Abstract
The lower morbidity and mortality of reduced-intensity conditioning (RIC) regimens have allowed allogeneic hematopoietic cell transplantation (HCT) in older patients. Unrelated umbilical cord blood (UCB) has been investigated as an alternative stem cell source to suitably HLA matched related (MRD) and adult volunteer unrelated donors. We hypothesized that RIC HCT using UCB would be safe and efficacious in older patients and compared the transplant related mortality (TRM) and overall survival of RIC HCT in patients older than 55 years using either MRD (n=47) or, in patients with no 5/6 or 6/6 HLA compatible related donors, UCB (n=43). RIC regimen consisted of total-body irradiation (200 cGy) and either cyclophosphamide and fludarabine (n=69), or busulfan and fludarabine (n=16) or busulfan and cladribine (n=5). The median age of MRD and UCB cohorts was 58 (range, 55-70) and 59 (range, 55-69) years, respectively. AML/MDS (50%) was the most common diagnosis. All MRD grafts were 6 of 6 HLA matched to the recipient. Among patients undergoing UCB HCT, 88% received two UCB units to optimize cell dose and 93% received 1-2 HLA mismatched grafts. The median followup for survivors was 27 (range, 12-61) months. The 3-year probabilities of progression-free survival (30% vs. 34%, p=0.98) and overall survival (43% vs. 34%, p=0.57) were similar for recipients of MRD and UCB. The cumulative incidence of grade 2-4 acute graft-versus-host disease (42% vs. 49%, p=0.20) and TRM at 180-days (23% vs. 28%, p=0.36) were comparable. However, UCB recipients had a lower incidence of chronic graft-versus-host disease at 1-year (40% vs. 17%, p=0.02). On multivariate analysis, graft type had no impact on TRM or survival and HCT comorbidity index score was the only factor independently predictive for these endpoints. Our study supports the use of HLA mismatched UCB as an alternative graft source for older patients who need a transplant but do not have a MRD. The use of RIC and UCB extends the availability of transplant therapy to older patients previously excluded on the basis of age and lack of a suitable MRD. A careful review of existing comorbidities is necessary when considering older patients for HCT.
Keywords: Allogeneic Stem Cell Transplantation, Umbilical Cord Blood Transplantation, Non-myeloablative Conditioning Regimen, Reduced Intensity Conditioning Regimen
INTRODUCTION
Allogeneic hematopoietic cell transplantation (HCT) is an effective therapy for a variety of malignant and non-malignant hematological disorders. The high rates of transplant related morbidity and mortality observed with traditional myeloablative conditioning regimens have typically restricted the use of allogeneic HCT to young and relatively healthy patients. Many adult hematologic malignancies manifest clinically in the sixth or seventh decade of life and because of advanced age and high-risk for transplant related mortality (TRM), these patients are usually excluded from most conventional myeloablative allogeneic HCT protocols. The advent of reduced-intensity (RIC) conditioning regimens that primarily rely on the graft-versus-tumor effect has allowed allogeneic HCT as a treatment option for these older patients. This approach has been shown to be feasible, lead to long-term engraftment, exhibit a graft-versus-malignancy effect, and result in acceptable TRM.1-3
Although a human leukocyte antigen (HLA) matched related donor (MRD) is the preferred donor source, it is available for less than 30% of patients requiring allogeneic HCT.4,5 Transplantation from adult volunteer unrelated donors also remains limited by the availability of fully HLA matched grafts, and increased HLA disparity in this setting adversely affects overall survival and increases the risk of graft-versus-host disease (GVHD).5,6 Unrelated umbilical cord blood (UCB) has been investigated as an alternative source for hematopoietic stem cells. We have previously reported that UCB HCT using a RIC regimen produces high rates of engraftment with tolerable toxicity.7-9
We hypothesized that for older patients without a MRD, UCB could lead to comparable survival and TRM, thus extending the availability of HCT as a therapeutic option for patients who would otherwise be ineligible for transplantation because of lack of a suitable donor. We therefore conducted a study comparing the safety and efficacy of allogeneic HCT after RIC regimen in patients older than 55 years using either MRD or UCB in patients with no MRD.
METHODS
Patient characteristics
Eligibility criteria for HCT using RIC at our institution include older age (≥55 years for MRD and ≥45 years for UCB), presence of significant comorbidity, and history of previous autologous transplant or extensive therapy. Data were collected prospectively on 90 consecutive patients between 55-70 years of age who received RIC HCT between January 2000 and December 2005 using either MRD (n=47) or, in patients with no 5/6 or 6/6 HLA compatible related donors, UCB (n=43). The primary indication for using RIC instead of a conventional myeloablative preparative regimen for all patients was age ≥ 55 years. Patients were considered for UCB HCT if they had no HLA-compatible related donors (5/6 or 6/6 HLA-A, B or DRB1 matches).
