Abstract
The amount of time asleep varies greatly in mammals, from 3 h in the donkey to 20 h in the armadillo. Previous comparative studies have suggested several functional explanations for interspecific variation in both the total time spent asleep and in rapid-eye movement (REM) or “quiet” (non-REM) sleep. In support of specific functional benefits of sleep, these studies reported correlations between time in specific sleep states (NREM or REM) and brain size, metabolic rate, and developmental variables. Here we show that estimates of sleep duration are significantly influenced by the laboratory conditions under which data are collected and that, when analyses are limited to data collected under more standardized procedures, traditional functional explanations for interspecific variation in sleep durations are no longer supported. Specifically, we find that basal metabolic rate correlates negatively rather than positively with sleep quotas, and that neither adult nor neonatal brain mass correlates positively with REM or NREM sleep times. These results contradict hypotheses that invoke energy conservation, cognition, and development as drivers of sleep variation. Instead, the negative correlations of both sleep states with basal metabolic rate and diet are consistent with trade-offs between sleep and foraging time. In terms of predation risk, both REM and NREM sleep quotas are reduced when animals sleep in more exposed sites, whereas species that sleep socially sleep less. Together with the fact that REM and NREM sleep quotas correlate strongly with each other, these results suggest that variation in sleep primarily reflects ecological constraints acting on total sleep time, rather than the independent responses of each sleep state to specific selection pressures. We propose that, within this ecological framework, interspecific variation in sleep duration might be compensated by variation in the physiological intensity of sleep.
Keywords: NREM, phylogeny, REM, sleep architecture
Unlike activities such as mating, foraging, and seeking shelter, the functional benefits of sleep are unclear, whereas the costs appear to be substantial. Sleeping animals sacrifice foraging and mating opportunities, they may experience increased risk of predation, and they are less able to detect and respond to changing environmental conditions. Some as-yet unknown benefit of sleep presumably outweighs these costs. Mammalian sleep is generally characterized by two major components, rapid-eye-movement (REM) and non–rapid-eye-movement (NREM) states, which alternate in a “sleep cycle” that is repeated one or more times during a sleep bout (Zepelin 1989; Zepelin et al. 2005). An adult sleep cycle typically starts with an episode of NREM, characterized by physical quiescence and high-amplitude, synchronized, slow waves in electroencephalographic (EEG) measurements of brain activity. REM sleep follows and can be distinguished by eye movements, muscle twitches, and low voltage fast waves in the brain (Zepelin 1989; Hobson 2005; Zepelin et al. 2005). The physiological distinctiveness of these two sleep states is thought to reflect functional differences (Zepelin 1989; Zepelin et al. 2005).
One approach to understanding the costs and benefits of sleep is to systematically investigate variation across species. In mammals, the amount of time asleep varies greatly, from 3 h in the donkey (Equus asinus; Ruckebush 1963) to 20 h in the armadillo (Chaetophractus villous; Affani et al. 2001), as does the amount of time devoted to NREM and REM sleep, referred to as “sleep quotas” (Zepelin 1989; Zepelin et al. 2005). These great interspecific differences could reflect either variation in the need for specific functional benefits of sleep (Zepelin et al. 2005; Lesku et al. 2006) or variation in constraints on sleep time (Allison and Cicchetti 1976). Allison and Cicchetti (1976), for example, found that species under higher risk of predation spend less time in this relatively vulnerable state. Elgar et al. (1988, 1990) suggested that species with higher foraging requirements are forced to trade sleep and foraging times. To what extent such ecological constraints drive species differences in sleep durations, hence affecting how the functional benefits of sleep can be acquired in species exposed to different ecological pressures, remains an open question (Siegel 2005).
Early comparative studies showed that sleep requirements are greater in smaller species, and found that NREM and REM sleep correlated with different traits (Zepelin and Rechtschaffen 1974; Elgar et al. 1988, 1990, Zepelin 1989), emphasizing their different functions (Rechtschaffen 1998; Siegel 2005; Zepelin et al. 2005). Because NREM sleep develops alongside thermoregulatory capacities in altricial species, is associated with a reduction in body temperature, and is the state through which torpor and hibernation are entered, it was argued that a major role of NREM sleep is to conserve energy and help balance the costs of endothermy (Berger 1975; Berger and Phillips 1995). In support of this hypothesis, NREM sleep quotas increased with mass-specific basal metabolic rate in mammals (Zepelin and Rechtschaffen 1974). The energy conservation hypothesis is controversial, however, as experimental evidence suggests that energy savings during NREM sleep are trivial (reviewed in Zepelin 1989). Furthermore, animals awaking from hibernation appear to require increased amounts of NREM sleep (e.g., Trachsel et al. 1991), which suggests that the physiological function of NREM sleep differs from that of energy saving performed during hibernation.
REM sleep is believed to be beneficial to the brain, because EEG patterns during REM sleep indicate that the brain is in a highly activated state (Maquet and Phillips 1999). Specifically, REM sleep might play a role in memory consolidation and learning, suggesting that species with greater cognitive abilities would require more REM sleep (reviewed in Hobson 2005; Stickgold 2005; Zepelin et al. 2005; Walker and Stickgold 2006). More recently, however, different studies have suggested that memory consolidation and learning may also require some involvement of NREM sleep (Stickgold 1998, 2005; Ambrosini and Giuditta 2001; Huber et al. 2004; Clemens et al. 2005), but the importance of sleep in both the processes is still hotly debated (Siegel 2001; Vertes 2004).
REM sleep might also be involved in brain maturation (Roffwarg et al. 1966; Zepelin 1989). This hypothesis is supported by the decline in REM sleep from birth to adulthood, indicating that REM sleep might act as an endogenous stimulus that aids the development of the neonatal brain (Roffwarg et al. 1966; Jouvert-Monier et al. 1970). Comparative studies supported this hypothesis, showing that REM sleep quotas increase with surrogate measures of neonatal brain development (Table 1; Zepelin and Rechtschaffen 1974; Elgar et al. 1988; 1990; Zepelin 1989; Zepelin et al. 2005).
Table 1.
