Abstract
Adenosine A2A receptors are involved in the regulation of several behavioral functions. Adenosine A2A antagonists exert antiparkinsonian effects in animal models, and adenosine A2A agonists suppress locomotion and impair various aspects of motor control. The present experiments were conducted to study the effects of low doses of the adenosine A2A agonist CGS 21680 on lever pressing, specific parameters of food intake, and sedation. In the first experiment, the effects of CGS 21680 on fixed ratio 5 lever pressing were assessed. In the second experiment, rats were tested in 30 min feeding sessions, and also were observed for drug-induced sedation using a sedation rating scale. CGS 21680 (0.025, 0.05, 0.1 mg/kg IP) produced a dose related suppression of lever pressing, and also reduced the amount of food consumed. The feeding effect was largely dependent upon a slowing of the rate of feeding, and there was only a modest suppression of time spent feeding. Doses of CGS 21680 that suppressed lever pressing and feeding also were associated with sedation/drowsiness. In conjunction with other studies, the present results suggest that sedative effects may play an important role in some of the behavioral effects produced by systemic administration of adenosine A2A agonists.
Keywords: Motor, Motivation, Sleep, Operant, Basal Ganglia
1. Introduction
Over the last few years, interest in the behavioral significance of adenosine receptor function has grown dramatically. Minor stimulants such as caffeine, theophylline and theobromine are known to act as relatively non-selective adenosine antagonists. Although there are at least four types of adenosine receptors, adenosine A2A receptors are primarily localized in striatal regions (DeMet and Chicz-DeMet, 2002; Jarvis and Williams, 1989), especially on the dendritic spines of GABAergic striatopallidal neurons (Ferré et al., 2004; Schiffmann et al., 1991). Considerable evidence indicates that there is a functional interaction between DA and adenosine A2A receptors in both dorsal and ventral striatal areas (Chen et al., 2001; Hettinger et al., 2001; Svenningsson et al., 1999; Wang et al., 2000). This interaction often has been studied in the context of animal models related to parkinsonism, which typically focus on neostriatal motor functions (Ferré et al., 1997, 2001; Hauber et al., 2001; Ishiwari et al., 2007; Jenner, 2003, 2005; Morelli and Pinna, 2001; Pinna et al., 2005; Svenningsson et al., 1999). In these studies, adenosine A2A receptor antagonists have been shown to exert effects consistent with antiparkinsonian actions in animal models (Correa et al., 2004; Ferré et al., 1997, 2001; Hauber et al., 2001; Pinna et al., 2005; Wardas et al., 2001). Based upon the results of these animal studies, adenosine A2A receptor antagonists are now being evaluated for their antiparkinsonian effects in human clinical trials (Jenner, 2005). In addition to this involvement in motor function, adenosine A2A receptors also are thought to be involved in other behavioral functions. For example, it was recently demonstrated that the adenosine A2A antagonist MSX-3 could reverse the effect of haloperidol on a concurrent lever pressing/feeding task that measures aspects of motivation related to response allocation and effort-related choice behavior (Farrar et al., 2007). Further studies have implicated adenosine A2A receptors in cognitive function (Takahashi et al., 2008) and sleep (Hong et al., 2005; Porkka-Heiskanen et al., 2000; Satoh et al., 1998; Scammell et al., 2001; Stenberg, 2007).
In addition to the pharmacological interaction between A2A and D2 receptors, there also is evidence indicating that adenosine A2A agonists can produce effects that resemble those produced by DA antagonists or DA depletions (Ferré, 1997). For example, intraventricular administration of the adenosine A2A receptor agonist CGS 21680 inhibited acquisition and expression of wheel running behavior (Cabeza de Vaca et al., 2007). CGS 21680 depressed locomotor activity when infused directly into the nucleus accumbens (Barraco et al., 1993; Hauber and Munkel, 1997). Stimulation of adenosine A2A receptors with high doses of CGS 21680 also was shown to induce catalepsy (Wardas et al., 2003). Although it seems clear that stimulation of adenosine A2A receptors can suppress motor activity, less is known about the effects of low doses of adenosine A2A agonists on other aspects of behavioral function. Based upon studies with adenosine A2A antagonists, it has been suggested that adenosine A2A receptors could be involved in reserpine-induced behavioral depression in rats (Minor et al., 2003), motor readiness (O’Neill and Brown, 2006), cocaine reinstatement (Weerts and Griffiths, 2003), and effort-related choice behavior (Farrar et al., 2007). Nevertheless, relatively little is known about the effects of adenosine A2A agonists on food-motivated behavior. The present studies were undertaken to investigate the effects of systemic administration of the adenosine A2A agonist CGS 21680 on food-reinforced lever pressing and feeding behavior. In the first experiment, the effects of CGS 21680 on operant responding were assessed. The fixed ratio 5 (FR5) lever pressing schedule was used because it generates a high rate of responding (i.e. greater than 1000 lever presses in 30 min) that is very sensitive to the response suppressant properties of drugs (Chuck et al., 2006; Salamone et al., 1993a). This schedule has been shown to be highly sensitive to the rate-decreasing effects of several classes of drugs, including DA antagonists (Salamone et al., 1993a, 1996, 2002), the acetylcholinesterase inhibitor tacrine (Carriero et al., 1998), cannabinoid CB1 agonists (Arizzi et al., 2004; Carriero et al., 1998; McLaughlin et al., 2005a) and ethanol (Chuck et al., 2006). In the second experiment, rats were observed in 30 min sessions that allowed for the measurement of food intake, time spent feeding, and feeding rate. This type of measurement of feeding behavior has been used previously by our laboratory to assess the effects of DA antagonists (Salamone et al., 1990), striatal DA depletions (Salamone et al., 1993b), and cannabinoid CB1 antagonists (McLaughlin et al., 2005b). As well as being assessed for aspects of feeding behavior, rats in the second experiment also were observed for drug-induced sedation using a sedation rating scale (Chuck et al., 2006; Salamone et al., 1996). Sedation was examined in experiment 2 because of the considerable body of evidence indicating that stimulation of adenosine A2A receptors could induce sedative effects, torpor, and drowsiness (Hong et al., 2005; Porkka-Heiskanen et al., 2000; Satoh et al., 1998; Scammell et al., 2001; Stenberg, 2007), and also because sedative effects of CGS 21680 were noted during experiment 1 (see below). It is important to examine these sedative effects because of the possibility that drug-induced sedation is an important factor in the suppression of lever pressing or feeding produced by systemic administration of adenosine A2A receptor agonists.