Pre-transplantation comorbidities were scored retrospectively for all patients using the HCT-specific comorbidity index (HCT CI) described by Sorror et al.10 The comorbidities captured by this tool include cardiac disorders, cerebrovascular disease, diabetes, altered hepatic function, infection, inflammatory bowel disease, obesity, peptic ulcer disease, psychiatric disturbance, pulmonary abnormalities, renal insufficiency and rheumatologic disorders. Scores are assigned to various comorbidities based on their severity and a final composite score is then calculated and patients can be assigned to one of three risk groups: low-risk (score 0), intermediate-risk (score 1-2) and high-risk (score ≥ 3).
Patients were classified as having standard or high risk disease. Standard risk disease included acute leukemia in first complete remission (CR), chronic myeloid leukemia in first chronic phase, myelodysplastic syndrome (MDS) — refractory anemia, and nonmalignant hematologic disorders; all other diagnoses were categorized as high risk disease.
Treatment plan
The majority of patients (n=69) underwent conditioning with a regimen that included cyclophosphamide (50 mg/kg intravenously on day -6), fludarabine (40 mg/m2 intravenously daily from days -6 through -2) and 200 cGy total body irradiation (TBI, on day -1). Prior to September 2001, patients received a regimen (n=16) using busulfan (2 mg/kg orally every 12 hours for 4 doses on days -8 and -7) with the same doses of fludarabine and TBI, or a regimen (n=5) using the same dose of busulfan and TBI with cladribine (10 mg/m2 intravenously daily from days -6 through -2). Equine anti-thymocyte globulin (ATG) 15 mg/kg intravenously every 12 hours for six doses from days -3 to -1 was added to a subgroup of patients who had not received chemotherapy within 3 months of HCT or a previous autologous transplant (n=23). All patients received GVHD prophylaxis with cyclosporine (days -3 to at least +100) and mycophenolate mofetil (days -3 to at least +30). Granulocyte colony-stimulating factor (G-CSF) 5 μg/kg/day intravenously was administered to all patients until the absolute neutrophil count (ANC) was more than 2.5 × 109/L for two days. The treatment protocols were approved by the University of Minnesota institutional review board and all patients gave informed consent prior to transplantation.
MRD and UCB grafts
All related donor grafts were 6 of 6 HLA matched to the recipient and the target cell dose was at least 3 × 108 NC/kg. Forty-four patients undergoing MRD HCT received G-CSF mobilized peripheral blood stem cell (PBSC) grafts and one patient received a bone marrow graft. In addition, due to inadequate donor mobilization, two patients received grafts derived from both PBSC and bone marrow.
Our UCB selection criteria for adults have been previously published.7,8,11 UCB grafts were matched at least 4 of 6 HLA-A,-B (antigen level) and -DRB1 (allele level) to the recipient, and in patients receiving two UCB units, to each other. Thirty-eight (88%) patients undergoing UCB HCT received grafts consisting of two UCB units to optimize cell dose and 40 (93%) received at least 1-2 HLA mismatched units. The median total cryopreserved nucleated cell dose was 3.7 × 107 NC/kg (range, 1.6-7.8 × 107 NC/kg) and CD34+ dose was 4.2 × 105 cells/kg (range, 1.3-16.6 × 105 cells/kg). UCB units were thawed using the method described by Rubinstein et al.12
Donor chimerism analysis
Donor chimerism was determined serially on marrow and/or blood samples on days +21-28, +60, +100, 6 months and annually after HCT. Chimerism analysis was performed using quantitative PCR of informative polymorphic variable-number tandem repeat (VNTR) or short tandem repeat (STR) regions in recipient and donor13,14 and has been described previously.7
Study definitions and statistical analysis
The primary endpoint was probability of progression-free survival (PFS). Other study endpoints included probability of overall survival (OS) and cumulative incidences of sustained donor engraftment, acute and chronic GVHD and TRM. PFS was defined as survival in CR or stable partial remission (PR); recurrence after achieving a CR or an increase in existing or new disease sites following a stable PR was considered disease progression. CR was defined as complete absence of disease on clinical, radiologic, and if indicated, pathologic evaluation. PR was defined as ≥ 50% reduction in sites of known disease. Failure to achieve a PR was defined as persistent disease. Sustained donor engraftment was defined as neutrophil recovery with donor hematopoiesis by day 42 after transplant. Time of neutrophil engraftment was defined as first of three consecutive days with an ANC greater than 0.5 × 109/L. Complete donor chimerism was defined as marrow reconstitution of donor origin of at least 90%. Ten patients (UCB=5, MRD=5) were censored from engraftment analysis because of death prior to day 21 and before the status of donor chimerism could be ascertained. Standard clinical criteria were used to diagnose and grade GVHD.15,16 TRM was defined as death within 180 days following HCT without disease progression or relapse.
