Abstract
Intrauterine inflammation has been implicated in developmental brain injuries, including the development of periventricular leukomalacia (PVL) and cerebral palsy (CP). Previous studies in our rat model of intrauterine inflammation demonstrated apoptotic cell death in fetal brains within the first 5 days after lipopolysaccharide (LPS) administration to mothers and eventual dysmyelination. Cysteine-containing, aspartate-specific proteases, or caspases, are proteins involved with apoptosis through both intracellular (intrinsic pathway) and extracellular (extrinsic pathway) mechanisms. We hypothesized that cell death in our model would occur mainly via activation of the extrinsic pathway. We further hypothesized that Fas, a member of the tumor necrosis factor receptor (TNFR) superfamily, would be increased and the death inducing signaling complex (DISC) would be detectable. Pregnant rats were injected intracervically with LPS at E15 and immunoblotting, immunohistochemical and immunoprecipitation analyses were performed. The presence of the activated form of the effector caspase (caspase-3) was observed 24 h after LPS administration. Caspase activity assays demonstrated rapid increases in (i) caspases-9 and -10 within 1 h, (ii) caspase-8 at 2 h and (iii) caspase-3 at 4 h. At 24 h after LPS, activated caspase-3+/Fas+ cells were observed within the developing white matter. Lastly, the DISC complex (caspase-8, Fas and Fas-associated Death Domain (FADD)) was observed within 30 min by immunoprecipitation. Apoptosis in our model occurs via both extrinsic and intrinsic pathways, and activation of Fas may play a role. Understanding the mechanisms of cell death in models of intrauterine inflammation may affect development of future strategies to mitigate these injuries in children.
Keywords: white matter disease, apoptosis, caspase, Fas, lipopolysaccharide
1. Introduction
Injury to the developing white matter of the brain, leading to the clinical syndromes of periventricular leukomalacia (PVL) and cerebral palsy (CP), has been associated with intrauterine inflammation and infection[12,17,40,47]. In these syndromes, maturing oligodendrocytes are injured and undergo apoptotic cell death[2,19,22]. Apoptosis can be initiated in response to cellular stress (intrinsic pathway), via transmission of the death signal through membrane receptors (extrinsic pathway) or a combination of the two pathways[1,6,43].
Apoptosis involves many cellular processes including the sequential activation of cysteine-containing, aspartate-specific proteases, caspases[11,36,50]. Two caspase cascades, initiated by either intracellular (intrinsic) or extracellular (extrinsic) stimuli lead to activation of additional, effector caspases that lead to DNA fragmentation, membrane blebbing and cell death. The intrinsic caspase cascade, initiated by reactive oxygen (ROS) or reactive nitrogen species (RNS), decreased oxygen supply and others, involves activation of caspase-9. In this cascade, procaspase-9, cytochrome c (released from injured mitochondria) and apoptosis protease activating factor-1 (Apaf-1) bind to form the apoptosome complex. When the apoptosome complex dimerizes, procaspase-9 is cleaved to its active form and this leads to activation of effector caspases. Classically, the extrinsic caspase cascade involves activation of caspases-8[43], while some studies have implicated caspase-10 as well[45]. Binding of circulating ligands to specific transmembrane receptors leads to intracellular aggregation of proteins within the cell called death domains. The receptor and death domain are then associated with procaspase-8 (or -10) and this death-induced signaling complex (DISC) leads to cleavage of the procaspase to its activated form. Activated caspase-8 or -10 then activates the effector caspase resulting in cell death. For many mammalian species and disorders, caspase-3 is the effector caspase that is activated caspases-8, -9 and -10. Activation of both the intrinsic and extrinsic caspase cascades has been described within brain injuries[5], but a thorough understanding of these pathways in either developmental brain injuries in children or in clinically-relevant models has yet to be demonstrated and may lead to novel therapeutic strategies.
We have developed a rat model of experimental intrauterine inflammation by administering lipopolysaccharide (LPS) into the cervix at a critical period of embryonic brain development (E15)[3,4]. We have previously demonstrated that this injury results in (i) increased apoptotic cell death (as evidenced by increased terminal dUTP nick end labeling (TUNEL) at early time points, (ii) dysmyelination in the developing corpus callosum (as manifested by a decrease of two oligodendrocyte-specific markers) at three weeks of life and (iii) increased expression of the pro-inflammatory cytokine tumor necrosis factor (TNF) within hours of our stimulus. TNF can bind to one of two receptors (TNFR1 and TNFR2) and this stimulus can, under certain conditions, lead to cell death or cell proliferation of oligodendrocyte progenitors in vitro. Activation of another member of the TNF superfamily of receptors, the transmembrane receptor Fas, leads only to cell death by binding of intracellular death domains with procaspases and the apoptosome complex. Activation of Fas, by binding of Fas with its ligand (FasL), occurs during normal development aw well as in brain injuries and systemic immune responses.
We hypothesized that our inflammatory stimulus causes apoptotic cell death via the caspase activation. Specifically, we hypothesized that cell death via activation of caspase-3 would be present after our inflammatory stimulus and we sought to demonstrate the contribution of both the extrinsic and intrinsic caspase cascades (through activation of caspases-8, -10 and caspase-9, respectively). We further hypothesized that our inflammatory stimulus would increase expression of Fas within the brain and that this activation would associated with the formation of the death-domain complex.