2. Methods
2.1. Subjects
Male Sprague–Dawley rats (Harlan Sprague–Dawley, Indianapolis, IN) weighing between 300–360 g at the beginning of the study (n=16), were housed in a colony maintained at 23 °C with a 12h light/dark cycle (lights on at 08:00 h). Rats were food restricted to 12 g food per day prior to training, and throughout the experiment animals received supplemental food (up to 12 g a day) and allowed modest growth. Water was available ad libitum in the home cages at all times. Experimental methods were in accordance with the Guide for the Care and Use of Laboratory Animals, National Research Council, National Academy Press, 1996.
2.2. Drugs
CGS 21680 was purchased from Tocris (Ellisville, Missouri). CGS 21680 was dissolved in 2% dimethyl sulfoxide solution (DMSO, Fisher Scientific, Hampton, New Hampshire, USA). This solution also served as the vehicle control.
2.3. Behavioral procedure — acquisition phase of operant behavior
The lever pressing experiment was conducted in operant chambers (28 × 23 × 23 cm; Med Associates) that contained one lever and a food magazine that was recessed into the wall of the chamber to the right of the lever. Animals were initially trained to lever press for 4 days (30 min sessions; 45 mg pellets, Bioserve Inc., Frenchtown, NJ) on a fixed ratio (FR) of 1 schedule of reinforcement. In this schedule for each lever press the animals receive one operant pellet (45 mg pellets, Bioserve Inc., Frenchtown, NJ). After this initial training, the animals were trained on a FR 5 schedule (30 min sessions, 5 days/week) for 4 additional weeks.
2.4. Behavioral procedure — acquisition of feeding behavior
Animals were trained to eat lab chow in an observation test chamber for 3 weeks before testing. Animals were allowed to eat the pre-weighed food for 30 min. The test chamber had wire-mesh floor that allowed for collection of spillage after each session. Food was weighed before and after each session, and sufficient food was provided to allow for ad-lib feeding during the session (16 to 19 g). Intake was defined as the difference between pre- and post-session food weight, including spillage, which was collected on paper sheets below the wire-mesh floor of the test chamber.
2.4.1. Experiment 1: effects of systemic administration of the selective adenosine A2A agonist CGS 21680 on FR5 lever-pressing behavior
All animals were tested after 4 weeks of training on the FR 5 schedule of reinforcement as described above. For this and all the following experiments, the 2% DMSO vehicle solution (see above) was also used as the vehicle control treatment. Rats (n=8) received i.p. injections of the following doses of CGS 21680: vehicle, 0.025, 0.05, 0.1 mg/kg. This experiment used a within-groups design, with all rats receiving all drug treatments in a randomly varied order (one treatment per week). Baseline training (i.e., non-drug) sessions were conducted four additional days per week. All injections were given 15 min before the animals were put in the in operant chambers for a 30 min session.
2.4.2. Experiment 2: effects of systemic administration of the selective adenosine A2A agonist CGS 21680 on feeding behavior and sedation
A separate group of rats was trained to eat lab chow in the test chambers for 30 min as previously described. On the test day, all animals received i.p. injections of the following doses of CGS 21680: vehicle, 0.025, 0.05 and 0.1 mg/kg (n=8). This experiment used a within-groups design, with all rats receiving all drug treatments in a randomly varied order (one treatment per week using a Latin-square design). Baseline training (i.e., non-drug) sessions were conducted four additional days per week. All injections were given 15 min before the animals were put in an observation test chambers with pre-weighed amounts of lab chow. During this test phase an observer blind to treatment manipulated a computer-controlled timing program. Observers depressed a switch while subjects were either eating or engaged in nonvacuous chewing (i.e., chewing initiated with pellet contact), and released the lever when subjects ceased eating or chewing. Temporal recording was controlled via a custom-written program in QBasic with a resolution of 1 s. Following a 30-minute session, remaining food and spillage was collected and weighed. Differences between pre- and post-session weights were taken as a measure of food intake. Feeding rate was calculated as food intake (g) divided by time spent feeding (min). These behavioral methods are similar to those used previously to study antagonists of DA or CB1 cannabinoid receptors (McLaughlin et al., 2005b; Salamone et al., 1990).
During the 30-min sessions the blind observer also assessed the behavior of the animals, and assigned the numerical score according to a Sedation Rate Scale previously described in Chuck et al. (2006). Briefly, the Sedation Rating Scale consisted of a 6-point scale ranging from 0 to 5. The ratings were as follows: 5—awake, active: engaged in locomotion, rearing, head movements or grooming; 4—awake, inactive: eyes fully open, head up, little to no locomotion, rearing or grooming, normal posture; 3—mild sedation: eyes partly closed, head somewhat down, impaired locomotion including abnormal posture, use of only some limbs, paw dragging and stumbling; 2—moderate sedation: head mostly or completely down, eyes partly closed, flattened posture, no spontaneous movement; 1—heavy sedation: eyes mostly closed, flattened posture, head down, no spontaneous movement; 0—asleep: eyes fully closed, body relaxed, asleep. In a reliability test, two independent observers who rated these behaviors in the same animal showed >90% agreement on the specific ratings.
2.5. Data analysis
The total number of lever presses and the feeding measures (food consumption, time spent feeding, feeding rate) were analyzed with repeated measures analysis of variance (ANOVA). Non-orthogonal planned comparisons using the overall error term were used, with the number of comparisons being restricted to the number of treatments minus one (Keppel, 1991). In addition, the lever pressing and feeding data were analyzed using a nonlinear regression analysis (GraphPad Prism version5). This method was used to estimate the effective dose 50 (ED50) and provide 95% confidence interval values. The dose–response curve was fit to an exponential one-phase decay function, and constrained to a minimum of zero and a maximum of the control vehicle mean. The ED50 was estimated from the curve as the dose that produced a response that was 50% of the control mean. The ED50 values and the confidence intervals are reported as arithmetic doses (mg/kg).
For the sedation rating scale results, the nonparametric Friedman’s test was used to analyze the overall effect. Post-hoc analyses between each drug dose and vehicle were performed using the Wilcoxon Signed Ranks test (α=.05 for all tests).
