Abstract
Interneurons expressing the calcium-binding protein parvalbumin (PV) are a critical component of the inhibitory circuitry of the basolateral nuclear complex (BLC) of the mammalian amygdala. These neurons form interneuronal networks interconnected by chemical and electrical synapses, and provide a strong perisomatic inhibition of local pyramidal projection neurons. Immunohistochemical studies in rodents have shown that most PV-positive (PV+) cells are GABAergic interneurons that co-express the calcium-binding protein calbindin (CB), but exhibit no overlap with interneuronal subpopulations containing the calcium-binding protein calretinin (CR) or neuropeptides. Despite the importance of identifying interneuronal subpopulations for clarifying the major players in the inhibitory circuitry of the BLC, very little is known about these subpopulations in primates. Therefore, in the present investigation dual-labeling immunofluorescence histochemical techniques were used to characterize PV+ interneurons in the basal and lateral nuclei of the monkey amygdala. These studies revealed that 90–94% of PV+ neurons were GABA+, depending on the nucleus, and that these neurons constituted 29–38 % of the total GABAergic population. CB+ and CR+ interneurons constituted 31–46% and 23–27%, respectively, of GABAergic neurons. Approximately one-quarter of PV+ neurons contained CB, and these cells constituted one-third of the CB+ interneuronal population. There was no colocalization of PV with the neuropeptides somatostatin or cholecystokinin, and virtually no colocalization with CR. These data indicate that the neurochemical characteristics of the PV+ interneuronal subpopulation in the monkey BLC are fairly similar to those seen in the rat, but there is far less colocalization of PV and CB in the monkey. These findings suggest that PV+ neurons are a discrete interneuronal subpopulation in the monkey BLC and undoubtedly play a unique functional role in the inhibitory circuitry of this brain region.
Keywords: gamma-aminobutyric acid (GABA), calbindin, calretinin, somatostatin, cholecystokinin
The basolateral nuclear complex of the amygdala (BLC) plays an important role in forebrain circuits involved in emotional behavior and learning (Aggleton, 2000, Shinnick and Gallagher, 2003). Understanding neuronal mechanisms mediating emotional information processing in the BLC will require knowledge of the anatomy and physiology of its main cell types. Previous studies have shown that there are two major cell classes in the BLC: pyramidal neurons and nonpyramidal neurons. Although these cells do not exhibit a laminar or columnar organization, their anatomical and electrophysiological characteristics are remarkably similar to those of their counterparts in the cerebral cortex (McDonald, 1992a; Washburn and Moises, 1992; Rainnie et al., 1993; Pare et al., 2003). Thus, the principal neurons in the BLC are spiny pyramidal-like projection neurons that utilize glutamate as an excitatory neurotransmitter (Fuller et al., 1987; McDonald, 1992, 1996a), whereas most nonpyramidal neurons in the BLC are spine-sparse interneurons that utilize gamma-aminobutyric acid (GABA) as an inhibitory neurotransmitter (McDonald, 1982; Carlsen, 1988; McDonald and Pearson, 1989).
As in the cerebral cortex, subpopulations of GABAergic interneurons in the rat BLC contain calcium-binding proteins (parvalbumin [PV], calbindin [CB], and calretinin [CR]) and neuropeptides (vasoactive intestinal peptide [VIP], somatostatin [SOM], neuropeptide Y, and cholecystokinin [CCK]; McDonald and Pearson, 1989; Kemppainen and Pitkänen, 2000; McDonald and Mascagni, 2001a). The results of recent double-labeling studies suggest that the lateral and basolateral nuclei of the rat BLC contain at least four distinct subpopulations of interneurons: 1) PV+/CB+ neurons, 2) SOM+/CB+ neurons, 3) large multipolar CCK+ neurons that are often CB+, and 4) small bipolar and bitufted interneurons that exhibit extensive colocalization of VIP, CR, and CCK (Kemppainen and Pitkänen, 2000; McDonald and Mascagni, 2001a, 2002; McDonald and Betette, 2001; Mascagni and McDonald, 2003).
Interneurons expressing PV are a critical component of the inhibitory circuitry of the BLC. PV+ interneurons constitute 19–43% of the GABAergic interneurons in the rodent BLC, depending on the nucleus, and form interneuronal networks interconnected by chemical and electrical synapses (McDonald and Mascagni, 2001a; Muller et al., 2005;Woodruff and Sah, 2007a). As in the cortex, many PV+ interneurons in both the rodent and primate BLC appear to be basket or chandelier cells that provide a strong perisomatic inhibition of local pyramidal neurons (Pitkänen and Amaral, 1993a; Sorvari et al., 1995, 1996b; McDonald and Betette, 2001; McDonald and Mascagni, 2001a; Rainnie et al., 2006; Woodruff and Sah, 2007a,b). Most excitatory inputs to PV+ interneurons arise from axon collaterals of local BLC pyramidal cells (Smith et al., 2000; McDonald et al., 2005). The interconnections of PV+ interneuronal networks with pyramidal cells appear to constitute the anatomical substrates for the generation of synchronized rhythmic oscillations related to arousal and emotional memory in the BLC (Paré and Collins, 2000; Paré et al., 2002; Seidenbecher et al., 2003; Pape et al., 2005; Narayanan et al., 2007).
Since anatomical and physiological studies have shown that distinct interneuronal subpopulations in the rodent basolateral nucleus exhibit unique connections and electrophysiological characteristics (Muller et al., 2003, 2005, 2006, 2007; Rainnie et al., 2006; Woodruff and Sah, 2007a), each subpopulation undoubtedly plays a unique functional role in the intrinsic circuitry of this brain region. Despite the importance of identifying interneuronal subpopulations for clarifying the major players in the inhibitory circuitry of the BLC, very little is known about these subpopulations in primates. Therefore, in the present investigation dual localization of PV with GABA, CB, CR, SOM and CCK using immunofluorescence histochemistry was performed to characterize PV-immunoreactive interneurons in the lateral and basolateral nuclei of the macaque amygdala. In addition, dual localization of CB and CR with GABA was carried out to determine the relative sizes of these GABAergic interneuronal subpopulations.
EXPERIMENTAL PROCEDURES
Tissue preparation
These immunohistochemical experiments were performed on amygdalas obtained from four macaque monkeys housed at Yerkes National Primate Research Center at Emory University, Atlanta, Georgia (one amygdala from each animal). Two of the monkeys were rhesus macaques (Macaca mulatta) and two were pigtail macaques (Macaca nemestrina) (Table 1). Animals were deeply anesthetized with an overdose of sodium pentobarbital (100 mg/kg), and then perfused with one of two fixatives: (1) a mixture of 4% paraformaldehyde/0.2% glutaraldehyde/0.2% picric acid (Zamboni’s fixative) in phosphate buffer (0.1 M, pH 7.4; PB), or (2) 4% paraformaldehyde in PB (Table 1). The care of the animals and all anesthesia and euthanasia procedures in this study were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of Emory University. All efforts were made to minimize animal suffering and to use the minimum number of animals necessary to produce reliable scientific data.
Table 1.
Macaques used in this study.
| Animal # | Species | Age, Sex | Fixative |
|---|---|---|---|
| YMZ-1 | Pigtail | 6.5 y.o., female | Zamboni’s |
| YMZ-2 | Pigtail | 2.0 y.o., male | Zamboni’s |
| YMP-2 | Rhesus | 1.2 y.o., female | 4% Paraformaldehyde |
| YMP-3 | Rhesus | 9.0 y.o., male | 4% Paraformaldehyde |
Coronal 50μm vibratome sections were cut and processed for immunohistochemistry, or were stored frozen in 15% sucrose in PB until immunohistochemical labeling was performed. A one-in-ten series of sections through each amygdala were processed for each double-labeling combination (see below), which generated 6–7 equally spaced sections at 500 μm intervals through each amygdala. In addition, a one-in-ten series of sections through each amygdala was Nissl-stained (cresyl violet) so that borders of amygdalar nuclei could be recognized in neighboring sections processed for immunofluorescence, using blood vessels and fiber bundles as landmarks.