Comparison of patient and transplant characteristics was performed using chi-square, Fisher’s exact or Wilcoxon’s rank sum test as appropriate. Cumulative incidence of engraftment, TRM and GVHD was calculated by treating deaths from other causes as competing risks.17 The Kaplan-Meier method was used to plot survival curves for PFS and OS.18 Both univariate and multivariate Cox regressions were performed with TRM, PFS and OS as the outcomes.19 Variables were included in the multivariate model if they were conceptually important, or if they approached or obtained statistical significance in the univariate regression. All multivariate models included donor type (UCB vs. MRD) and were adjusted for age at transplant, gender, time from diagnosis to transplant, history of previous transplant, disease risk, recipient-donor HLA disparity, recipient-donor cytomegalovirus (CMV) serologic status, conditioning regimen, use of ATG, acute GVHD, chronic GVHD and HCT CI score. Acute GVHD was treated as a time dependent variable. Event times were measured from date of transplantation to date of death or last contact. All p-values were two sided. Analyses were performed in SAS 8.2 and SAS 9.1 (Cary, North Carolina, USA). The analysis was based on followup through June 2007.
RESULTS
Patient characteristics
Patient, disease and transplant characteristics are summarized in table 1. The median age at transplant for the entire cohort was 59 years (range, 55-70 years). A significantly higher proportion of patients undergoing UCB HCT received ATG with their conditioning. All UCB grafts were CMV seronegative. UCB units also had a significantly lower cryopreserved cell dose and CD34+ cell dose.
Table 1.
Patient, disease and transplant characteristics
| Characteristics | MRD (n=47) | UCB (n=43) | P-value |
|---|---|---|---|
| Median age, years (range) | 58 (55-70) | 59 (55-69) | 0.22 |
| Gender, male | 33 (70%) | 32 (74%) | 0.66 |
| Median weight, kilograms (range) | 78 (55-120) | 79 (50-130) | 0.96 |
| Race | 0.88 | ||
| White | 43 (91%) | 38 (88%) | |
| Other | 4 (9%) | 5 (12%) | |
| Diagnosis | 0.07 | ||
| Acute myeloid leukemia | 10 (21%) | 19 (44%) | |
| Acute lymphoblastic leukemia | 2 (4%) | 2 (5%) | |
| Chronic myeloid leukemia | 2 (4%) | 1 (2%) | |
| Chronic lymphocytic leukemia | 3 (7%) | 2 (5%) | |
| Myelodysplastic syndrome | 6 (13%) | 10 (23%) | |
| Non-Hodgkin’s lymphoma | 14 (30%) | 7 (16%) | |
| Hodgkin’s lymphoma | 2 (4%) | 0 | |
| Multiple myeloma | 6 (13%) | 0 | |
| Other 1 | 2 (4%) | 2 (5%) | |
| Disease risk 2 | 0.33 | ||
| Standard | 9 (19%) | 12 (28%) | |
| High | 38 (81%) | 31 (72%) | |
| Prior HCT 3 | 9 (19%) | 3 (7%) | 0.09 |
| Median time from diagnosis to HCT, months (range) | 25 (3-154) | 13 (3-250) | 0.24 |
| HCT-specific comorbidity index score 4 | 0.10 | ||
| 0 | 7 (16%) | 15 (36%) | |
| 1-2 | 19 (43%) | 13 (31%) | |
| ≥ 3 | 18 (41%) | 14 (33%) | |
| Conditioning regimen | 0.13 | ||
| Cy/Flu/TBI | 33 (70%) | 36 (84%) | |
| Other 5 | 14 (30%) | 7 (16%) | |
| ATG used in conditioning | 6 (13%) | 17 (40%) | <0.01 |
| HLA compatibility6 | <0.01 | ||
| 6/6 antigen match | 47 (100%) | 3 (7%) | |
| 5/6 antigen match | 0 | 13 (30%) | |
| 4/6 antigen match | 0 | 27 (63%) | |
| Recipient-donor CMV serologic status | 0.02 | ||
| Recipient negative-donor negative | 16 (34%) | 18 (42%) | |
| Recipient negative-donor positive | 8 (17%) | 0 | |
| Recipient positive | 23 (49%) | 25 (58%) | |
| Median cell dose, x108NC/kg (range) | 9.2 (3.0-21.2) | 0.4 (0.2-0.8) | <0.01 |
| Median CD34+ cell dose, x106cells/kg (range) | 5.3 (1.2-15.5) | 0.4 (0.1-1.7) | <0.01 |
| Median follow up, months (range) | 37 (18-61) | 24 (12-44) | 0.33 |
MRD — matched related donor; UCB — unrelated umbilical cord blood donor; MDS — myelodysplastic syndrome; HCT — hematopoietic cell transplantation; ATG — anti-thymocyte globulin; Cy/Flu/TBI — cyclophosphamide/fludarabine/total body irradiation; HLA — human leukocyte antigen; CMV — cytomegalovirus; NC — nucleated cells
Includes one patient each with renal cell carcinoma and myelofibrosis in MRD group and renal cell carcinoma and aplastic anemia in UCB group.
