Abstract
Ethanol has complex effects on memory performance, although hippocampus-dependent memory may be especially vulnerable to disruption by acute ethanol intoxication occurring during or shortly after a training episode. In the present experiments, the effects of post-training ethanol on delay and trace fear conditioning were examined in adolescent rats. In Experiment 1, 30-day-old Sprague-Dawley rats were given delay or trace conditioning trials in which a 10 s flashing light CS was paired with a 0.5mA shock US. For trace groups, the trace interval was 10 s. On days 31-33, animals were administered ethanol once daily (0.0 or 2.5 g/kg via intragastric intubation), and on day 34 animals were tested for CS-elicited freezing. Results showed that post-training ethanol affected the expression of trace, but had no effect on delay conditioned fear. Experiment 2 revealed that this effect was dose-dependent; doses lower than 2.5 g/kg were without effect. Experiment 3 evaluated whether proximity of ethanol to the time of training or testing was critical. Results show that ethanol administration beginning 24 h after training was more detrimental to trace conditioned freezing than administration that was delayed by 48 h. Finally, in Experiment 4 animals were trained with one of three different trace intervals: 1, 3 or 10 s. Results indicate that post-training administration of 2.5 g/kg ethanol disrupted trace conditioned fear in subjects trained with a 10 s, but not with a 1 or 3 s, trace interval. Collectively the results suggest that ethanol administration impairs post-acquisition memory processing of hippocampus-dependent trace fear conditioning.
Keywords: alcohol, fear conditioning, delay conditioning, trace conditioning, hippocampus, amygdala
It has long been recognized that ethanol can have profound effects on learning and memory (Ryback, 1971). The effects of ethanol however, depend on several factors, including when ethanol is administered relative to training, the dose, and the type of task used. Research with human participants, for example, has suggested that moderate doses of ethanol given just prior to acquisition impair selective aspects of memory retrieval. Retrieval of declarative memory is generally impaired (Duka, Weissenborn & Dienes, 2001; Lister, Gorenstein & Risher-Flowers, 1991). In contrast, measures of implicit memory seem more impervious to acute ethanol and are generally immune to ethanol-induced amnesia.
In other species too, acute intoxication can interfere with some, but not all, types of learning and memory. Recent reviews of this extensive literature are now available to suggest that ethanol may have particularly detrimental effects on hippocampus-dependent forms of memory (Ryabinin, 1998; White, Matthews & Best, 2000; White & Swartzwelder, 2004). For example, acute pre-training ethanol administration to rodents dose-dependently compromises trace fear conditioning (Weitemier & Ryabinin, 2003), contextual fear conditioning (Gould, 2003; Melia, Ryabinin, Corodimas, Wilson & LeDoux, 1996) and spatial navigation (Acheson, Ross & Swartzwelder, 2001; Markwiese, Acheson, Levin, Wilson & Swartzwelder, 1998), all considered to be hippocampally-mediated tasks. In contrast, performance on non-hippocampal tasks is less severely affected (Gulick & Gould, 2007; Melia et al., 1996, Weitemier & Ryabinin, 2003), although very high doses can produce generalized disruptions in performance (e.g. McKinzie, Lee, Bronfen, Spear & Spear, 1994; Ryback, 1971; Weitemier and Ryabinin, 2003).
In addition to reports of pre-training administration, post-training ethanol also can have effects on later memory performance. Ethanol administered shortly after training to human participants often results in enhancement of memory (Bruce and Pihl, 1997; Hewitt, Holder & Laird, 1996; Lamberty, Beck & Petros, 1990; Parker, Birnbaum, Weingartner, Harltey, Stillman & Wyatt, 1980). Possible reasons for improved retention that have been postulated include a direct facilitative effect on memory consolidation processes (Parker et al., 1980) or a reduction in retroactive interference from events experienced after the target episode (Mueller, Lisman & Spear, 1983). Research examining the effects of post-training ethanol administration in rodents has yielded conflicting results. Some reports indicate that post-training administration of ethanol dose-dependently decreases avoidance performance in mice (Aversano, Ciamei, Cestari, Passino, Middei & Castellano, 2002; Castellano & Pavone, 1988) while others indicate that even very high doses of ethanol (4.5 g/kg) administered immediately after training improve retention (Alkana and Parker, 1979; Colbern, Sharek & Zimmermann, 1986). Still other research has reported no effects of post-training ethanol on retention of two types of avoidance tasks in rats (Prado de Carvolho, Vendite & Izquierdo, 1978). Gulick and Gould (2007) have shown that immediate post-training injection of moderate ethanol doses to mice has little effect on context and cued fear conditioning. Land & Spear (2004a, b) have reported impairments in odor discrimination and Pavlovian fear conditioning in rats administered ethanol shortly after training, but these effects were dependent upon the age of the animals. Thus, the effects of post-training ethanol in a variety of tasks are quite complex.
Ethanol’s amnesic effects, when they occur, have been linked to its suppressive actions within the hippocampus, which may be why hippocampal memory is generally more affected by acute ethanol intoxication than is non-hippocampal memory. Ethanol is known to suppress the spontaneous firing rate of hippocampal pyramidal neurons, diminish experience-dependent c-fos expression in hippocampal neurons, and inhibit the induction of hippocampal long-term potentiation (LTP) (Givens & McMahon, 1995; for review see Ryabinin, 1998; White et al., 2000). The question posed in the present research was whether hippocampus-dependent memory would be especially vulnerable to amnesia induced by ethanol administered after training. Here, delay and trace fear conditioning procedures were used to further understand ethanol’s relative selectivity for hippocampus-dependent memory processes. In delay conditioning procedures the unconditioned stimulus (US) is presented at the termination of a conditioned stimulus (CS), such as a light or tone. The amygdala is the brain structure most often associated with delay fear conditioning (LeDoux, 2000). For trace conditioning, a temporal gap (trace interval) separates the offset of the CS from the onset of the US. Trace fear conditioning requires hippocampal circuits in addition to the amygdala (McEchron, Tseng & Disterhoft, 2003; Quinn, Oommen, Morrison & Fanselow, 2002). If hippocampus-dependent learning is especially sensitive to ethanol-induced amnesia then administration of this drug will produce relatively selective effects on later trace conditioned responding, while sparing delay conditioned responding.
In contrast to previous studies that implemented a single administration of ethanol shortly after a learning episode, which often results in no noticeable impairment in later test performance, a repeated drug administration regimen was employed here. A previous report from our lab (Yttri, Burk & Hunt, 2004) revealed that a series of ethanol exposures (every other day for one week) prior to training resulted in substantial impairments in trace fear conditioning in adolescent subjects that were trained one week after the final ethanol administration. In the present experiments a variation of this procedure was used to assess whether ethanol-induced amnesia would result for trace, but not delay, fear conditioning when drug exposure occurred for several days after training.
A growing body of evidence indicates that performance on hippocampus-dependent tasks can be compromised by manipulations occurring after a learning episode. Kim, Clark and Thompson (1995), for example, showed that lesions of the hippocampus made 24 h after trace eyeblink conditioning in rabbits resulted in impairments in eyeblink responding when subjects were later tested. The lesions did not affect delay conditioned responding. Kim and Fanselow (1992) reported that contextual fear conditioning was also impaired by hippocampal lesions made 1 day after training. These and other findings (e.g. Quinn et al., 2002; Takehara, Kawahara & Kirino, 2003) lend support to the hypothesis that post-training memory processing that relies on the hippocampus may be malleable for some extended time (Knowlton & Fanselow, 1998).