Immunoperoxidase staining of parvalbumin
In two of the macaques a series of sections was stained for parvalbumin using a monoclonal primary antibody (see below) and the avidin-biotin immunoperoxidase (ABC) technique. Sections were processed for immunohistochemistry in tissue culture chamber slides. All antibodies were diluted in PBS containing Triton X-100 (0.5%) and 1% normal goat serum. Sections were incubated in primary antibody overnight at 4° C and then processed for the avidin-biotin immunoperoxidase technique using a biotinylated goat anti-mouse secondary antibody (1:500; Jackson ImmunoResearch Laboratories, West Grove PA) and a Standard Vectastain ABC kit (Vector Laboratories, Burlingame, CA). DAB (3, 3′-diaminobenzidine-4HCl, Sigma Chemical Co., St. Louis, MO) was used as a chromogen to generate a brown reaction product. Following the immunohistochemical procedures, sections were mounted on gelatinized slides, dried overnight, dehydrated in ethanol, cleared in xylene, and coverslipped in Permount (Fisher Scientific, Pittsburgh, PA).
Immunofluorescence experiments
Colocalization of GABA with PV, CB, or CR was performed in the two macaques that were perfused with Zamboni’s fixative (YMZ-1 and YMZ-2). The following antibodies were used for these studies: rabbit anti-GABA (1:1000; Sigma), mouse anti-PV (1:5000; Swant, Bellinzona, Switzerland), mouse anti-CB (1:8000; Sigma), and mouse anti-CR (1:2000, Chemicon International, Inc., Temecula, CA, USA). Sections were incubated in a cocktail of two primary antibodies (either GABA/PV, GABA/CB, or GABA/CR) overnight at 4°C, rinsed in three changes of PBS (10 min each), and then incubated in a cocktail of Alexa 488-labeled goat anti-rabbit IgG (1:200; Molecular Probes, Eugene, OR, USA) and Alexa 568-labeled goat anti-mouse IgG (1:400, Molecular Probes) for 4 h at room temperature. As in all other experiments (see below), both secondary antibodies were highly cross-adsorbed by the manufacturer to insure specificity for primary antibodies raised in rabbit or mouse. Sections were then rinsed in three changes of PBS (10 min each) and mounted on glass slides using Vectashield mounting medium (Vector Laboratories).
Colocalization of PV with CB or CR was performed in two macaques (YMZ-1 and YMP-2). The following antibodies were used for these studies: mouse anti-PV (1:5000; Swant), rabbit anti-calbindin-D-28K (1:8000, antiserum R-8701 obtained from Dr. Kenneth Baimbridge, University of British Columbia), and rabbit anti-CR (1:1000, Chemicon). Sections were incubated in a cocktail of primary antibodies (either PV/CB or PV/CR) overnight at 4°C, rinsed in three changes of PBS (10 min each), and then incubated in a cocktail of Alexa 488-labeled goat anti-rabbit IgG (1:400; Molecular Probes) and Alexa 568-labeled goat anti-mouse IgG (1:400, Molecular Probes) for 4 h at room temperature. Sections were then rinsed in three changes of PBS (10 min each) and mounted on glass slides using Vectashield mounting medium (Vector Laboratories).
Colocalization of PV with somatostatin (SOM) or cholecystokinin (CCK) was performed in two macaques (YMP-2 and YMP-3). The mouse anti-PV antibody (1:5000; Swant) and a rabbit polyclonal somatostatin-28 antibody (1:500; Peninsula Laboratories, San Carlos, CA) were used for the PV/SOM study, whereas a rabbit anti-PV antibody (antiserum R-301, obtained from Dr. Kenneth Baimbridge, University of British Columbia) and a mouse anti-CCK antibody (1:500; monoclonal antibody No. 9303, obtained from Dr. J.H. Walsh, UCLA) were used for the PV/CCK study. Sections were incubated in a cocktail of primary antibodies overnight at 4°C, rinsed in three changes of PBS (10 min each), and then incubated in a cocktail of Alexa 488-labeled goat anti-rabbit IgG (1:400; Molecular Probes) and Alexa 568-labeled goat anti-mouse IgG (1:400, Molecular Probes) for 4 h at room temperature. Sections were then rinsed in three changes of PBS (10 ml each) and mounted on glass slides using Vectashield mounting medium (Vector Laboratories).
Sections were examined with a Bio-Rad MRC-1024 confocal laser scanning system equipped with an argon-krypton laser attached to a Nikon Eclipse E800M microscope. Fluorescence of Alexa 488 (green) and Alexa 568 (red) dyes was analyzed using filter configurations for sequential excitation/imaging via 488-nm and 568-nm channels. Since GABA-like immunoreactivity was only seen on the outer surfaces of the sections, analysis of the colocalization of GABA with calcium-binding proteins was accomplished by focusing on the upper and lower surfaces of each section using the lowest iris aperture of the confocal microscope (which results in the thinnest ‘optical section’). This step was not necessary for experiments involving colocalization of PV with other calcium binding proteins or peptides, since immunostaining of these substances extended throughout the entire thickness of the section. In each of the confocal immunofluorescence cases some control sections were processed with one of the two primary antibodies omitted. In all cases only the color of the corresponding secondary fluorescent antibody was observed, and only on the appropriate channel. These results indicated that the secondary antibodies were specific for rabbit or mouse IgGs, and that there was no “crosstalk” between the red and green channels (Wouterlood et al., 1998).
All portions of the macaque BLC were examined for qualitative analysis of colocalization of interneuronal markers, and cell counts were performed in the dorsal intermediate subdivision of the lateral nucleus (Ldi) and the magnocellular subdivision of the basal nucleus (Bmc) near the junction of the middle and caudal thirds of the amygdala (i.e., approximately at the level where the “dogleg” of the basal nucleus contacts the fibers of the temporal limb of the anterior commissure; Fig. 1A). Both the Ldi and Bmc have a high density of PV+ neurons at this level (see Fig. 2C in Pitkänen and Amaral, 1998). Counts of single-labeled and double-labeled neurons in each nucleus were performed in 2 sections per animal for each dual localization combination. At 200× magnification, cell counts were made from the image of a 400×400-μm field displayed for merged (red/green) channels on the computer screen (double-labeled cells appear yellow). Images of the non-merged red and green channels were also displayed. Only somata of non-pyramidal neurons were counted. As in the rat, some pyramidal cells in the monkey BLC exhibit low levels of CB or CR. However, as in rat, they were easily distinguished from the intensely stained non-pyramidal neurons at the antibody dilutions used in this study.