Significant predictors for daily total sleep time (TST), NREM, and REM sleep in Zepelin and Rechtschaffen (1974), Elgar et al. (1988, 1990), Allison and Cicchetti (1974), and Lesku et al. (2006). Direction of the relationship is indicated in brackets.
| TST | NREM | REM | |
|---|---|---|---|
| ABM (−) | |||
| ABrM (−) | |||
| Zepelin and Rechtschaffen (1974) | ABM (−) | ABM (−) | GL (−) |
| ABrM (−) | ABrM (−) | % NBrM/ABrM (−) | |
| Mass-specific BMR (+) | Mass-specific BMR (+) | LS (+) | |
| AP (−) | |||
| Elgar et al. (1988, 1990) | ABM (−) | ABM (−) | |
| Total BMR (−) | Total BMR (−) | AP (−) | |
| Allison and Cicchetti (1974) | – | ABW (−) | GL (−) |
| – | Predation risk (−) | Predation risk (−) | |
| ABrM (+)1 | |||
| Lesku et al. (2006) | Relative BMR (−) | Relative BMR (−) | GL (−) |
| Trophic level (−) | Sleep exposure (−) | ||
| Trophic level (−) |
ABM, adult body mass; ABrM, adult brain mass; mass specific BMR, total basal metabolic rate divided by body mass; total BMR, total basal metabolic rate; relative BMR, total BMR relative to body size using residuals; GL, gestation length; NBrM, neonatal brain mass; LS, litter size, AP, altricial-precocial at birth; Predation risk, index of overall danger for a species; Trophic level index, from more carnivorous to more herbivorous diets; Sleep exposure, index on the vulnerability of sleeping sites.
Adult brain mass correlated with percentage of REM sleep on total sleep but not with hours spent in REM sleep per day.
Although these studies called attention to the value of comparative analyses for improving our understanding of the function of sleep, they suffered from two key problems. First, they did not account for the similarity between species due to their common ancestry, an omission that can lead to erroneous conclusions (Felsenstein 1985; Harvey and Pagel 1991; Martins and Garland 1991). Second, these studies used data whose comparability has repeatedly been questioned (Campbell and Tobler 1984; Berger 1990).
A recent phylogenetic analysis of sleep architecture (Lesku et al. 2006) addressed the first issue but not the second. The results supported a functional role for REM sleep in brain development and cognitive performance, but contradicted the energy conservation hypothesis for NREM sleep. Moreover, Lesku et al. (2006) argued that REM sleep—but not NREM sleep—decreased in species under higher predation risk. Data quality is, however, particularly important, as different laboratory conditions and measurement procedures may confound estimates of sleep parameters (Campbell and Tobler 1984; Siegel 2005). Although the issue of data quality has been raised previously (e.g., Campbell and Tobler 1984; Berger 1990; Elgar et al. 1990; Siegel 2005), no study has examined existing data to determine whether particular laboratory procedures systematically affect estimates of sleep.
In this article, we present three major sets of analyses. First, we assessed the influence of different laboratory conditions on estimates of sleep and developed standards for data inclusion. From this analysis, we identified a “restricted” dataset that holds constant laboratory conditions that we identify as affecting sleep quotas. We used this dataset in all subsequent analyses. Second, we investigated whether sleep quotas show phylogenetic signal (Blomberg and Garland 2002; Freckleton et al. 2002; Blomberg et al. 2003). If so, this would indicate that sleep quotas are characteristic of different evolutionary lineages and that phylogenetic methods are needed to investigate the correlates of sleep. Finally, based on the discussion presented above, we investigated the following hypotheses and predictions: (1) more encephalized species exhibit more sleep—particularly REM sleep—because of their greater cognitive abilities, predicting a positive association between REM (and possibly NREM) sleep quotas and encephalization (relative brain mass); (2) NREM sleep serves to conserve energy, predicting a positive association between NREM (but not REM) sleep and energy expenditure (basal metabolic rate); (3) REM sleep promotes brain development, leading to the expectation that REM (but not NREM) sleep quotas will be higher in species with less-developed brains at birth; (4) species under greater risk of predation will be constrained to spend less time in both sleep states, predicting reduced sleep time in species that sleep in more exposed sites, in species that sleep alone relative to species that sleep socially, and in “predators” relative to “prey.”
Methods
SLEEP DATA AND THE INFLUENCE OF LABORATORY CONDITIONS
We performed an extensive literature search to gather data on NREM and REM sleep durations (hours/day) in mammals (protocol described in [McNamara et al. 2008]). Total daily sleep time (TST) was calculated as the sum of NREM and REM sleep time. We found data on adults or presumed adults for 130 species, after excluding studies that clearly used juveniles and/or neonates. For each study we compiled a dataset (available at http://www.bu.edu/phylogeny/index.html) including information on the following laboratory procedures:
Recording time (categorical three-state variable). Studies were assigned to one of three categories: < 12 h recording time, between 12 and < 24 h, and recording time ≥ 24 h.
Recording method (EEG or behavior). Sleep time was estimated using EEG or behavioral observations.
Habituation (yes or no). Whether the experimental animals were habituated to sleep recording and laboratory conditions prior to data collection.
Restraint (yes or no). Animals were restrained or they could move freely.
We also recorded information on diet, photoperiod, and ambient temperature, as these factors may affect REM and NREM sleep durations, and as a consequence TST (Campbell and Tobler 1984; Siegel 2005). However, sample sizes for these laboratory variables were too small to assess their impact on sleep. We found multiple estimates from studies that differed for at least one laboratory procedure for 25 species. We used a paired t-test to compare estimates from studies on the same species that differed for a given condition. When multiple references were available for one species, we averaged sleep times estimated under identical laboratory conditions for a given factor (e.g., all studies with restrained subjects) and compared this with the average from studies that differed for that condition (e.g., all studies with unrestrained subjects).
We restricted the comparative analysis of sleep evolution to terrestrial mammals because aquatic mammals (Cetacea, Pinnipedia, and Sirenia) exhibit different sleep patterns relative to terrestrial mammals, having facultative or obligatory unihemispheric sleep with little or no REM sleep (Rattenborg and Amlaner 2002; Siegel 2004). We also excluded monotremes because the distinction between REM and NREM sleep in these species is not as clear as in the other terrestrial mammals (Zepelin et al. 2005). We calculated weighted means of sleep quotas for species that had multiple sleep estimates, with weights based on the number of animals studied.
FUNCTIONAL AND ECOLOGICAL TRAITS
We extracted data from the primary literature to ensure that both sleep data and data on the independent variables were comparable. Data on adult body mass (all species) were compiled from an extensive literature search to control for allometric effects of size on the independent variables (see below). In contrast to previous studies (Elgar et al. 1988; Lesku et al. 2006), we used only direct estimates of brain mass (in grams; 43 species), and avoided indirect measures (e.g., cranial capacity, brain volume) that cannot be considered equivalent (Von Rohrs and Ebinger 2001).
We used basal metabolic rate (BMR; in ml02/hour) relative to body size (see below) as a measure of total daily energy expenditure. Data on BMR with matching data on the body mass of the experimental animals (43 species) were taken from a published dataset (White et al. 2006) collected from adult nonreproducing individuals, tested when postabsorptive and resting but not asleep (see McNab 1997, 1999), and integrated with new data that satisfied the same requirements. Contrary to previous studies (Elgar et al. 1988, 1990; Lesku et al. 2006), we did not use other estimates of metabolic rates (i.e., when one or more of the above conditions was not satisfied) or results that were reported with insufficient details on experimental conditions.