The subjects in these experiments were adolescent rats. There were two principle reasons for using animals of this age. First, following training using procedures similar to those employed here, equivalent levels of freezing to the CS are evident following delay or trace conditioning trials (Barnet & Hunt, 2005; Yttri et al., 2004). One difficulty often inherent in studies that directly compare the effects of a manipulation on trace and delay conditioning is that trace conditioning often results in weaker responding than delay conditioning (Moye & Rudy, 1987). If this were the case, outcomes could be interpreted as reflecting an effect of the manipulation on weaker learning, as opposed to a specific effect on hippocampus-dependent memory processes per se (e.g. Beylin, Gandhi, Wood, Talk, Matzel & Shors, 2001). The second reason for a focus on this particular age group is that adolescent may be particularly vulnerable to ethanol-induced impairments in memory (e.g. Land & Spear, 2004a; Markwiese et al., 1998; Spear, 2002; but see Land & Spear, 2004b; Rajendran & Spear, 2004), and this may in part relate to developmental changes in ethanol’s disruption of hippocampal activity. Swartzwelder and colleagues, for example, have shown that hippocampal slices from juvenile and adolescent rats showed greater sensitivity to ethanol inhibition of NMDA-mediated synaptic plasticity and induction of LTP than slices obtained from adults (Pyapali, Turner, Wilson & Schwartzwelder, 1999; Swartzwelder, Wilson & Tayyeb, 1995). Thus, the adolescent brain may be especially susceptible to ethanol-induced disruption of hippocampal memory processes. Further, given the increasing prevalence of drinking, and in particular binge drinking, among adolescents (e.g. Johnston, O’Malley, Bachman & Schulenberg, 2007; Wechsler, Davenport, Dowdall, Moeykens & Castillo, 1994), and studies suggesting that early exposure to ethanol may have life-long consequences for alcohol abuse and dependence (Grant, 1998), there is currently great interest in evaluating the consequences of ethanol ingestion in this age group, and animal models of adolescent ethanol exposure are of utmost importance (Smith, 2003; Spear, 2000, 2002; Witt, 1994).
Experiment 1
The purpose of Experiment 1 was to examine the effects of post-training ethanol administration on the later expression of conditioned fear in adolescent rats. Animals were trained with either delay or trace CS-US pairings. Subjects were then administered ethanol (or water) intragastrically for three consecutive days beginning 24 h after training. Subjects were tested for CS-elicited freezing 24 h after the final ethanol administration. Thus, both training and testing occurred in a drug-free state. The prediction was that trace conditioned responding would be compromised in ethanol-exposed animals, but that delay conditioned responding would be less affected.
Method
Subjects
Forty-eight 30-day-old Sprague-Dawley-derived rats representing 6 litters were randomly assigned to one of four groups, designated according to training condition (delay or trace) and post-training ethanol dose (0.0 or 2.5 g/kg). Two animals per litter (one male and one female) were assigned to each group. The subjects were born and reared in the Psychology Department vivarium at the College of William and Mary. Males and females used for breeders were obtained from Charles River Laboratories (Wilmington, MA) and were housed in pairs in 50.8 × 40.6 × 21.6 cm polycarbonate cages with wire lids and pine chip bedding. Food (LabDiet Formula 5008) and water were available ad libitum. Cages were checked daily for pups and the day of birth was designated as postnatal day (PD) 0. Litters were culled to 8-10 pups on PD 2. Pups were weaned on PD 21 and maintained in a cage with siblings throughout the experiment. The vivarium was maintained on a 14:10 h light:dark cycle with light onset at 0600 h, and all training and testing procedures occurred during the light portion of the cycle. Procedures were approved by the Institutional Animal Care and Use Committee at the College of William and Mary and conformed to the guidelines established by the National Institutes of Health (1996).
Apparatus
Delay and trace conditioning trials occurred in two identical modified Skinner boxes, each measuring 38.0 × 26.0 × 22.0 cm. The two shorter walls were made of aluminum and the two longer walls and top were made of Plexiglas. The floor was constructed of 5-mm stainless-steel bars spaced 1.5 cm apart (center-to-center). The grid floor was connected to a custom made constant current shock generator that delivered the 0.5 mA 1 s shock US. Each chamber was located in a custom-built sound-attenuating shell measuring 67.0 × 71.5 × 71.0 cm. A 4-W red bulb was mounted on an inner wall of the sound-attenuating shell to provide constant low-level illumination. The visual CS was produced by a 25-W white bulb, the center of which was located 12 cm above the floor and 8.5 cm from the rear of the training chamber. The CS flashed at a rate of 2/s. All stimulus presentations were controlled by a PC that interfaced Coulbourn Instruments (Allentown, PA) software and hardware.
Testing occurred in one of two novel contexts that were similar except for the dimensions of the sound-attenuating shells. For both, a 29.0 × 21.5 × 46.5 cm clear Plexiglas chamber with an open top and bottom rested on a Plexiglas floor covered with brown paper. The lower 11 cm of the chambers were constructed of horizontally-mounted stainless-steel rods, 5 mm diameter and spaced 1.5 cm apart (center-to-center). The Plexiglas chamber was housed in a sound-attenuating shell (IAC; Industrial Acoustics, New York, NY) with inner walls that were painted black. A 7-W white light was mounted on an inner wall of each IAC to provide constant low-level illumination. Behavior during the test session was videotaped using Sony video cameras (Model CCD-TRV67).
Procedure
Animals were placed into the conditioning chamber for an initial 5 min period of adaptation. This was followed by five CS-US pairings. For delay conditioning, subjects were exposed to presentations of the 10 s light CS that terminated with the onset of the shock US. For the trace conditioning groups, a 10 s trace interval separated CS offset from US onset. Inter-trial intervals ranged from 200 to 300 s, and the CS-to-CS interval was equated across groups. Animals were removed from the chamber 90 s after the final shock and returned to the home cage. The single training session lasted 30 min.
On PD 31-33 animals were removed from the home cage, weighed, and administered one of two doses of ethanol via intragastric (i.g.) intubation: 0.0 or 2.5 g/kg. The 2.5 g/kg dose was selected on the basis of previous research (Acheson et al., 2001; Yttri et al., 2004). The ethanol solution was 20% v/v, made from 95% ethanol (Sigma Chemicals, St. Louis, MO) dissolved in tap water. All animals were intubated with a volume of .0158 ml/g body weight. Intubations were achieved using 15-cm lengths of polyethylene tubing (PE-50; Intramedic) attached to 5-ml disposable syringes. Ethanol or water was administered on each of the three days, at about the same time of day (between 1100 and 1300h). Immediately after i.g. administration, animals were returned to the home cage.
Testing occurred approximately 24 h after the final ethanol administration, on PD 34. Animals were placed into a novel test chamber and given 5 min of adaptation. Subjects were then presented with three nonreinforced 10 s light CSs separated by 90-120 s intervals. The test sessions were videotaped and tapes were later scored by an observer blind to subject group, using a time-sampling procedure. For 10 s prior to each stimulus and during the 10 s of the CS the subject was briefly observed once every 2 s for the presence/absence of freezing. Freezing was defined as the absence of observable movements except those necessary for respiration (Fanselow, 1980).
Statistical analyses
The freezing data were first converted into a percentage of intervals scored as freezing (ranging from 0 to 100%) during the pre-CS and CS periods. The data were then converted a Change score (% CS freezing – % preCS freezing). Change scores were used to give a relatively “pure” measure of CS-elicited freezing because nonspecific (background) freezing was removed (Barnet & Hunt, 2006; Hunt, Hess & Campbell, 1997; Thompson & Rosen, 2000; see Walker & Davis, 2002 for discussion on the utility of %Change scores as indices of learning). To assess whether there were group differences in levels of pre-CS freezing the data recorded during the 10 s prior to CS presentation on all three trials were averaged and analyzed using between-groups Analyses of Variance (ANOVA). The Change scores were analyzed similarly in each experiment. It should be noted that pre-CS freezing was generally low and did not differ between groups in any of the experiments. Thus, the Change scores were not erroneously influenced by group differences in baseline responding.