3. Results
3.1. Experiment 1: effects of systemic administration of the selective adenosine A2A agonist CGS 21680 on lever-pressing behavior
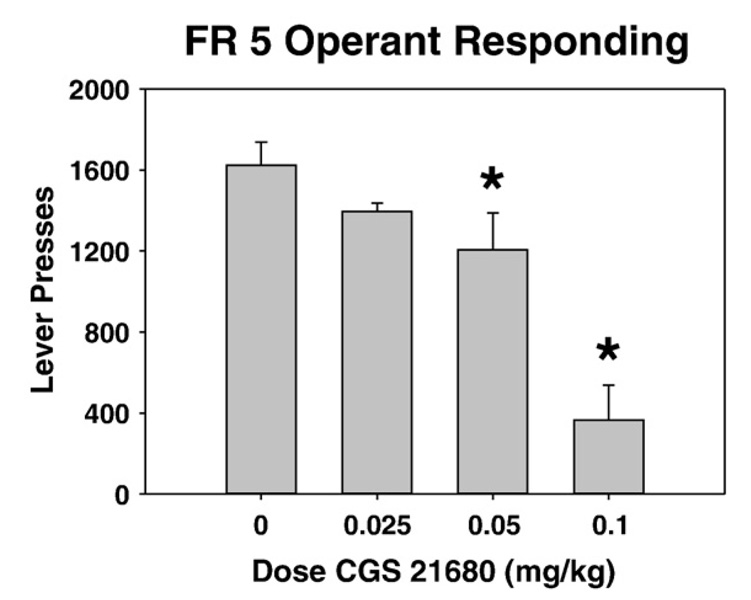
As shown in Fig. 1, systemic administration of CGS 21680 significantly decreased lever pressing in rats trained on an FR5 schedule of reinforcement. ANOVA revealed a significant overall effect of treatment (F(3, 21)=16.8, p<0.001). Planned comparisons revealed that 0.05 and 0.1 mg/kg doses of CGS 21680 significantly decreased lever pressing relative to vehicle control (p<0.05). In animals that were used in experiment 1, it was noted by experimenters that rats treated with CGS 21680 also showed overt signs of sedation when they were being taken in and out of the operant chambers. Based upon these observations, rats were explicitly observed for sedative effects in experiment 2 (see below).
Fig. 1.
Effects of systemic injections of the adenosine A2A agonist CGS 21680 on lever pressing performance (experiment 1). Rats received treatment with vehicle or various doses of CGS 21680. Mean (± SEM) number of lever presses (FR 5 schedule) during the 30 min session are shown. (* p<0.05, different from vehicle).
3.2. Experiment 2: effects of systemic administration of the selective adenosine A2A agonist CGS 21680 on feeding and sedation
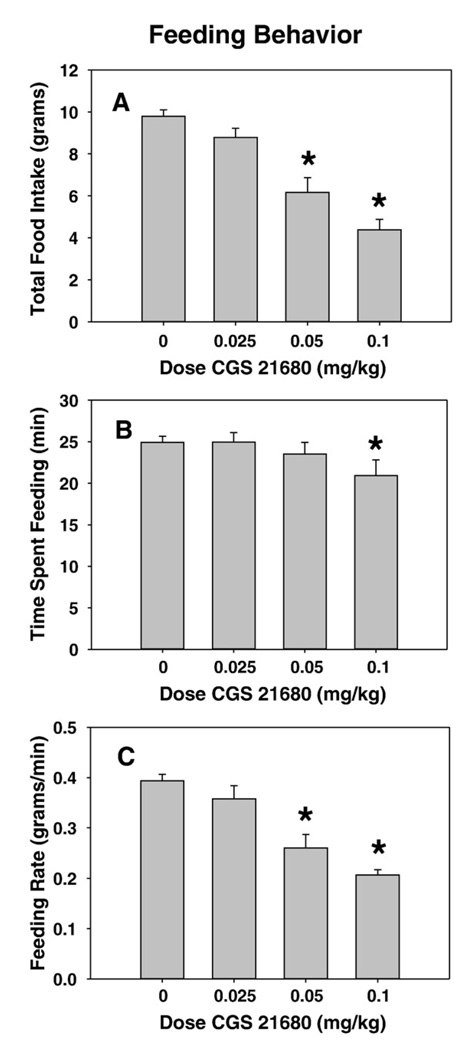
CGS 21680 impaired food intake in a dose-dependent manner. ANOVA revealed a significant overall treatment effect (F(3, 21)=24.1, p<0.001; Fig. 2A), and planned comparisons revealed that 0.05 and 0.1 mg/kg doses of CGS 21680 significantly decreased food intake relative to vehicle control (p<0.05). Time spent feeding was minimally impaired (dose effect: F(3, 21)=3.2, p=0.043;), with only the highest dose showing a small reduction (Fig. 2B). Additional analyses showed that CGS 21680 substantially reduced the feeding rate at doses that suppressed intake (see Fig. 2C). ANOVA revealed a significant effect of dose (F(3, 21)=23.7, p<0.001) and planned comparisons revealed that 0.05 and 0.1 mg/kg doses of CGS 21680 significantly decrease food intake relative to vehicle control (p<0.05).
Fig. 2.
Effects of systemic injections of the adenosine A2A agonist CGS 21680 on feeding behavior (experiment 2). Rats received treatment with vehicle or various doses of CGS 21680. A. Mean (± SEM) gram quantity of chow intake. B. Mean (± SEM) time spent feeding (in min). C. Mean (± SEM) rate of feeding (in g/min). (* p<0.05, different from vehicle).