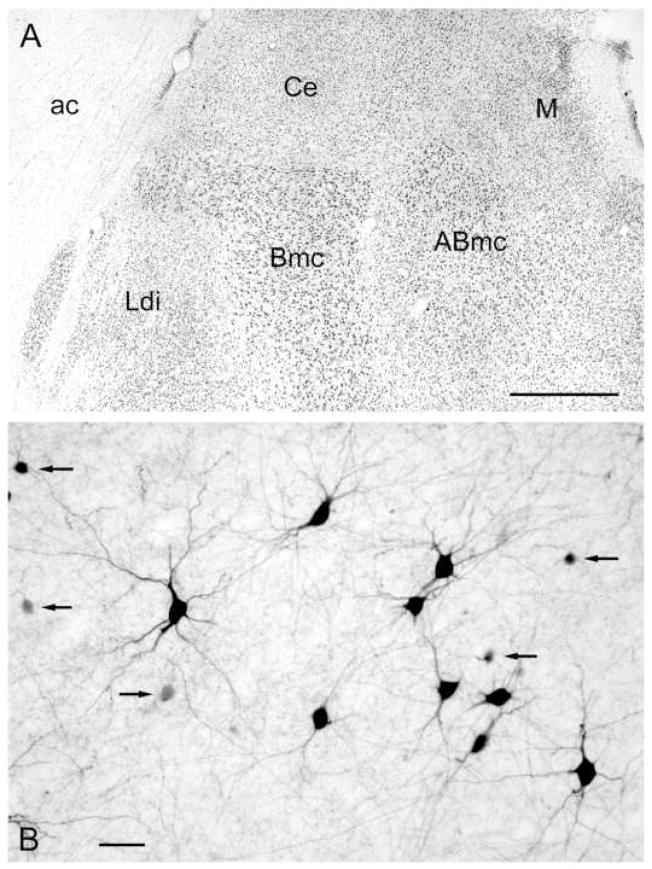
Fig. 1.
A. Photomicrograph of the dorsal half of the macaque amygdala in a Nissl-stained coronal section showing the two nuclei of the BLC from which cell counts were performed in immunofluorescence experiments: Ldi (dorsal intermediate subdivision of the lateral nucleus); and Bmc (magnocellular subdivision of the basal nucleus). Orientation: the Bmc is medial to the Ldi, and ventral to the central nucleus (Ce). Abbreviations: ABmc, magnocellular subdivision of the accessory basal nucleus; ac, anterior commissure; Ce, central amygdalar nucleus; M, medial amygdalar nucleus. B. Photomicrograph of PV+ neurons in the lateral amygdalar nucleus stained by immunoperoxidase histochemistry. Note the various sizes and shapes of PV+ neurons that characterize all nuclei of the BLC. Some of the cells are large with well-stained dendritic arborizations whereas others are small to medium-sized (arrows). Scale bars = 1 mm in A, 50 μm in B.
There were no noticeable differences in the cytoarchitecture or chemoarchitecture of the BLC in the two macaque species used in this study. Chi-square statistical tests indicated that there were no significant differences (P > 0.05) in the cell counts obtained from the two different animals used for each dual-labeling immunohistochemical combination. Therefore, cell counts for each nucleus were pooled from the two animals used for each dual localization combination.
Because of differences in the sizes of somata in each of the interneuronal populations, the percentages of colocalization obtained in this study are approximations of the actual percentages that exist in each nucleus. All of the interneuronal subpopulations investigated in the present study were morphologically heterogeneous, with perikaryal diameters varying from about 10 to 20 μm. However, qualitative observations revealed that the CB+ and CR+ interneuronal subpopulations had a greater number of smaller neurons, compared to the PV+ subpopulation. Since for all of the GABA colocalization experiments in the present study, somata were sampled from the upper or lower surfaces of the sections, the number of smaller neurons would tend to be underestimated. Thus, the proportions of CR+/GABA+ and CB+/GABA+ neurons obtained in this study might be slightly less than the true proportions, since on average these cell were smaller than most PV+/GABA+ neurons.
Antibody specificity
The antibodies used in this study were all markers for specific neuronal subpopulations in the BLC. Each produced the characteristic pattern of marker immunostaining seen in previous studies of the monkey BLC (PV: Pitkänen and Amaral, 1993a; GABA: McDonald and Augustine, 1993; Pitkänen and Amaral, 1994; CB: Pitkänen and Amaral, 1993b; CR: McDonald, 1994; SOM: Amaral et al., 1989; McDonald et al., 1995). The mouse monoclonal PV antibody utilized in this study (Swant #235) is one of the most widely used PV antisera in studies of the central nervous system. The immunogen used to generate the antibody was carp-II PV. The specificity of this antibody has been well documented (Celio et al., 1988). The polyclonal PV antiserum (antiserum R-301, generously donated by Dr. Kenneth Baimbridge, University of British Columbia) is also one of the most widely used PV antisera in studies of the central nervous system. It was raised in rabbit against rat muscle PV. Previous immunohistochemical adsorption studies have shown that it recognizes PV, but not CR or CB (Conde et al., 1994).
The mouse monoclonal calbindin-D-28K antibody (Sigma, clone CB-955, cat. # C9848) was raised against bovine kidney calbindin-D-28K. Western blot studies conducted by the manufacturer using Madin Darby Bovine Kidney cell extract showed a single band of 28 kD. Additional studies conducted by the manufacturer have shown that this antibody does not react with other members of the EF-hand family. The rabbit polyclonal anti-calbindin-D-28K (antiserum R-8701 obtained from Dr. Kenneth Baimbridge, University of British Columbia) was raised against bovine cerebellar calbindin-D-28K. Previous immunohistochemical adsorption studies have shown that it recognizes CB, but not PV or CR (Conde et al., 1994). The mouse monoclonal anti-calretinin antibody (Chemicon, clone 6B8.2, cat. # MAB1568) was raised against recombinant rat calretinin. Western blot studies conducted by the manufacturer using rat cerebellum cytosolic extract showed a single band of 31kD. The rabbit polyclonal antibody to calretinin (# AB5054, Chemicon) was raised against recombinant rat calretinin. Western blot studies conducted by the manufacturer indicate that it is specific for calretinin and recognizes both calcium-bound and calcium-unbound conformations of this protein.
The mouse monoclonal CCK antibody (antibody # 9303, generously donated by Dr. J.H. Walsh, UCLA) was raised against gastrin, but recognizes cholecystokinin because of homologies in the terminal pentapeptide shared by these peptides. Gastrin is not found in the telencephalon (Rehfeld, 1978; Rehfeld and Lundberg, 1983), and this antibody has been used in numerous studies of the forebrain to study the distribution of cholecystokinin. Preadsorption of this antibody with CCK-8 (25 μg/ml; Sigma) abolished all immunostaining in the rat amygdala and other forebrain regions (McDonald and Mascagni, 2001b). The polyclonal antibody to somatostatin (# T-4547; Peninsula Laboratories) was raised in rabbit against somatostatin-28. Studies conducted by the manufacturer indicate that it recognizes somatostatin-28 but does not react with various other neuropeptides including somatostatin-14, substance P, CCK, or VIP. The rabbit polyclonal GABA antibody (#A-2052, Sigma) was raised against GABA conjugated to bovine serum albumin. In studies conducted by the manufacturer it showed positive binding with GABA in a dot blot assay, and negative binding with bovine serum albumin.
RESULTS
Colocalization of GABA with parvalbumin, calbindin, and calretinin
Immunohistochemical staining for GABA, PV, CB and CR was identical to that seen in previous studies of the monkey BLC (McDonald and Augustine, 1993; Pitkänen and Amaral, 1993a,b; Pitkänen and Amaral, 1994; McDonald, 1994). Thus, GABA-immunoreactive (GABA+) and PV+ neurons were morphologically heterogeneous (Figs. 1–3), and their densities varied in different nuclei. All appeared to be spine-sparse nonpyramidal neurons. Whereas the most intensely-stained CB+ and CR+ neurons in the BLC were nonpyramidal neurons, there were some large lightly-stained neurons with piriform or pyramidal somata that appeared to be pyramidal cells, as demonstrated in previous studies of the monkey BLC (McDonald, 1994; Pitkänen and Amaral, 1993b). These immunopositive pyramidal cells were not included in the cell counts.