Results and Discussion
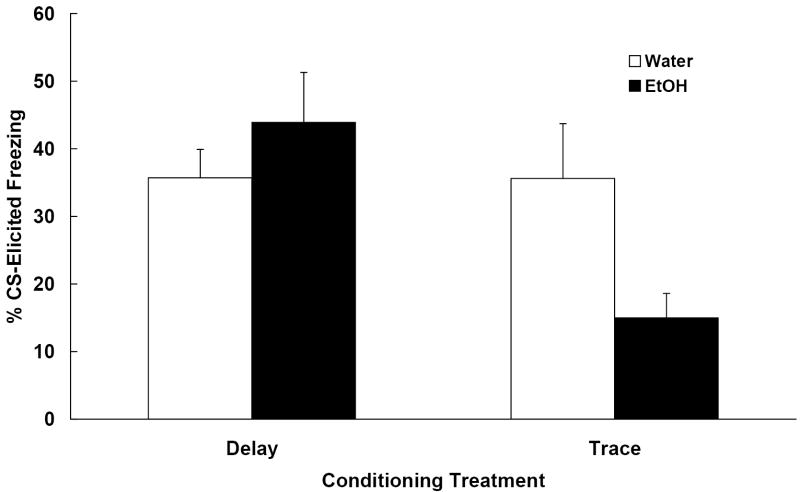
One subject assigned to the 0.0 g/kg-delay group died from an improper intubation. The data from the remaining 47 subjects were analyzed (ns = 11-12/group). Subjects administered ethanol i.g. on the three consecutive days following training exhibited less CS-elicited freezing than animals administered water, but this was only true for subjects that had been given trace CS-US pairings. Post-training ethanol had no effect on delay conditioned responding. These results are depicted in Figure 1 and were confirmed statistically. First, a 2 (ethanol dose) × 2 (conditioning treatment) ANOVA was conducted on the average pre-CS freezing data and yielded no significant effects. All groups showed equivalent and relatively low levels of freezing in the novel test context (M = 14.5 +/- 4.6%). The 2 × 2 ANOVA conducted on the Change scores yielded a significant main effect of conditioning treatment [F (1, 43) = 5.91, p < .05] and an Ethanol Dose × Conditioning Treatment interaction [F (1, 43) = 5.75, p < .05]. To further explore the interaction, post hoc comparisons were made using Newman-Keuls tests (p < .05). Subjects administered the water vehicle (0.0 g/kg) following either delay or trace conditioning exhibited freezing to the CS, the magnitude of which was equivalent. A different pattern was observed in subjects administered 2.5 g/kg ethanol; while delay conditioned responding was unaffected by post-training ethanol administration, trace conditioned responding was substantially reduced. Indeed, the freezing scores obtained from the latter group (trace-2.5 g/kg) are comparable to those seen in animals that have been given explicitly unpaired presentations of the CS and US (Barnet & Hunt, 2005). It thus appears that ethanol selectively disrupted the expression of conditioned freezing when administered after training in the hippocampus-dependent trace fear conditioning procedure.
Figure 1.
Mean (+/- SEM) change in freezing (%CS freezing - %pre-CS freezing) observed during the test session on postnatal day (PD) 34 from Experiment 1. Animals were trained with delay or trace conditioning procedures on PD 30. On PD 31-33, half of the subjects were given a single daily dose of 2.5 g/kg i.g. ethanol and the other half was given water (0.0 g/kg). Post-training ethanol disrupted trace conditioned responding, but had no effect on delay.
Experiment 2
Experiment 2 was conducted to assess the consequences of more modest doses of ethanol on trace conditioned responding. Alcohol’s effects on memory are typically dose-dependent (Acheson et al., 2001; Castellano & Pavone, 1988; Land & Spear, 2004a, b; Markwiese et al., 1998). In general, moderate to high doses of ethanol ingested prior to or shortly after a training session can impair learning, especially in hippocampal tasks, while low doses in some cases can enhance later responding (Acheson et al., 2001; Gulick & Gould, 2007; Hernandez, Valentine & Powell, 1986).
Method
Subjects
The subjects in this experiment were 60 30-day-old Sprague-Dawley rats representing 6 litters. Animals were reared as described in Experiment 1. One male and one female from each litter were assigned to each of the five dose groups.
Procedure
The procedures for trace conditioning and testing were the same as those described in Experiment 1. Trace conditioning occurred on PD 30. Animals were subsequently given 0.0, 0.5, 1.0, 1.5 or 2.5 g/kg ethanol i.g. once daily on PD 31-33. The ethanol solution was 20% v/v, and the doses were achieved through different volumes of the solution to the groups. The volume of the tap water vehicle given to the 0.0 g/kg group was equal to that of the highest (2.5 g/kg) dose of ethanol. All animals were tested for CS-elicited freezing 24 h following the final ethanol dose, on PD 34.
Results and Discussion
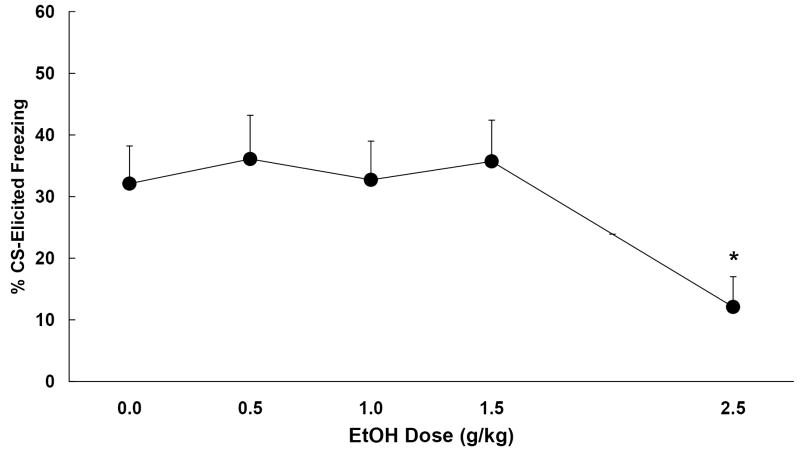
Four animals died during the intubation procedure, yielding a total of 56 subjects that were tested (ns = 11-12/group). The data are depicted in Figure 2. The one-way ANOVA conducted on the pre-CS freezing data obtained from the five dose groups revealed no significant effects. All groups exhibited low levels of pre-CS freezing during the test (M = 9.2 +/- 3.0%). The one-way ANOVA conducted on the Change scores yielded a significant main effect of ethanol dose, F (4, 51) = 2.71, p < .05. Newman-Keuls post-hoc tests revealed that only the group that was administered 2.5 g/kg ethanol exhibited significantly less freezing at test than the group administered water (0.0 g/kg). Lower doses of ethanol had little effect on subsequent conditioned freezing.
Figure 2.
Mean (+/- SEM) change in freezing (%CS freezing - %pre-CS freezing) observed in Experiment 2. Subjects were given trace conditioning trials on PD 30, and administered ethanol (range 0.0-2.5 g/kg i.g.) once daily for three consecutive days. Post-training ethanol disrupted the subsequent expression of trace conditioned freezing, but only at a relatively high (2.5 g/kg) dose. Lower doses did not significantly disrupt conditioned freezing. * indicates group differs significantly from 0.0 g/kg.