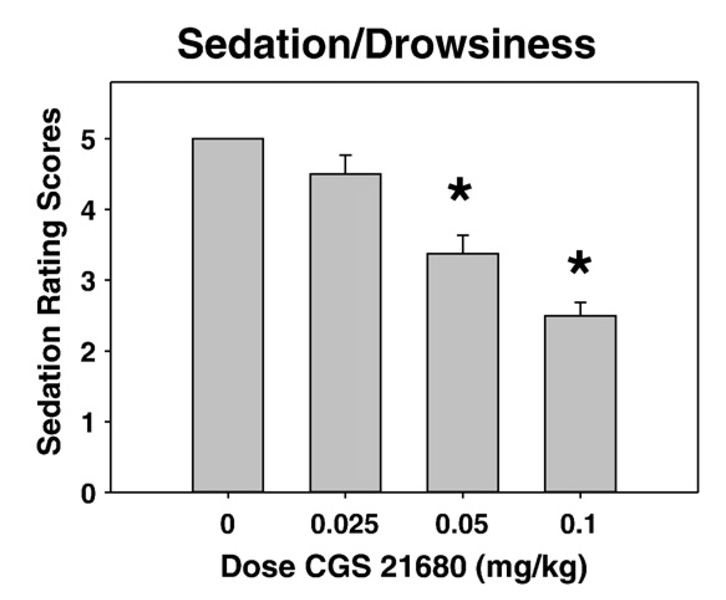
CGS 21680 also dose-dependently induced behavioral markers of sedation (see Fig. 3). Nonparametric analyses using Friedman’s test revealed an overall treatment effect (α=p<0.05), and post-hoc analyses between each drug dose and vehicle using the Wilcox on Signed Ranks test revealed that 0.05 and 0.1 mg/kg doses of CGS 21680 induced significant (α=p<0.05) signs of sedation (e.g., eyes partly closed, lowered head, flattened posture, paw dragging).
Fig. 3.
Effects of CGS 21680 on sedation/drowsiness. Mean (± SEM) sedation rating score is shown for each condition. (* p<0.05, different from vehicle).
3.3. Potency analyses: ED50 values for the effects of CGS 21680 on various behavioral measures in experiments 1 and 2
Table 1 lists the ED50 values and 95% confidence intervals for the effects of CGS 21680 on each of the behavioral measures obtained in experiments 1 and 2. Suppression of lever pressing, reductions in food intake and feeding rate, and induction of sedation, all occurred in roughly the same range of doses (i.e., ED50s from 0.07 to 0.1 mg/kg, with overlapping confidence intervals). In contrast, the ED50 for reduction of time spent feeding had to be extrapolated outside the range of doses tested (i.e., >0.4 mg/kg).
Table 1.
Potency analyses: ED50 values and 95% confidence intervals (C.I.) for the effects of CGS 21680 on various behavioral measures in experiments 1 and 2
| Behavior task | Behavior measure | ED50 (mg/kg) | 95% C.I. (mg/kg) |
|---|---|---|---|
| Operant (FR5) | Lever presses | 0.070 | 0.052 to 0.109 |
| Feeding task | Food intake | 0.087 | 0.071 to 0.112 |
| Time Feeding | 0.457 | 0.292 to 1.508 | |
| Rate of Feeding | 0.106 | 0.085 to 0.141 | |
| Sedation Rating Scale | 0.100 | 0.084 to 0.124 |
4. Discussion
These experiments demonstrate that the adenosine A2A agonist CGS 21680 could suppress food-reinforced lever pressing, as well as consumption of lab chow, in the same dose range (i.e., 0.05–0.1 mg/kg). The decreases in lab chow intake were characterized by reductions in both the rate of feeding and the time spent feeding. These drug-induced changes in feeding behavior were accompanied by overt signs of sedation. Taken together, the present results provide a further characterization of the behavioral effects of adenosine A2A receptor stimulation, and allow for comparisons between the effects of CGS 21680 and previously reported actions of DA antagonists.
The results of experiment 1 demonstrated that CGS 21680 suppressed food-reinforced FR5 lever pressing at relatively low doses (i.e., 0.05 and 0.1 mg/kg). This finding is consistent with a previous report showing that CGS 21680 could reduce operant response rates in rats responding on cocaine and methamphetamine drug discrimination tasks (Justinova et al., 2003). In the same dose range, this adenosine A2A agonist also suppressed consumption of lab chow (experiment 2). The specific pattern of results suggests that there are similarities and differences between the effects of adenosine A2A receptor stimulation and effects of DA antagonists that have been reported in the literature. The fact that CGS 21680 suppressed lever pressing and chow intake indicates that adenosine A2A receptor stimulation can produce effects that superficially resemble those of the DA antagonist haloperidol (Salamone et al., 1990, 1993a). Furthermore, the suppression of feeding produced by CGS 21680 was characterized by a modest reduction in time spent feeding, but a substantial suppression of feeding rate. This pattern of results suggests that the effects of CGS 21680 on feeding behavior are somewhat similar to the effects of DA antagonists such as haloperidol, pimozide and raclopride (Blundell and Latham, 1980; Blundell, 1987; Clifton et al., 1991; Salamone et al., 1990; Lee and Clifton, 2002), or striatal DA depletions (Salamone et al., 1990, 1993b). In addition to these similarities, there also appear to be differences between the effects of CGS 21680 and those of most DA antagonists. For example, DA antagonists such as haloperidol and spiroperidol generally have been reported to suppress food-reinforced lever pressing at doses that are considerably lower than those that suppress feeding (Fibiger et al., 1976; Rolls et al., 1974; Rusk and Cooper, 1994). The same pattern has been reported for the effects of DA antagonists on water-reinforced behavior compared to water intake as well (Ljungberg, 1987, 1988, 1990). In contrast, the present results indicate that CGS 21680 produced effects upon lever pressing and chow intake at roughly the same range of doses (i.e., there were similar potencies based upon the ED50 values).