To test the hypothesis that REM sleep promotes neonatal brain development, we extracted data on neonatal brain mass (mass of the brain at birth; 26 species; sources: Mangold-Wirz 1966; Sacher and Staffeldt 1974), and neonatal body mass (as measured at birth; 55 species), to account for size effects (sources: Hayssen et al. 1993; monographs of Mammalian Species). Because the sample size of neonatal brain mass was small, we followed previous studies and used neonatal body mass and gestation length (in days; 59 species; data from Hayssen et al. 1993). The length of gestation influences the developmental states of neonates and therefore is associated with neonatal body and brain mass (Pagel and Harvey 1990). However, neonatal body mass is more strongly related to neonatal brain mass (t24 = 17.43, R2 = 0.93, P < 0.0001) than gestation length (t24 = 8.49, R2 = 0.75, P < 0.0001) and therefore should be a better proxy for neonatal brain mass than gestation length. We calculated weighted means for all these variables when multiple references were available.
We quantified predation risk using two variables: social sleep behavior (45 species) and exposure of sleeping sites (60 species). We developed a sleep site exposure index on a three-point scale using information from the literature. Fully enclosed sleeping sites, such as burrows and tree holes, were classified as least exposed; sites with partial closure, such as vegetation on the ground or in trees, were coded as intermediate; and sites in open habitats with no protection were considered as most exposed. In comparison to indices developed in previous studies (Allison and Cicchetti 1976; Lesku et al. 2006), our index makes fewer assumptions regarding relative safety of sleeping sites (such as sleeping in the tree canopy being safer than below the canopy at branch junctions, which may be invalid because exposure to aerial predators may increase for some species). We classified species according to their social sleep behavior on a three-point index, and categorized them as solitary (both males and females sleep alone), partially social (females but not males sleep socially, with other females), and social (both sexes sleep socially). Sleeping with offspring was not considered social sleep unless it was prolonged into adulthood. The data on sleep site exposure and social sleep behavior were coded by three and two independent observers, respectively, who were unaware of the hypotheses and aims of the study. For both indices average scores were calculated when intraspecific variability was present.
Finally, we used an index developed in a previous study based on diet composition to reflect each species’ trophic level (Lesku et al. 2006). This diet index ranged between 1 (diet based exclusively on vertebrates) and 4 (entirely herbivorous; details in Lesku et al. 2006). Diet may reflect the vulnerability of animals resulting from their position in the trophic chain, because predators should be less vulnerable to predation risk and thus may have greater opportunity for sleep (Lesku et al. 2006); alternatively, it may reflect ecological constraints due to trade-offs between foraging and sleeping time (Allison and Cicchetti 1976; Elgar et al. 1988, 1990).
NREM sleep was normally distributed and, because TST consists mostly of NREM sleep, TST was also normally distributed. REM sleep quotas and all functional traits were log-transformed to meet assumptions of normality. We acknowledge that TST is not independent of REM and NREM sleep. We chose to present results for TST for comparison to previous studies, and because ecological constraints may act most strongly on total sleep time, especially if different species adjust the amount of both REM and NREM sleep in the context of ecological constraints.
INFLUENCE OF PHYLOGENY
Siegel (1995, p. 29; 2004, pp. 164 and 174; 2005, p. 1266) argued that sleep quotas are unrelated to phylogeny as some species in different mammalian orders exhibited similar amount of total daily sleep. Lesku et al. (2006) employed phylogenetic independent contrasts under the assumption that sleep exhibits phylogenetic signal. However, sleep time might evolve quickly so that the phylogenetic signal is weakened or lost, or high intraspecific variability could mask phylogenetic differences, thus calling into question the use of phylogenetic independent contrasts. Therefore, testing whether sleep traits exhibit phylogenetic signal is not merely a statistical exercise but has important implications for understanding the evolution of sleep and for choosing the most appropriate phylogeny-based comparative method (Pagel 1997, 1999; Blomberg and Garland 2002; Freckleton et al. 2002; Blomberg et al. 2003; Garland et al. 2005).
We used the computer program “Continuous” (Available from M. Pagel, University of Reading, Reading, U. K.) to quantify the strength of the phylogenetic signal (lambda, λ) for NREM and REM sleep quotas, based on the method of phylogenetic generalized least squares models (PGLS; Pagel 1997; 1999; Freckleton et al. 2002). Based on previous suggestions in the literature that total sleep times are not correlated with phylogeny (Siegel 1995, 2004, 2005), we also examined total sleep time. As acknowledged above, however, this test is not necessarily independent from analyses of NREM and REM sleep durations. A λ value equal to 0 (or not statistically different from 0) indicates species independence, thus no need to control for phylogeny. Conversely, a λ value equal to 1 (or not statistically different from 1) indicates a strong phylogenetic signal, such that the pattern of similarity in the trait values among species is correctly predicted by the phylogenetic relationships under a Brownian motion (random walk) model of evolution (Pagel 1997, 1999; Freckleton et al. 2002).
ANALYSIS OF CORRELATED EVOLUTION AND ALLOMETRY
To run the phylogenetic tests, we created a composite phylogeny assembled from different published trees (phylogenetic tree and references in Appendix). Branch lengths were unavailable for the entire tree. We therefore used different branch length options and chose the set of branch lengths that performed best in diagnostic tests of assumption violations (no correlation between the absolute values of contrasts and their standard deviations; Garland et al. 1992). When branch lengths were set equal, no assumption violations for the sleep traits and other continuous variables were found, with the sole exception of neonatal body mass. Neonatal body mass exhibited violations against standard deviation (SD) that could not be resolved; therefore results involving this trait need to be taken with caution.
To account for similarity between species due to their common ancestry (Felsenstein 1985; Harvey and Pagel 1991; Garland et al. 1992; Nunn and Barton 2001), we calculated phylogenetically independent contrasts using the computer program CAIC based on the CRUNCH algorithm (Purvis and Rambaut 1995). Analyses of independent contrasts were performed using linear regression with the regression line forced through the origin (Felsenstein 1985; Harvey and Pagel 1991; Garland et al. 1992).
With the exceptions of diet and the index of social sleep behavior, the independent variables covaried with body mass. We removed allometric effects from the nonsleep variables using residuals from regression through the origin of the nonsleep trait contrasts on body mass contrasts (Garland et al. 1992; Purvis and Rambaut 1995). We then tested the correlations between sleep and these residuals, which we hereafter call “relative,” that is, relative body mass. All statistical tests were two-tailed with significance level (α) set at 0.05.
Finally, we checked that our conclusions were not affected by multiple testing by using the false discovery rate (FDR) control test (Bejamini and Hochberg 1995; Verhoeven et al. 2005). This method assesses the proportion of type I errors among all the significant results, rather than reducing the probability of making type I errors (e.g., as in the Bonferroni correction). FDR therefore has greater power (lower type II error rates) than the commonly used Bonferroni correction, even when the number of tests performed is high (Verhoeven et al. 2005). Here we report only if results differed after controlling for multiple testing.