Experiment 3
The previous experiments revealed that ethanol administered once daily during the interval between training and testing can impair the later expression of trace conditioned fear. What remains to be determined, however, is whether ethanol administration is most detrimental to test performance if given closer to the time of training or to the time of test. In the previous experiments ethanol treatment began 24 h following training and ended 24 h prior to the test and therefore did not allow analysis of the temporal dynamics of ethanol’s effects on trace fear conditioning. The question addressed in this experiment was whether ethanol results in amnesia by impairing post-acquisition processing of information that occurs shortly after the learning episode. It could also be that ethanol interferes with later stages of memory processing or that lingering effects of ethanol (“hangover”) on the day of test may have influenced trace conditioned responding (e.g. Spiers & Fusco, 1992). The design of the present experiment afforded examination of these possibilities.
In this experiment all animals were trained on PD 30 and were tested on PD 35. Two groups of rats (E31-33 and E32-34) were given three daily doses of 2.5 g/kg ethanol i.g. Group E31-33 was given ethanol on postnatal days 31 through 33, identical to the treatment used in the previous experiments. For this group, ethanol was proximal to the time of training and the final dose was given 48 h prior to test. Group E32-34 was also given ethanol once daily for three consecutive days. However, for this group the first ethanol administration did not occur until 48 h after training and the final ethanol dose was given the day before test. If ethanol is detrimental to the expression of trace conditioning by impacting early consolidation process (Parker et al., 1980) or reducing retroactive interference (Mueller et al., 1983), then ethanol administered closer to the time of training in group E31-33 would be expected to have the greater effect. In contrast, if ethanol is having its debilitating effects by affecting later processing of the acquired information, or the expression of the memory at test, then the opposite pattern of findings should result. Two additional groups of ethanol-exposed subjects were included. These groups, E31 and E34, were administered a single dose of 2.5 g/kg ethanol i.g., either 24 h after training or 24 h prior to test. Finally, the control group (W31-34) was administered an equivalent volume of the water vehicle on PD 31-34.
Methods
Subjects
Subjects in this experiment were 50 Sprague-Dawley rats, 30 days of age at the beginning of the experiment and representing 5 litters. One male and one female rat per litter were assigned to each of five treatment groups: E31, E31-33, E32-34, E34 or W31-34.
Procedure
All subjects were given five trace conditioning trials on PD 30, as previously described, and were tested for CS-elicited freezing on PD 35. Animals in group E31 were administered a single dose of 2.5 g/kg ethanol i.g. 24 h after trace conditioning. Group E31-33 was administered 2.5 g/kg once daily for three consecutive days beginning 24 h after training. Group E32-34 also was given three consecutive days of ethanol (2.5 g/kg) but treatment did not begin until 48 h after training, on PD 32. Group E34 was administered a single dose of 2.5 g/kg ethanol 24 h prior to test. On days without drug administration the animals remained in the home cage and were not subjected to the intubation procedure. Finally, group W31-34 was administered the water vehicle for each of the four days between training and test.
Results and Discussion
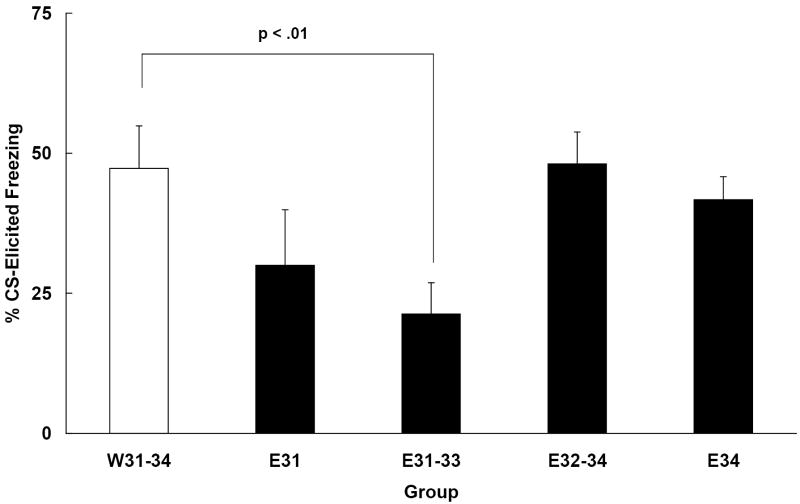
One subject assigned to group E32-34 died from an improper intubation, leaving 49 subjects for which data were collected (ns = 9-10/group). The one-way ANOVA conducted on the pre-CS data indicated no group differences (M = 18.6 +/- 3.6%). The ANOVA conducted on the Change scores yielded a main effect of group, F (4, 44) = 3.13, p < .05. Results are shown in Figure 3. Post hoc comparisons (Newman-Keuls tests) revealed that group E31-33 exhibited significantly less freezing at test than group W31-34. No other group differed from water controls. Group E31’s performance was intermediate, not differing from E31-33 or W31-34. These data suggest that ethanol administration occurring nearer to the time of training, as opposed to that closer to the time of test, is more detrimental to the later expression of trace conditioned fear, and that multiple daily doses of ethanol have a greater effect than a single dose. Furthermore, ethanol given for the three days prior to test, but not beginning until 48 h post-training, had no effect on CS-elicited freezing, as indicated by the similarity in the levels of freezing exhibited by groups E32-34 and W31-34.
Figure 3.
Mean (+/- SEM) change in freezing (%CS freezing - %pre-CS freezing)recorded during a test session that occurred on PD 35 in Experiment 3. Subjects were all given trace conditioning trials, in which a 10 s trace interval was used, on PD 30. Animals differed in the timing of ethanol administration post-training. Some subjects were given ethanol beginning 24 h after training (E31 and E31-33) while some subjects were given ethanol closer to the day of test (E32-34 and E34). The control group (W31-34) was given water on each of the four days between training and test.
Experiment 4
Results from the previous experiments reveal that ethanol given daily for three consecutive days following training disrupts trace conditioned responding. This effect is likely due to ethanol’s disruption of activity within the hippocampus (Givens & McMahon, 1995; White et al., 2000). However, the dependence of trace conditioning on the hippocampus is determined by the relative length of the trace interval. Hippocampal lesions disrupt trace eyeblink conditioning (Port, 1986; Moyer, Deyo & Disterhoft, 1990) and trace fear conditioning (Chowdhury, Quinn & Fanselow, 2005), but only when the trace interval used during training is relatively long. Training with short trace intervals is generally much less affected by lesions to the hippocampus. This suggests that the presence of a trace interval per se is not a sufficient condition to observe disruption by hippocampal lesions. A similar temporal dependence on trace fear conditioning has been observed following lesions of the medial septum that provides cholinergic input to the hippocampus (McAlonan, Wilkinson, Robbins & Everitt,1995) as well as pre-training administration of NMDA receptor antagonists (Misane, Tovote, Meyer, Spiess, Ögren & Steidl, 2005; Takatsuki, Kawahara & Takehara, 2001). In light of these findings, the final experiment was designed to evaluate the effects of post-training ethanol administration on responding to a CS that was trained using one of three different trace interval durations (1, 3 or 10 s). The specific trace intervals chosen were largely based on those used by Chowdhury et al. (2005).
Method
Subjects
Sixty 30-day-old rats representing ten different litters were used as subjects. No more than one male and one female rat per litter were assigned to each of the six groups. Groups were designated on the basis of post-training ethanol dose (0.0 or 2.5 g/kg) and duration of the trace interval used for training (1, 3 or 10 s).