Another difference between the effects of CGS 21680 and haloperidol appears to be the presence or absence of sedation in the dose range that also suppresses lever pressing. Previous results indicate that doses of haloperidol ranging from 0.05–0.15 mg/kg, whether administered acutely or repeatedly for 14 days, were able to substantially suppress lever pressing at doses that did not produce appreciable changes in sedation (Salamone et al., 1996). The results of experiment 2 indicated that administration of CGS 21680 at doses that suppressed lever pressing and feeding led to observable signs of sedation/drowsiness that included paw dragging, stumbling, lowered head, flattened posture and partially closed eyes. The magnitude of the sedation effect produced by 0.1 mg/kg CGS 21680 in the present experiment was comparable to that shown previously for 2.0 g/kg ethanol in rats assessed using the same scale (Chuck et al., 2006). The sedative effects of adenosine have been widely reported in previous studies (Hong et al., 2005; Porkka-Heiskanen et al., 2000; Satoh et al., 1998; Scammell et al., 2001; Stenberg, 2007). Sleep can be induced by administration of adenosine either systemically, into the ventricles, or locally into the basal forebrain (Stenberg, 2007). Extracellular levels of adenosine are increased by sleep deprivation, and non-selective adenosine antagonists such as caffeine are routinely used to promote wakefulness (Stenberg, 2007). Stenberg (2007) suggested that both adenosine A1 and A2A receptors are involved in the regulation of sleep, though probably through different brain areas and mechanisms. Previous studies have reported that systemic administration of CGS 21680, or local injections into the basal forebrain, induce sleep (Hong et al., 2005; Porkka-Heiskanen et al., 2000; Satoh et al., 1998; Scammell et al., 2001; Stenberg, 2007). Adenosine A2A receptor knockout mice showed a loss of sensitivity to the sedative effects of adenosine A2A agonists compared to wild-type mice, and in these studies it was clearly shown that an adenosine A2A agonist could induce sleep in the wild-type mice (Satoh et al., 1998). Thus, the presence of overt signs of sedation in animals treated with relatively low doses of CGS 21680 is consistent with much of the published literature, and suggests that sedative effects could be an important factor related to the suppression of lever pressing and feeding rate that also were observed in experiments 1 and 2.
The atypical antipsychotic clozapine also has been shown to produce observable behavioral signs of sedation at doses that suppress lever pressing (Salamone et al., 1996). Although this could be viewed as consistent with the idea that adenosine A2A agonists produce behavioral effects in animals that resemble those of atypical antipsychotics (Andersen et al., 2002; Ferré, 1997; Wardas et al., 2003), such comparisons should be treated with considerable caution (Wardas, in press). There still are not any clinical reports indicating that selective adenosine A2A agonists produce therapeutic antipsychotic effects in humans. In addition, data on the ability of non-selective adenosine antagonists to promote psychotic symptoms are rather mixed, with some studies suggesting that caffeine can worsen schizophrenic symptoms (De Freitas and Schwartz, 1979; Lucas et al., 1990; Mikkelsen, 1978), but other studies being unable to observe this finding (Gurpegui et al., 2004; Hughes et al., 1998; Koczapski et al., 1989; Mayo et al., 1993). In view of the fact that sedation is generally seen as an undesirable side effect of clozapine administration (Burke and Sebastian, 1993; Chesler and Salamone, 1996; Safferman et al., 1991; Salamone et al., 1996), and not as a marker of the therapeutic effect, the present results should probably be interpreted as indicating that CCS 21680 and clozapine both share the ability to produce overt signs of sedation and drowsiness at doses that also induce other behavioral effects; any interpretation in terms of possible antipsychotic activity of adenosine A2A agonists must await specific clinical findings.
In summary, the present experiments demonstrated that the adenosine A2A agonist CGS 21680 could suppress food-reinforced lever pressing and lab chow intake in the same dose range. There were drug-induced reductions in both the rate of feeding and the time spent feeding, though the feeding rate effect was more potent. The suppression of feeding behavior produced by CGS 21680 was accompanied by measurable signs of sedation and drowsiness. These results suggest that there are both similarities and differences between the effects of CGS 21680 and previously reported effects of DA antagonists. Clearly, drug-induced sedation is an important factor that contributes to the suppressive effects of CGS 21680 on lever pressing and feeding. It also is possible that the suppression of lever pressing and feeding rate produced by systemic injections of CGS 21680 results from a combination of sedation and other behavioral effects, including actions on striatal mechanisms that partially resemble the effects of interference with DA transmission. The effects of CGS 21680 did not closely resemble those produced by the cannabinoid CB1 antagonists/inverse agonist AM251, which has been reported to have greater effects on time spent feeding rather than feeding rate (McLaughlin et al., 2005b). However, possible appetite suppressant or food aversion effects of CGS 21680 cannot be completely ruled out based solely upon the present data. Additional studies involving intracranial administration of CGS 21680 (e.g. Salamone et al., 2007) may be useful for disentangling some of the distinct behavioral effects produced by adenosine A2A receptor stimulation.
Acknowledgements
This research was supported by grants to JDS from the United States NIH/NINDS and NIH/NIMH. Many thanks to Kelly Sink for her help with the interrater reliability measurement.