2. Results
Activation of Caspases
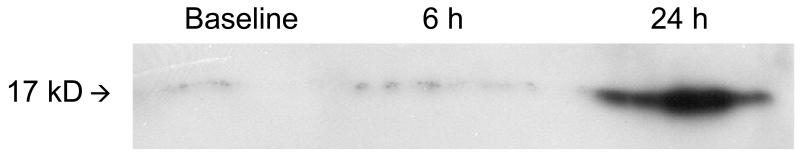
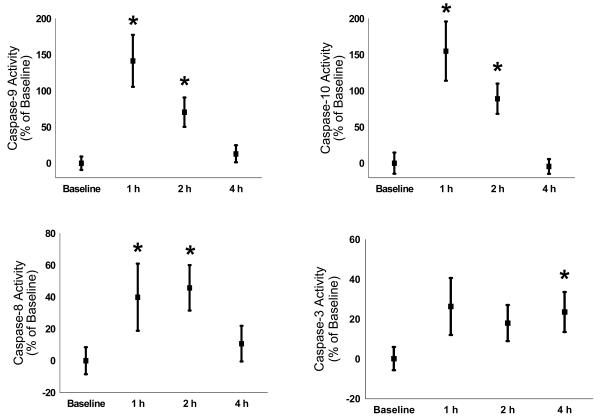
Using immunohistochemistry, we found that activated caspase-3 is expressed in protein homogenates of LPS brains (n = 9 per time point) by 24 h but not at 6 h (see Figure 1 for representative immunoblot). In performing immunoblotting for caspases-8, -9, and -10, we were observed multiple bands representing cleaved fragments of the procaspases making definitive interpretation difficult. Therefore, we performed caspase activity assays to determine the time course of activation of these proteins. We observed that the activity of caspase-9 and caspase-10 were maximal at 1 h after LPS (n = 20 per time point from 4 different mothers; increase from baseline ± SEM; caspase-9: 1 h – 141 ± 35%, 2 h – 70 ± 20%, 4 h – 13 ± 11%; caspase-10: 1 h – 155 ± 40%, 2 h – 89 ± 21%, 4 h - −5 ± 10%; p < 0.05 for 1 h and 2 h for both caspase-9 and -10, see Figure 2). Caspase-8 activity, while increased greater than baseline at 1 h, was maximal at 2 h after LPS administration (1 h – 40 ± 21%, 2 h – 46 ± 14%, 4 h – 10 ± 11%; p < 0.05 for 1 h and 2 h, see Figure 2). For the effector caspase, caspase-3, the activity was increased greater than control only at 4 h after LPS administration (1 h – 26 ± 15%, 2 h – 18 ± 11%, 4 h – 24 ± 9%; p < 0.05 only for 4 h, see Figure 2).
Figure 1.
Representative immunoblot demonstrating that activated caspase-3 is detectable at 24 h after LPS administration.
Figure 2.
Caspase activities in brain homogenates increased following LPS administration with caspase-9 and caspase-10 activity maximal at 1 h after LPS (increase from baseline ± SEM; caspase-9: 1 h – 141 ± 35%, 2 h – 70 ± 20%, 4 h – 13 ± 11%; caspase-10: 1 h – 155 ± 40%, 2 h – 89 ± 21%, 4 h - −5 ± 10%; p < 0.05 for 1 h and 2 h for both caspase-9 and -10), caspase-8 activity maximal at 2 h (1 h – 40 ± 21%, 2 h – 46 ± 14%, 4 h – 10 ± 11%; p < 0.05 for 1 h and 2 h) and caspase-3 maximal at 4 h (1 h – 26 ± 15%, 2 h – 18 ± 11%, 4 h - 24 ± 9%; p < 0.05 only for 4 h).
Effect of LPS on Fas Expression
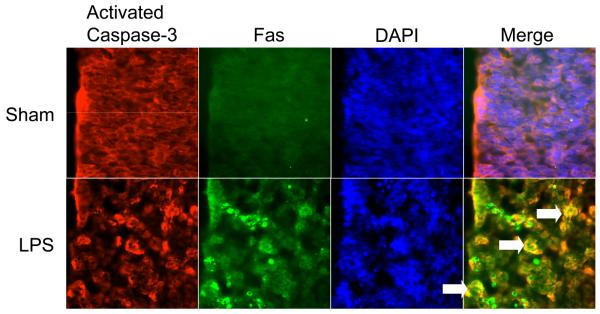
Immunohistochemically, we found Fas and activated caspase-3 expression within the developing subventricular zone 24 h after LPS administration but not in sham injected animals (n = 9 per experimental group from 3 different mothers, 3 slides per pup were analyzed to survey the majority of the subventricular zone, see Figure 3 for representative photomicrographs). In sham animals, we found only non-specific staining of secondary antibodies while co-localized Fas+/activated Caspase-3+ cells were seen extensively after LPS administration (double labeled cells indicated by white arrows in LPS panel).
Figure 3.
Representative micrographs of the developing white matter that were immunostained for Fas and activated caspase-3. At 24 h after LPS administration, activated caspase-3+/Fas+ cells are demonstrable within the developing white matter, which is not observed in shams.