Procedure
The procedures were similar to those used in the previous experiments. On PD 30 all animals were given 5 trace conditioning trials during a 30-min session. The trace interval separating CS offset from US onset was 1, 3 or 10 s, and the CS-to-CS interval was equated for all groups. On PD 31-33 animals were administered a single daily dose of 0.0 or 2.5 g/kg ethanol i.g. Testing for CS-elicited freezing occurred on PD 34 using the procedures described in Experiment 1.
Results and Discussion
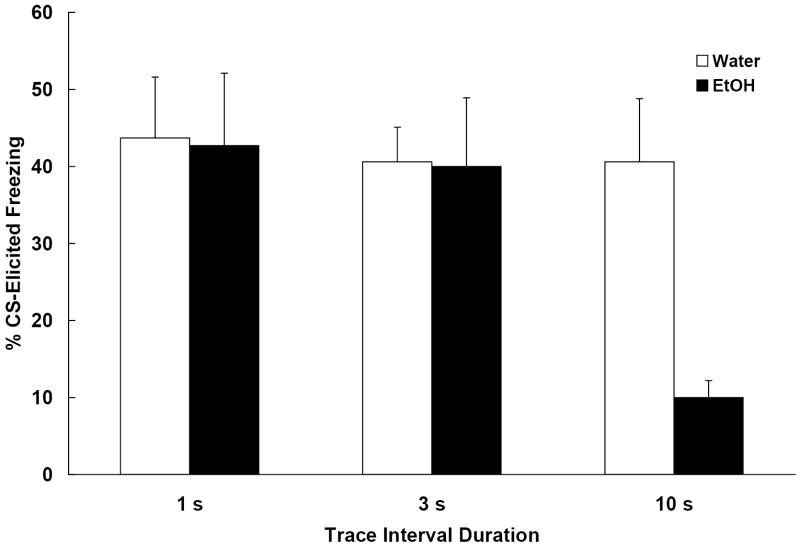
One subject assigned to the 1 s trace interval-2.5 g/kg ethanol group died from an improper intubation. The data obtained from the remaining 59 animals were analyzed (ns = 9-10/group). The results, shown in Figure 4, indicate that post-training ethanol affected conditioned freezing only in the subjects trained with the 10 s trace interval. Responding by subjects that were trained with either the 1 or 3 s trace interval was unaffected by post-training ethanol. The 2 (ethanol dose) × 3 (trace interval) ANOVA conducted on the pre-CS freezing data revealed no significant effects. All groups showed equivalent levels of pre-CS freezing (M = 23.6 +/- 3.5%). The ANOVA conducted on the Change scores yielded a significant main effect of trace interval [F (2, 53) = 3.85, p < .05] and an Ethanol Dose × Trace Interval interaction [F (2, 53) = 3.71, p < .05]. Newman-Keuls post hoc tests indicated that the group exposed to ethanol following training with a 10 s trace interval exhibited significantly less CS-elicited freezing at test than the corresponding water controls. Groups trained with the shorter trace intervals, 1 and 3 s, were not affected by ethanol. Thus, post-training ethanol disrupted the expression of trace conditioned fear, but only when the prior training involved a relatively long trace interval. These data parallel the effects of hippocampal lesions on trace fear conditioning (Chowdhury et al., 2005).
Figure 4.
Mean (+/- SEM) change in freezing (%CS freezing - %pre-CS freezing) observed during the test on postnatal day (PD) 34 from Experiment 4. All animals were trained with trace CS-US pairings on PD 30. The trace interval varied depending on group (1, 3 or 10 s). On PD 31-33, subjects were administered one of two doses of ethanol (0.0 or 2.5 g/kg). Post-training ethanol disrupted trace conditioning, but only for subjects that were trained with the longest (10 s) trace interval duration.
General Discussion
The results of these experiments indicate that memory processing following Pavlovian trace conditioning is malleable and subject to disruption by repeated ethanol administration beginning 24 h after fear conditioning. This effect was specific for the hippocampus-dependent trace conditioning task, as delay fear conditioning was unaffected by post-training ethanol exposure. The present data are in accord with those of Kim et al. (1995) and Quinn et al. (2002) demonstrating that lesions of the hippocampus impair trace conditioned responding when made 24 h after training and extend these finding to the amnesic effects of ethanol.
Collectively the behavioral results reported here support the suggestion that ethanol’s memory-impairing effects are primarily due to its actions on hippocampal function (for reviews see Ryabinin, 1998; White et al., 2000; White & Swartzwelder, 2004). In Experiment 1 it was shown that delay fear conditioning, which is hippocampus-independent (e.g. Quinn et al., 2002), was not affected by post-training ethanol. In contrast, trace conditioning which is hippocampus-dependent, was adversely affected. Results of pre-training administration of ethanol have likewise reported impaired trace fear conditioning (Weitemier and Ryabinin, 2003) and contextual fear conditioning (Gulick & Gould, 2007; Melia et al., 1996), with a notable lack of effect on performance of delay conditioning. Results demonstrating relatively selective effects of ethanol intoxication on measures of declarative memory in humans (Duka et al., 2001; Lister et al., 1991) also support the hypothesis that the hippocampus is a primary target for ethanol-induced memory deficits.
The memory impairing effects of ethanol were shown to be dose-dependent in Experiment 2. Post-training administration of doses ranging from 0.5 to 1.5 g/kg had little effect on later conditioned freezing to the trace CS, whereas responding was significantly reduced following exposure to the highest (2.5 g/kg) dose. This dose of ethanol has also been found to impair trace conditioning when repeatedly administered to adolescent rats prior to training (Yttri et al., 2004). These latter findings suggest that repeated exposure to ethanol for even a relatively short period of time may, in some circumstances, result in a relatively long-lasting alteration in hippocampal function.
Experiment 3 results indicated that ethanol-induced disruption in trace conditioned freezing depended on ethanol administration beginning 24 h after training. In this experiment it was also found that a single post-training exposure to ethanol given one day after conditioning had no effect on conditioned freezing, a result that is similar to that reported by other laboratories in which a single dose of ethanol was administered immediately after a training session (Gould & Lommock, 2003; Gulick & Gould, 2007; Land & Spear, 2004b). A closer examination of the differences between single and repeated ethanol exposures on hippocampal cellular activity and function (Roberto, Nelson & Ur, 2002) could provide insights into the mechanism of ethanol’s consequences for hippocampus-dependent memory.
Results from Experiment 4 demonstrate that ethanol-induced disruption of trace conditioning depended on the duration of the trace interval used during training. The only group to display impaired conditioned freezing by post-training ethanol was the one trained with a relatively long (10 s) trace interval. Groups trained with shorter trace intervals were unaffected by post-training ethanol. These findings are in agreement with other data showing that drug-and lesion-induced disruptions of hippocampal function likewise have effects that are dependent on the length of the trace interval (Chowdhury et al., 2005; Misane et al., 2005). It seems that hippocampus involvement in trace conditioning is not a ubiquitous phenomenon, and that the relative contribution of the hippocampus to trace conditioning lies along a continuum that is determined by trace interval length.
The role of the hippocampus for establishing trace conditioning is not entirely clear. Acquisition of trace conditioning is assumed to require the maintenance of a memorial representation of the CS that spans the length of the trace interval during CS-US pairings, and research has shown that hippocampal neurons exhibit altered firing rates during the trace interval that may serve as a neural code/memory of the CS (e.g. Green & Arenos, 2007; McEchron et al., 2003; Rodriguez and Levy, 2001). However, the effects of post-training manipulations suggest that the hippocampus also contributes to memory processing occurring after training, perhaps by promoting the consolidation of the trace memory (Kim et al., 1995; Quinn et al., 2002). Given the available data, it is likely that the hippocampus importantly contributes to both. Ethanol as well may induce amnesia via effects on both processes, given that ethanol administration occurring either pre- (Weitemier and Ryabinin, 2003) or post-training (present experiments) results in substantial deficits in trace conditioned responding. The findings of Experiment 3, that ethanol is much more disruptive to later trace performance when administered proximal to the time of training further implicates effects on processing of information that occurs around the time of acquisition.
