke University Animal Care and Use Committee.
(b) Behaviour
One eye of each bird was covered with several layers of black vinyl electrical tape; the innermost layer was placed so that the smooth surface covered the eye to prevent irritation. The tape was sealed at the edges with super glue to the surrounding skin and feathers, to prevent light leakage. We alternated covering of the preferred (right) and non-preferred (left) eye in different birds to prevent potential biases in the results; when allowed to use only one eye, zebra finch males tend to sing slightly more songs to females when viewing them with their right eye (George et al. 2006). Birds were then isolated at least overnight in individual cages that were in sound attenuation boxes. They were divided into three groups: female-directed singing (n=8; right eye covered n=4 and left eye covered n=4); silent alone (n=5; right eye covered n=3 and left eye covered n=2); and silent in the dark (n=3; right eye covered n=1 and left eye covered n=2). We also used another control group of female-directed singers with both eyes open (n=3). For the female-directed singing groups, a female was placed in the cage with the male in the dark on the night before the recording session, but separated by a cage wall barrier. The cage wall barrier was made from the same metal bar material as the rest of the cage. Thus, with the lights on, the male and female could interact visually, but not physically. From a slit outside the sound box, we placed a thick, opaque paper barrier attached to a string alongside the cage wall barrier. In the morning, the lights were turned on, and then for every 5–10 min the paper barrier was removed so that the male could see the female and become stimulated to sing to her. This was done for 45 min. The intermittent removal of the paper barrier and the presence of the cage barriers induced more singing behaviour than normally occurs with continuous sight of the female and with tactile interactions with the female. The behaviour was videotaped, and songs were recorded using an Avisoft Recorder (Avisoft Bioacoustics, Berlin, Germany). The silent alone birds remained alone with the lights on for 45 min and did not sing; the silent dark birds remained silent alone in the dark, during waking hours. After the sessions, birds were killed, their brains were removed and embedded in optimum cutting temperature compound (Sakura Fine Technical), frozen and stored at −80°C. For the female-directed group, we used birds that sang approximately 20 or more song motifs (range 17–88).
(c) Gene expression analysis
Serial coronal brain sections (12 μm) were cut throughout the telencephalon and brainstem, and mounted on silanated glass slides. Radioactive in situ hybridization was performed with a zebra finch egr-1 clone containing the full-length coding region (obtained from Dr Osceola Whitney, Duke University), using a previously described procedure (Wada et al. 2004). We performed two separate in situ experiments: one in which sections from an original five female-directed singers (right eye covered n=3 and left eye covered n=2) were hybridized together with all brain sections from all other animals, and another where we replicated the female-directed group with three additional animals to test for possible statistical differences in IEG activation depending on which eye was covered (right eye covered n=4 total and left eye covered n=4 total). Two pictures per brain region in each hemisphere from the same section were taken with a Leica DMXRA microscope under a 60× objective. We carefully chose the areas using lamina boundaries, Nissl stain and overall gene expression profiles, so that the same areas were measured from animal to animal. The density slice and analyse functions of Scion Image (NIH) were used to count silver grains, as previously described (Hara et al. 2007), in visual areas (PH, PMD, TeO, Ne, MVe, Ste and E), song nuclei (LAreaX, LMAN, HVC and RA), motivation-related (rostral VTA (rVTA) and caudal VTA (cVTA)), movement-associated (AMV and AN) and auditory midbrain (MLd) areas (see abbreviations list). To generate representative figures of gene expression profiles, darkfield pictures were taken with a Wild M420 microscope and processed in Adobe Photoshop (San Jose, CA, USA).
(d) Anatomy
The anatomical terminology used in this paper follows the new avian brain nomenclature (Reiner et al. 2004; Jarvis et al. 2005) with modifications (Feenders et al. 2008; see abbreviations list). For visually activated areas adjacent to and near the entopallium (E) of the tectofugal pathway that have been called lateral nidopallium and lateral ventral mesopallium, we called them nidopallium adjacent to the entopallium (Ne) and ventral mesopallium near the entopallium (MVe). For those of the thalamofugal pathway, we called them posterior hyperpallium (PH) and posterior dorsal mesopallium (PMD; figure 1c). For the connectivity of the vocal motor pathway, HVC (a letter-based name) projects to RA, and RA projects onto the midbrain dorsomedial nucleus (DM) and motor neurons of the 12th tracheosyringeal nucleus (nXIIts, black arrows, figure 1b). The vocal pallial-basal-ganglia loop consists of the lateral magnocellular nucleus of the nidopallium (LMAN), which projects to AreaX in the striatum, AreaX projects to the dorsolateral medial nucleus (DLM) in the dorsal thalamus, which in turn projects back to LMAN, forming a loop (white arrows, figure 1b). The movement-associated areas are regions in the anterior nidopallium (AN) and anterior dorsal mesopallium directly adjacent to the song nuclei LMAN and the mesopallium oval nucleus, respectively, and show movement-associated gene expression when birds hop (Feenders et al. 2008), which zebra finches normally do during directed singing.
(e) Statistics
The numbers of silver grains from two pictures per brain region per animal were averaged and used for statistical tests with SigmaStat v. 3.1 (Systat Software). We performed two types of statistical tests. (i) We compared the average number of silver grains (non-normalized values) between hemispheres within a group and in each hemisphere across groups by two-way repeated-measures ANOVA (factors: hemispheres and groups) with a Holm–Sidak post hoc test; statistical results within a group are in the figures and across groups are described in the main text. (ii) We compared ratios of silver grains on the side contralateral to the open eye relative to the side contralateral to the covered eye in each brain area across groups also by two-way repeated-measures ANOVA (factors: areas and groups). With the first test, the raw values, we can determine the direction of change for one group relative to another. With the second test, the ratios, we theoretically cannot determine the direction of change for either hemisphere, but we can use the ratios to internally normalize the data for variability between individual animals and for the amount of singing (Jarvis et al. 1998); therefore, the ratios should provide a more sensitive assay to detect small quantitative differences. The ratios also allow us to compare data from different experiments, as they can normalize out experimental variation. When performing ratio analyses between hemispheres or brain areas within the same animal as its own control, sample sizes of three to six per group have been sufficient to detect statistically significant small differences in singing-regulated gene expression in vocal nuclei among groups (Jarvis & Nottebohm 1997; Hara et al. 2007; Kubikova et al. 2007).