Where there was evidence that the distributional assumptions of linear regression may not be met, potentially yielding erroneous results, we reassessed the significance of independent variables using bootstrapped estimates of effects with 1000 bootstrap samples and 95% confidence intervals. These statistics do not make strict distributional assumptions about the data, and reduce bias caused by outliers (Efron and Tibshirani 1993). Bootstrap analyses were implemented in Genstat version 8 (VSN International, Hemel Hempstead, U. K.). Results did not differ qualitatively from those presented here.
Results
INFLUENCE OF LABORATORY PROCEDURES ON SLEEP DURATION ESTIMATES
Compared to studies that recorded subjects for at least 24 h, sleep times from studies with recording time less than 12 h significantly underestimated REM and NREM sleep time, and as a consequence also TST (REM: t8 = −5.88, P = 0.0004; NREM: t8 = −6.37, P = 0.0002; TST: t8 = −6.42, P = 0.0002; Fig. 1A). Observation periods of between 12 and < 24 h provided estimates that were intermediate to < 12 h and ≥ 24 h recording, although did not differ statistically from the latter group (TST: t8 = −1.67, P = 0.133; REM: t6 = −0.45, P = 0.667; NREM: t6 = −0.59, P = 0.580; Fig. 1A). Sample sizes for comparisons with < 12 h were too small for statistical testing (n = 3). For the following analyses of other laboratory conditions, we controlled for this effect by making comparisons only among studies within the same class of recording time.
Figure 1.
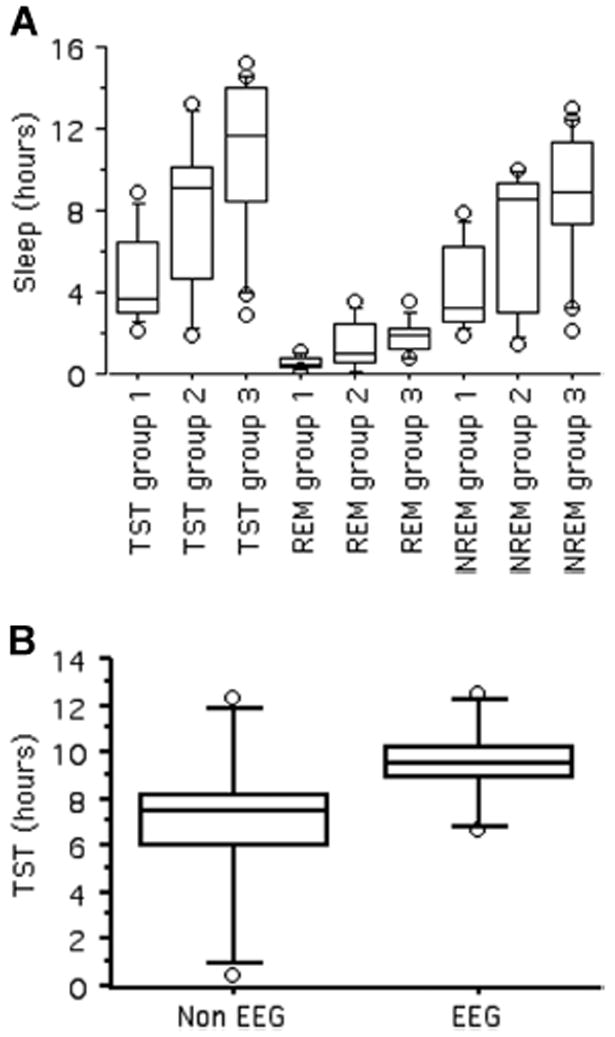
Laboratory conditions: (A) Total daily sleep time (TST), REM, and NREM sleep quotas in studies with < 12 h recording time (group 1), between 12 and < 24 recording time (including 12 h; group 2), and in studies with ≥ 24 h recording time (group 3; see text). (B) TST as estimated by EEG and nonEEG studies. Boxes show upper and lower quartiles with the horizontal line indicating the median, whiskers outside the box delimit are drawn at 1.5 interquartile range, and circles represent outliers.
EEG estimates of TST tended to be higher than non-EEG estimates, but the difference was not significant (t5 = −2.07, P = 0.093; Fig. 1B). This analysis could not be replicated for individual sleep states because all studies that reported REM and NREM quotas, except one, were conducted using EEG. Sleep did not differ significantly between studies in relation to habituation (TST: t7 = −0.42, P = 0.688; REM: t5 = −1.31, P = 0.247; NREM: t5 = −1.18, P = 0.292) or restraint (TST: t6 = 1.80, P = 0.126; REM: t5 = −1.24, P = 0.270; NREM: t5 = 1.08, P = 0.331).
Based on these results, we restricted our analyses on the evolution of sleep durations to data collected under comparable laboratory conditions and, from the dataset available in our website, we selected studies that used EEG recording of sleep for at least 12 h or longer. Our final “restricted” database included 61 terrestrial mammals (the list of species used for the analysis is available in online Supplementary Fig. S1, which also indicates the phylogenetic relationships among the species in our dataset).
INFLUENCE OF PHYLOGENY
The PGLS analysis revealed strong phylogenetic signal for each of the sleep traits analyzed individually, as the estimated maximum-likelihood score of λ was not statistically different from 1 (Table 2). These results demonstrate the need to control for phylogeny in comparative studies of mammalian sleep quotas and indicate that phylogenetically independent contrasts are suitable and appropriate for investigating the evolution of sleep.
Table 2.
PGLS analysis to estimate the phylogenetic signal, lambda (λ), of each sleep trait (TST is total sleep time).
| Log-likelihood for λ = 0 | Log-likelihood for λ = ML | Likelihood-ratio test | P | ML λ | Lower CI of ML λ | |
|---|---|---|---|---|---|---|
| TST | −179.76 | −167.42 | 12.34 | < 0.001 | 1.00 | 0.83 |
| NREM | −156.83 | −149.07 | 7.77 | < 0.001 | 0.861 | 0.46–1.00 |
| REM | −66.08 | −52.79 | 13.29 | < 0.001 | 1.00 | 0.90 |
The first two columns give the Log-likelihood values for a model with λ = 0 (species independence) and for a model with λ equal to the estimate from Continuous with the highest likelihood score (ML, maximum likelihood). The third and forth columns give the likelihood-ratio test and its associated P-value. The last columns indicate the estimated ML λ value for the sleep traits and its associated lower confidence interval (when ML λ equal 1, no upper CI can be reported).
The ML λ value for NREM is not significantly different from 1 (P = 0.183).