Standard risk disease - acute leukemia in first complete remission, chronic myeloid leukemia in first chronic phase, myelodysplastic syndrome refractory anemia, non-malignant hematological disorder; High risk disease — all other disease categories.
All had prior autologous HCT except for 1 patient in MRD group who had received a MRD myeloablative allogeneic HCT for MDS 8 years prior.
Excludes 4 patients (MRD=3, UCB=1) for whom sufficient data was not available to calculate the HCT-specific comorbidity score.
Includes busulfan/fludarabine/TBI (n=16) and busulfan/cladribine/TBI (n=5)
Worst HLA match for patients undergoing UCB transplantation using two UCB units
Sixty eight (76%) patients had at least one comorbid condition; however, the distribution of HCT CI scores among the two groups was comparable. The common pre-transplant comorbidities observed in our cohort were pulmonary disorders (DLCO and/or FEV1 ≤ 80%, dyspnea or requiring oxygen; n=47), hepatic abnormalities (chronic hepatitis, liver cirrhosis or bilirubin, serum transaminase levels more than the upper limit of normal; n=17), cardiac abnormalities (coronary artery disease, congestive heart failure, myocardial infarction or LVEF ≤ 50%; n=16), history of prior malignancy (n=11), psychiatric disturbances (depression or anxiety needing treatment; n=10), diabetes needing treatment (n=6), obesity (body mass index > 35 kg/m2; n=6) and infections (n=5).
Survival
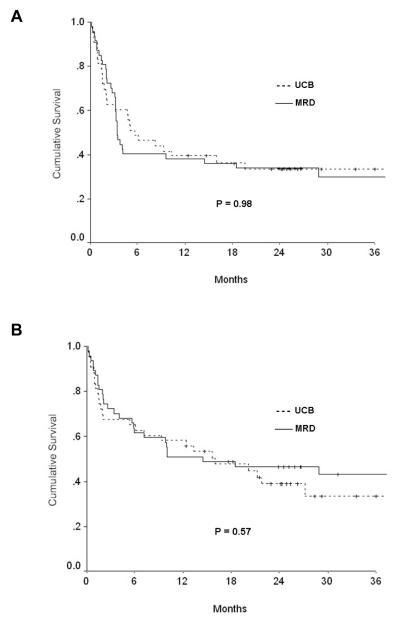
The median followup of survivors was 27 months (range, 12-61 months). The probabilities of 3-year PFS and OS were comparable between the two groups (Table 2). The probability of PFS at 3 years was 30% (95% confidence intervals [CI], 16-44%) for recipients of MRD and 34% (95% [CI], 19-48%) for UCB recipients (p=0.98). The probability of OS at 3 years was 43% (95% CI, 29-58%) for MRD and 34% (95% CI, 17-50%) for UCB recipients (p=0.57) (Figure 1).
Table 2.