3. Results
(a) Visual areas
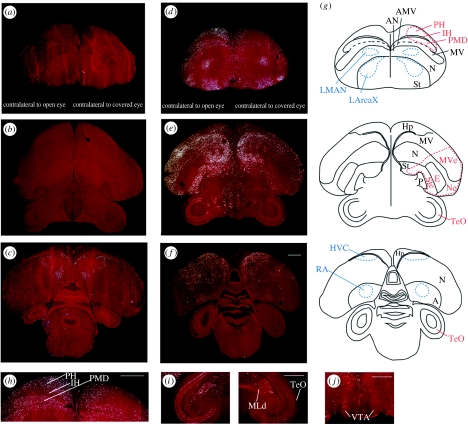
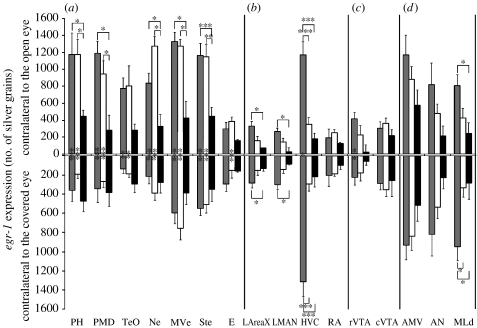
Occlusion of one eye blocked induction of egr-1 expression in the visual pathways of the contralateral brain hemisphere (figure 2). In the two groups exposed to light, silent alone and female directed, there was significantly less egr-1 expression in both the thalamofugal (PH and PMD) and tectofugal (TeO, Ne, MVe and Ste) pathways contralateral to the covered eye relative to the open eye (figure 2d,e,h,i; quantification in figure 3a). Furthermore, although there remained a low level of expression in these areas in some animals on the side contralateral to the covered eye (figure 2e), these levels were not significantly different from that seen in the silent dark group (lower half of graph, figure 3a). In the silent dark group, the level of egr-1 expression in visual areas was low and similar in both hemispheres (figures 2a,b and 3a). The first-order thalamic recipient neurons of the tectofugal pathway in the pallium, called the entopallium (E; figure 1c), normally do not express high levels of egr-1 (Mello & Clayton 1995), but they had low egr-1 levels that were significantly higher on the side contralateral to the open eye in the silent alone group (figure 3a; but see ratio analysis).
Figure 2.
Expression of egr-1 mRNA (white, silver grains) in frontal sections (counter stained with cresyl violet) in the (a–c) silent dark (rostral to caudal) and (d–f) female-directed singing groups. (g) Schematics of the brain sections in (a–f), including visual areas (red; thalamofugal pathway: PH, IH and PMD; tectofugal pathway: TeO, MVe, Ne, Ste and E), song nuclei (blue; LAreaX, LMAN, HVC and RA) and motor-related areas (AMV and AN). (h) Higher power view of the PH, IH and PMD regions from (d). (i) Higher power view of the midbrain, showing TeO and MLd from an animal of the female-directed group. (j) Section with rVTA from an animal of the female-directed group. Scale bars, 1 mm.
Figure 3.
Quantification of absolute levels of egr-1 expression in (a) visual areas, (b) song nuclei, (c) motivation-related areas and (d) motor-associated and auditory areas, contralateral to open (top bars) and covered (bottom bars) eyes. n=5 for female-directed singing (FD, grey bars); n=5 for silent alone (SA, white bars); n=3 for silent dark (SD, black bars). Each bar shows an average value±s.e.m. *p<0.05, **p<0.01, ***p<0.001. The asterisks inside the bars are significant differences between hemispheres within a group; the asterisks above or below the bars are significant differences among groups (two-way repeated-measures ANOVA; hemisphere F=1.782–92.382; group F=1.015–22.672). In some comparisons of the ANOVA, the accepted α-level was less than 0.05, and, in these cases, we indicated only the significance with the appropriate asterisk if the p-value was below that α-level cut-off.
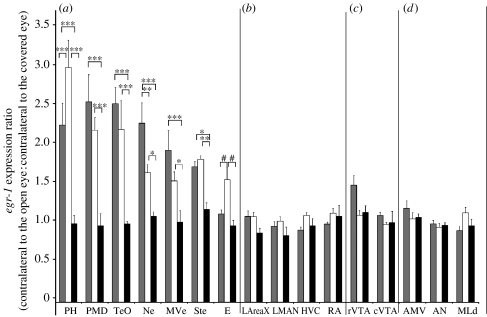
Next, we determined whether there were differences in brain areas among groups, using the ratio measures (contralateral to the open eye: contralateral to the covered eye). Except for the entopallium, all measured visual areas had significantly higher ratios of egr-1 expression in the silent alone and female-directed groups relative to the silent dark group (figure 4a), supporting the non-normalized results. (The comparison for the entopallium approached significance, p=0.022, α-level=0.017; two-way repeated-measures ANOVA.) Furthermore, we observed a higher ratio of expression in the PH of the silent alone relative to the female-directed group, but higher expression in the Ne of the female-directed relative to the silent alone group (figure 4a). In general, high-induced contralateral egr-1 expression was blocked throughout the rostral–caudal extent of the PH and PMD, but not in more anterior and medially adjacent areas within the hyperpallium and dorsal mesopallium of the known somatosensory parts of these brain subdivisions (figure 2d,h,g; Wild 1997; Wild & Williams 2000; Feenders et al. 2008); and it was blocked in the entire MVe, Ne and Ste surrounding E (figure 2e,g) and in all layers of the TeO (figure 2e,f, i,g).