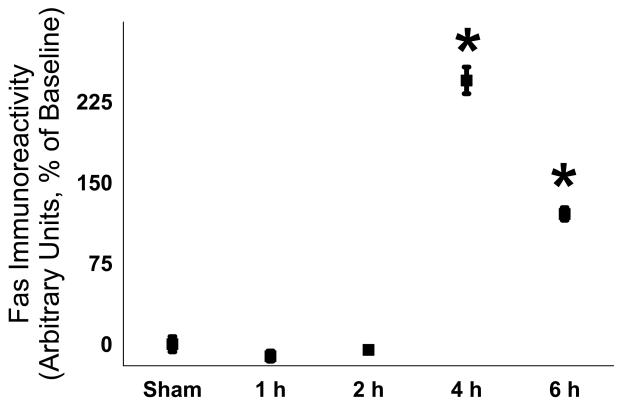
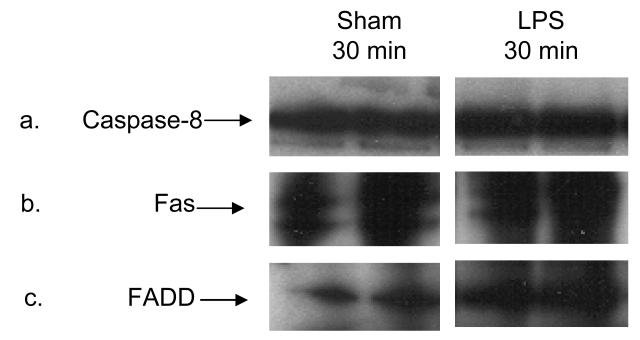
To quantify the temporal effect of LPS administration on Fas expression, we determined that Fas protein in brain homogenates. Fas protein was detected by immunoblotting and was increased after 4 h and remained so for up to 24 h (n = 9 per time point from 3 different mothers; 1 h - −11 ± 5%, 2 h - −5 ± 2%, 4 h – 245 ± 12%, 6 h – 120 ± 6%; p < 0.05 for 4 h and 6 h, see Figure 4). To determine if the Fas-associated death complex (DISC) was assembled, we sequentially immunoprecipitated caspase-8, Fas and FADD after our stimulus. After sham injection of saline as well as after LPS, caspase-8 was readily detectable on the gel for both sham and LPS conditions and Fas co-localized with caspase-8 in both conditions as well (n = 6 for each group, representative immunoblot shown as Figure 5). However, FADD co-localized with caspase-8 with much greater intensity only in the LPS treated group.
Figure 4.
LPS caused increased expression of Fas in brain homogenates (n = 9 per time point from 3 different mothers; 1 h - −11 ± 5%, 2 h - −5 ± 2%, 4 h – 245 ± 12%, 6 h – 120 ± 6%; p < 0.05 for 4 h and 6 h).
Figure 5.
The Fas DISC complex (caspase-8, Fas and Fas-associated death domain (FADD)) is detectable 30 min after LPS administration compared to sham by serial immunoprecipitation with caspase-8, then Fas and finally FADD (greater intensity of immunostaining in panel c of LPS, not quantified).
3. Discussion
We found that our model of perinatal brain injury causes (i) activation of caspases from both the intrinsic and extrinsic pathway within hours, (ii) activation of the effector caspase-3 in brains and within the developing white matter at 24 h and (iii) an increase in Fas receptor expression and assembly of the Fas-DISC complex. Since both intrinsic and extrinsic caspase pathways are activated, protective strategies must be developed to mitigate intracellular stresses and extracellular signals from ligand/receptors such as FasL/Fas and TNF/TNFR in our model, which may indicate that both pathways may need to be targeted in children with intrauterine inflammation/infection.
Caspases and Brain Injury
Caspase activation is an integral process of programmed cell death during development[7,23,26,46], during various brain injuries[10,15,48,49] as well as hypoxia and ischemia – processes believed to be at least as contributive to perinatal brain injury as inflammation. In mature animals, Namura and colleagues showed that activated caspase-3 protein was detected within 12 h after stroke while peak caspase-3 activity was observed within 1 h[30]. In a similar model, Benchoua and colleagues demonstrated that caspase-8 activation occurs in the acute time periods after stroke while delayed cell death (after 24 h) occurs concurrently with activation of caspase-9, suggesting differential activation of the two caspase cascades[5]. In a perinatal stroke model, Northington and colleagues found cell death was mediated through caspase-8 (within 3 h) and caspase-3 (within 24 h)[31] while Feng and colleagues demonstrated that caspase-8 activity increased within 12 h after injury[14]. Moreover, caspase-8 inhibition led to diminished stroke volume and improved functional outcome in this model. Lastly, in newborn rats exposed to 100% N2 for 20 minutes, Grojean and colleagues found caspase-3 activation at 3 d after injury[18]. In general, all these studies generally showed a time course with some similarity to our model (although many did not test at our earliest time points), with only the Namura study demonstrating a more rapid activation of caspase-3. This might be expected since our inflammatory stimulus necessarily needs to traverse the placenta-fetal barrier and then damage the developing brain. The other models also generally only investigated a single caspase cascade and did not investigate early time points. From their data, it appears that the extrinsic caspase cascade (via activation of caspase-8) appears to be important after stroke/hypoxia.
In inflammatory-based perinatal brain injury models, caspase-3 activation has been extensively demonstrated but evidence for the initiation of the cascade is not as extensively studied. In guinea pigs, Patrick and colleagues demonstrated increased numbers of activated caspase-3 cells at 48 – 72 h after inoculation of bacteria at 70% gestation[34]. In rats, Cai demonstrated activated caspase-3 cells within 24 h after intraventricular injection of LPS into neonatal brain[8]. Finally, several groups have demonstrated caspase-3 expression in pups on days 1 after LPS administration to pregnant females[33,39]. However, our data is the first to demonstrate that the initiator caspases are activated prior to the detection of the effector caspase (caspase-3). It will be important to determine if modulation of the actions of the two pathways leads to better functional or histological outcome in future studies.