ALLOMETRY OF SLEEP AND CORRELATED EVOLUTION OF SLEEP QUOTAS
REM and NREM sleep were positively associated with each another (t58 = 4.47, R2 = 0.26, P < 0.0001; Fig. 2) and, although this relationship appeared to be stronger for intermediate values of contrasts in sleep quotas, the association between REM and NREM sleep quotas remained highly significant after bootstrapping. Because TST was calculated as the sum of REM and NREM sleep time, both REM and NREM sleep quotas were strongly correlated with TST (NREM sleep: t58 = 20.60, R2 = 0.88, P < 0.0001; REM sleep: t58 = 7.62, R2 = 0.50, P < 0.0001). Sleep quotas, and thus also TST, did not correlate with body mass (TST: t58 = −1.46, R2 = 0.04, P = 0.149; NREM: t58 = −1.43, R2 = 0.03, P =0.158; REM: t58 = −1.27, R2 =0.02, P =0.209). Based on these findings, we did not statistically correct sleep traits for body mass.
Figure 2.
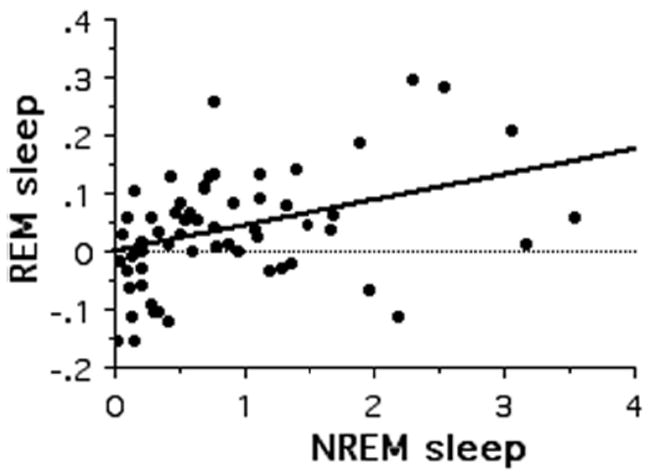
Bivariate relationships between phylogenetically independent contrasts of NREM and REM sleep quotas in mammals.
SLEEP AND COGNITIVE FUNCTIONS
Encephalization was quantified using residuals of brain mass on body mass (relative brain mass; see Methods). Counter to predictions, relative brain mass, and total sleep were significantly negatively correlated (t41 = −2.23, R2 = 0.11, P = 0.031), whereas a similar negative correlation between NREM quotas and relative brain mass approached significance (t41 = −1.92, R2 = 0.08, P = 0.061). Relative brain mass was unrelated to REM sleep (t41 = −1.61, R2 = 0.06, P = 0.114), the sleep state most commonly linked to cognitive functions. Previous studies argued that the percentage of REM sleep on total sleep time would better detect trade-offs between NREM and REM sleep than sleep quotas per se, and found that percentage of REM sleep, but not REM sleep quotas, was positively associated with brain mass after accounting for allometry (Lesku et al. 2006). However, as with REM quotas, percentage of REM sleep was unrelated to relative brain mass in our analyses (t41 = −0.67, R2 = 0.01, P = 0.505).
ENERGY CONSERVATION
We accounted for allometry in BMR using residuals (through the origin) of BMR on the experimental animal body mass as given in the study from which BMR data were extracted. Contrary to the prediction of the energy conservation hypothesis, relative BMR was negatively correlated with all sleep traits (TST: t40 = −2.59, R2 = 0.15, P = 0.013; NREM: t40 = −2.32, R2 = 0.12, P = 0.026; REM: t40 = −2.08, R2 = 0.10, P = 0.044; Fig. 3). After controlling for multiple testing, however, the relationship between REM sleep and relative BMR did not retain significance (FDR α threshold = 0.033).
Figure 3.
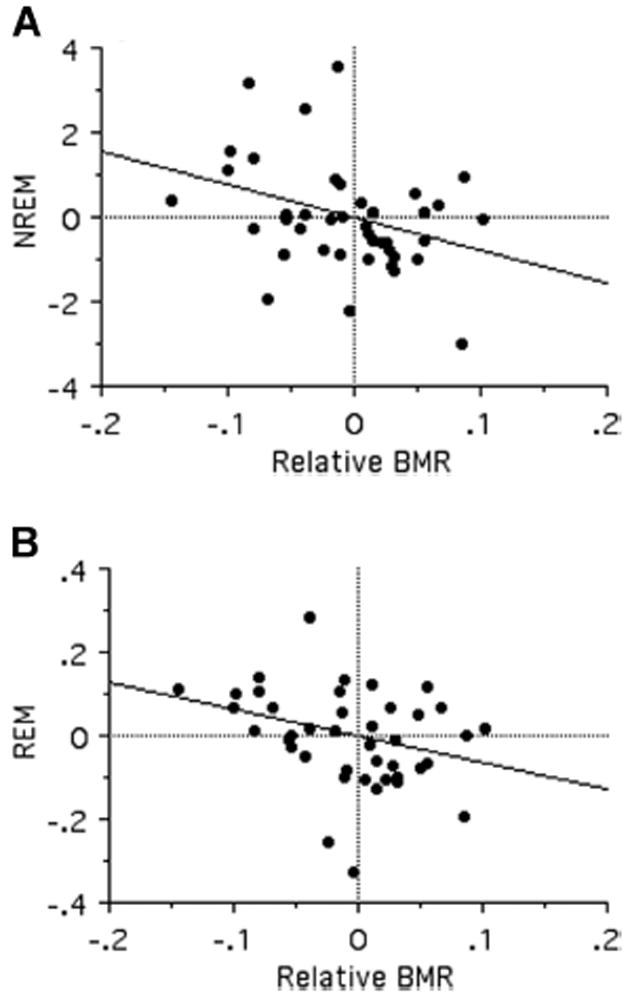
Bivariate relationships between phylogenetically independent contrasts in NREM (A) and REM (B) with contrasts in relative BMR.
BRAIN DEVELOPMENT
There was no evidence of an association between sleep and brain development at birth, as no sleep traits were significantly correlated with relative neonatal brain mass (Table 3). This result, however, may be due to small sample size; we thus tested the hypothesis using surrogate measures of brain development (neonatal body mass and gestation length; see Methods). All sleep traits were significantly negatively associated with relative gestation length, and both TST and REM sleep decreased with increasing relative neonatal body mass, (Table 3; Fig. 4).
Table 3.
Bivariate independent contrast analysis of sleep (TST is total sleep time) and relative neonatal brain mass, relative gestation length, and relative neonatal body mass. Neonatal brain mass was corrected for allometry using neonatal body mass.
| Predictor | Dependent variable | Linear regression |
|---|---|---|
| Relative neonatal brain mass | REM | t24=0.01 R2< 0.01 P=0.920 |
| NREM | t24=0.67 R2=0.02 P=0.508 | |
| TST | t24=0.28 R2=0.01 P=0.782 | |
| Relative gestation length | REM | t56=−4.02 R2=0.22 P<0.001 |
| NREM | t56=−3.20 R2=0.15 P=0.002 | |
| TST | t56=−3.92 R2=0.22 P<0.001 | |
| Relative neonatal body mass | REM | t52=−3.58 R2=0.20 P=0.001 |
| NREM | t52=−1.67 R2=0.05 P=0.102 | |
| TST | t52=−2.78 R2=0.13 P=0.008 |
Figure 4.