Ethanol has widespread effects within the central nervous system, and although hippocampal function is altered by ethanol, this structure is not necessarily the only site of ethanol’s deleterious consequences on trace conditioned responding. Another possible site for ethanol’s amnesic effects that could lead to impaired responding in this paradigm is the frontal cortex. Recent studies have implicated the medial prefrontal cortex (mPFC) as a storage site for trace memories, and lesions of the mPFC made prior to training result in impairments in trace, but not delay, eyeblink conditioning (Kronforst-Collins & Disterhoft, 1998; McLaughlin, Skaggs, Churchwell & Powell, 2002; Runyan, Moore & Dash, 2004). Frontal function is compromised by acute ethanol intoxication (Devenport & Hale, 1989; Peterson, Rothfleisch & Zelazo, 1990) and damage to the frontal lobe can result from long-term alcohol use (Crews, Braun, Hoplight, Switzer & Knapp, 2000). It is thus possible that the memory impairment observed in the present experiments is due to ethanol’s actions in the frontal cortex as opposed to, or in addition to, its known actions within the hippocampus (cf. Takahara et al., 2003).
The adolescent brain may be especially sensitive to the depressive actions of ethanol on neural activity (Slawecki, Betancourt, Cole & Ehlers, 2001; Swartzwelder et al., 1995). Also, regions of the adolescent brain, such as the hippocampus and frontal cortex, may be quite vulnerable to pathology arising from repeated ethanol administration because these regions continue to undergo substantial maturational changes during this time (Dahl & Spear, 2004; De Bellis et al., 2000). It is well established that periods of exposure to high blood-ethanol concentrations and repeated withdrawals may exacerbate ethanol’s neurotoxicity (Becker & Hale, 1993; Hunt, 1993). Importantly such intermittent exposure to high doses of ethanol, intermixed with periods of abstinence, is a hallmark feature of adolescent drinking (Spear, 2002; Wechsler et al., 1994). Although the present research did not specifically compare memory impairment by ethanol at different ages, other studies have shown that adolescent rats may be more susceptible to the amnesia effects of ethanol in some tasks (Land & Spear, 2004a; Markwiese et al., 1998; but see Land & Spear, 2004b). The present results do, however, show a profound decrement in memory for prior trace conditioning resulting from intermittent, post-training ethanol intoxication in adolescent rats. Future research specifically comparing performance of adolescents with other age groups should yield important information about the neurobehavioral consequences of binge-like ethanol ingestion (Dahl & Spear, 2004; Grant, 1998; Spear, 2002; White & Swartzwelder, 2004).
Acknowledgments
This research was supported by National Institute on Alcohol Abuse and Alcoholism grant AA015343 and by a Howard Hughes Medical Institute grant through the Undergraduate Biological Sciences Education Program to the College of William and Mary.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acheson SK, Ross EL, Swartzwelder HS. Age-independent and dose-response effects of ethanol on spatial memory in rats. Alcohol. 2001;23:167–175. doi: 10.1016/s0741-8329(01)00127-6. [DOI] [PubMed] [Google Scholar]
- Alkana RL, Parker ES. Memory facilitation by post-training injection of ethanol. Psychopharmacology. 1979;66:117–119. doi: 10.1007/BF00427617. [DOI] [PubMed] [Google Scholar]
- Aversano M, Ciamei A, Cestari V, Passino E, Middei S, Castellano C. Effects of MK-801 and ethanol combinations on memory consolidation in CD1 mice: Involvement of GABAergic mechanisms. Neurobiology of Learning and Memory. 2002;77:327–337. doi: 10.1006/nlme.2001.4029. [DOI] [PubMed] [Google Scholar]
- Barnet RC, Hunt PS. Trace and long-delay fear conditioning in the developing rat. Learning and Behavior. 2005;33:437–443. doi: 10.3758/bf03193182. [DOI] [PubMed] [Google Scholar]
- Barnet RC, Hunt PS. The expression of fear-potentiated startle during development: Integration of learning and response systems. Behavioral Neuroscience. 2006;120:861–872. doi: 10.1037/0735-7044.120.4.861. [DOI] [PubMed] [Google Scholar]
- Becker HC, Hale RL. Repeated episodes of alcohol withdrawal potentiate the severity of subsequent withdrawal seizures: An animal model of alcohol withdrawal “kindling.”. Alcoholism: Clinical and Experimental Research. 1993;17:94–98. doi: 10.1111/j.1530-0277.1993.tb00731.x. [DOI] [PubMed] [Google Scholar]
- Beylin AV, Gandhi CC, Wood GE, Talk AC, Matzel LD, Shors TJ. The role of the hippocampus in trace conditioning: Temporal discontinuity or task difficulty? Neurobiology of Learning and Memory. 2001;76:447–461. doi: 10.1006/nlme.2001.4039. [DOI] [PubMed] [Google Scholar]
- Bruce K, Pihl RO. Forget “drinking to forget:” Enhanced consolidation of emotionally charged memory by alcohol. Experimental Clinical Psychopharmacology. 1997;5:242–250. doi: 10.1037//1064-1297.5.3.242. [DOI] [PubMed] [Google Scholar]
- Castellano C, Pavone F. Effects of ethanol on passive avoidance behavior in the mouse: Involvement of GABAergic mechanisms. Pharmacology, Biochemistry & Behavior. 1988;29:321–324. doi: 10.1016/0091-3057(88)90163-3. [DOI] [PubMed] [Google Scholar]
- Chowdhury N, Quinn JJ, Fanselow MS. Dorsal hippocampus involvement in trace fear conditioning with long, but not short, trace intervals in mice. Behavioral Neuroscience. 2005;119:1396–1402. doi: 10.1037/0735-7044.119.5.1396. [DOI] [PubMed] [Google Scholar]
- Colbern DL, Sharek P, Zimmermann EG. The effect of home or novel environment on the facilitation of passive avoidance by post-training ethanol. Behavioral and Neural Biology. 1986;46:1–12. doi: 10.1016/s0163-1047(86)90850-2. [DOI] [PubMed] [Google Scholar]
- Crews FT, Braun CJ, Hoplight B, Switzer RC, Knapp DJ. Binge ethanol consumption causes differential brain damage in young adolescent rats compared with adult rats. Alcoholism: Clinical and Experimental Research. 2000;24:1712–1723. [PubMed] [Google Scholar]
- Dahl RE, Spear LP. Adolescent brain development: Vulnerabilities and opportunities. New York Academy of Sciences. 2004;1021 doi: 10.1196/annals.1308.001. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Clark DB, Beers SR, Soloff PH, Boring AM, Hall J, Kersh A, Keshaven MS. Hippocampal volume in adolescent-onset alcohol use disorders. American Journal of Psychiatry. 2000;157:737–744. doi: 10.1176/appi.ajp.157.5.737. [DOI] [PubMed] [Google Scholar]
- Devenport LD, Hale RL. Contributions of hippocampus and neocortex to the expression of ethanol’s effects. Psychopharmacology. 1989;99:337–344. doi: 10.1007/BF00445554. [DOI] [PubMed] [Google Scholar]
- Duka T, Weissenborn R, Dienes Z. State-dependent effects of alcohol on recollective experience, familiarity and awareness of memories. Psychopharmacology. 2001;153:295–306. doi: 10.1007/s002130000564. [DOI] [PubMed] [Google Scholar]