Figure 4.
Quantification of ratios of egr-1 expression between hemispheres in (a) visual areas, (b) song nuclei, (c) motivation-related areas and (d) motor-associated and auditory areas. n=5 for female-directed singing (FD, grey bars); n=5 for silent alone (SA, white bars); n=3 for silent dark (SD, black bars). Each bar shows an average value±s.e.m. *p<0.05, **p<0.01, ***p<0.001, representing differences between hemispheres (contralateral to the open eye: contralateral to the covered eye) across groups (two-way repeated-measures ANOVA). The hash symbol represents the p-value that approached significance (p=0.022, α-level=0.017).
Although we found a comparable reduction of egr-1 in the visual pathway areas regardless of which eye was covered, we further tested whether our results could have been significantly skewed by potential biases in lateralization, as portions of the TeO show higher levels of egr-1 induction in the left hemisphere (driven by right eye input) when both eyes are open (George et al. 2006). Thus, we measured egr-1 induction in a separate set of birds that sang to females with both eyes open. In these birds, there were no apparent differences in gene expression between hemispheres in the cerebral visual areas (PH, PMD, Ne, MVe and Ste; paired t-test, p=0.193–0.911; n=3). The overall results indicate that unilateral eye occlusion reduces egr-1 expression in the contralateral visual areas to the levels that are similar to birds lacking light exposure, and that it does so throughout the contralateral visual pathways.
(b) Song nuclei
Tract tracing and electrophysiology studies suggest that visual information may enter the song system from the ipsilateral TeO to the thalamic nucleus uvaeformis (UVa) to the HVC (Wild 1994). It could also potentially enter the system from the TeO through the VTA. Thus, because the visual pathways in birds with laterally positioned eyes are nearly completely crossed (Weidner et al. 1985) and unilateral eye covering blocks egr-1 activation in the contralateral visual pathways, it could potentially block visually driven activation of the song system. Therefore, we examined whether unilateral removal of visual input prevented social context modulation of egr-1 expression during social interaction with a female bird.
Males with one eye covered still approached and sang directed songs to females, while viewing them with the open eye. In this group, robust social context-dependent singing-regulated egr-1 expression was seen in the song nuclei of both hemispheres: high egr-1 expression levels in the HVC and low levels in the LAreaX, LMAN and RA (figure 2d,f). Furthermore, there were no hemispheric differences in the LAreaX, LMAN or RA (female-directed group, figure 3b). The level of expression in the HVC was slightly lower in the hemisphere contralateral to the open eye side (female-directed group, figure 3b; but see ratio analysis below).
The non-normalized values across groups showed that in the female-directed compared with the silent alone and silent dark groups, there was a high level of egr-1 expression in the song nucleus HVC contralateral to the open eye (upper bars, figures 2f and 3b; three to four fold increases relative to the silent alone group), as expected (Jarvis et al. 1998). In the LAreaX and LMAN, there was a low level of induced egr-1 expression in the female-directed group (figure 3b; LAreaX 1.4–1.67-fold and LMAN 1.3–1.7-fold), as expected. We did not see a significant increase in the central portion of the RA, also as expected. The song nuclei contralateral to the covered eye showed similar results (lower bars, figure 3b); the female-directed singing birds had high induced expression in the HVC and some induced expression in the LAreaX and LMAN relative to the silent groups. Thus, in the absence of unilateral visual input, there is still a dramatic social context difference in the song system activation of both hemispheres when birds sing to females. We do not believe that visual input would have made a difference for undirected singing, as the undirected singing-driven gene expression pattern is the same whether or not a male sees a female (Jarvis et al. 1998).
To be certain that blocking contralateral visual input did not result in a small difference in the social context regulation in the song nuclei of the female-directed group, we next assessed whether there were group differences using ratio analysis (contralateral to the open eye: contralateral to the covered eye), which also eliminates the amount of singing as a factor (Jarvis et al. 1998). The ratio analysis confirmed that there were no significant differences among groups in the song nuclei (figure 4b), including in HVC, suggesting that the small difference noted above in the HVC within the female-directed group is not significantly different from the differences between hemispheres at basal levels without singing. We repeated this experiment with an additional three animals in our second experiment, and still found no significant differences in the song nuclei with all animals combined (AreaX, p>0.340; LMAN, p>0.407; HVC, p>0.386; RA, p>0.384; n=8 female directed, n=5 silent alone and n=3 silent alone in the dark, two-way repeated-measures ANOVA).
Another sensitive measure that detects social context differences in the song nuclei during singing is the ratio of expression between a nucleus that shows a social context difference (e.g. AreaX) and one that does not (e.g. HVC). This measure is sensitive to the amount of directed and undirected singing an individual animal performs, and it follows a logarithmic sigmoidal function (Jarvis et al. 1998); when birds sing 100 per cent directed song, the AreaX : HVC egr-1 expression ratio is below 0.4; when they sing 100 per cent undirected song, the ratio is above 0.6; when they sing a combination of directed and undirected songs, the ratios fall between 0.3 and 0.6. We thus measured the ratios of expression between AreaX and HVC in our female-directed singing birds with one eye covered to determine whether there were differences in either hemisphere. We found that the AreaX : HVC ratios were within the expected range for directed singing (0.14–0.41) and were not significantly different between hemispheres (p=0.996; paired t-test, n=8).