References
- Andersen MB, Fuxe K, Werge T, Gerlach J. The adenosine A2A receptor agonist CGS 21680 exhibits antipsychotic-like activity in Cebus apella monkeys. Behav Pharmacol. 2002;13:639–644. doi: 10.1097/01.fbp.0000047148.28986.67. [DOI] [PubMed] [Google Scholar]
- Arizzi MN, Cervone KM, Aberman JE, Betz A, Liu Q, Lin S, et al. Behavioral effects of inhibition of cannabinoid metabolism: The amidase inhibitor AM374 enhances the suppression of lever pressing produced by exogenously administered anandamide. Life Sci. 2004;74:1001–1011. doi: 10.1016/j.lfs.2003.07.024. [DOI] [PubMed] [Google Scholar]
- Barraco RA, Martens KA, Parizon M, Normile HJ. Adenosine A2a receptors in the nucleus accumbens mediate locomotor depression. Brain Res Bull. 1993;31(3–4):397–404. doi: 10.1016/0361-9230(93)90233-2. [DOI] [PubMed] [Google Scholar]
- Blundell JE. Structure, process and mechanism: case studies in the psychopharmacology of feeding. In: Iversen LL, Iversen SD, Snyder SH, editors. Handbook of Psychopharmacology. vol. 19. NY: Plenum Press; 1987. pp. 123–182. [Google Scholar]
- Blundell JE, Latham CJ. Characterisation of adjustments to the structure of feeding behaviour following pharmacological treatment: effects of amphetamine and fenfluramine and the antagonism produced by pimozide and methergoline. Pharmacol Biochem Behav. 1980;12:717–722. doi: 10.1016/0091-3057(80)90155-0. [DOI] [PubMed] [Google Scholar]
- Burke M, Sebastian C. Treatment of clozapine sedation. Am J Psychiatr. 1993;150:1900–1901. doi: 10.1176/ajp.150.12.1900. [DOI] [PubMed] [Google Scholar]
- Cabeza de Vaca S, Kannan P, Pan Y, Jiang N, Sun Y, Carr KD. The adenosine A2A receptor agonist, CGS-21680, blocks excessive rearing, acquisition of wheel running, and increases nucleus accumbens CREB phosphorylation in chronically food-restricted rats. Brain Res. 2007;1142:100–109. doi: 10.1016/j.brainres.2007.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carriero D, Aberman J, Lin SY, Hill A, Makriyannis A, Salamone JD. A detailed characterization of the effects of four cannabinoid agonists on operant lever pressing. Psychopharmacology (Berl) 1998;137:147–156. doi: 10.1007/s002130050604. [DOI] [PubMed] [Google Scholar]
- Chen JF, Moratalla R, Impagnatiello F, Grandy DK, Cuellar B, Rubinstein M, et al. The role of the D2 dopamine receptor (D2R) in A2a adenenosine-receptor (a2ar) mediated behavioral and cellular responses as revealed by A2a and D2 receptor knockout mice. Proc Natl Acad Sci. 2001;98:1970–1975. doi: 10.1073/pnas.98.4.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesler EJ, Salamone JD. Effects of acute and repeated clozapine injections on cholinomimetic-induced vacuous jaw movements. Pharmacol Biochem Behav. 1996;54(3):619–624. doi: 10.1016/0091-3057(95)02280-5. [DOI] [PubMed] [Google Scholar]
- Chuck TL, McLaughlin PJ, Arizzi-LaFrance MN, Salamone JD, Correa M. Comparison between multiple behavioral effects of peripheral ethanol administration in rats: sedation, ataxia, and bradykinesia. Life Sci. 2006;79(2):154–161. doi: 10.1016/j.lfs.2005.12.045. [DOI] [PubMed] [Google Scholar]
- Clifton PG, Rusk IN, Cooper SJ. Effects of dopamine D1 and dopamine D2 antagonists on the free feeding and drinking patterns of rats. Behav Neurosci. 1991;105:272–281. doi: 10.1037//0735-7044.105.2.272. [DOI] [PubMed] [Google Scholar]
- Correa M, Wisniecki A, Betz A, Dobson DR, O’Neill MF, O’Neill MJ, Salamone JD. The adenosine A2A antagonist KF 17837 reverses the locomotor suppression and tremulous jaw movements induced by haloperidol in rats: possible relevance to parkinsonism. Behav Brain Res. 2004;148:47–54. doi: 10.1016/s0166-4328(03)00178-5. [DOI] [PubMed] [Google Scholar]
- De Freitas B, Schwartz G. Effects of caffeine in chronic psychiatric patients. Am J Psychiatr. 1979;136:1337–1338. doi: 10.1176/ajp.136.10.1337. [DOI] [PubMed] [Google Scholar]
- DeMet EM, Chicz-DeMet A. Localization of adenosine A2A-receptors in rat brain with [3H]ZM-241385. Naunyn-Schmiedeberg’s Arch Pharmacol. 2002;366:478–481. doi: 10.1007/s00210-002-0613-3. [DOI] [PubMed] [Google Scholar]
- Farrar AM, Pereira M, Velasco F, Hockemeyer J, Muller CE, Salamone JD. Adenosine A(2A) receptor antagonism reverses the effects of dopamine receptor antagonism on instrumental output and effort-related choice in the rat: implications for studies of psychomotor slowing. Psychopharmacology (Berl) 2007;191:579–586. doi: 10.1007/s00213-006-0554-5. [DOI] [PubMed] [Google Scholar]
- Ferré S. Adenosine–dopamine interactions in the ventral striatum. Implications for the treatment of schizophrenia. Psychopharmacology (Berl) 1997;133(2):107–120. doi: 10.1007/s002130050380. [DOI] [PubMed] [Google Scholar]
- Ferré S, Freidholm BB, Morelli M, Popoli P, Fuxe K. Adenosine–dopamine receptor–receptor interactions as an integrative mechanism in the basal ganglia. Trends Neurosci. 1997;20:482–487. doi: 10.1016/s0166-2236(97)01096-5. [DOI] [PubMed] [Google Scholar]
- Ferré S, Popoli P, Gimenez-Llort L, Rimondini R, Müller CE, Stromberg I, et al. Adenosine/dopamine interaction: implications for the treatment of Parkinson’s disease. Parkinsonism Relat Disord. 2001;7:235–241. doi: 10.1016/s1353-8020(00)00063-8. [DOI] [PubMed] [Google Scholar]
- Ferré S, Ciruela F, Canals M, Marcellino D, Burgueno J, Casado V, et al. Adenosine A2A–dopamine D2 receptor–receptor heteromers. Targets for neuro-psychiatric disorders. Parkinsonism Relat Disord. 2004;10:265–271. doi: 10.1016/j.parkreldis.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Fibiger HC, Carter DA, Phillips AG. Decreased intracranial self-stimulation after neuroleptics or 6-hydroxydopamine: evidence for mediation by motor deficits rather than by reduced reward. Psychopharmacology (Berl) 1976;47:21–27. doi: 10.1007/BF00428696. [DOI] [PubMed] [Google Scholar]