Fas and Brain Injury
Similar to caspases, Fas-mediated apoptosis has also been demonstrated in both normal development and during various injuries including hypoxia/ischemia. In spinal cord ischemia, Matsushita and colleagues found that (i) Fas expression was increased within hours, (ii) caspase-8 was cleaved within 1.5 h and (iii) the Fas DISC complex was detectable in these early time periods[29]. These findings are strikingly similar to ours and indicate that Fas-related mechanisms of cell death may be common between these two models. Northington and colleagues, in the paper cited above, also demonstrated that perinatal hypoxia/ischemia led to 300% increase in Fas expression within the thalamus that preceded the cleavage of caspases[31]. Again, these findings are quite similar to ours regarding both caspase and Fas expression. In contrast, while Jin and colleagues found Fas expression increased after global cerebral ischemia, they found that the Fas DISC complex was associated with caspase-10, instead of caspase-8[21]. Finally, several groups have reported that Fas-knockout mice are protected from hypoxia/ischemia in mature and perinatal animals, respectively[16,27,28].
We are the first group to demonstrate Fas-related changes in an inflammation-based, perinatal brain injury model. In other brain models of inflammation, the results support the hypothesis that Fas-mediated mechanisms are is associated with worsening symptoms or neurological scores. Peripherally-administered LPS led to increased Fas expression within the thalamus for up to 2 weeks but the rats suffered no documented neurological sequelae[42]. Administration of FasL to mice with experimental autoimmune encephalomyelitis (EAE), an inflammatory-demyelinating disease, resulted in worse symptoms and prolonged recovery[9]. Using knockout mice for Fas (strain lpr), the anticipated neuroprotective effects without the death-mediating receptor were more equivocal. Lpr mice demonstrated decreased mortality in encephalitis[25] and decreased susceptibility to EAE[32]. However, lpr mice were not protected from bacterial meningitis[35] so it is clear that Fas is not the only mechanism of cell damage related to inflammation.
The role of Fas activation in human development and brain diseases has emerged over the past decade. Fas is detectable in fetuses at 9 to 10 weeks gestation and in vitro studies demonstrate the receptor-cell death machinery is functional at that time[24]. FADD is associated with severity of injury in patients with Parkinson's disease[20], Fas and FasL are readily detectable in senile plaques in patients with Alzheimer's Disease[41] and the Fas DISC complex is detectable after brain trauma[38]. In children, Fas was increased in the cerebrospinal fluid of children who developed PVL[13] and was detectable in the brains of children with severe perinatal asphyxia[44]. Taken as a whole, it appears that studying Fas-related mechanisms of injury in animal models may prove fruitful in improving our understanding of developmental brain injuries in children.
Limitations
Our study has several limitations. While we observed the various caspases activated within the first few hours after LPS, we did not localize caspases-8, -9 and -10 directly within the white matter as we did with activated caspase-3. Without specific antibodies for activated products of caspases-8, -9 and -10, we could not identify the cellular location of these processes. We considered microdissecting only the white matter of developing fetuses to determine if there was a regional localization to the caspase activation. However, because of the small size of the developing white matter and the possibility of introducing sampling bias, we instead chose whole brain homogenates for the activity assays. In making this decision, we obviously decreased the sensitivity of our assays since unaffected regions would be included in the homogenates along with affected regions, yet we still found significant differences. This decision may also explain why the standard error bars for the assays are relatively broad and there is a non-significant increase in caspase-3 activity at early time periods (especially when compared to the vigorous increase in the initiator caspases). Additionally, we did not attempt to mitigate the roles of either Fas or the intrinsic caspase cascade in order to determine if this therapy would diminish apoptosis. We believe that this goal is important, but could be accomplished by using wild-type and mutant mice such as the lpr strain or ones deficient in caspases with more definitive results.
In conclusion, this is the first study to demonstrate the roles of intrinsic and extrinsic caspase cascade as well as the role Fas-mediated apoptosis in an inflammation-based perinatal injury model. Our data suggest that both intrinsic caspase activation and extrinsic cellular signaling pathways may need to be mitigated for optimal neuroprotection after inflammation. Determining the role of these pathways in human perinatal injuries may lead to significant advances in our knowledge of cell death in infants exposed to intrauterine inflammation.
4. Experimental Procedures
Experimental protocol
All experiments were performed with the approval of the Institutional Animal Care and Use Committee of Children's Research Institute of Children's National Medical Center. Pregnant rats were studied at E15 using a protocol we have previously described[3]. Briefly, rats were anesthetized with 4% isoflurane, the cervix was visualized, and 0.1 mg/kg of LPS was administered in a mixture of 0.1 ml of saline. In pilot experiments to develop our model, we found that the cervical wall could be easily visualized using a surgical microscope and a small speculum. We found that we could reliably inject 0.1 mL of dye (Evans blue) within the cervical muscle with high reliability and without leakage into the surrounding tissue. Once injected with LPS, rats recovered from anesthesia and the procedure within 2 min of induction. We have previously established that this dose of LPS is associated with minimal fetal mortality (4%) when animals are allowed to survive to delivery. At a designated time after the injection, rats were again anesthetized with 4% isoflurane. A laparotomy was performed and the uterus exposed. Fetal sacs were isolated and fetal brains were processed based on the procedures outlined below.