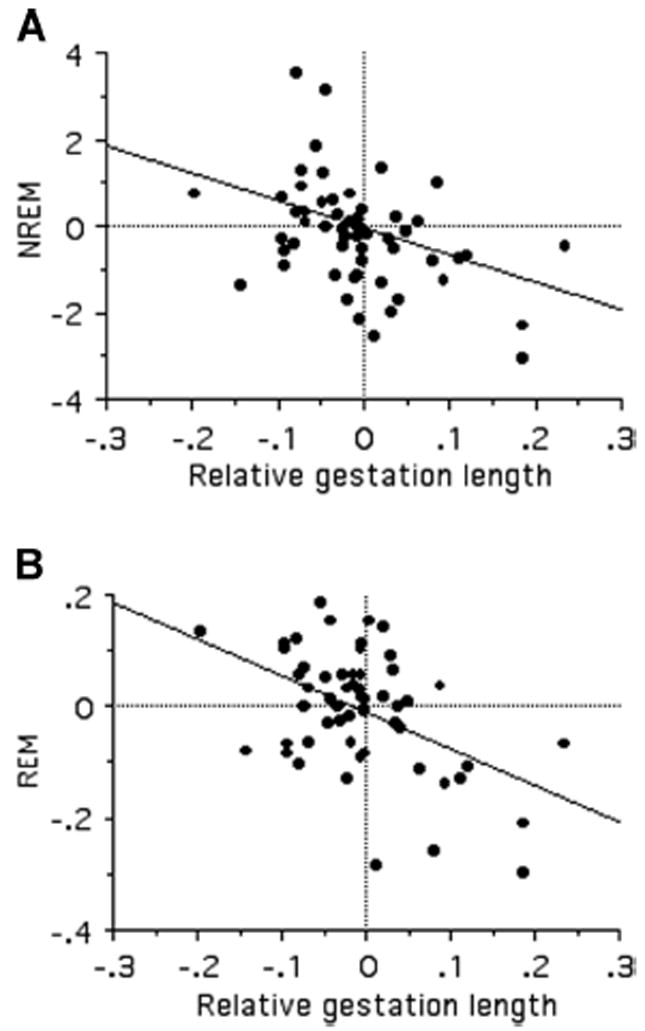
Bivariate relationship between contrasts in NREM (A) and REM (B) with relative gestation length.
Given these results, we carried out a multiple regression analysis with both relative gestation length and relative neonatal body mass as predictors of TST and REM sleep time, predicting that neonatal body mass would better explain variation in REM sleep quotas than gestation length. However, relative gestation length was the best predictor of both REM sleep (F2,50 = 9.38, R2 = 0.27, P < 0.001, relative gestation length P = 0.047, relative neonatal body mass P = 0.199) and TST (F2,50 = 7.49, R2 = 0.23, P = 0.001, relative gestation length P = 0.019, relative neonatal body mass P = 0.654). Therefore REM, NREM, and TST were better explained by gestation length than by neonatal body mass, a closer proxy for neonatal brain mass, undermining the idea of a functional role of REM sleep in neonatal brain maturation.
PREDATION RISK
Whereas a previous study found that only REM sleep is influenced by vulnerability to predators while asleep (Lesku et al. 2006), we found that both sleep quotas (REM and NREM sleep) decreased when exposure of the sleep site was greater (NREM: t57 = −3.16, R2 = 0.15, P = 0.003; REM: t57 = −2.91, R2 = 0.13, P = 0.005). As a consequence also TST, being the sum of REM and NREM sleep durations, was inversely related to sleep-site exposure (TST: t57 = −3.40, R2 = 0.17, P = 0.001). The sleep exposure index was positively correlated with body mass (t57 = 2.75, R2 = 0.12, P = 0.008) indicating that larger species sleep in more dangerous sites. Therefore, because smaller species are more vulnerable to predation and invest in searching for secure sleeping sites (Caro 2005), sleep exposure index relative to body mass would better estimate predation risk while sleeping. We thus corrected sleep exposure index for body mass and found that all sleep traits remained negatively correlated with relative sleep exposure (TST: t57 = −2.99, R2 = 0.13, P = 0.004; NREM: t57 = −2.76, R2 = 0.12, P = 0.008; REM: t57 = −2.57, R2 = 0.10, P = 0.013; Fig. 5A, B). Finally, contrary to predictions, sleep time was lower with increased degree of social sleep behavior (TST: t42 = −3.03, R2 = 0.18, P = 0.004; NREM: t42 = −2.39, R2 = 0.12, P = 0.021; REM: t42 = −3.09, R2 = 0.19, P = 0.004; Fig. 5C, D).
Figure 5.
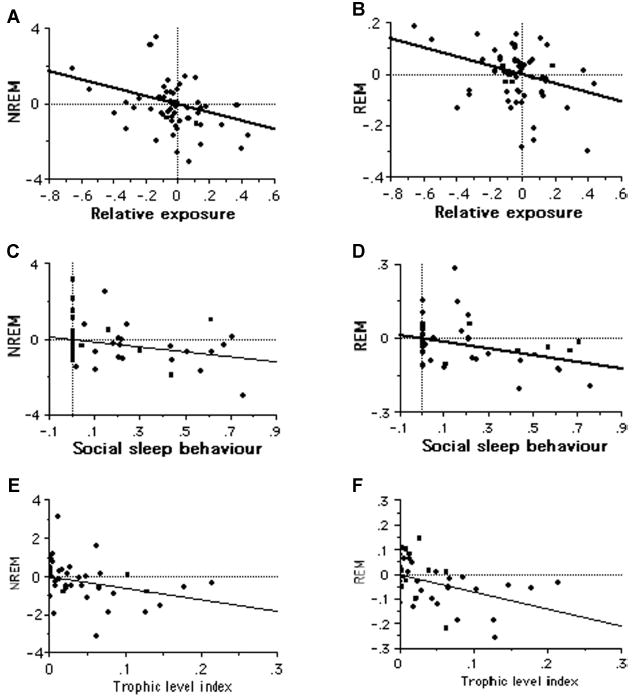
Bivariate relationship of contrasts in NREM and REM with relative sleep site exposure (A and B), social sleep behavior (C and D), and diet (E and F).
DIET
NREM and REM sleep quotas, and therefore TST, decreased in more herbivorous species (TST: t39 = −3.39, R2 = 0.23, P = 0.002; NREM: t39 = −2.61, R2 = 0.15, P = 0.013; REM: t39 = −3.71, R2 = 0.26, P < 0.0001; Fig. 5E, F). These results are consistent with both increased predation pressure in herbivorous mammals, and greater foraging requirements in herbivorous species constraining the time available for sleep.