We next assessed whether a possible eye preference had any small effect on the social context differences in the song system nuclei that could have been masked by inclusion of birds that used the non-preferred eye in our analysis. We found that there were no detectable significant differences in the ratios of expression between hemispheres between birds that used the assumed preferred eye (right) and the non-preferred (left) eye (AreaX p=0.456; LMAN p=0.195; HVC p=0.503; RA p=0.059; t-test; n=4 per group). These findings are consistent with a previous report that found no significant differences in singing-driven egr-1 expression in the right and left song nuclei during directed or undirected singing with both eyes open (Hara et al. 2007). Therefore, our results show that although covering either eye reduces or eliminates the activation of the visual pathway in the contralateral hemisphere, it has no major effect on the social context differences in the song nuclei of either hemisphere.
(c) Other brain areas
Next, we investigated whether the lack of a visual blocking effect on the song nuclei reflected a lack of visual influence on the non-visual brain areas generally or on the song nuclei specifically. We examined expression in a non-vocal brain area that shows social context differences, the VTA (Hara et al. 2007), in the motor-related areas of the AN and anterior ventral mesopallium (AMV) that are adjacent to the song nuclei of the pallial-basal-ganglia loop (Feenders et al. 2008) and in an auditory area, MLd.
Interestingly, in the female-directed group, blocking visual input prevented high levels of egr-1 induction in the rVTA contralateral to the covered eye (figures 2j and 3c); the cVTA showed no difference between hemispheres (figure 3c). The difference in the rVTA was also apparent in the ratio analysis (figure 4c), and statistically stronger when including birds from both experiments (p>0.0001 for the female-directed group (n=8) relative to the silent alone (n=5) or silent alone in the dark (n=3) group, two-way repeated-measures ANOVA). In the motor-associated areas AN and AMV, egr-1 was expressed in the female-directed and silent alone groups (figure 2a), as expected, since they were actively moving in their cages, but there were no significant differences between hemispheres or among groups (figures 3d and 4d). In the auditory midbrain nucleus MLd adjacent to the TeO, there was induced expression in both hemispheres of the female-directed group (figures 2i and 3d, female directed relative to silent alone), as expected due to the males hearing their own songs and the female calls, but there were no significant differences between hemispheres (figures 3d and 4d). Finally, we observed reduced egr-1 expression contralateral to the covered eye in other brain areas that are not known to be part of the visual pathway, such as the nidopallium far lateral to the HVC (figure 2f,g), but we did not quantify them further as the functions of these areas are not known.
Similar to the visual areas and song nuclei, the results in the rVTA were not affected by which eye was covered (p=0.918; t-test; n=4 per group), consistent with the previous findings of no significant differences in singing-associated egr-1 expression in the right and left VTA during directed singing with both eyes open (Hara et al. 2007). Taken together, these findings indicate that although activation of some non-visual areas (such as rVTA) can be reduced by blocking visual input from the contralateral eye, the modulation of song nuclei activation by social context is not affected.
4. Discussion
This paper is the first that we are aware of to test the role of vision in modulating social context-dependent activation of brain and the first to demonstrate a requirement of light for induced IEG expression in the visual pathways of the avian brain. We demonstrate that covering one eye is sufficient to block IEG expression in all known regions of both the visual pathways of the contralateral hemisphere and is sufficient to reduce expression in a contralateral motivation-related brain area, but does not prevent social context-dependent gene regulation in the contralateral song nuclei. Below, we discuss the implications of these findings for social context-dependent modulation of brain systems and for understanding functional organization of the visual pathways.
Studies conducted in different species have shown that visual cues are one of the key factors for female mate choice (Summers et al. 1999; Waitt et al. 2003; Horth 2007). In addition, male birds and lizards view females more often with a preferred eye during courtship (Hews & Worthington 2001; George et al. 2006), further highlighting the importance of vision in mate choice. In zebra finches, egr-1 is strongly expressed in several visual areas after courtship behaviours (Sadananda & Bischof 2002, 2006; George et al. 2006). These results suggest that processing in the visual pathways is important for courtship behaviours of male zebra finches.
We hypothesized that blocking visual input might prevent the social context-dependent modulation in the song nuclei, but found that unilateral eye covering did not alter the pattern of egr-1 expression in the contralateral song nuclei. This lack of a detectable effect in the song nuclei is intriguing. It could reflect bilateral modulation of song system activation by visual input arriving via a yet unknown pathway. Another possibility is that visual cues, similar to auditory cues (Jarvis et al. 1998; Hessler & Doupe 1999), are not necessary for generating the social context modulation of song nuclei. Sensory cues alone are not sufficient for producing the social context differences, in that although several song nuclei (LMAN, LAreaX and RA) and the VTA are differentially activated when a male sees a female and sings to her, this does not occur when a male sees a female and does not sing (Yanagihara & Hessler 2006; Hara et al. 2007). The combined results of past studies with this study indicate that males may simply need to sense through at least one sensory modality (vision or hearing) that a female is present. Once known, then a subsequent sensory-independent mechanism may bilaterally modulate the social context differences in the song nuclei. This idea can be tested further by simultaneously blocking visual input to one eye and blocking hearing by deafening, and then assess song nuclei activation during directed singing.
While activation of forebrain song nuclei was not altered by blocking contralateral visual input, activation of an area thought to receive more direct visual input, VTA, was reduced by blocking visual input. The VTA in mammals receives short-latency visual input from the superior colliculus (Comoli et al. 2003; McHaffie et al. 2006), the avian homologue of the TeO. The TeO has been previously shown to be strongly activated during directed singing, but not during undirected singing (George et al. 2006). Based on our hypothesis that dopamine from the VTA is an important source of song system modulation, and an observed reduction of singing-related egr-1 expression occurs in the AreaX by ipsilateral VTA and substantia nigra pars compacta (SNc) lesions (Hara et al. 2007), we had expected that unilateral reduction of VTA activation by blocking visual input would alter ipsilateral AreaX activation. We did not observe such an effect here. It may be that since monocular occlusion affected only the rostral VTA, that not enough of VTA–SNc was affected to influence gene regulation in AreaX.