Study outcomes
| End point | All patients (n=90) |
MRD (n=47) |
UCB (n=43) |
P-value1 |
|---|---|---|---|---|
| 3-year PFS (95% CI) | 32% (22-43) | 30% (16-44) | 34% (19-48) | 0.98 |
| 3-year OS (95% CI) | 39% (28-50) | 43% (29-58) | 34% (17-50) | 0.57 |
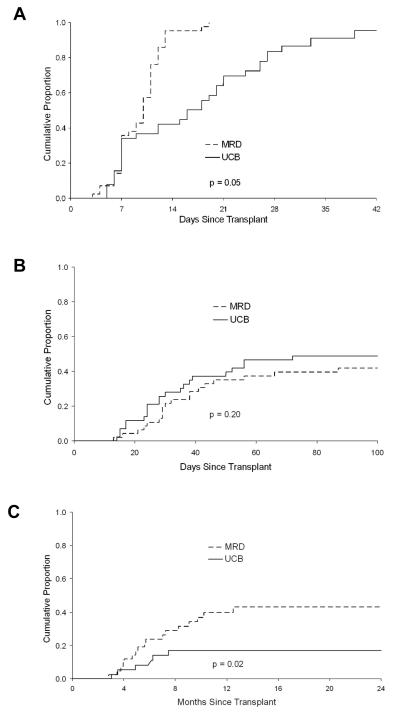
| Sustained donor engraftment at 42 days (95% CI)2,3 | 95% (93-97) | 100% | 89% (80-99) | 0.05 |
| Grade 2-4 acute GVHD (95% CI)2 | 45% (34-47) | 42% (27-57) | 49% (32-65) | 0.20 |
| Chronic GVHD at 1 year (95% CI)2 | 29% (18-40) | 40% (23-56) | 17% (5-29) | 0.02 |
| 180-day TRM (95% CI)2 | 26% (16-35) | 23% (11-36) | 28% (14-41) | 0.36 |
MRD — matched related donor; UCB — unrelated umbilical cord blood donor; TRM — transplant related mortality; GVHD — graft-versus-host disease; PFS — progression free survival; OS — overall survival; CI — confidence intervals
P-value for comparison between MRD and UCB
Cumulative incidence
Ten patients (MRD=5, UCB=5) were censored for engraftment analysis because of death prior to analysis for donor chimerism
Figure 1.
Probability of (A) progression-free and (B) overall survival. The probability of progression-free and overall survival at 3 years between the two graft sources was comparable.
On univariate analysis, PFS was significantly worse in patients who had received a previous transplant (hazard ratio [HR] 2.1, 95% CI 1.0-4.1, p=0.04), and was significantly better in recipients of Cy/Flu/TBI conditioning regimen (HR 0.5, 95% CI, 0.3-0.9, p=0.03) and in patients with a pre-transplant HCT CI score of < 3 (HR 0.5, 95% CI, 0.3-0.8, p=0.009). The same variables also affected OS; OS was significantly worse in patients who had received a previous transplant (HR 2.6, 95% CI, 1.3-5.2, p=0.007) and was significantly better in patients transplanted using the Cy/Flu/TBI regimen (HR 0.4, 95% CI, 0.2-0.8, p=0.006) or with pre-transplant HCT CI score of < 3 (HR 0.4, 95% CI, 0.2-0.7, p=0.001). Pre-transplant HCT CI score was the only independent predictor for both PFS and for OS on Cox-regression analysis (Table 3). PFS at 3 years was 50% (95% CI, 29-71%), 34% (95% CI, 17-50%) and 16% (95% CI, 0-33%) for HCT CI scores of 0, 1-2 and ≥3, respectively (p=0.03). OS at 3-years for the three risk groups was 56% (95% CI, 33-78%), 46% (95% CI, 29-64%) and 23% (95% CI, 5-40%), respectively (p=0.01).
Table 3.
Adjusted Cox-regression analysis for factors predicting progression-free and overall survival
| Variable | Progression Free Survival |
Overall Survival |
||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Donor Type | ||||
| MRD | 1.0 | 1.0 | ||
| UCB | 1.31 (0.73-2.32) | 0.36 | 1.78 (0.93-3.42) | 0.08 |
| HCT CI Score | ||||
| ≥ 3 | 1.0 | 1.0 | ||
| < 3 | 0.54 (0.30-0.96) | 0.04 | 0.45 (0.24-0.85) | 0.01 |
MRD — matched related donor; UCB — unrelated umbilical cord blood donor; HCT CI — hematopoietic cell transplantation-specific comorbidity index; HR — hazard ratio; CI — confidence intervals
Engraftment, GVHD and TRM
The cumulative incidence of TRM and grade 2-4 acute GVHD was also similar, whereas the incidence of sustained donor engraftment and chronic GVHD was lower in the UCB group (Table 2 and Figure 2).
Figure 2.
Cumulative incidence of (A) sustained donor engraftment, (B) acute grade 2-4 graft-versus-host disease (GVHD), and (C) chronic GVHD. The cumulative incidence of acute GVHD was comparable between the two graft sources, while that for donor engraftment and chronic GVHD was lower in UCB recipients.
TRM was higher among patients with HCT CI score of ≥ 3. The cumulative incidence of TRM at 180-days was 14% (95% CI, 0-28%), 19% (95% CI, 5-32%) and 44% (95% CI, 26-62%) for patients with HCT CI scores of 0, 1-2 and ≥3, respectively (p<0.01). On Cox regression analysis, HCT CI score was the only independent predictor of TRM; compared to patients with a higher score, those with a score of < 3 had HR for TRM of 0.3 (95% CI, 0.1-0.7, p=0.006).