Protein Concentration Determination
For further experiments, protein concentrations of sample supernatants were determined using reagents from BCA following the directions from the manufacturer (Pierce Chemical, Rockford, IL). In brief, 2 μL of supernatant was mixed with 48 μL of deionized water and 1 mL of the BCA protein assay mixture. Standards were prepared similarly using BSA as a protein source. Samples were heated to 60°C in a water bath for 30 min and protein concentrations were determined using a spectrophotometer (Beckman DU Series 500, Beckman Coulter, Inc., Fullerton, CA) at 562 nm.
Immunoblotting
Brains were dissected and placed in 600 μL of iced lysis buffer (RIPA buffer containing 50 mM Tris-HCl, 1% NP-40, 0.25% Na-deoxycholate, 150 mM NaCl, and 1 mM EDTA; recipe from Upstate, Charlottesville VA). The tissue was dissociated, sonicated and centrifuged for 10 minutes at 14,000 rpm at 4°C. Supernatants were collected and stored at −80°C until analysis. Protein concentrations were determined as described above and 50 μg of total brain protein per lane was separated by SDS-PAGE electrophoresis on a 4-20% gel (Bioexpress, Kayesville UT). Proteins were transferred to a PVDF membrane and probed with a variety of antibodies (anti-cleaved-caspase-3, anti-Fas at 1:5000 (BD Biosciences, San Jose, CA) and anti-actin at 1:5000 (Chemicon, Temecula, CA)). Immunoblots were probed with the appropriate monoclonal antibody in 4% milk overnight at 4°C. Following washing in Tris buffered saline (Biosource, Camarillo CA) with 0.1% Tween-20 (Sigma Aldrich, Milwaukee WI), blots were probed with an appropriate horseradish-peroxidase conjugated antibody and developed chemiluminescently (ECLplus, Amersham Biosciences, Piscataway, NJ). Bands were quantified with commercially available software (Image-J, NIH).
Caspase Activity Assays
Activity of caspases-3, 8, -9 and -10 were determined using commercially available kits (Calbiochem, SanDiego CA) following manufacturer's instructions. Briefly, brains were placed in 400 μL of an iced lysis buffer provided by the manufacturer. The tissue was then dissociated, sonicated, and centrifuged at 14,000 rpm at room temperature and supernatants were stored at −70°C until analysis. Protein concentrations were determined as above and 300 to 400 μg of protein was exposed to a caspase-specific substrate that was conjugated to fluorometrically active AFC. The signal was measured on a Cytofluor 4000 spectrofluorometer (Applied Biosystems, San Diego, CA) at an excitation wavelength of 400/30 and emission wavelength of 508/20. All experiments were carried out in a 96 well microplate at 37°C. For caspase assay specificity was confirmed with the appropriate recombinant caspase as a positive control. Additionally, caspase inhibitor was added to both recombinant caspase and unknown samples, resulting in complete absence of activity in all cases (data not shown). Additional negative controls (absence of substrate and absence of any unknown sample) similarly resulted in absence of detection or signal (data not shown).
Immunohistochemical Assessment
At 24 h after LPS or sham injection, the mother rats were re-anesthetized and a hysterotomy was performed. Fetal brains were immersion fixed in buffered 4% paraformaldehyde overnight and frozen in methylbutane. Brains were sectioned on a cryostat at 10 μm and sections including the subventricular zone were chosen for analysis. Slides were boiled for 10 min in citrate solution and blocking serum (5%) was applied. Anti-Fas (1:200, BD Biosciences) and anti-activated caspase-3 (1:100, Cell Signalling, Beverly, MA) antibody was applied overnight at 4°C followed by a fluorescently-conjugated secondary antibody for 1 h at 37°C.
Immunoprecipitation of DISC Complex
In order to detect if caspase-8, Fas, and Fas-associated death domain became bound after our stimulus, we performed immunoprecipitation experiments as previously described with some modifications[37]. Briefly, fetal brains were dissociated and sonicated in buffer (20 mM Tris-HCl, 140 mM NaCl, 1% Triton X-100, 2 mM EDTA, 1 mM p-amidinophenyl methanesulfonyl fluoride hydrochloride, 50 mM NaF, 0.7 μM aprotinin and 10% glycerol). Suspensions were centrifuged at 14,000 rpm at 4°C and protein concentration of the supernatant was determined as previously described. Samples were pre-cleared in Protein G Plus-Agarose (Santa Cruz Technologies, Santa Cruz, CA) and normal mouse antibody, and immunoprecipitated overnight at 4°C with anti-caspase-8 antibody (Santa Cruz Technologies). Following addition of Protein-G Agarose for 2 h at 4°C and washing twice with wash buffer (50 mM Tris-HCl, 0.1% SDS, 0.5% deoxycorticosterone, 1% NP-40 and 62.5 mM NaCl), the complexes were dissociated by boiling for 5 min. Protein was then separated by SDS-PAGE electrophoresis on a 4-20% gel, transferred to a PVDF membrane and probed with antibodies against Fas and FADD.
Statistical Analysis
For immunoblotting experiments, band intensities (standardized for actin) were compared to baseline conditions and are reported in relative units. The individual caspase activities are expressed as a percent change from baseline and reported as activated fluorescent units (AFU). Data between time points were compared by One-Way Anova with Dunn's correction (Sigma Stat, SPSS). Statistical significance was defined as p < 0.05.