- Gurpegui M, Aguilar MC, Martínez-Ortega JM, Diaz FJ, de Leon J. Caffeine intake in outpatients with schizophrenia. Schizophr Bull. 2004;30:935–945. doi: 10.1093/oxfordjournals.schbul.a007143. [DOI] [PubMed] [Google Scholar]
- Hauber W, Munkel M. Motor depressant effects mediated by dopamine D2 and adenosine A2A receptors in the nucleus accumbens and the caudate-putamen. Eur J Pharmacol. 1997;323(2–3):127–131. doi: 10.1016/s0014-2999(97)00040-x. [DOI] [PubMed] [Google Scholar]
- Hauber W, Neuscheler P, Nagel J, Muller CE. Catalepsy induced by a blockade of dopamine D1 or D2 receptors was reversed by a concomitant blockade of adenosine A2a receptors in the caudate putamen of rats. Eur J Neurosci. 2001;14:1287–1293. doi: 10.1046/j.0953-816x.2001.01759.x. [DOI] [PubMed] [Google Scholar]
- Hettinger BD, Lee A, Linden J, Rosin DL. Ultrastructural localization of adenosine A2A receptors suggests multiple cellular sites for modulation of gabaergic neurons in rat striatum. J Comp Neurol. 2001;431:331–346. doi: 10.1002/1096-9861(20010312)431:3<331::aid-cne1074>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Hong ZY, Huang ZL, Qu WM, Eguchi N, Urade Y, Hayaishi O. An adenosine A receptor agonist induces sleep by increasing GABA release in the tuberomammillary nucleus to inhibit histaminergic systems in rats. J Neurochem. 2005;92:1542–1549. doi: 10.1111/j.1471-4159.2004.02991.x. [DOI] [PubMed] [Google Scholar]
- Hughes JR, McHugh P, Holtzman S. Caffeine and schizophrenia. Psychiatr Serv. 1998;49:1415–1417. doi: 10.1176/ps.49.11.1415. [DOI] [PubMed] [Google Scholar]
- Ishiwari K, Madson LJ, Farrar AM, Mingote SM, Valenta JP, DiGianvittorio MD, et al. Injections of the selective adenosine A2A antagonist MSX-3 into the nucleus accumbens core attenuate the locomotor suppression induced by haloperidol in rats. Behav Brain Res. 2007;178(2):190–199. doi: 10.1016/j.bbr.2006.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis MF, Williams M. Direct autoradiographic localization of adenosine A2 receptors in the rat brain using the A2-selective agonist, [3H]CGS 21680. Eur J Pharmacol. 1989;168:243–246. doi: 10.1016/0014-2999(89)90571-2. [DOI] [PubMed] [Google Scholar]
- Jenner P. A2A antagonists as novel non-dopaminergic therapy for motor dysfunction in PD. Neurology. 2003;61:S32–S38. doi: 10.1212/01.wnl.0000095209.59347.79. [DOI] [PubMed] [Google Scholar]
- Jenner P. Istradefylline, a novel adenosine A2A receptor antagonist, for the treatment of Parkinson’s disease. Exp Opin Investig Drugs. 2005;14:729–738. doi: 10.1517/13543784.14.6.729. [DOI] [PubMed] [Google Scholar]
- Justinova Z, Ferré S, Segal PN, Antoniou K, Solinas M, Pappas LA, et al. Involvement of adenosine A1 and A2A receptors in the adenosinergic modulation of the discriminative-stimulus effects of cocaine and methamphetamine in rats. J Pharmacol Exp Ther. 2003;307:977–986. doi: 10.1124/jpet.103.056762. [DOI] [PubMed] [Google Scholar]
- Keppel G. Design and analysis: a researcher’s handbook. Englewood Cliffs, NJ: Prentice-Hall; 1991. [Google Scholar]
- Koczapski A, Paredes J, Kogan C, Ledwidge B, Higenbottam J. Effects of caffeine on behavior of schizophrenic inpatients. Schizophr Bull. 1989;15(2):339–344. doi: 10.1093/schbul/15.2.339. [DOI] [PubMed] [Google Scholar]
- Lee MD, Clifton PG. Meal patterns of free feeding rats treated with clozapine, olanzapine, or haloperidol. Pharmacol Biochem Behav. 2002;71:147–154. doi: 10.1016/s0091-3057(01)00630-x. [DOI] [PubMed] [Google Scholar]
- Ljungberg T. Blockade by neuroleptics of water intake and operant responding in the rat: anhedonia, motor deficit or both? Pharmacol Biochem Behav. 1987;27:341–350. doi: 10.1016/0091-3057(87)90578-8. [DOI] [PubMed] [Google Scholar]
- Ljungberg T. Scopolamine reverses haloperidol-attenuated lever pressing for water but not haloperidol-attenuated water intake in the rat. Pharmacol Biochem Behav. 1988;29:205–211. doi: 10.1016/0091-3057(88)90298-5. [DOI] [PubMed] [Google Scholar]
- Ljungberg T. Differential attenuation of water intake and water- rewarded operant responding by repeated administration of haloperidol and SCH 23390 in the rat. Pharmacol Biochem Behav. 1990;35:111–115. doi: 10.1016/0091-3057(90)90213-2. [DOI] [PubMed] [Google Scholar]
- Lucas PB, Pickar D, Kelsoe J, Rapaport M, Pato C, Hommer D. Effects of the acute administration of caffeine in patients with schizophrenia. Biol Psychiatry. 1990;28:35–40. doi: 10.1016/0006-3223(90)90429-6. [DOI] [PubMed] [Google Scholar]
- Mayo KM, Falkowski W, Jones CA. Caffeine: use and effects in long-stay psychiatric patients. Br J Psychiatry. 1993;162:543–545. doi: 10.1192/bjp.162.4.543. [DOI] [PubMed] [Google Scholar]
- McLaughlin PJ, Lu D, Winston KM, Thakur G, Swezey LA, Makriyannis A, et al. Behavioral effects of the novel cannabinoid full agonist AM411. Pharmacol Biochem Behav. 2005a;81(1):78–88. doi: 10.1016/j.pbb.2005.02.005. [DOI] [PubMed] [Google Scholar]
- McLaughlin PJ, Winston KM, Limebeer CL, Parker LA, Makriyannis A, Salamone JD. The cannabinoid CB1 antagonist AM251 produces food avoidance and behaviors associated with nausea but does not impair feeding efficiency in rats. Psychopharmacology (Berl) 2005b;180:286–293. doi: 10.1007/s00213-005-2171-0. [DOI] [PubMed] [Google Scholar]
- Mikkelsen EJ. Caffeine and schizophrenia. J Clin Psychiatry. 1978;39:732–736. [PubMed] [Google Scholar]
- Minor TR, Huang Q, Foley EA. Cytokine–purine interactions in behavioral depression in rats. Integr Physiol Behav Sci. 2003;38:189–202. doi: 10.1007/BF02688853. [DOI] [PubMed] [Google Scholar]
- Morelli M, Pinna A. Interaction between dopamine and adenosine A2A receptors as a basis for the treatment of Parkinson’s disease. Neurol Sci. 2001;22:71–72. doi: 10.1007/s100720170052. [DOI] [PubMed] [Google Scholar]