Discussion
We assessed the putative functional benefits and ecological constraints that might have shaped interspecific variation in mammalian sleep, while accounting for data comparability and phylogenetic history. In contrast to previous studies, we found that REM and NREM sleep quotas exhibit similar correlations, and that ecological constraints, acting on total sleep time rather than on a specific sleep state, appear to be the primary drivers of variation in mammalian sleep architecture. Conversely, none of the traditional explanations of the potential benefits associated with either REM or NREM sleep (cognition, energy conservation and brain development) were supported after we controlled for laboratory conditions and phylogeny.
More specifically, we showed that:
REM and NREM sleep quotas do not increase significantly with encephalization (relative brain mass). These findings undermine the hypothesized cognitive benefits of sleep, at least as an explanation for sleep durations.
The energy conservation hypothesis is not supported because sleep quotas correlated negatively with relative BMR. This result supports the alternative hypothesis of a trade-off between time available for sleep and foraging in species with greater energy expenditure for their size.
The lack of a significant association between REM sleep and relative neonatal brain mass suggests that the major role of REM sleep is not linked to brain development. This conclusion is further strengthened by the presence of significant correlations involving both NREM and REM sleep and surrogate measures of neonatal development, and are probably a byproduct of predation pressure.
The effect of predation pressure on sleep evolution is more complex than previously appreciated. Although sleep time is reduced in species that sleep in more exposed sites, sleep time is also reduced in species that sleep socially (and should therefore obtain benefits of reduced predation risk). In addition, sleep quotas decreased in more herbivorous species, in agreement with both the predation risk hypothesis and the sleep-foraging trade-offs hypothesis.
An assumption of comparative studies is that data from different species must be directly comparable (Campbell and Tobler 1984; Siegel 2005). Our analyses caution against pooling comparative data on sleep quotas derived from different types of study. We found clear evidence that data from studies that recorded sleep for < 12 h significantly underestimated sleep quotas, and as a consequence TST, and we caution against adjusting such estimates up to 24 h, as pointed out by Berger (1990). Non-EEG and EEG studies did not differ significantly, but, given a trend at P < 0.10 and small sample sizes, it would be premature to conclude that measurement method has no effect. Contrary to the expectation that sleep would be systematically disturbed and underestimated in restrained and nonhabituated animals, we failed to find evidence that these conditions systematically impact estimates of sleep quotas, and therefore of TST, across species. This surprising conclusion, however, must again be tempered by the small sample sizes for this particular comparison, and the issue deserves further investigation when additional data become available.
Our analysis showed that there is a strong phylogenetic signal in REM and NREM sleep quotas, and as a result also in TST, meaning that closely related species exhibit similar sleep durations. These findings run counter to suggestions that phylogeny has no importance in determining sleep times in mammals (Siegel 1995, 2004, 2005) and emphasize the importance of accounting for phylogenetic similarity between species when investigating the evolutionary correlates of mammalian sleep. Like Lesku et al. (2006), we failed to detect a significant association between sleep and body mass in phylogenetic tests. Together with the finding of strong phylogenetic signal in sleep quotas, the results suggest that the association between sleep and body mass reported in previous studies (Zepelin 1989; Zepelin and Rechtschaffen 1974; Zepelin et al. 2005; Savage and West 2007) was a phylogenetic artifact, and thus conclusions based on interpretation of allometric exponents of correlations with no phylogenetic correction and control for data quality are potentially misleading.
We also did not find evidence that REM sleep increases with encephalization in mammals. This conclusion differs from that of a previous study (Lesku et al. 2006) and is probably due to our use of both sleep and brain size data collected under more consistent conditions. Our results do not rule out the possibility that one or both of the sleep states performs some primary neural function such as memory consolidation (reviewed in Walker and Stickgold 2006) or repairs damage due to oxidative stress (Siegel 2001, 2003), but neither do they support such hypotheses. Further studies are needed to test if sleep correlates with specific brain components given that encephalization is a crude measure of cognitive abilities.
Our results do not support the energy conservation hypothesis for the evolution of NREM sleep but are consistent with the idea that there are trade-offs between foraging and sleep time in species with high energy demands for their size and a more herbivorous diet. Contrary to previous studies (Elgar et al 1988, 1990; Lesku et al. 2006), however, our results emphasize that both NREM and REM sleep (and therefore TST, because this is the sum of sleep quotas) were negatively associated with relative BMR and diet (see below), suggesting that such ecological constraints act on total sleep time and affect both sleep states equally. Although after controlling for multiple testing, the association between relative BMR and REM sleep was marginally nonsignificant, basal metabolic rate is a relatively poor predictor of total daily energy expenditure in mammals (I. Capellini and R. A. Barton, unpubl. ms.). Hence, this relationship should be reevaluated once more data on field metabolic rate become available.
We found no support for the hypothesis that REM sleep promotes neonatal brain development, as REM sleep requirements were not significantly higher in species with smaller neonatal brain mass after controlling for allometry. Contrary to the predictions and results of previous studies (Zepelin and Rechtschaffen 1974; Elgar et al. 1988; Zepelin 1989; Elgar et al. 1990; Lesku et al. 2006), both REM and NREM sleep exhibited significant negative correlations with gestation length and neonatal body mass, indicating that associations with developmental traits are not REM specific. We suggest that these results reflect a constraint on the overall amount of time spent sleeping due to predation risk, because precocial offspring are often found in species that cannot hide and protect newborns from predators (Eisenberg 1981). It is important to note that the sleep exposure index and the degree of offspring development at birth (as inferred by gestation length and neonatal body mass) are associated with different aspects of predation risk. Although the former was an estimate of vulnerability at the sleeping site, the latter would be more closely related to the offspring’s vulnerability during the entire 24-h period.
Although we did not find a significant correlation between adult REM quotas and relative neonatal brain mass, REM sleep may help in the development of specific brain structures rather than the brain as a whole (e.g., the visual system; Marks et al. 1995), a hypothesis that has yet to be tested comparatively. Furthermore, all comparative tests of the neurodevelopmental hypotheses of REM sleep were based on the assumption that REM quotas during development are reflected in REM quotas in the adult. Tests of hypotheses linking REM sleep neonatal brain development should ideally use REM and NREM quotas in neonates, rather than adult sleep quotas. Unfortunately such data are currently not available.