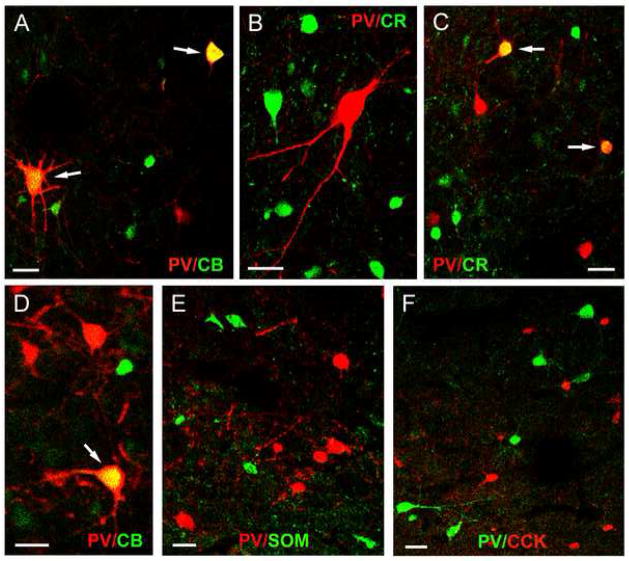
Fig. 3.
A) Dual localization of PV and interneuronal markers in merged images (double-labeled neurons are indicated in yellow). A) Dual localization of PV (red) and CB (green) in the Ldi. Two neurons (arrows) are double-labeled in this field (yellow). B) Dual localization of PV (red) and CR (green) in the Bmc. No neurons are double-labeled in this field. C) Dual localization of PV (red) and CR (green) in the Ldi. Two neurons (arrows) are double-labeled in this field (yellow). D) Dual localization of PV (red) and CB (green) in the Bmc. One neuron (arrow) is double-labeled in this field (yellow). E) Dual localization of PV (red) and SOM (green) in the Ldi. No neurons are double-labeled. F) Dual localization of PV (green) and CCK (red) in the Bmc. No neurons are double-labeled. Scale bars = 25 μm for all images.
The great majority of PV+ and CB+ nonpyramidal cells in all nuclei of the BLC, including the Ldi and Bmc, were also GABA+ (Fig. 2; Table 2). The level of GABA immunoreactivity in many CR+ cells was somewhat less than that seen in PV+ and CB+ subpopulations, and the percentage of CR+ cells that were GABA+ was also slightly lower (Table 2). Approximately one-quarter to one-half of GABA+ neurons were co-labeled with each calcium-binding protein antibody (Table 2).
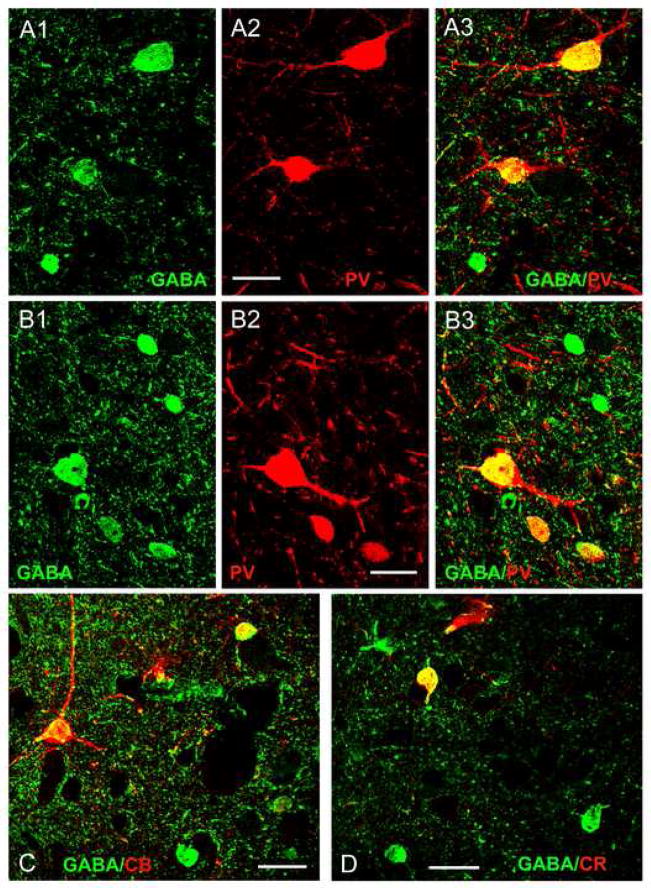
Fig. 2.
Colocalization of GABA and calcium-binding proteins using immunofluorescence confocal laser scanning microscopy. (A1–A3) Colocalization of GABA and PV in the Bmc: (A1) Three GABA+ somata (green). (A2) Two PV+ somata (red) in the same field. (A3) Merging of the red and green channels indicates that both of the PV+ somata express GABA (yellow). (B1–B3) Colocalization of GABA and PV in the Ldi: (B1) Several GABA+ somata (green). (B2) Three PV+ somata (red) in the same field. (B3) Merging of the red and green channels indicates that all three of the PV+ somata express GABA (yellow). C) Colocalization of GABA (green) and CB (red) in the Ldi. Colocalization of GABA and CB is indicated in yellow in this merged image. D) Colocalization of GABA (green) and CR (red) in the Bmc. Colocalization of GABA and CR is indicated in yellow in this merged image. Scale bars = 25 μm for all images.
Table 2.
Dual localization of three different calcium-binding proteins (CaBP: PV, CB or CR) with GABA in the monkey BLC. Cell counts for each nucleus were pooled from two animals for each dual localization combination.
| CaBP | Nucleus | Single-labeled CaBP | Single-labeled GABA | Double-labeled GABA/CaBP | % CaBP Double-labeled | % GABA Double-labeled |
|---|---|---|---|---|---|---|
| PV | Ldi | 3 | 108 | 44 | 93.6% | 28.9% |
| Bmc | 6 | 88 | 53 | 89.9% | 37.6% | |
| CB | Ldi | 1 | 101 | 45 | 97.8% | 30.8% |
| Bmc | 11 | 71 | 61 | 84.7% | 46.2% | |
| CR | Ldi | 7 | 95 | 29 | 80.5% | 23.4% |
| Bmc | 4 | 102 | 38 | 90.5% | 27.1% |
Colocalization of parvalbumin with calbindin and calretinin
There was fairly extensive colocalization of PV and CB in the BLC (Figs. 3A, D). In the Ldi approximately one quarter of both PV+ neurons (25.6%) and CB+ neurons (23.0%) also contained the other calcium-binding protein (Table 3). Similarly, in the Bmc, approximately one third of both PV+ neurons (33.8%) and CB+ neurons (33.7%) also contained the other calcium-binding protein. There was virtually no colocalization of PV and CR in both the Ldi and Bmc. Only an occasional neuron (0–2 per section) was double-labeled (Fig. 3B, C).
Table 3.
Dual localization of PV and CB in the monkey BLC. Cell counts for each nucleus were pooled from two animals.
| Nucleus | Single-labeled PV | Single-labeled CB | Double-labeled PV/CB | % PV Double-labeled | % CB Double-labeled |
|---|---|---|---|---|---|
| Ldi | 122 | 141 | 42 | 25.6% | 23.0% |
| Bmc | 131 | 132 | 67 | 33.8% | 33.7% |
Lack of colocalization of parvalbumin with the neuropeptides somatostatin and CCK
Consistent with previous studies of the macaque BLC, SOM+ neurons were morphologically heterogeneous, but most were medium-sized, bitufted, nonpyramidal neurons with fusiform somata (Amaral et al., 1989; McDonald et al., 1995). All CCK+ neurons in the BLC were nonpyramidal neurons, and the great majority were small (6–10 μm in diameter) bipolar or multipolar neurons. However, a few CCK+ neurons were large (about 20 μm in diameter) multipolar nonpyramidal cells. These two types of CCK+ neurons closely resembled the small type S CCK+ neurons and the large type L CCK+ neurons, respectively, observed in the rat BLC (Mascagni and McDonald, 2003). No colocalization of PV with SOM or CCK was observed in the macaque BLC (Figs. 3E, F).