Acknowledgments
The author (MJB) was supported by K08 HD044716 and a grant from United Cerebral Palsy.
Abbreviations
- CP
Cerebral palsy
- LPS
Lipopolysaccharide
- PVL
Periventricular leukomalacia
- ROS
Reactive oxygen species
- RNS
Reactive nitrogen species
- TNF
Tumor necrosis factor
- TUNEL
terminal dUTP nick end labeling
- FADD
Fas-associated death domain
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Annunziato L, Amoroso S, Pannaccione A, Cataldi M, Pignataro G, D'Alessio A, Sirabella R, Secondo A, Sibaud L, Di Renzo GF. Apoptosis induced in neuronal cells by oxidative stress: role played by caspases and intracellular calcium ions. Toxicol Lett. 2003;139:125–133. doi: 10.1016/s0378-4274(02)00427-7. [DOI] [PubMed] [Google Scholar]
- 2.Back S, Luo N, Borenstein N, Levine J, Volpe J, Kinney H. Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. J Neurosci. 2001;21:1302–1312. doi: 10.1523/JNEUROSCI.21-04-01302.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bell M, Hallenbeck J. Effects of intrauterine inflammation on developing rat brain. J Neurosci Res. 2002;70:570–579. doi: 10.1002/jnr.10423. [DOI] [PubMed] [Google Scholar]
- 4.Bell M, Hallenbeck J, Gallo V. Determining the fetal inflammatory response in an experimental model of intrauterine inflammation in rats. Pediatr Res. 2004;56:541–546. doi: 10.1203/01.PDR.0000139407.89883.6B. [DOI] [PubMed] [Google Scholar]
- 5.Benchoua A, Guegan C, Couriaud C, Hosseini H, Sampaio N, Morin D, Onteniente B. Specific caspase pathways are activated in the two stages of cerebral infarction. J Neurosci. 2001;21:7127–7134. doi: 10.1523/JNEUROSCI.21-18-07127.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boatright KM, Salvesen GS. Mechanisms of caspase activation. Curr Opin Cell Biol. 2003;15:725–731. doi: 10.1016/j.ceb.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 7.Boonman Z, Isacson O. Apoptosis in neuronal development and transplantation: role of caspases and trophic factors. Exp Neurol. 1999;156:1–15. doi: 10.1006/exnr.1999.7056. [DOI] [PubMed] [Google Scholar]
- 8.Cai Z, Pang Y, Lin S, Rhodes P. Differential roles of TNF and IL-1B in lipopolysaccharide-induced brain injury in the neonatal rat. Brain Res. 2003;975:37–47. doi: 10.1016/s0006-8993(03)02545-9. [DOI] [PubMed] [Google Scholar]
- 9.Ciusani E, Gelati M, Frigerio S, Pollo B, Massa G, Sacerdote P, Panerai AE, Salmaggi A. Modulation of experimental allergic encephalomyelitis in Lewis rats by administration of a peptide of Fas ligand. J Autoimmun. 2001;17:273–280. doi: 10.1006/jaut.2001.0554. [DOI] [PubMed] [Google Scholar]
- 10.Clark RS, Kochanek PM, Chen M, Watkins SC, Marion DW, Chen J, Hamilton RL, Loeffert JE, Graham SH. Increases in Bcl-2 and cleavage of caspase-1 and caspase-3 in human brain after head injury. Faseb J. 1999;13:813–821. doi: 10.1096/fasebj.13.8.813. [DOI] [PubMed] [Google Scholar]
- 11.Cornelis S, Kersse K, Festjens N, Lamkanfi M, Vandenabeele P. Inflammatory caspases: targets for novel therapies. Curr Pharm Des. 2007;13:365–383. doi: 10.2174/138161207780163006. [DOI] [PubMed] [Google Scholar]
- 12.Dammann O, Kuban K, Leviton A. Perinatal infection, fetal inflammatory response, white matter damage and cognitive limitations in children born preterm. Ment Retard Dev Disabil Res Rev. 2002;8:46–50. doi: 10.1002/mrdd.10005. [DOI] [PubMed] [Google Scholar]
- 13.Felderhoff-Mueser U, Buhrer C, Groneck P, Obladen M, Bartmann P, Heep A. Soluble Fas (CD95/Apo-1), soluble Fas ligand, and activated caspase 3 in the cerebrospinal fluid of infants with posthemorrhagic and nonhemorrhagic hydrocephalus. Pediatr Res. 2003;54:659–664. doi: 10.1203/01.PDR.0000084114.83724.65. [DOI] [PubMed] [Google Scholar]
- 14.Feng Y, Fratkin JD, LeBlanc MH. Inhibiting caspase-8 after injury reduces hypoxic-ischemic brain injury in the newborn rat. Eur J Pharmacol. 2003;481:169–173. doi: 10.1016/j.ejphar.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 15.Garden GA, Budd SL, Tsai E, Hanson L, Kaul M, D'Emilia DM, Friedlander RM, Yuan J, Masliah E, Lipton SA. Caspase cascades in human immunodeficiency virus-associated neurodegeneration. J Neurosci. 2002;22:4015–4024. doi: 10.1523/JNEUROSCI.22-10-04015.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Graham EM, Sheldon RA, Flock DL, Ferriero DM, Martin LJ, O'Riordan DP, Northington FJ. Neonatal mice lacking functional Fas death receptors are resistant to hypoxic-ischemic brain injury. Neurobiol Dis. 2004;17:89–98. doi: 10.1016/j.nbd.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 17.Grether JK, Nelson KB. Maternal infection and cerebral palsy in infants of normal birth weight. JAMA. 1997;278:207–211. [PubMed] [Google Scholar]