- Fanselow MS. Conditional and unconditional components of post-shock freezing. Pavlovian Journal of Biological Science. 1980;15:177–182. doi: 10.1007/BF03001163. [DOI] [PubMed] [Google Scholar]
- Givens B, McMahon K. Ethanol suppresses the induction of long-term potentiation in vivo. Brain Research. 1995;688:27–33. doi: 10.1016/0006-8993(95)00499-g. [DOI] [PubMed] [Google Scholar]
- Gould TJ. Ethanol disrupts fear conditioning in C57BL/6 mice. Journal of Psychopharmacology. 2003;17:77–81. doi: 10.1177/0269881103017001702. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Lommock JA. Nicotine enhances contextual fear conditioning and ameliorates ethanol-induced deficits in contextual fear conditioning. Behavioral Neuroscience. 2003;117:1276–1282. doi: 10.1037/0735-7044.117.6.1276. [DOI] [PubMed] [Google Scholar]
- Grant BF. The impact of a family history of alcoholism on the relationship between age at onset of alcohol use and DSM-IV alcohol dependence: Results from the National Longitudinal Alcohol Epidemiologic Survey. Alcohol Health and Research World. 1998;22:144–147. [PMC free article] [PubMed] [Google Scholar]
- Green JT, Arenos JD. Hippocampal and cerebellar single-unit activity during delay and trace eyeblink conditioning in the rat. Neurobiology of Learning and Memory. 2007;87:269–284. doi: 10.1016/j.nlm.2006.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulick D, Gould TJ. Acute ethanol has biphasic effects on short-and long-term memory in both foreground and background contextual fear conditioning in C57BL/6 mice. Alcoholism: Clinical and Experimental Research. 2007;31:1528–1537. doi: 10.1111/j.1530-0277.2007.00458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez LL, Valentine JD, Powell DA. Ethanol enhancement of Pavlovian conditioning. Behavioral Neuroscience. 1986;100:494–503. doi: 10.1037//0735-7044.100.4.494. [DOI] [PubMed] [Google Scholar]
- Hewitt GP, Holder M, Laird J. Retrograde enhancement of human kinesthetic memory by alcohol: Consolidation or protection against interference? Neurobiology of Learning and Memory. 1996;65:269–277. doi: 10.1006/nlme.1996.0032. [DOI] [PubMed] [Google Scholar]
- Hunt WA. Are binge drinkers more at risk of developing brain damage? Alcohol. 1993;10:559–561. doi: 10.1016/0741-8329(93)90083-z. [DOI] [PubMed] [Google Scholar]
- Hunt PS, Hess MF, Campbell BA. Conditioned cardiac and behavioral response topography to an olfactory CS dissociates with age. Animal Learning and Behavior. 1997;25:53–61. [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future National Survey Results on Drug Use, 1975-2006: Vol 1, Secondary School Students (NIH Pub No 07-6205) National Institute of Drug Abuse; Bethesda, MD: 2007. [Google Scholar]
- Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Clark RE, Thompson RE. Hippocampectomy impairs the memory of recently, but not remotely, acquired trace eyeblink conditioned responses. Behavioral Neuroscience. 1995;109:195–203. doi: 10.1037//0735-7044.109.2.195. [DOI] [PubMed] [Google Scholar]
- Knowlton BJ, Fanselow MS. The hippocampus, consolidation and on-line memory. Current Opinion in Neurobiology. 1998;8:293–296. doi: 10.1016/s0959-4388(98)80154-2. [DOI] [PubMed] [Google Scholar]
- Kronforst-Collins MA, Disterhoft JF. Lesions of the caudal area of rabbit medial prefrontal cortex impair trace eyeblink conditioning. Neurobiology of Learning and Memory. 1998;69:147–162. doi: 10.1006/nlme.1997.3818. [DOI] [PubMed] [Google Scholar]
- Lamberty GJ, Beckwith BE, Petros TV. Posttrial treatment with ethanol enhances recall of prose narratives. Physiology & Behavior. 1990;48:653–658. doi: 10.1016/0031-9384(90)90206-j. [DOI] [PubMed] [Google Scholar]
- Land C, Spear NE. Ethanol impairs memory of a simple discrimination in adolescent rats at doses that leave adult memory unaffected. Neurobiology of Learning and Memory. 2004a;81:75–81. doi: 10.1016/j.nlm.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Land C, Spear NE. Fear conditioning is impaired in adult rats by ethanol doses that do not affect periadolescents. International Journal of Developmental Neuroscience. 2004b;22:355–362. doi: 10.1016/j.ijdevneu.2004.04.008. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lister RG, Gorenstein C, Risher-Flowers D. Dissociation of the acute effects of alcohol on implicit and explicit memory processes. Neuropsychologia. 1991;29:1205–1212. doi: 10.1016/0028-3932(91)90034-6. [DOI] [PubMed] [Google Scholar]
- Markwiese BJ, Acheson SK, Levin ED, Wilson WA, Swartzwelder HS. Differential effects of ethanol on memory in adolescent and adult rats. Alcoholism: Clinical and Experimental Research. 1998;22:416–421. [PubMed] [Google Scholar]
- McAlonan GM, Wilkinson LS, Robbins TW, Everitt BJ. The effects of AMPA-induced lesions of the septohippocampal cholinergic projection on aversive conditioning to explicit and contextual cues and spatial learning in the water maze. European Journal of Neuroscience. 1995;7:281–292. doi: 10.1111/j.1460-9568.1995.tb01064.x. [DOI] [PubMed] [Google Scholar]
- McEchron MD, Tseng W, Disterhoft JF. Single neurons in CA1 hippocampus encode trace interval duration during trace heart rate (fear) conditioning in rabbit. Journal of Neuroscience. 2003;23:1535–1547. doi: 10.1523/JNEUROSCI.23-04-01535.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinzie DJ, Lee J, Bronfen JH, Spear LP, Spear NE. Context and tone conditioning are selectively impaired by ethanol in the preweanling rat: Effects of dose and time of administration. Behavioral and Neural Biology. 1994;62:201–209. doi: 10.1016/s0163-1047(05)80018-4. [DOI] [PubMed] [Google Scholar]
- McLaughlin J, Skaggs H, Churchwell J, Powell DA. Medial prefrontal cortex and Pavlovian conditioning: Trace versus delay conditioning. Behavioral Neuroscience. 2002;116:37–47. [PubMed] [Google Scholar]
- Melia KR, Ryabinin AE, Corodimas KP, Wilson MC, LeDoux JE. Hippocampal-dependent learning and experience-dependent activation of the hippocampus are preferentially disrupted by ethanol. Neuroscience. 1996;74:313–322. doi: 10.1016/0306-4522(96)00138-8. [DOI] [PubMed] [Google Scholar]
- Misane I, Tovote P, Meyer M, Spiess J, Ögren SO, Stiedl O. Time-dependent involvement of the dorsal hippocampus in trace fear conditioning in mice. Hippocampus. 2005;15:418–426. doi: 10.1002/hipo.20067. [DOI] [PubMed] [Google Scholar]
- Moye TB, Rudy JW. Ontogenesis of trace conditioning in young rats: Dissociation of associative and memory processes. Developmental Psychobiology. 1987;20:405–414. doi: 10.1002/dev.420200405. [DOI] [PubMed] [Google Scholar]
- Moyer JR, Deyo RA, Disterhoft JF. Hippocampectomy disrupts trace eye-blink conditioning in rabbits. Behavioral Neuroscience. 1990;104:243–252. doi: 10.1037//0735-7044.104.2.243. [DOI] [PubMed] [Google Scholar]