DISCUSSION
Colocalization of calcium-binding proteins with GABA in BLC interneurons
The overwhelming majority of PV+ neurons in the macaque BLC (90–95%, depending on the nucleus) were also GABA+. Overlap in these neuronal subpopulations in the monkey BLC was not surprising since virtually all PV+ neurons in the rat BLC are GABA+ (Kemppainen and Pitkänen, 2000; McDonald and Mascagni, 2001a). As in previous studies in the rat (Kemppainen and Pitkänen, 2000; McDonald and Bettete 2001; McDonald and Mascagni 2001a) and macaque BLC (Pitkänen and Amaral, 1993b; McDonald 1994), two types of CB+ and CR+ neurons were observed in the present study: (1) large lightly-stained presumptive pyramidal cells, and (2) intensely-stained nonpyramidal interneurons. Consistent with previous studies in the rat demonstrating that pyramidal cells are glutamatergic (Fuller et al., 1987; McDonald, 1996a), none of the lightly-stained presumptive pyramidal cells were GABA+. In contrast, the great majority of CB+ and CR+ nonpyramidal interneurons in the macaque BLC were GABA+.
The percentages of PV+ and CB+ interneurons in the macaque BLC that were GABA+ were similar to those obtained in the rat (McDonald and Mascagni, 2001a), but the overall percentages of CR+ neurons that were GABA+ (80–90%, depending on the nucleus) were slightly greater than those seen in the rat BLC (67–81%, depending on the nucleus). A Chi-square statistical test indicated that the percentages of CR+ neurons that were GABA+ in the Bmc of the monkey (90.5%) and the homologous anterior subdivision of the basolateral nucleus of the rat (67.0%; McDonald and Mascagni, 2001a) were statistically different (P = 0.0052). However, there was a not a statistical difference in the percentages of CR+ neurons that were GABA+ in the Ldi of the monkey (80.5%) and the lateral nucleus of the rat (76.5%; McDonald and Mascagni, 2001a) (P = 0.6299). It is possible that the subpopulations of PV+, CB+ and CR+ interneurons that were classified as GABA-negative in these investigations of the rat and macaque BLC are actually GABAergic, but may have levels of GABA that are below the level of detectability of the immunofluorescence methods used in these investigations. However, in the rat cortex there is a subpopulation of CR+ interneurons that is cholinergic, but not GABAergic (von Engelhardt et al., 2007). Whereas the rat BLC may contain a similar interneuronal subpopulation (Carlsen et al., 1986), cholinergic interneurons in the BLC were not observed in a previous study of the macaque (Amaral and Bassett, 1989).
The percentages of the GABA+ neuronal population stained by antibodies to PV, CB, or CR in basal and lateral nuclei of the macaque BLC were fairly similar to the percentages obtained in these nuclei in the rat BLC. Thus, in the macaque BLC 28–37% of GABA+ neurons were PV+, 30–46% were CB+, and 23–27% were CR+, depending on the nucleus analyzed. In the rat BLC 19–43% of GABA+ neurons were PV+, 41–57% were CB+, and 17–21% were CR+ (McDonald and Mascagni, 2001a).
Colocalization of PV and CB in BLC interneurons
One major difference in the rat versus the macaque BLC is the percentage of PV+ neurons that also express CB. In the lateral and basal nuclei of the rat 80–83% of PV+ neurons were CB+ (McDonald and Betette, 2001), whereas in the present study only 25–34% of PV+ neurons in these nuclei of the macaque BLC were CB. Similar to the pattern in non-human primates, only 25% of PV+ neurons co-express CB in the human BLC (Pantazopoulos et al., 2006). This relative lack of coexistence of PV and CB in the primate BLC had been predicted in previous single-labeling studies which noted differences in the distribution of PV+ and CB+ structures in the BLC of both the macaque (Pitkänen and Amaral, 1993a,b) and human (Sorvari et al., 1995, 1996a). Related to this less extensive colocalization of PV and CB in the macaque versus the rat BLC, the percentage of CB+ neurons that also exhibited PV immunoreactivity in the macaque (23–34%) was less than that seen in the rat (62%; McDonald and Betette, 2001).
The rodent BLC contains several subpopulations of PV+ interneurons that differ in electrophysiological characteristics and connectivity (Rainnie et al., 2006; Woodruff and Sah, 2007a). It has been suggested that specific electrophysiologically-defined subpopulations may correlate with specific anatomically-defined subpopulations that innervate various pyramidal cell domains, including cell bodies (PV+ basket cells), axon initial segments (PV+ chandelier cells), or distal dendrites (PV+ dendrite-targeting cells). The species differences in the extent of PV/CB colocalization suggests that one or more subpopulations of PV+/CB+ interneurons in the rat BLC may not co-express CB in the primate BLC. The finding that pericellular basket-like arrays of immunoreactive axon terminals surrounding pyramidal cell somata are prominent in both CB-and PV-stained preparations of the rat BLC (McDonald, 1997; Kemppainen and Pitkänen, 2000; McDonald and Betette 2001), but only in PV-stained preparations of the primate BLC (Pitkänen and Amaral, 1993a,b; Sorvari et al., 1995, 1996a), suggests that PV+ basket cells may co-express CB in the rat, but not in primates. In addition, the finding that PV+ axonal cartridges enveloping pyramidal cell axon initial segments are only seen in PV-stained preparations in both the rat and primate BLC suggests that chandelier cells do not co-express CB (McDonald and Betette, 2001; Pitkänen and Amaral, 1993a,b; Sorvari et al., 1995, 1996a,b). Thus, most PV+/CB+ neurons in the monkey BLC appear to be dendrite-targeting interneurons. It is of interest in this regard that there is little colocalization of PV and CB in the cortex, but a distinct type of dendrite-targeting cortical PV+ interneuron does co-express CB (Blatow et al., 2003b).
Since little is known about the exact functions of calcium-binding proteins, it is difficult to predict the functional significance of less co-expression of CB in subpopulations of PV+ neurons of the primate versus the rodent BLC. However, it is known that PV and CB have different calcium binding rates and affinities, and can therefore have differential effects on calcium transients in both axons and dendrites (Schwaller et al., 2002). PV is a slow calcium buffer which does not affect the peak amplitude of calcium transients, but does accelerate the initial decay of calcium levels. In axon terminals of PV+ hippocampal basket cells, PV limits facilitation of IPSC amplitude that could occur with prolonged high-frequency firing of fast-spiking interneurons (Vreugdenhil et al., 2003). Thus, the strength of synapses of PV+ basket cells with pyramidal cells is stabilized during high frequency trains. CB is a fast calcium buffer which has a profound effect on the peak amplitude of calcium transients (Schwaller et al., 2002). Experiments on multipolar bursting interneurons in the neocortex indicate that the normal co-expression of CB in these PV+ interneurons enhances paired-pulse facilitation via calcium buffer saturation (Blatow et al., 2003a). Therefore, expression of either one or both of these calcium binding proteins in BLC interneurons could differentially modulate presynaptic short-term plasticity.
Experiments on cerebellar Purkinje cells, which co-express PV and CB, demonstrate that the expression of CB in these cells also has dramatic effects on synaptically-evoked calcium transients in dendrites, including those mediated by calcium-permeable GluR1 AMPA receptors (Schmidt et al., 2003; Barski et al., 2003). Since PV+ and CB+ interneurons are the cells with the highest levels of these AMPA receptors in the BLC (McDonald, 1996b), it seems likely that the expression of CB in particular subpopulations of PV+ neurons could influence long-term potentiation mediated by these receptors (Mahanty and Sah, 1998) by altering the peak amplitude of the calcium transient.