Lesions of VTA–SNc also reduce a zebra finch males' motivation to sing directed song to females, but do not affect the motivation to sing undirected song (Hara et al. 2007). In mammals, the VTA is specifically known to be involved in modulating motivation-related behaviours, including romantic love in humans (Young & Wang 2004; Aron et al. 2005; Esch & Stefano 2005). Perhaps the visual dependence of rostral VTA activation during directed singing in songbirds is part of a mechanism that links a motivating visual stimulus (a female) to activation of the sexually motivated behaviour (directed singing). This idea can be further tested by stimulating the rostral VTA and assessing the motivation to sing directed song to conspecific females and other animals and/or objects.
In regard to visual systems, our unilateral visual occlusion experiments confirmed that the avian visual pathway encompasses portions of the nidopallium (Ne) and ventral mesopallium (MVe) adjacent to the entopallium for the tectofugal pathway and of the PH and PMD for the thalamofugal pathway (Shimizu & Bowers 1999; Krutzfeldt & Wild 2004). These findings support the previous suggestion that the nidopallium is functionally associated with the ventral mesopallium and the hyperpallium is functionally associated with the dorsal mesopallium (Mouritsen et al. 2005; Feenders et al. 2008). They also support the recent suggestion based on connectivity that a large portion of the striatum adjacent to the entopallium (Ste) is a part of the tectofugal pathway (Krutzfeldt & Wild 2004).
Several previous reports examined IEG activation in known visual pathway regions in zebra finches and chickens (Sadananda & Bischof 2002, 2006; Lieshoff et al. 2004; George et al. 2006), but they did not demonstrate that vision was required for this induction nor did they study the effects in the song nuclei. Other studies used monocular eye covering, but they examined non-visual areas in chickens (Anokhin et al. 1991) or night-vision brain areas in migratory songbirds (Mouritsen et al. 2005; Liedvogel et al. 2007). One of the prior studies that examined daylight visual areas in zebra finches (Sadananda & Bischof 2006) found that in males courting females for the first time (singing was not determined) compared with males being chased by the fingers of an experimenter, c-fos expression was higher in the Ne (tectofugal pathway) but not in the PH (thalamofugal pathway). Birds housed alone with the lights on did not show high induction levels of expression in visual areas, but dark-housed animals were not included. In our study, we compared light-stimulated animals with dark-housed animals, and found that silent alone birds simply exposed to light for 30–45 min after an overnight period of darkness showed IEG induction in both visual pathways. Furthermore, we also found that the Ne had higher activation in the female-directed animals relative to the silent alone animals, but that the PH had lower expression in the female-directed animals relative to the silent alone animals that did not see females but were still exposed to light. These differences in the Ne and PH between studies could be due to the differences in the activation of different IEGs (egr-1 and c-fos), the behaviours studied, or the long (Sadananda & Bischof 2006) versus acute (this study) exposure to light. We also found that the entopallium had low but significantly higher expression with acute exposure to light in the silent alone group. In another study, George et al. (2006) found that egr-1 was higher in the left TeO (tectofugal pathway) relative to the right one when males saw females. In that study, however, the differences only occurred for a medial proportion of the TeO in 75 per cent of the birds, and the preference for singing directed song with the right eye was relatively small (20–67% more song was produced when the right eye was left open). Taken together, these findings indicate that different parts of each visual pathway may be more engaged for visual processing when courting females (Ne of tectofugal) versus when simply exposed to light for waking activities (PH of thalamofugal and entopallium of tectofugal). This idea may reconcile opposing hypotheses that have proposed different functions for each pathway (Gunturkun & Hahmann 1999; Shimizu & Bowers 1999)—stimulus localization and identification versus visuomotor processing.
In summary, our findings contribute to a growing body of knowledge which shows that neural activation in the brain, whether in motor, sensory or motivation-related areas, can be dependent on the social context in which a behaviour is performed and independent of direct sensory or motor processing, including fishes, birds and mammals (Jarvis et al. 1998; Hessler & Doupe 1999; Burmeister et al. 2005; Vignal et al. 2005; Sassa et al. 2007; Weaver et al. 2007). In songbirds, not only the song system shows social context-dependent gene regulation, but so does the auditory pathway when a bird hears song depending on who else is listening (Vignal et al. 2005). In cichlid fishes, the preoptic area shows differential gene activation in response to the social opportunity to become dominant, but not with simple movements, and to sensory stimuli associated with dominance behaviour (Burmeister et al. 2005). In mice, differential maternal care of pups, and thus environmental context, alters the methylation status of egr-1 promoters in the hippocampus, leading to long-term changes in its functional activation due to context (Weaver et al. 2007). In humans, although some variables were not controlled for, fRMI studies have shown that speech directed towards another individual relative to descriptive undirected speech is associated with higher activation in a focal region of the medial prefrontal cortex and in the auditory temporal cortex that have been proposed to be analogous to songbird LMAN and the auditory forebrain, respectively (Jarvis 2004). The authors proposed that their findings in humans suggest that these areas could be involved in theory of mind processing, e.g. ‘mentalization during speech production (Sassa et al. 2007)’. Whether future experiments in humans or birds would support such a hypothesis, our findings indicate that the social context modulation of the vocal motor pathway in songbirds is less sensory driven than initially considered. These findings open up new questions on mechanisms of social context regulation for a behaviour with parallels to human speech.
Acknowledgments
All experiments were performed according to the RIKEN BSI guidelines and were approved by the RIKEN Animal Experiments Committee and the Duka University Animal Care and Use Committee.