- O’Neill M, Brown VJ. The effect of the adenosine A2A antagonist KW-6002 on motor and motivational processes in the rat. Psychopharmacology. 2006;184:46–55. doi: 10.1007/s00213-005-0240-z. [DOI] [PubMed] [Google Scholar]
- Pinna A, Wardas J, Simola N, Morelli M. New therapies for the treatment of Parkinson’s disease: adenosine A2A receptor antagonists. Life Sci. 2005;77:3259–3267. doi: 10.1016/j.lfs.2005.04.029. [DOI] [PubMed] [Google Scholar]
- Porkka-Heiskanen T, Strecker RE, McCarley RW. Brain site-specificity of extracellular adenosine concentration changes during sleep deprivation and spontaneous sleep: an in vivo microdialysis study. Neuroscience. 2000;99(3):507–517. doi: 10.1016/s0306-4522(00)00220-7. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Rolls BJ, Kelly PH, Shaw SG, Wood RJ, Dale R. The relative attenuation of self-stimulation, eating and drinking produced by dopamine-receptor blockade. Psychopharmacologia. 1974;38:219–230. doi: 10.1007/BF00421374. [DOI] [PubMed] [Google Scholar]
- Rusk IN, Cooper SJ. Parametric studies of selective D1 and D2 antagonists: effects on appetitive and feeding behavior. Behav Pharmacol. 1994;5:615–622. doi: 10.1097/00008877-199410000-00007. [DOI] [PubMed] [Google Scholar]
- Safferman A, Lieberman JA, Kane JM, Szymansky S, Kinon B. Update on the clinical efficacy of clozapine. Schizophr Bull. 1991;17:247–261. doi: 10.1093/schbul/17.2.247. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Zigmond MJ, Stricker EM. Characterization of the impaired feeding behavior in rats given haloperidol or dopamine-depleting brain lesions. Neuroscience. 1990;39:17–24. doi: 10.1016/0306-4522(90)90218-s. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Kurth PA, McCullough LD, Sokolowski JD, Cousins MS. The ole of brain dopamine in response initiation: effects of haloperidol and regionally specific dopamine depletions on the local rate of instrumental responding. Brain Res. 1993a;628:218–226. doi: 10.1016/0006-8993(93)90958-p. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Mahan K, Rogers S. Ventrolateral striatal dopamine depletions impair feeding and food handling in rats. Pharmacol Biochem Behav. 1993b;44:605–610. doi: 10.1016/0091-3057(93)90174-r. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Cousins MS, Maio C, Champion M, Turski T, Kovach J. Different behavioral effects of haloperidol, clozapine and thioridazine in an instrumental lever pressing/feeding procedure. Psychopharmacology (Berl) 1996;125:105–112. doi: 10.1007/BF02249408. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Arizzi M, Sandoval MD, Cervone KM, Aberman JE. Dopamine antagonists alter response allocation but do not suppress appetite for food in rats: contrast between the effects of SKF 83566, raclopride and fenfluramine on a concurrent choice task. Psychopharmacology (Berl) 2002;160:371–380. doi: 10.1007/s00213-001-0994-x. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Font L, Mingote S, Worden L, Stopper C, Farrar AM. Neuroscience meeting planner. San Diego, CA: Society for Neuroscience; 2007. Forebrain circuitry involved in effort-related functions: intra-accumbens injections of the adenosine A2A agonist CGS 21680 produce an impairment in response allocation similar to that produced by accumbens dopamine depletions. Program No. 310.18. [Google Scholar]
- Satoh S, Matsumura H, Hayaishi O. Involvement of adenosine A2A receptor in sleep promotion. Eur J Pharmacol. 1998;351:155–162. doi: 10.1016/s0014-2999(98)00302-1. [DOI] [PubMed] [Google Scholar]
- Scammell TE, Gerashchenko DY, Mochizuki T, McCarthy MT, Estabrooke IV, Sears CA, et al. An adenosine A2a agonist increases sleep and induces Fos in ventrolateral preoptic neurons. Neuroscience. 2001;107(4):653–663. doi: 10.1016/s0306-4522(01)00383-9. [DOI] [PubMed] [Google Scholar]
- Schiffmann SN, Jacobs O, Vanderhaeghen JJ. Striatal restricted adenosine A2 receptor (RDC8) is expressed by enkephalin but not by substance P neurons: an in situ hybridization histochemistry study. J Neurochem. 1991;57:1062–1067. doi: 10.1111/j.1471-4159.1991.tb08257.x. [DOI] [PubMed] [Google Scholar]
- Stenberg D. Neuroanatomy and neurochemistry of sleep. Cell Mol Life Sci. 2007;64:1187–1204. doi: 10.1007/s00018-007-6530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsson P, Le Moine C, Fisone G, Fredholm BB. Distribution, biochemistry and function of striatal adenosine A2A receptors. Prog Neurobiol. 1999;59:355–396. doi: 10.1016/s0301-0082(99)00011-8. [DOI] [PubMed] [Google Scholar]
- Takahashi RN, Pamplona FA, Prediger RD. Adenosine receptor antagonists for cognitive dysfunction: a review of animal studies. Front Biosci. 2008;13:2614–2632. doi: 10.2741/2870. [DOI] [PubMed] [Google Scholar]
- Wang WF, Ishiwata K, Nonaka H, Ishii S, Kiyosawa M, Shimada J, et al. Carbon-11-labeled KF21213: a highly selective ligand for mapping CNS adenosine A(2A) receptors with positron emission tomography. Nucl Med Biol. 2000;27:541–546. doi: 10.1016/s0969-8051(00)00126-8. [DOI] [PubMed] [Google Scholar]
- Wardas J. Potential role of adenosine A2A receptors in the treatment of schizophrenia. Fontiers Biosci. doi: 10.2741/2995. (in press) [DOI] [PubMed] [Google Scholar]
- Wardas J, Konieczny J, Lorenc-Koci E. SCH 58261, an A(2A) adenosine receptor antagonist, counteracts parkinsonian-like muscle rigidity in rats. Synapse. 2001;41:160–171. doi: 10.1002/syn.1070. [DOI] [PubMed] [Google Scholar]
- Wardas J, Konieczny J, Pietraszek M. Influence of CGS 21680, a selective adenosine A(2A) agonist, on the phencyclidine-induced sensorimotor gating deficit and motor behaviour in rats. Psychopharmacology (Berl) 2003;168:299–306. doi: 10.1007/s00213-003-1439-5. [DOI] [PubMed] [Google Scholar]
- Weerts EM, Griffiths RR. The adenosine receptor antagonist CGS15943 reinstates cocaine-seeking behavior and maintains self-administration in baboons. Psychopharmacology. 2003;168:155–163. doi: 10.1007/s00213-003-1410-5. [DOI] [PubMed] [Google Scholar]