Lack of colocalization of PV with CR, SOM and CCK in BLC interneurons
There was very limited expression of CR in PV+ neurons in Ldi and Bmc of the macaque BLC in the present study. This differs slightly from the rat BLC, where no CR/PV colocalization was observed (McDonald and Mascagni, 2001a). As in the rat (McDonald and Mascagni, 2002; Mascagni and McDonald, 2003), there was no colocalization of PV with SOM or CCK in the macaque BLC. PV, SOM and CCK are each contained in separate interneuronal subpopulations in the rat BLC, but colocalization of SOM and CCK has yet to be investigated in the macaque BLC.
Interneuronal subpopulations in the primate BLC
Since anatomical and physiological studies in the rodent BLC and cortex have shown that distinct interneuronal subpopulations exhibit unique connections and electrophysiological characteristics (Freund and Buzsáki, 1996; Kawaguchi and Kondo, 2002; Muller et al., 2003, 2005, 2006, 2007; Markram et al., 2004; Rainnie et al., 2006; Woodruff and Sah, 2007a), each interneuronal subpopulation in the monkey BLC probably plays a unique function role in the intrinsic circuitry of the BLC. The lack of overlap of PV with SOM and CCK, and the virtual lack of overlap of PV with CR, indicates that PV+ interneurons constitute a separate and distinct subpopulation of GABAergic interneurons in the monkey BLC. Our findings also demonstrate more limited overlap of PV with CB compared to the rat BLC, where the great majority of PV+ interneurons are CB+ (McDonald and Mascagni, 2001a). As discussed above, these data suggest that there may be at least two separate subpopulations of PV+ interneurons in the monkey BLC: (1) PV+/CB− interneurons that provide most of the perisomatic innervation of pyramidal cells, and (2) PV+/CB+ interneurons that mainly innervate dendrites.
There is also evidence for a population of PV−/CB+ neurons that provide a robust innervation of dendrites in the primate BLC. Thus, in CB-stained preparations, but not PV-stained preparations, there are “bundles” of axons surrounding individual unstained dendrites (Pitkanen and Amaral, 1993b; Sorvari et al., 1995). These bundles closely resemble the axonal configurations formed by CB+ double-bouquet cells of the monkey neocortex, which innervate the shafts and spines of apical dendrites of pyramidal cells (DeFelipe et al., 1989). Cortical SOM+ interneurons also selectively target distal dendrites (Hendry et al., 1984; Wang et al., 2004), and their axons form bundles surrounding pyramidal cell dendrites in some portions of the primate neocortex (deLima and Morrison, 1989). Because SOM+ interneurons in the rat BLC, the majority of which also express CB (McDonald and Mascagni, 2002), mainly target pyramidal cell distal dendrites and spines (Muller et al., 2007), it seems likely that SOM+ interneurons may provide a similar innervation in the monkey BLC. It will be interest to determine whether SOM+ interneurons in the monkey BLC are a subpopulation of CB+ neurons.
Differential targeting of perisomatic versus dendritic compartments of pyramidal cells by distinct subpopulations of primate BLC interneurons has profound functional significance. PV+/CB− basket cells and chandelier cells can regulate pyramidal cell firing via their perisomatic innervation. This is critical for the timing of synchronous rhythmic oscillations involved in processing fear-related information in the BLC (Woodruff and Sah, 2007b; Paré et al., 2002). In contrast, dendritic inhibition provided by presumptive PV+/CB+ and SOM+ interneurons can shunt excitatory inputs to dendrites (Buhl et al., 1994), affect the generation of calcium-dependent action potentials in dendrites (Miles et al., 1996), and prevent back-propagation of action potentials from somatic to dendritic compartments (Stuart et al., 1997). All of these functions can regulate long-term potentiation (LTP) involved in emotional learning (Blair et al., 2001).
Acknowledgments
The authors are grateful to Dr. Kenneth Baimbridge (University of British Columbia) for the donation of the rabbit PV and rabbit CB antibodies, and to Dr. John Walsh (CURE/Digestive Diseases Research Center Antibody/RIA Core, NIH Grant #DK41301, Los Angeles, CA, USA) for the donation of the mouse CCK/gastrin antibody. We would also like to thank Dr. Jay Muller for comments on an earlier draft of this manuscript. This work was supported by NIH Grant R01-NS38998 to AJM, the Yerkes Base Grant (RR00165) and a Merit Award, Department of Veterans Affairs to ECM, and NIMH grant MH069852, the Center for Behavioral Neuroscience (NSF agreement IBN-9876754), and an NIH/NCRR base grant (P51RR000165) to Yerkes National Primate Research Center to DGR.
Abbreviations
- BLC
basolateral nuclear complex of the amygdala
- Bmc
basal magnocellular amygdalar nucleus
- CB
calbindin
- CCK
cholecystokinin
- CR
calretinin
- GABA
gamma-aminobutyric acid
- Ldi
dorsal intermediate subdivision of the lateral amygdalar nucleus
- PV
parvalbumin
- SOM
somatostatin
- VIP
vasoactive intestinal peptide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggleton JP. The Amygdala: Second Edition A Functional Analysis. Oxford: Oxford University Press; 2000. [Google Scholar]
- Amaral DG, Bassett JL. Cholinergic innervation of the monkey amygdala: an immunohistochemical analysis with antisera to choline acetyltransferase. J Comp Neurol. 1989;281:337–361. doi: 10.1002/cne.902810303. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Avendaño C, Benoit R. Distribution of somatostatin-like immunoreactivity in the monkey amygdala. J Comp Neurol. 1989;284:294–313. doi: 10.1002/cne.902840211. [DOI] [PubMed] [Google Scholar]
- Barski JJ, Hartmann J, Rose CR, Hoebeek F, Mörl K, Noll-Hussong M, De Zeeuw CI, Konnerth A, Meyer M. Calbindin in cerebellar Purkinje cells is a critical determinant of the precision of motor coordination. J Neurosci. 2003;23:3469–3477. doi: 10.1523/JNEUROSCI.23-08-03469.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair HT, Schafe GE, Bauer EP, Rodrigues SM, LeDoux JE. Synaptic plasticity in the lateral amygdala: a cellular hypothesis of fear conditioning. Learn Mem. 2001;8:229–2242. doi: 10.1101/lm.30901. [DOI] [PubMed] [Google Scholar]
- Blatow M, Caputi A, Burnashev N, Monyer H, Rozov A. Ca2+ buffer saturation underlies paired pulse facilitation in calbindin-D28k-containing terminals. Neuron. 2003a;38:79–88. doi: 10.1016/s0896-6273(03)00196-x. [DOI] [PubMed] [Google Scholar]
- Blatow M, Rozov A, Katona I, Hormuzdi SG, Meyer AH, Whittington MA, Caputi A, Monyer H. A novel network of multipolar bursting interneurons generates theta frequency oscillations in neocortex. Neuron. 2003b;38:805–817. doi: 10.1016/s0896-6273(03)00300-3. [DOI] [PubMed] [Google Scholar]
- Buhl EH, Halasy K, Somogyi P. Diverse sources of hippocampal unitary inhibitory postsynaptic potentials and the number of synaptic release sites. Nature. 1994;368:823–8. doi: 10.1038/368823a0. [DOI] [PubMed] [Google Scholar]