We thank Maurice Anderson for assistance in processing brain sections, Alyssa Zhu for assistance in quantification and Dr Osceola Whitney and Dr Isabelle George for their useful comments on the manuscript. Support was contributed by NIH R01MH62083 and an NIH Director's Pioneer Award to E.D.J. and the RIKEN Brain Science Institute to N.A.H.
References
- Anokhin K.V., Mileusnic R., Shamakina I.Y., Rose S.P. Effects of early experience on c-fos gene expression in the chick forebrain. Brain Res. 1991;544:101–107. doi: 10.1016/0006-8993(91)90890-8. doi:10.1016/0006-8993(91)90890-8 [DOI] [PubMed] [Google Scholar]
- Aron A., Fisher H., Mashek D.J., Strong G., Li H., Brown L.L. Reward, motivation, and emotion systems associated with early-stage intense romantic love. J. Neurophysiol. 2005;94:327–337. doi: 10.1152/jn.00838.2004. doi:10.1152/jn.00838.2004 [DOI] [PubMed] [Google Scholar]
- Burmeister S.S., Jarvis E.D., Fernald R.D. Rapid behavioral and genomic responses to social opportunity. PLoS Biol. 2005;3:e363. doi: 10.1371/journal.pbio.0030363. doi:10.1371/journal.pbio.0030363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comoli E., Coizet V., Boyes J., Bolam J.P., Canteras N.S., Quirk R.H., Overton P.G., Redgrave P. A direct projection from superior colliculus to substantia nigra for detecting salient visual events. Nat. Neurosci. 2003;6:974–980. doi: 10.1038/nn1113. doi:10.1038/nn1113 [DOI] [PubMed] [Google Scholar]
- Dunn A.M., Zann R.A. Undirected song encourages the breeding female zebra finch to remain in the nest. Ethology. 1996;102:540–548. [Google Scholar]
- Esch T., Stefano G.B. The neurobiology of love. Neuro Endocrinol. Lett. 2005;26:175–192. [PubMed] [Google Scholar]
- Feenders G., Liedvogel M., Rivas M., Zapka M., Horita H., Hara E., Wada K., Mouritsen H., Jarvis E.D. Molecular mapping of movement-associated areas in the avian brain: a motor theory for vocal learning origin. PLoS ONE. 2008;3:e1768. doi: 10.1371/journal.pone.0001768. doi:10.1371/journal.pone.0001768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii N., Hihara S., Iriki A. Dynamic social adaptation of motion-related neurons in primate parietal cortex. PLoS ONE. 2007;2:e397. doi: 10.1371/journal.pone.0000397. doi:10.1371/journal.pone.0000397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George I., Hara E., Hessler N.A. Behavioral and neural lateralization of vision in courtship singing of the zebra finch. J. Neurobiol. 2006;66:1164–1173. doi: 10.1002/neu.20273. doi:10.1002/neu.20273 [DOI] [PubMed] [Google Scholar]
- Gunturkun O., Hahmann U. Functional subdivisions of the ascending visual pathways in the pigeon. Behav. Brain Res. 1999;98:193–201. doi: 10.1016/s0166-4328(98)00084-9. doi:10.1016/S0166-4328(98)00084-9 [DOI] [PubMed] [Google Scholar]
- Haesler S., Wada K., Nschdejan A., Morrisey E., Lints E.K.T., Jarvis E.D., Scharff C. FoxP2 expression in avian vocal learners and non-learners. J. Neurosci. 2004;24:3164–3175. doi: 10.1523/JNEUROSCI.4369-03.2004. doi:10.1523/JNEUROSCI.4369-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haesler S., Rochefort C., Georgi B., Licznerski P., Osten P., Scharff C. Incomplete and inaccurate vocal imitation after knockdown of FoxP2 in songbird basal ganglia nucleus Area X. PLoS Biol. 2007;5:e321. doi: 10.1371/journal.pbio.0050321. doi:10.1371/journal.pbio.0050321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara E., Kubikova L., Hessler N.A., Jarvis E.D. Role of the midbrain dopaminergic system in modulation of vocal brain activation by social context. Eur. J. Neurosci. 2007;25:3406–3416. doi: 10.1111/j.1460-9568.2007.05600.x. doi:10.1111/j.1460-9568.2007.05600.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimovics S.A., Riters L.V. Immediate early gene activity in song control nuclei and brain areas regulating motivation relates positively to singing behavior during, but not outside of, a breeding context. J. Neurobiol. 2005;65:207–224. doi: 10.1002/neu.20181. doi:10.1002/neu.20181 [DOI] [PubMed] [Google Scholar]
- Hessler N.A., Doupe A.J. Social context modulates singing-related neural activity in the songbird forebrain. Nat. Neurosci. 1999;2:209–211. doi: 10.1038/6306. doi:10.1038/6306 [DOI] [PubMed] [Google Scholar]
- Hews D.K., Worthington R.A. Fighting from the right side of the brain: left visual field preference during aggression in free-ranging male tree lizards (Urosaurus ornatus) Brain Behav. Evol. 2001;58:356–361. doi: 10.1159/000057576. doi:10.1159/000057576 [DOI] [PubMed] [Google Scholar]
- Horth L. Sensory genes and mate choice: evidence that duplications, mutations, and adaptive evolution alter variation in mating cue genes and their receptors. Genomics. 2007;90:159–175. doi: 10.1016/j.ygeno.2007.03.021. doi:10.1016/j.ygeno.2007.03.021 [DOI] [PubMed] [Google Scholar]