Twenty-six MRD and 27 UCB HCT recipients have died. The primary causes of death were disease progression (MRD=14, UCB=12), infections (MRD=6, UCB=8), acute GVHD (MRD=5, UCB=1) and multi-organ failure (MRD=1, UCB=3). Two patients from the UCB cohort died of primary graft failure; the cause of death could not be determined for one UCB recipient. Four patients (MRD=1, UCB=3) have developed second malignancies following transplantation with a resultant 2-year cumulative incidence of 13% (95% CI, 0-26%). These include two patients with non-melanoma skin cancer, one patient with malignant melanoma in situ and one patient who developed Epstein-Barr virus related post-transplantation lymphoproliferative disorder 5 months after UCB transplant.
DISCUSSION
We report comparable TRM and survival following allogeneic HCT after RIC using either MRD or mismatched UCB in patients between 55-70 years of age. Our results are also comparable to other reports of RIC HCT using either a MRD or matched volunteer adult unrelated donor in older patients.2,3,20-22 The limitation of advanced age for patients who could potentially benefit by allogeneic HCT has been significantly overcome by the introduction of RIC regimens.2,20-23 However, the lack of suitable donors remains a major deterrent to transplantation in older patients. Compared to their younger counterparts, the already limited probability of finding a MRD is even more restricted as their siblings may not be healthy enough to undergo graft collections or may poorly mobilize hematopoietic cells. The use of UCB, however, expands the donor pool and offers the possibility of allogeneic HCT in older patients who otherwise lack a suitable related or unrelated donor.
Cell dose is among the most critical determinants of engraftment and transplant outcome following UCB transplantation and the limited availability of hematopoietic stem cells from a single UCB unit has been the main barrier to widespread use of UCB as a donor source, especially in adults.24,25 We have been investigating the utilization of two UCB units as a strategy to increase the total cell dose and have previously reported on the safety and efficacy of double unit UCB transplantation in both myeloablative and RIC settings.7,8,26 In this study, the majority (88%) of UCB recipients received two UCB units with acceptable rates of engraftment and transplant related deaths, supporting the use of this approach in older patients.
Despite the use of grafts with greater HLA disparity, UCB recipients in our study had a similar incidence of acute GVHD and a significantly lower incidence of chronic GVHD. Other studies have suggested that UCB might be associated with a lower risk of chronic GVHD.8,27-32 However, a significantly larger proportion of patients undergoing UCB HCT in our study also received ATG as a part of their conditioning regimen and ATG has been reported to decrease the risk of chronic GVHD after allogeneic HCT with both myeloablative and RIC regimens.33-35
We found the HCT CI to be a useful tool for pre-transplant risk assessment in this cohort of older patients. In comparison to the original cohort (median age 45 years) used by Sorror et al to describe this index,10 our relatively older cohort (median age 58 years) had a higher proportion of patients with 3 or more comorbidites (37% vs. 28%). Although HCT CI score of ≥ 3 (high-risk group) was independently predictive of PFS, TRM and OS, no significant difference was observed between the outcomes of low-risk (score 0) and intermediate-risk (score 1 and 2) groups. Though age remains an important factor determining eligibility for transplantation, the incorporation of risk assessment models such as the HCT CI into decision algorithms will further assist in selecting suitable older candidates for RIC HCT.
In conclusion, our study supports the use of HLA mismatched UCB as an alternative graft source for older patients who need a transplant but do not have a MRD. Together, use of RIC and UCB markedly extends the availability of transplant therapy to older patients previously excluded on the basis of age and lack of a suitable MRD. Healthy older HCT recipients have a low and acceptable risk of TRM and a careful review of existing comorbidities is necessary when considering older patients for HCT.