- Carlsen J, Heimer L. Correlated light and electron microscopic immunocytochemical study of cholinergic terminals and neurons in the rat amygdaloid body with special emphasis on the basolateral amygdaloid nucleus. J Comp Neurol. 1986;244:121–136. doi: 10.1002/cne.902440110. [DOI] [PubMed] [Google Scholar]
- Carlsen J, Heimer L. The basolateral amygdaloid complex as a cortical-like structure. Brain Res. 1988;441:377–380. doi: 10.1016/0006-8993(88)91418-7. [DOI] [PubMed] [Google Scholar]
- Conde F, Lund JS, Jacobowitz DM, Baimbridge KG, Lewis DA. Local circuit neurons immunoreactive for calretinin, calbindin D-28k or parvalbumin in monkey prefrontal cortex: distribution and morphology. J Comp Neurol. 1994;341:95–116. doi: 10.1002/cne.903410109. [DOI] [PubMed] [Google Scholar]
- DeFelipe J, Hendry SH, Jones EG. Synapses of double bouquet cells in monkey cerebral cortex visualized by calbindin immunoreactivity. Brain Res. 1989;503:49–54. doi: 10.1016/0006-8993(89)91702-2. [DOI] [PubMed] [Google Scholar]
- de Lima AD, Morrison JH. Ultrastructural analysis of somatostatin-immunoreactive neurons and synapses in the temporal and occipital cortex of the macaque monkey. J Comp Neurol. 1989;283:212–227. doi: 10.1002/cne.902830205. [DOI] [PubMed] [Google Scholar]
- Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Fuller TA, Russchen FT, Price JL. Sources of presumptive glutamatergic/aspartergic afferents to the rat ventral striatopallidal region. J Comp Neurol. 1987;258:317–338. doi: 10.1002/cne.902580302. [DOI] [PubMed] [Google Scholar]
- Hendry SH, Jones EG, Emson PC. Morphology, distribution, and synaptic relations of somatostatin- and neuropeptide Y-immunoreactive neurons in rat and monkey neocortex. J Neurosci. 1984;4:2497–517. doi: 10.1523/JNEUROSCI.04-10-02497.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Kondo S. Parvalbumin, somatostatin and cholecystokinin as chemical markers for specific GABAergic interneuron types in the rat frontal cortex. J Neurocytol. 2002;31:277–287. doi: 10.1023/a:1024126110356. [DOI] [PubMed] [Google Scholar]
- Kemppainen S, Pitkänen A. Distribution of parvalbumin, calretinin, and calbindin-D(28k) immunoreactivity in the rat amygdaloid complex and colocalization with gamma-aminobutyric acid. J Comp Neurol. 2000;426:441–467. doi: 10.1002/1096-9861(20001023)426:3<441::aid-cne8>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Mahanty NK, Sah P. Calcium-permeable AMPA receptors mediate long-term potentiation in interneurons in the amygdala. Nature. 1998;394:683–687. doi: 10.1038/29312. [DOI] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- Mascagni F, McDonald AJ. Immunohistochemical characterization of cholecystokinin containing neurons in the rat basolateral amygdala. Brain Res. 2003;976:171–184. doi: 10.1016/s0006-8993(03)02625-8. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Neurons of the lateral and basolateral amygdaloid nuclei: a Golgi study in the rat. J Comp Neurol. 1982;212:293–312. doi: 10.1002/cne.902120307. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Cell types and intrinsic connections of the amygdala. In: Aggleton JP, editor. The Amygdala. New York: Wiley-Liss; 1992a. pp. 67–96. [Google Scholar]
- McDonald AJ. Projection neurons of the basolateral amygdala: a correlative Golgi and retrograde tract tracing study. Brain Res Bull. 1992b;28:179–185. doi: 10.1016/0361-9230(92)90177-y. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Calretinin immunoreactive neurons in the basolateral amygdala of the rat and monkey. Brain Res. 1994;667:238–242. doi: 10.1016/0006-8993(94)91501-6. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Glutamate and aspartate immunoreactive neurons of the rat basolateral amygdala: colocalization of excitatory amino acids and projections to the limbic circuit. J Comp Neurol. 1996a;365:367–379. doi: 10.1002/(SICI)1096-9861(19960212)365:3<367::AID-CNE3>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Localization of AMPA glutamate receptor subunits in subpopulations of non-pyramidal neurons in the rat basolateral amygdala. Neurosci Lett. 1996b;208:175–178. doi: 10.1016/0304-3940(96)12585-4. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Calbindin-D28k immunoreactivity in the rat amygdala. J CompNeurol. 1997;383:231–244. [PubMed] [Google Scholar]
- McDonald AJ, Pearson JC. Coexistence of GABA and peptide immunoreactivity in non-pyramidal neurons of the basolateral amygdala. Neurosci Lett. 1989;100:53–58. doi: 10.1016/0304-3940(89)90659-9. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Augustine JR. Localization of GABA-like immunoreactivity in the monkey amygdala. Neuroscience. 1993;52:281–294. doi: 10.1016/0306-4522(93)90156-a. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F, Augustine JR. Neuropeptide Y and somatostatin-like immunoreactivity in neurons of the monkey amygdala. Neuroscience. 1995;66:959–982. doi: 10.1016/0306-4522(94)00629-j. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Betette RL. Parvalbumin-containing neurons in the rat basolateral amygdala: morphology and co-localization of calbindin-D(28k) Neuroscience. 2001;102:413–425. doi: 10.1016/s0306-4522(00)00481-4. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F. Colocalization of calcium-binding proteins and GABA in neurons of the rat basolateral amygdala. Neuroscience. 2001a;105:681–693. doi: 10.1016/s0306-4522(01)00214-7. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F. Localization of the CB1 type cannabinoid receptor in the rat basolateral amygdala: high concentrations in a subpopulation of cholecystokinin-containing interneurons. Neuroscience. 2001b;107(4):641–652. doi: 10.1016/s0306-4522(01)00380-3. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F. Immunohistochemical characterization of somatostatin containing interneurons in the rat basolateral amygdala. Brain Res. 2002;943:237–244. doi: 10.1016/s0006-8993(02)02650-1. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F, Mania I, Rainnie DG. Evidence for a perisomatic innervation of parvalbumin-containing interneurons by individual pyramidal cells in the basolateral amygdala. Brain Res. 2005;1035:32–40. doi: 10.1016/j.brainres.2004.11.052. [DOI] [PubMed] [Google Scholar]
- Miles R, Toth K, Gulyas AI, Hajos N, Freund TF. Differences between somatic and dendritic inhibition in the hippocampus. Neuron. 1996;16:815–823. doi: 10.1016/s0896-6273(00)80101-4. [DOI] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Synaptic connections of distinct interneuronal subpopulations in the rat basolateral amygdalar nucleus. J Comp Neurol. 2003;456:217–236. doi: 10.1002/cne.10435. [DOI] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Coupled networks of parvalbumin-immunoreactive interneurons in the rat basolateral amygdala. J Neurosci. 2005;25:7366–7376. doi: 10.1523/JNEUROSCI.0899-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Pyramidal cells of the rat basolateral amygdala: synaptology and innervation by parvalbumin-immunoreactive interneurons. J Comp Neurol. 2006;494:635–50. doi: 10.1002/cne.20832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Postsynaptic targets of somatostatin-containing interneurons in the rat basolateral amygdala. J Comp Neurol. 2007;500:513–529. doi: 10.1002/cne.21185. [DOI] [PubMed] [Google Scholar]