- 18.Grojean S, Schroeder H, Pourie G, Charriaut-Marlangue C, Koziel V, Desor D, Vert P, Daval JL. Histopathological alterations and functional brain deficits after transient hypoxia in the newborn rat pup: a long term follow-up. Neurobiol Dis. 2003;14:265–278. doi: 10.1016/s0969-9961(03)00082-2. [DOI] [PubMed] [Google Scholar]
- 19.Hargitai B, Szabo V, Hajdu J, Harmath A, Pataki M, Farid P, Papp Z, Szende B. Apoptosis in various organs of preterm infants: histologic study of lung, kidney, liver and brain of ventilated infants. Pediatr Res. 2001;50:110–114. doi: 10.1203/00006450-200107000-00020. [DOI] [PubMed] [Google Scholar]
- 20.Hartmann A, Mouatt-Prigent A, Faucheux BA, Agid Y, Hirsch EC. FADD: A link between TNF family receptors and caspases in Parkinson's disease. Neurology. 2002;58:308–310. doi: 10.1212/wnl.58.2.308. [DOI] [PubMed] [Google Scholar]
- 21.Jin K, Graham SH, Mao X, Nagayama T, Simon RP, Greenberg DA. Fas (CD95) may mediate delayed cell death in hippocampal CA1 sector after global cerebral ischemia. J Cereb Blood Flow Metab. 2001;21:1411–1421. doi: 10.1097/00004647-200112000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Kinney HC, Back SA. Human oligodendroglial development: Relationship to periventricular leukomalacia. Sem Ped Neurol. 1998;5:180–189. doi: 10.1016/s1071-9091(98)80033-8. [DOI] [PubMed] [Google Scholar]
- 23.Kuida K, Haydar TF, Kuan CY, Gu Y, Taya C, Karasuyama H, Su MS, Rakic P, Flavell RA. Reduced apoptosis and cytochrome c-mediated caspase activation in mice lacking caspase 9. Cell. 1998;94:325–337. doi: 10.1016/s0092-8674(00)81476-2. [DOI] [PubMed] [Google Scholar]
- 24.Lautrette C, Giraud S, Vermot-Desroches C, Preud'homme JL, Jauberteau MO. Expression of a functional Fas death receptor by human foetal motoneurons. Neuroscience. 2003;119:377–385. doi: 10.1016/s0306-4522(03)00034-4. [DOI] [PubMed] [Google Scholar]
- 25.Licon Luna RM, Lee E, Mullbacher A, Blanden RV, Langman R, Lobigs M. Lack of both Fas ligand and perforin protects from flavivirus-mediated encephalitis in mice. J Virol. 2002;76:3202–3211. doi: 10.1128/JVI.76.7.3202-3211.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marks N, Berg MJ. Recent advances on neuronal caspases in development and neurodegeneration. Neurochem Int. 1999;35:195–220. doi: 10.1016/s0197-0186(99)00061-3. [DOI] [PubMed] [Google Scholar]
- 27.Martin-Villalba A, Hahne M, Kleber S, Vogel J, Falk W, Schenkel J, Krammer P. Therapeutic neutralization of CD95-ligand and TNF attenuates brain damage in stroke. Cell Death Differ. 2001;8:679–686. doi: 10.1038/sj.cdd.4400882. [DOI] [PubMed] [Google Scholar]
- 28.Martin-Villalba A, Herr I, Jeremias I, Hahne M, Brandt R, Vogel J, Schenkel J, Herdegen T, Debatin K. CD95 ligand (FasL-APO1L) and Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand Mediate Ischemia-Induced Apoptosis in Neurons. J Neurosci. 1999;19:3809–3817. doi: 10.1523/JNEUROSCI.19-10-03809.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsushita K, Wu Y, Qiu J, Lang-Lazdunski L, Hirt L, Waeber C, Hyman BT, Yuan J, Moskowitz MA. Fas receptor and neuronal cell death after spinal cord ischemia. J Neurosci. 2000;20:6879–6887. doi: 10.1523/JNEUROSCI.20-18-06879.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Namura S, Zhu J, Fink K, Endres M, Srinivasan A, Tomaselli KJ, Yuan J, Moskowitz MA. Activation and cleavage of caspase-3 in apoptosis induced by experimental cerebral ischemia. J Neurosci. 1998;18:3659–3668. doi: 10.1523/JNEUROSCI.18-10-03659.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Northington FJ, Ferriero DM, Martin LJ. Neurodegeneration in the thalamus following neonatal hypoxia-ischemia is programmed cell death. Dev Neurosci. 2001;23:186–191. doi: 10.1159/000046141. [DOI] [PubMed] [Google Scholar]
- 32.Okuda Y, Bernard CC, Fujimura H, Yanagihara T, Sakoda S. Fas has a crucial role in the progression of experimental autoimmune encephalomyelitis. Mol Immunol. 1998;35:317–326. doi: 10.1016/s0161-5890(98)00049-2. [DOI] [PubMed] [Google Scholar]