Acknowledgments
This study was supported in part by grants from the National Cancer Institute (P01CA65493) (J.E.W, J.S.M, P.B.M) and Children’s Cancer Research Fund (C.G.B, J.E.W)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Baron F, Maris MB, Sandmaier BM, et al. Graft-versus-tumor effects after allogeneic hematopoietic cell transplantation with nonmyeloablative conditioning. J Clin Oncol. 2005;23:1993–2003. doi: 10.1200/JCO.2005.08.136. [DOI] [PubMed] [Google Scholar]
- 2.Maris MB, Niederwieser D, Sandmaier BM, et al. HLA-matched unrelated donor hematopoietic cell transplantation after nonmyeloablative conditioning for patients with hematologic malignancies. Blood. 2003;102:2021–2030. doi: 10.1182/blood-2003-02-0482. [DOI] [PubMed] [Google Scholar]
- 3.Giralt S, Logan B, Rizzo D, et al. Reduced-intensity conditioning for unrelated donor progenitor cell transplantation: long-term follow-up of the first 285 reported to the national marrow donor program. Biol Blood Marrow Transplant. 2007;13:844–852. doi: 10.1016/j.bbmt.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 4.Beatty PG, Boucher KM, Mori M, Milford EL. Probability of finding HLA-mismatched related or unrelated marrow or cord blood donors. Hum Immunol. 2000;61:834–840. doi: 10.1016/s0198-8859(00)00138-5. [DOI] [PubMed] [Google Scholar]
- 5.Dodson KL, Coppo PA, Confer DL. The National Marrow Donor Program: improving access to hematopoietic stem cell transplantation. Clin Transpl. 1999:121–127. [PubMed] [Google Scholar]
- 6.Davies SM, Shu XO, Blazar BR, et al. Unrelated donor bone marrow transplantation: influence of HLA A and B incompatibility on outcome. Blood. 1995;86:1636–1642. [PubMed] [Google Scholar]
- 7.Barker JN, Weisdorf DJ, DeFor TE, Blazar BR, Miller JS, Wagner JE. Rapid and complete donor chimerism in adult recipients of unrelated donor umbilical cord blood transplantation after reduced-intensity conditioning. Blood. 2003;102:1915–1919. doi: 10.1182/blood-2002-11-3337. [DOI] [PubMed] [Google Scholar]
- 8.Brunstein CG, Barker JN, Weisdorf DJ, et al. Umbilical cord blood transplantation after nonmyeloablative conditioning: impact on transplant outcomes in 110 adults with hematological disease. Blood. 2007 doi: 10.1182/blood-2007-04-067215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Majhail NS, Weisdorf DJ, Wagner JE, Defor TE, Brunstein CG, Burns LJ. Comparable results of umbilical cord blood and HLA-matched sibling donor hematopoietic stem cell transplantation after reduced-intensity preparative regimen for advanced Hodgkin lymphoma. Blood. 2006;107:3804–3807. doi: 10.1182/blood-2005-09-3827. [DOI] [PubMed] [Google Scholar]
- 10.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Majhail NS, Brunstein CG, Wagner JE. Double umbilical cord blood transplantation. Curr Opin Immunol. 2006;18:571–575. doi: 10.1016/j.coi.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 12.Rubinstein P, Dobrila L, Rosenfield RE, et al. Processing and cryopreservation of placental/umbilical cord blood for unrelated bone marrow reconstitution. Proc Natl Acad Sci U S A. 1995;92:10119–10122. doi: 10.1073/pnas.92.22.10119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schichman SA, Suess P, Vertino AM, Gray PS. Comparison of short tandem repeat and variable number tandem repeat genetic markers for quantitative determination of allogeneic bone marrow transplant engraftment. Bone Marrow Transplant. 2002;29:243–248. doi: 10.1038/sj.bmt.1703360. [DOI] [PubMed] [Google Scholar]
- 14.Scharf SJ, Smith AG, Hansen JA, McFarland C, Erlich HA. Quantitative determination of bone marrow transplant engraftment using fluorescent polymerase chain reaction primers for human identity markers. Blood. 1995;85:1954–1963. [PubMed] [Google Scholar]
- 15.Sullivan KM, Agura E, Anasetti C, et al. Chronic graft-versus-host disease and other late complications of bone marrow transplantation. Semin Hematol. 1991;28:250–259. [PubMed] [Google Scholar]
- 16.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 17.Lin DY. Non-parametric inference for cumulative incidence functions in competing risks studies. Stat Med. 1997;16:901–910. doi: 10.1002/(sici)1097-0258(19970430)16:8<901::aid-sim543>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 18.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 19.Cox DR. Regression models and life tables. J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 20.McSweeney PA, Niederwieser D, Shizuru JA, et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood. 2001;97:3390–3400. doi: 10.1182/blood.v97.11.3390. [DOI] [PubMed] [Google Scholar]