- Mueller CW, Lisman SA, Spear NE. Alcohol enhancement of human memory: Tests of consolidation and interference hypotheses. Psychopharmacology. 1983;80:226–230. doi: 10.1007/BF00436158. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health. Principles of Laboratory Animal Care (pub No 85-23) 1996 [Google Scholar]
- Parker ES, Birnbaum IM, Weingartner H, Hartley JT, Stillman RC, Wyatt RJ. Retrograde enhancement of human memory with alcohol. Psychopharmacology. 1980;69:219–222. doi: 10.1007/BF00427653. [DOI] [PubMed] [Google Scholar]
- Peterson JB, Rothfleisch J, Zelazo PD. Acute alcohol intoxication and cognitive functioning. Journal of Studies on Alcohol. 1990;51:114–122. doi: 10.15288/jsa.1990.51.114. [DOI] [PubMed] [Google Scholar]
- Port RL. Retention and acquisition of classical trace conditioned responses by rabbits with hippocampal lesions. Behavioral Neuroscience. 1986;100:745–752. doi: 10.1037//0735-7044.100.5.745. [DOI] [PubMed] [Google Scholar]
- Prado de Carvalho L, Vendite DA, Izquierdo I. A near-lethal dose of ethanol, given intraperitoneally, does not affect memory consolidation of two different avoidance tasks. Psychopharmacology. 1978;59:71–74. doi: 10.1007/BF00428033. [DOI] [PubMed] [Google Scholar]
- Pyapali GK, Turner DA, Wilson WA, Swartzwelder HS. Age and dose-dependent effects of ethanol on the induction of hippocampal long-term potentiation. Alcohol. 1999;19:107–111. doi: 10.1016/s0741-8329(99)00021-x. [DOI] [PubMed] [Google Scholar]
- Quinn JJ, Oommen SS, Morrison GE, Fanselow MS. Post-training excitotoxic lesions of the dorsal hippocampus attenuate forward trace, backward trace, and delay fear conditioning in a temporally specific manner. Hippocampus. 2002;12:495–504. doi: 10.1002/hipo.10029. [DOI] [PubMed] [Google Scholar]
- Rajendran P, Spear LP. The effects of ethanol on spatial and nonspatial memory in adolescent and adult rats studied using an appetitive paradigm. Annals of the New York Academy of Sciences. 2004;1021:441–444. doi: 10.1196/annals.1308.060. [DOI] [PubMed] [Google Scholar]
- Roberto M, Nerlson TE, Ur CL. Long-term potentiation in the rat hippocampus is reversibly depressed by chronic intermittent ethanol exposure. Journal of Neurophysiology. 2002;87:2385–2397. doi: 10.1152/jn.2002.87.5.2385. [DOI] [PubMed] [Google Scholar]
- Rodriguez P, Levy WB. A model of hippocampal activity in trace conditioning: Where’s the trace? Behavioral Neuroscience. 2001;115:1224–1238. doi: 10.1037//0735-7044.115.6.1224. [DOI] [PubMed] [Google Scholar]
- Runyan JD, Moore AN, Dash PK. A role for prefrontal cortex in memory storage for trace fear conditioning. Journal of Neuroscience. 2004;24:1288–1295. doi: 10.1523/JNEUROSCI.4880-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryabinin AE. Role of hippocampus in alcohol-induced memory impairment: Implications from behavioral and immediate early gene studies. Psychopharmacology. 1998;139:34–43. doi: 10.1007/s002130050687. [DOI] [PubMed] [Google Scholar]
- Ryback RS. The continuum and specificity of the effects of alcohol on memory. Quarterly Journal of Studies on Alcohol. 1971;32:995–1016. [PubMed] [Google Scholar]
- Slawecki CJ, Betancourt M, Cole M, Ehlers CL. Periadolescent alcohol exposure has lasting effects on adult neurophysiological function in rats. Developmental Brain Research. 2001;128:63–72. doi: 10.1016/s0165-3806(01)00150-x. [DOI] [PubMed] [Google Scholar]
- Smith RF. Animal models of periadolescent substance abuse. Neurotoxicology and Teratology. 2003;25:291–301. doi: 10.1016/s0892-0362(02)00349-5. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and the college drinker: Biological basis of propensity to use and misuse alcohol. Journal of Studies on Alcohol. 2002;Suppl. 14:71–81. doi: 10.15288/jsas.2002.s14.71. [DOI] [PubMed] [Google Scholar]
- Spiers DE, Fusco LE. Delayed thermoregulatory changes in the immature rat following a single injection of ethanol. Alcoholism: Clinical and Experimental Research. 1992;16:41–47. doi: 10.1111/j.1530-0277.1992.tb00633.x. [DOI] [PubMed] [Google Scholar]
- Swartzwelder HS, Wilson WA, Tayyeb MI. Differential sensitivity of NMDA receptor-mediated synaptic potentials to ethanol in immature versus mature hippocampus. Alcoholism: Clinical and Experimental Research. 1995;19:320–323. doi: 10.1111/j.1530-0277.1995.tb01509.x. [DOI] [PubMed] [Google Scholar]
- Takatsuki K, Kawahara S, Takehara K. Effects of the noncompetitive NMDA receptor antagonist MK-801 on classical eyeblink conditioning in mice. Neuropharmacology. 2001;41:618–628. doi: 10.1016/s0028-3908(01)00113-7. [DOI] [PubMed] [Google Scholar]
- Takehara K, Kawahara S, Kirino Y. Time-dependent reorganization of the brain components underlying memory retention in trace eyeblink conditioning. Journal of Neuroscience. 2003;23:9897–9905. doi: 10.1523/JNEUROSCI.23-30-09897.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson BL, Rosen JB. Effects of TRH on acoustic startle, conditioned fear and active avoidance in rats. Neuropeptides. 2000;34:38–44. doi: 10.1054/npep.1999.0785. [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M. Quantifying fear potentiated startle using absolute versus proportional increase scoring methods: Implications for the neurocircuitry of fear and anxiety. Psychopharmacology. 2002;164:318–328. doi: 10.1007/s00213-002-1213-0. [DOI] [PubMed] [Google Scholar]
- Wechsler H, Davenport A, Dowdall GW, Moeykens B, Castillo S. Health and behavioral consequences of binge-drinking in college: A national survey at 140 campuses. Journal of the American Medical Association. 1994;272:1672–1677. [PubMed] [Google Scholar]
- Weitemier AZ, Ryabinin AE. Alcohol-induced memory impairment in trace fear conditioning: A hippocampus-specific effect. Hippocampus. 2003;13:305–315. doi: 10.1002/hipo.10063. [DOI] [PubMed] [Google Scholar]
- White AM, Matthews DB, Best PJ. Ethanol, memory, and hippocampal function: A review of recent findings. Hippocampus. 2000;10:88–93. doi: 10.1002/(SICI)1098-1063(2000)10:1<88::AID-HIPO10>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- White AM, Swartzwelder HS. Hippocampal function during adolescence: A unique target of ethanol effects. Annals of the New York Academy of Sciences. 2004;1021:206–220. doi: 10.1196/annals.1308.026. [DOI] [PubMed] [Google Scholar]
- Witt ED. Mechanisms of alcohol abuse and alcoholism in adolescents: A case for developing animal models. Behavioral and Neural Biology. 1994;62:168–177. doi: 10.1016/s0163-1047(05)80015-9. [DOI] [PubMed] [Google Scholar]
- Yttri EA, Burk JA, Hunt PS. Intermittent ethanol exposure in adolescent rats: Dose-dependent impairments in trace conditioning. Alcoholism: Clinical and Experimental Research. 2004;28:1433–1436. doi: 10.1097/01.alc.0000147657.51745.a7. [DOI] [PubMed] [Google Scholar]