- 33.Pang Y, Rodts-Palenik S, Cai Z, Bennett WA, Rhodes PG. Suppression of glial activation is involved in the protection of IL-10 on maternal E. coli induced neonatal white matter injury. Brain Res Dev Brain Res. 2005;157:141–149. doi: 10.1016/j.devbrainres.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 34.Patrick LA, Gaudet LM, Farley AE, Rossiter JP, Tomalty LL, Smith GN. Development of a guinea pig model of chorioamnionitis and fetal brain injury. Am J Obstet Gynecol. 2004;191:1205–1211. doi: 10.1016/j.ajog.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 35.Paul R, Angele B, Sporer B, Pfister HW, Koedel U. Inflammatory response during bacterial meningitis is unchanged in Fas- and Fas ligand-deficient mice. J Neuroimmunol. 2004;152:78–82. doi: 10.1016/j.jneuroim.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 36.Prunell GF, Arboleda VA, Troy CM. Caspase function in neuronal death: delineation of the role of caspases in ischemia. Curr Drug Targets CNS Neurol Disord. 2005;4:51–61. doi: 10.2174/1568007053005082. [DOI] [PubMed] [Google Scholar]
- 37.Qiu J, Whalen MJ, Lowenstein P, Fiskum G, Fahy B, Darwish R, Aarabi B, Yuan J, Moskowitz MA. Upregulation of the Fas receptor death-inducing signaling complex after traumatic brain injury in mice and humans. J Neurosci. 2002;22:3504–3511. doi: 10.1523/JNEUROSCI.22-09-03504.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qui J, Whalen M, Lowenstein P, Fiskum G, Fahy B, Darwish R, Aarabi B, Yuan J, Moskowitz M. Upregulation of the Fas receptor death-inducing signaling complex after traumatic brain injury in mice and humans. J Neurosci. 2002;22:3504–3511. doi: 10.1523/JNEUROSCI.22-09-03504.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rousset CI, Chalon S, Cantagrel S, Bodard S, Andres C, Gressens P, Saliba E. Maternal exposure to LPS induces hypomyelination in the internal capsule and programmed cell death in the deep gray matter in newborn rats. Pediatr Res. 2006;59:428–433. doi: 10.1203/01.pdr.0000199905.08848.55. [DOI] [PubMed] [Google Scholar]
- 40.Schendel D, Schuchat A, Thorsen P. Public health issues related to infection and pregnancy and cerebral palsy. Ment Retard Dev Disabil Res Rev. 2002;8:39–45. doi: 10.1002/mrdd.10011. [DOI] [PubMed] [Google Scholar]
- 41.Su JH, Anderson AJ, Cribbs DH, Tu C, Tong L, Kesslack P, Cotman CW. Fas and Fas ligand are associated with neuritic degeneration in the AD brain and participate in beta-amyloid-induced neuronal death. Neurobiol Dis. 2003;12:182–193. doi: 10.1016/s0969-9961(02)00019-0. [DOI] [PubMed] [Google Scholar]
- 42.Terrazzino S, Bauleo A, Baldan A, Leon A. Peripheral LPS administrations up-regulate Fas and FasL on brain microglial cells: a brain protective or pathogenic event? J Neuroimmunol. 2002;124:45–53. doi: 10.1016/s0165-5728(02)00013-9. [DOI] [PubMed] [Google Scholar]
- 43.Thorburn A. Death receptor-induced cell killing. Cell Signalling. 2004;16:139–144. doi: 10.1016/j.cellsig.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 44.van Landeghem FK, Felderhoff-Mueser U, Moysich A, Stadelmann C, Obladen M, Bruck W, Buhrer C. Fas (CD95/Apo-1)/Fas ligand expression in neonates with pontosubicular neuron necrosis. Pediatr Res. 2002;51:129–135. doi: 10.1203/00006450-200202000-00003. [DOI] [PubMed] [Google Scholar]
- 45.Wajant H. The Fas signaling pathway: more than a paradigm. Science. 2002;296:1635–1636. doi: 10.1126/science.1071553. [DOI] [PubMed] [Google Scholar]
- 46.Wang J, Lenardo MJ. Roles of caspases in apoptosis, development, and cytokine maturation revealed by homozygous gene deficiencies. J Cell Sci. 2000;113(Pt 5):753–757. doi: 10.1242/jcs.113.5.753. [DOI] [PubMed] [Google Scholar]
- 47.Wu Y, Colford J. Chorioamnionitis as a risk factor for cerebral palsy: A meta-analysis. JAMA. 2000;284:1417–1424. doi: 10.1001/jama.284.11.1417. [DOI] [PubMed] [Google Scholar]
- 48.Yakovlev AG, Faden AI. Caspase-dependent apoptotic pathways in CNS injury. Mol Neurobiol. 2001;24:131–144. doi: 10.1385/MN:24:1-3:131. [DOI] [PubMed] [Google Scholar]
- 49.Yakovlev AG, Ota K, Wang G, Movsesyan V, Bao WL, Yoshihara K, Faden AI. Differential expression of apoptotic protease-activating factor-1 and caspase-3 genes and susceptibility to apoptosis during brain development and after traumatic brain injury. J Neurosci. 2001;21:7439–7446. doi: 10.1523/JNEUROSCI.21-19-07439.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang F, Yin W, Chen J. Apoptosis in cerebral ischemia: executional and regulatory signaling mechanisms. Neurol Res. 2004;26:835–845. doi: 10.1179/016164104X3824. [DOI] [PubMed] [Google Scholar]