- Jarvis E.D. Learned birdsong and the neurobiology of human language. Ann. NY Acad. Sci. 2004;1016:749–777. doi: 10.1196/annals.1298.038. doi:10.1196/annals.1298.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis E.D., Nottebohm F. Motor-driven gene expression. Proc. Natl Acad. Sci. USA. 1997;94:4097–4102. doi: 10.1073/pnas.94.8.4097. doi:10.1073/pnas.94.8.4097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis E.D., Scharff C., Grossman M.R., Ramos J.A., Nottebohm F. For whom the bird sings: context-dependent gene expression. Neuron. 1998;21:775–788. doi: 10.1016/s0896-6273(00)80594-2. doi:10.1016/S0896-6273(00)80594-2 [DOI] [PubMed] [Google Scholar]
- Jarvis E.D., et al. Avian brains and a new understanding of vertebrate brain evolution. Nat. Rev. Neurosci. 2005;6:151–159. doi: 10.1038/nrn1606. doi:10.1038/nrn1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao M.H., Brainard M.S. Lesions of an avian basal ganglia circuit prevent context-dependent changes to song variability. J. Neurophysiol. 2006;96:1441–1455. doi: 10.1152/jn.01138.2005. doi:10.1152/jn.01138.2005 [DOI] [PubMed] [Google Scholar]
- Karten H.J. Homology and evolutionary origins of the ‘neocortex’. Brain Behav. Evol. 1991;38:264–272. doi: 10.1159/000114393. doi:10.1159/000114393 [DOI] [PubMed] [Google Scholar]
- Krutzfeldt N.O., Wild J.M. Definition and connections of the entopallium in the zebra finch (Taeniopygia guttata) J. Comp. Neurol. 2004;468:452–465. doi: 10.1002/cne.10972. doi:10.1002/cne.10972 [DOI] [PubMed] [Google Scholar]
- Kubikova L., Turner E.A., Jarvis E.D. The pallial basal ganglia pathway modulates the behaviorally driven gene expression of the motor pathway. Eur. J. Neurosci. 2007;25:2145–2160. doi: 10.1111/j.1460-9568.2007.05368.x. doi:10.1111/j.1460-9568.2007.05368.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C.S.L., Fisher S.E., Hurst J.A., Vargha-Khadem F., Monaco A.P. A forkhead-domain gene is mutated in a severe speech and language disorder. Nature. 2001;413:519–523. doi: 10.1038/35097076. doi:10.1038/35097076 [DOI] [PubMed] [Google Scholar]
- Lewis J.W., Ryan S.M., Arnold A.P., Butcher L.L. Evidence for a catecholaminergic projection to area X in the zebra finch. J. Comp. Neurol. 1981;196:347–354. doi: 10.1002/cne.901960212. doi:10.1002/cne.901960212 [DOI] [PubMed] [Google Scholar]
- Liedvogel M., Feenders G., Wada K., Troje N.F., Jarvis E.D., Mouritsen H. Lateralized activation of cluster N in the brains of migratory songbirds. Eur. J. Neurosci. 2007;25:1166–1173. doi: 10.1111/j.1460-9568.2007.05350.x. doi:10.1111/j.1460-9568.2007.05350.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieshoff C., Grosse-Ophoff J., Bischof H.J. Sexual imprinting leads to lateralized and non-lateralized expression of the immediate early gene ZENK in the zebra finch brain. Behav. Brain Res. 2004;148:145–155. doi: 10.1016/s0166-4328(03)00189-x. doi:10.1016/S0166-4328(03)00189-X [DOI] [PubMed] [Google Scholar]
- Maney D.L., MacDougall-Shackleton E.A., MacDougall-Shackleton S.A., Ball G.F., Hahn T.P. Immediate early gene response to hearing song correlates with receptive behavior and depends on dialect in a female songbird. J. Comp. Physiol. A: Neuroethol. Sens. Neural Behav. Physiol. 2003;189:667–674. doi: 10.1007/s00359-003-0441-z. doi:10.1007/s00359-003-0441-z [DOI] [PubMed] [Google Scholar]
- McHaffie J.G., Jiang H., May P.J., Coizet V., Overton P.G., Stein B.E., Redgrave P. A direct projection from superior colliculus to substantia nigra pars compacta in the cat. Neuroscience. 2006;138:221–234. doi: 10.1016/j.neuroscience.2005.11.015. doi:10.1016/j.neuroscience.2005.11.015 [DOI] [PubMed] [Google Scholar]
- Mello C.V., Clayton D.F. Differential induction of the ZENK gene in the avian forebrain and song control circuit after metrazole-induced depolarization. J. Neurobiol. 1995;26:145–161. doi: 10.1002/neu.480260112. doi:10.1002/neu.480260112 [DOI] [PubMed] [Google Scholar]
- Mouritsen H., Feenders G., Liedvogel M., Wada K., Jarvis E.D. Night-vision brain area in migratory songbirds. Proc. Natl Acad. Sci. USA. 2005;102:8339–8344. doi: 10.1073/pnas.0409575102. doi:10.1073/pnas.0409575102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olveczky B.P., Andalman A.S., Fee M.S. Vocal experimentation in the juvenile songbird requires a basal ganglia circuit. PLoS Biol. 2005;3:e153. doi: 10.1371/journal.pbio.0030153. doi:10.1371/journal.pbio.0030153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner A., et al. Revised nomenclature for avian telencephalon and some related brainstem nuclei. J. Comp. Neurol. 2004;473:377–414. doi: 10.1002/cne.20118. doi:10.1002/cne.20118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riters L.V., Teague D.P., Schroeder M.B., Cummings S.E. Vocal production in different social contexts relates to variation in immediate early gene immunoreactivity within and outside of the song control system. Behav. Brain Res. 2004;155:307–318. doi: 10.1016/j.bbr.2004.05.002. doi:10.1016/j.bbr.2004.05.002 [DOI] [PubMed] [Google Scholar]