- Narayanan RT, Seidenbecher T, Kluge C, Bergado J, Stork O, Pape HC. Dissociated theta phase synchronization in amygdalo- hippocampal circuits during various stages of fear memory. Eur J Neurosci. 2007;25:1823–1831. doi: 10.1111/j.1460-9568.2007.05437.x. [DOI] [PubMed] [Google Scholar]
- Pantazopoulos H, Lange N, Hassinger L, Berretta S. Subpopulations of neurons expressing parvalbumin in the human amygdala. J Comp Neurol. 2006;496:706–722. doi: 10.1002/cne.20961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape HC, Narayanan RT, Smid J, Stork O, Seidenbecher T. Theta activity in neurons and networks of the amygdala related to long-term fear memory. Hippocampus. 2005;15:874–880. doi: 10.1002/hipo.20120. [DOI] [PubMed] [Google Scholar]
- Paré D, Collins DR. Neuronal correlates of fear in the lateral amygdala: multiple extracellular recordings in conscious cats. J Neurosci. 2000;20:2701–2710. doi: 10.1523/JNEUROSCI.20-07-02701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paré D, Collins DR, Pelletier JG. Amygdala oscillations and the consolidation of emotional memories. Trends Cogn Sci. 2002;6:306–314. doi: 10.1016/s1364-6613(02)01924-1. [DOI] [PubMed] [Google Scholar]
- Paré D, Royer S, Smith Y, Lang EJ. Contextual inhibitory gating of impulse traffic in the intra-amygdaloid network. Ann N Y Acad Sci. 2003;985:78–91. doi: 10.1111/j.1749-6632.2003.tb07073.x. [DOI] [PubMed] [Google Scholar]
- Pitkänen A, Amaral DG. Distribution of parvalbumin-immunoreactive cells and fibers in the monkey temporal lobe: the amygdaloid complex. J Comp Neurol. 1993a;331:14–36. doi: 10.1002/cne.903310103. [DOI] [PubMed] [Google Scholar]
- Pitkänen A, Amaral DG. Distribution of calbindin-D28k immunoreactivity in the monkey temporal lobe: the amygdaloid complex. J Comp Neurol. 1993b;331:199–224. doi: 10.1002/cne.903310205. [DOI] [PubMed] [Google Scholar]
- Pitkänen A, Amaral DG. The distribution of GABAergic cells, fibers, and terminals in the monkey amygdaloid complex: An immunohistochemical and in situ hybridization study. J Neurosci. 1994;14:2200–2224. doi: 10.1523/JNEUROSCI.14-04-02200.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkänen A, Amaral DG. Organization of the intrinsic connections of the monkey amygdaloid complex: projections originating in the lateral nucleus. J Comp Neurol. 1998;398:431–58. doi: 10.1002/(sici)1096-9861(19980831)398:3<431::aid-cne9>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Rainnie DG, Asprodini EK, Shinnick-Gallagher P. Intracellular recordings from morphologically identified neurons of the basolateral amygdala. J Neurophysiol. 1993;69:1350–1361. doi: 10.1152/jn.1993.69.4.1350. [DOI] [PubMed] [Google Scholar]
- Rainnie DG, Mania I, Mascagni F, McDonald AJ. Physiological and morphological characterization of parvalbumin-containing interneurons of the rat basolateral amygdala. J Comp Neurol. 2006;498:142–161. doi: 10.1002/cne.21049. [DOI] [PubMed] [Google Scholar]
- Rehfeld JF. Localisation of gastrins to neuro- and adenohypophysis. Nature. 1978;271:771–773. doi: 10.1038/271771a0. [DOI] [PubMed] [Google Scholar]
- Rehfeld JF, Lundberg JM. Cholecystokinin in feline vagal and sciatic nerves: concentration, molecular form and transport velocity. Brain Res. 1983;275:341–347. doi: 10.1016/0006-8993(83)90995-2. [DOI] [PubMed] [Google Scholar]
- Schmidt H, Stiefel KM, Racay P, Schwaller B, Eilers J. Mutational analysis of dendritic Ca2+ kinetics in rodent Purkinje cells: role of parvalbumin and calbindin D28k. J Physiol. 2003;551:13–32. doi: 10.1113/jphysiol.2002.035824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwaller B, Meyer M, Schiffmann S. ‘New’ functions for ‘old’ proteins: the role of the calcium-binding proteins calbindin D-28k, calretinin and parvalbumin, in cerebellar physiology. Studies with knockout mice. Cerebellum. 2002;1:241–258. doi: 10.1080/147342202320883551. [DOI] [PubMed] [Google Scholar]
- Seidenbecher T, Laxmi TR, Stork O, Pape HC. Amygdalar and hippocampal theta rhythm synchronization during fear memory retrieval. Science. 2003;301:846–850. doi: 10.1126/science.1085818. [DOI] [PubMed] [Google Scholar]
- Shinnick-Gallagher P, Pitkänen A, Shekhar A, Cahill L. Ann N Y Acad Sci. Vol. 985. New York: The New York Academy of Sciences; 2003. The Amygdala in Brain Function: Basic and Clinical Approaches. [Google Scholar]
- Smith Y, Paré J-F, Paré D. Differential innervation of paravalbumin-immunoreactive interneurons of the basolateral amygdaloid complex by cortical and intrinsic inputs. J Comp Neurol. 2000;416:496–508. [PubMed] [Google Scholar]
- Sorvari H, Soininen H, Paljärvi L, Karkola K, Pitkänen A. Distribution of parvalbumin-immunoreactive cells and fibers in the human amygdaloid complex. J Comp Neurol. 1995;360:185–212. doi: 10.1002/cne.903600202. [DOI] [PubMed] [Google Scholar]
- Sorvari H, Soininen H, Pitkänen A. Calbindin-D28K-immunoreactive cells and fibres in the human amygdaloid complex. Neuroscience. 1996a;75:421–443. doi: 10.1016/0306-4522(96)00296-5. [DOI] [PubMed] [Google Scholar]
- Sorvari H, Miettinin R, Soininen H, Pitkänen A. Parvalbumin-immunoreactive neurons make inhibitory synapses on pyramidal cells in the human amygdala: a light and electron microscopic study. Neurosci Lett. 1996b;217:93–96. [PubMed] [Google Scholar]
- Stuart G, Schiller J, Sakmann B. Action potential initiation and propagation in rat neocortical pyramidal neurons. J Physiol. 1997;505(3):617–632. doi: 10.1111/j.1469-7793.1997.617ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Engelhardt J, Eliava M, Meyer AH, Rozov A, Monyer H. Functional characterization of intrinsic cholinergic interneurons in the cortex. J Neurosci. 2007;27:5633–42. doi: 10.1523/JNEUROSCI.4647-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vreugdenhil M, Jefferys JG, Celio MR, Schwaller B. Parvalbumin-deficiency facilitates repetitive IPSCs and gamma oscillations in the hippocampus. J Neurophysiol. 2003;89:1414–1422. doi: 10.1152/jn.00576.2002. [DOI] [PubMed] [Google Scholar]
- Washburn MS, Moises HC. Electrophysiological and morphological properties of rat basolateral amygdaloid neurons in vitro. J Neurosci. 1992;12:4066–4079. doi: 10.1523/JNEUROSCI.12-10-04066.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff AR, Sah P. Networks of parvalbumin-positive interneurons in the basolateral amygdala. J Neurosci. 2007a;27:553–563. doi: 10.1523/JNEUROSCI.3686-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff AR, Sah P. Inhibition and synchronization of basal amygdala principal neuron spiking by parvalbumin-positive interneurons. J Neurophysiol. 2007b;98:2956–2961. doi: 10.1152/jn.00739.2007. [DOI] [PubMed] [Google Scholar]
- Wouterlood FG, Van Denderen JC, Blijleven N, Van Minnen J, Hartig W. Two-laser dual-immunofluorescence confocal laser scanning microscopy using Cy2- and Cy5-conjugated secondary antibodies: unequivocal detection of co-localization of neuronal markers. Brain Res Brain Res Protoc. 1998;2:149–159. doi: 10.1016/s1385-299x(97)00038-x. [DOI] [PubMed] [Google Scholar]