- 21.Shimoni A, Kroger N, Zabelina T, et al. Hematopoietic stem-cell transplantation from unrelated donors in elderly patients (age >55 years) with hematologic malignancies: older age is no longer a contraindication when using reduced intensity conditioning. Leukemia. 2005;19:7–12. doi: 10.1038/sj.leu.2403591. [DOI] [PubMed] [Google Scholar]
- 22.Tsirigotis P, Bitan RO, Resnick IB, et al. A non-myeloablative conditioning regimen in allogeneic stem cell transplantation from related and unrelated donors in elderly patients. Haematologica. 2006;91:852–855. [PubMed] [Google Scholar]
- 23.Kroger N, Shimoni A, Zabelina T, et al. Reduced-toxicity conditioning with treosulfan, fludarabine and ATG as preparative regimen for allogeneic stem cell transplantation (alloSCT) in elderly patients with secondary acute myeloid leukemia (sAML) or myelodysplastic syndrome (MDS) Bone Marrow Transplant. 2006;37:339–344. doi: 10.1038/sj.bmt.1705259. [DOI] [PubMed] [Google Scholar]
- 24.Wagner JE, Barker JN, DeFor TE, et al. Transplantation of unrelated donor umbilical cord blood in 102 patients with malignant and nonmalignant diseases: influence of CD34 cell dose and HLA disparity on treatment-related mortality and survival. Blood. 2002;100:1611–1618. doi: 10.1182/blood-2002-01-0294. [DOI] [PubMed] [Google Scholar]
- 25.Schoemans H, Theunissen K, Maertens J, Boogaerts M, Verfaillie C, Wagner J. Adult umbilical cord blood transplantation: a comprehensive review. Bone Marrow Transplant. 2006;38:83–93. doi: 10.1038/sj.bmt.1705403. [DOI] [PubMed] [Google Scholar]
- 26.Barker JN, Weisdorf DJ, DeFor TE, et al. Transplantation of 2 partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood. 2005;105:1343–1347. doi: 10.1182/blood-2004-07-2717. [DOI] [PubMed] [Google Scholar]
- 27.Rocha V, Cornish J, Sievers EL, et al. Comparison of outcomes of unrelated bone marrow and umbilical cord blood transplants in children with acute leukemia. Blood. 2001;97:2962–2971. doi: 10.1182/blood.v97.10.2962. [DOI] [PubMed] [Google Scholar]
- 28.Barker JN, Davies SM, DeFor T, Ramsay NK, Weisdorf DJ, Wagner JE. Survival after transplantation of unrelated donor umbilical cord blood is comparable to that of human leukocyte antigen-matched unrelated donor bone marrow: results of a matched-pair analysis. Blood. 2001;97:2957–2961. doi: 10.1182/blood.v97.10.2957. [DOI] [PubMed] [Google Scholar]
- 29.Rocha V, Wagner JE, Jr., Sobocinski KA, et al. Graft-versus-host disease in children who have received a cord-blood or bone marrow transplant from an HLA-identical sibling. Eurocord and International Bone Marrow Transplant Registry Working Committee on Alternative Donor and Stem Cell Sources. N Engl J Med. 2000;342:1846–1854. doi: 10.1056/NEJM200006223422501. [DOI] [PubMed] [Google Scholar]
- 30.Rocha V, Labopin M, Sanz G, et al. Transplants of umbilical-cord blood or bone marrow from unrelated donors in adults with acute leukemia. N Engl J Med. 2004;351:2276–2285. doi: 10.1056/NEJMoa041469. [DOI] [PubMed] [Google Scholar]
- 31.Majhail NS, Weisdorf DJ, Wagner JE, Defor TE, Brunstein CG, Burns LJ. Comparable results of umbilical cord blood and HLA matched sibling donor hematopoietic stem cell transplant after reduced-intensity preparative regimen for advanced hodgkin’s lymphoma. Blood. 2005 doi: 10.1182/blood-2005-09-3827. [DOI] [PubMed] [Google Scholar]
- 32.Arora M, Nagaraj S, Wagner JE, et al. Chronic Graft Versus Host Disease (cGVHD) Following Unrelated Donor Hematopoietic Stem Cell Transplantation (HSCT): Higher Response Rate in Recipients of Unrelated Donor (URD) Umbilical Cord Blood (UCB) ASH Annual Meeting Abstracts. 2005;106:1814. doi: 10.1016/j.bbmt.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 33.Nachbaur D, Eibl B, Kropshofer G, et al. In vivo T cell depletion with low-dose rabbit antithymocyte globulin results in low transplant-related mortality and low relapse incidence following unrelated hematopoietic stem cell transplantation. J Hematother Stem Cell Res. 2002;11:731–737. doi: 10.1089/15258160260194884. [DOI] [PubMed] [Google Scholar]
- 34.Nakai K, Mineishi S, Kami M, et al. Antithymocyte globulin affects the occurrence of acute and chronic graft-versus-host disease after a reduced-intensity conditioning regimen by modulating mixed chimerism induction and immune reconstitution. Transplantation. 2003;75:2135–2143. doi: 10.1097/01.TP.0000066453.32263.F7. [DOI] [PubMed] [Google Scholar]
- 35.Bacigalupo A, Lamparelli T, Barisione G, et al. Thymoglobulin prevents chronic graft-versus-host disease, chronic lung dysfunction, and late transplant-related mortality: long-term follow-up of a randomized trial in patients undergoing unrelated donor transplantation. Biol Blood Marrow Transplant. 2006;12:560–565. doi: 10.1016/j.bbmt.2005.12.034. [DOI] [PubMed] [Google Scholar]