- Sadananda M., Bischof H.J. Enhanced fos expression in the zebra finch (Taeniopygia guttata) brain following first courtship. J. Comp. Neurol. 2002;448:150–164. doi: 10.1002/cne.10232. doi:10.1002/cne.10232 [DOI] [PubMed] [Google Scholar]
- Sadananda M., Bischof H.J. C-fos induction in forebrain areas of two different visual pathways during consolidation of sexual imprinting in the zebra finch (Taeniopygia guttata) Behav. Brain Res. 2006;173:262–267. doi: 10.1016/j.bbr.2006.06.033. doi:10.1016/j.bbr.2006.06.033 [DOI] [PubMed] [Google Scholar]
- Sassa Y., Sugiura M., Jeong H., Horie K., Sato S., Kawashima R. Cortical mechanism of communicative speech production. Neuroimage. 2007;37:985–992. doi: 10.1016/j.neuroimage.2007.05.059. doi:10.1016/j.neuroimage.2007.05.059 [DOI] [PubMed] [Google Scholar]
- Shimizu T., Bowers A.N. Visual circuits of the avian telencephalon: evolutionary implications. Behav. Brain Res. 1999;98:183–191. doi: 10.1016/s0166-4328(98)00083-7. doi:10.1016/S0166-4328(98)00083-7 [DOI] [PubMed] [Google Scholar]
- Sossinka R., Bohner J. Song types in the zebra finch (Poephila guttata castanotis) Z. Tierpsychol. 1980;53:123–132. [Google Scholar]
- Summers K., Symula R., Clough M., Cronin T. Visual mate choice in poison frogs. Proc. R. Soc. B. 1999;266:2141–2145. doi: 10.1098/rspb.1999.0900. doi:10.1098/rspb.1999.0900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teramitsu I., White S.A. FoxP2 regulation during undirected singing in adult songbirds. J. Neurosci. 2006;26:7390–7394. doi: 10.1523/JNEUROSCI.1662-06.2006. doi:10.1523/JNEUROSCI.1662-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Bos W., McClure S.M., Harris L.T., Fiske S.T., Cohen J.D. Dissociating affective evaluation and social cognitive processes in the ventral medial prefrontal cortex. Cogn. Affect Behav. Neurosci. 2007;7:337–346. doi: 10.3758/cabn.7.4.337. [DOI] [PubMed] [Google Scholar]
- Vignal C., Andru J., Mathevon N. Social context modulates behavioural and brain immediate early gene responses to sound in male songbird. Eur. J. Neurosci. 2005;22:949–955. doi: 10.1111/j.1460-9568.2005.04254.x. doi:10.1111/j.1460-9568.2005.04254.x [DOI] [PubMed] [Google Scholar]
- Wada K., Sakaguchi H., Jarvis E.D., Hagiwara M. Differential expression of glutamate receptors in avian neural pathways for learned vocalization. J. Comp. Neurol. 2004;476:44–64. doi: 10.1002/cne.20201. doi:10.1002/cne.20201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waitt C., Little A.C., Wolfensohn S., Honess P., Brown A.P., Buchanan-Smith H.M., Perrett D.I. Evidence from rhesus macaques suggests that male coloration plays a role in female primate mate choice. Proc. R. Soc. B. 2003;270(Suppl. 2):S144–S146. doi: 10.1098/rsbl.2003.0065. doi:10.1098/rsbl.2003.0065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver I.C., D'Alessio A.C., Brown S.E., Hellstrom I.C., Dymov S., Sharma S., Szyf M., Meaney M.J. The transcription factor nerve growth factor-inducible protein A mediates epigenetic programming: altering epigenetic marks by immediate-early genes. J. Neurosci. 2007;27:1756–1768. doi: 10.1523/JNEUROSCI.4164-06.2007. doi:10.1523/JNEUROSCI.4164-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidner C., Reperant J., Miceli D., Haby M., Rio J.P. An anatomical study of ipsilateral retinal projections in the quail using radioautographic, horseradish peroxidase, fluorescence and degeneration techniques. Brain Res. 1985;340:99–108. doi: 10.1016/0006-8993(85)90778-4. doi:10.1016/0006-8993(85)90778-4 [DOI] [PubMed] [Google Scholar]
- Wild J.M. Visual and somatosensory inputs to the avian song system via nucleus uvaeformis (Uva) and a comparison with the projections of a similar thalamic nucleus in a nonsongbird, Columba livia. J. Comp. Neurol. 1994;349:512–535. doi: 10.1002/cne.903490403. doi:10.1002/cne.903490403 [DOI] [PubMed] [Google Scholar]
- Wild J.M. The avian somatosensory system: the pathway from wing to wulst in a passerine (Chloris chloris) Brain Res. 1997;759:122–134. doi: 10.1016/s0006-8993(97)00253-9. doi:10.1016/S0006-8993(97)00253-9 [DOI] [PubMed] [Google Scholar]
- Wild J.M., Williams M.N. Rostral wulst in passerine birds. I. Origin, course, and terminations of an avian pyramidal tract. J. Comp. Neurol. 2000;416:429–450. doi:10.1002/(SICI)1096-9861(20000124)416:4<429::AID-CNE2>3.0.CO;2-X [PubMed] [Google Scholar]
- Yanagihara S., Hessler N.A. Modulation of singing-related activity in the songbird ventral tegmental area by social context. Eur. J. Neurosci. 2006;24:3619–3627. doi: 10.1111/j.1460-9568.2006.05228.x. doi:10.1111/j.1460-9568.2006.05228.x [DOI] [PubMed] [Google Scholar]
- Yang E.J., Wilczynski W. Social experience organizes parallel networks in sensory and limbic forebrain. Dev. Neurobiol. 2007;67:285–303. doi: 10.1002/dneu.20347. doi:10.1002/dneu.20347 [DOI] [PubMed] [Google Scholar]
- Young L.J., Wang Z. The neurobiology of pair bonding. Nat. Neurosci. 2004;7:1048–1054. doi: 10.1038/nn1327. doi:10.1038/nn1327 [DOI] [PubMed] [Google Scholar]