Ecological constraints act on total sleep time rather than one specific state and, contrary to what previously suggested (Lesku et al. 2006), there is no specific cost associated with REM sleep. The effect of predation risk on sleep architecture appears to be more complex than previously appreciated. Although sleep quotas are reduced when exposure of the sleeping site is greater, contrary to predictions that species that sleep socially can sleep for longer, both NREM and REM sleep quotas are lower in species that sleep socially. This finding may indicate that social sleepers sleep more efficiently and acquire the benefits of sleep in a shorter time interval, because the protection derived from sleeping in larger groups may allow them to spend more time in deeper sleep stages. Alternatively, there might be trade-offs between sleep and time devoted to social interactions, with more social species sacrificing sleep to service social relationships. Finally, both REM and NREM sleep quotas are lower in more herbivorous species. This result is consistent with the predation risk hypothesis, because herbivores may be under greater predation pressure than nonherbivores, but it is also consistent with the idea that foraging strategy constrains the time available to sleep, because herbivores are believed to need greater foraging times to meet their energy requirements, as discussed above (see also Allison and Cicchetti 1974; Elgar et al. 1988, 1990; Siegel 2005).
Overall, our results suggest that, once phylogeny and data quality of both sleep and nonsleep data are controlled for, REM and NREM sleep are similarly constrained by ecological factors. Our conclusions differ from previous studies (Zepelin and Rechtschaffen 1974; Elgar et al. 1988; Lesku et al. 2006) that did not both employ phylogenetic comparative methods and control for data comparability and quality of both the sleep and the nonsleep variables (e.g., BMR, brain mass). Further differences with a recent phylogenetic study on the evolution of mammalian sleep (Lesku et al. 2006) reflect that, relative to the more rigid formulation of predictions in a path model (Petraitis et al. 1996; Shipley 2000), our more flexible approach allowed us to assess for a given hypothesis both predicted associations (e.g., NREM sleep increases with BMR if NREM sleep helps conserve energy) and unpredicted associations (e.g., REM sleep does not correlate with BMR if the energy conservation hypothesis is true). Thus, we could reveal that both REM and NREM sleep exhibit similar correlations with nonsleep variables (e.g., with BMR and gestation length), indicating that there are no specific costs or benefits associated with either state.
Our results also raise the intriguing question of whether sleep times can be reduced by increasing the “quality” of sleep. Specifically, interspecific variation in the physiological intensity of sleep could potentially compensate for the loss of sleep in species exposed to ecological pressures to remain active. If this is correct, we suggest that functional benefits of sleep will be evident in comparative studies of sleep evolution when measures of sleep intensity become more widely available. Finally, sleep quotas increased with one another. This result is consistent with studies showing that, although REM and NREM sleep have distinct neurophysiological characteristics, they exhibit functional interactions (Benington and Heller 1994, 1995; Van Cauter et al. 1998; Ambrosini and Giuditta 2001; Steiger 2003). We thus agree with suggestions (e.g., Benington and Heller 1994, 1995; Van Cauter et al. 1998) that variation in mammalian sleep might be better explained by an approach that integrates the two states functionally and ecologically.
In conclusion, sleep is a fundamental aspect of mammalian life, and understanding the drivers of sleep patterns is useful for understanding mammalian behavior and ecology. Previous research on mammalian sleep has produced a remarkably varied set of results, and surprisingly few conclusions have remained robust across studies. Here, we attempted to clarify the comparative patterns by acquiring the largest database yet constructed on mammalian sleep, pruning this dataset to a set of data collected under standardized conditions, and controlling for phylogeny. Our study suggests that ecological constraints on overall sleep time appear to be more important than previously thought (but see Lima et al. 2005). Further studies on the importance of ecological constraints that shape variation in sleep are therefore likely to provide new insights to mammalian sleep evolution.
Supplementary Material
Acknowledgments
We thank E. Harris and N. Patel for their work on the database and logistic support; L. Ruvinskaya for translating papers from Russian, P. Lindenfors, J. Chang, M. Mohneke, and T. Morrison for help collecting the data on the ecological traits; M. Pagel and A. Purvis for advice on the comparative analysis. This work was supported by NIMH grant number 1R01MH070415-01A1 and the Max Planck Society (CN).
Footnotes
Supplementary Material
The following supplementary material is available for this article:
Appendix S1. Phylogenetic tree and sources.
Figure S1. Phylogenetic tree of the mammalian species used in the analyses, assembled using published pylogenies (see text for details and sources).
This material is available as part of the online article from: http://www.blackwell-synergy.com/doi/abs/10.1111/j.1558-5646.2008.00393.x (This link will take you to the article abstract).
Please note: Blackwell Publishing is not responsible for the content or functionality of any supplementary materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
LITERATURE CITED
- Affani JM, Cervino CO, Marcos HJA. Absence of penile erections during paradoxical sleep. Peculiar penile events during wake-fulness and slow wave sleep in the armadillo. J Sleep Res. 2001;10:219–228. doi: 10.1046/j.1365-2869.2001.00259.x. [DOI] [PubMed] [Google Scholar]
- Allison T, Cicchetti DV. Sleep in mammals: ecological and constitutional correlates. Science. 1976;194:732–734. doi: 10.1126/science.982039. [DOI] [PubMed] [Google Scholar]
- Ambrosini MV, Giuditta A. Learning and sleep: the sequential hypothesis. Sleep Med Rev. 2001;5:477–490. doi: 10.1053/smrv.2001.0180. [DOI] [PubMed] [Google Scholar]
- Bejamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc B. 1995;57:289–300. [Google Scholar]
- Benington JH, Heller HC. Does the function of REM sleep concern non-REM sleep or waking? Progress Neurobiol. 1994;44:433–339. doi: 10.1016/0301-0082(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Benington JH, Heller HC. Restoration of brain energy metabolism as the function of sleep. Progress Neurobiol. 1995;45:347–360. doi: 10.1016/0301-0082(94)00057-o. [DOI] [PubMed] [Google Scholar]
- Berger RJ. Bioenergetic functions of sleep and activity rhythms and their possible relevance to aging. Fed Proc. 1975;34:97–102. [PubMed] [Google Scholar]
- Berger RJ. Relations between sleep duration, body weight and metabolic rate in mammals. Anim Behav. 1990;40:989–991. [Google Scholar]
- Berger RJ, Phillips NH. Energy conservation and sleep. Behav Brain Res. 1995;69:65–73. doi: 10.1016/0166-4328(95)00002-b. [DOI] [PubMed] [Google Scholar]
- Blomberg SP, Garland T. Tempo and mode in evolution: phylogenetic inertia, adaptation and comparative methods. J Evol Biol. 2002;15:899–910. [Google Scholar]
- Blomberg SP, Garland T, Ives A. Testing for phylogenetic signal in comparative data: behavioural traits are more labile. Evolution. 2003;57:717–745. doi: 10.1111/j.0014-3820.2003.tb00285.x. [DOI] [PubMed] [Google Scholar]
- Campbell SS, Tobler I. Animal sleep: a review of sleep duration across phylogeny. Neurosci Biobehav Rev. 1984;8:269–300. doi: 10.1016/0149-7634(84)90054-x. [DOI] [PubMed] [Google Scholar]
- Caro T. Antipredator defenses in birds and mammals. Univ. of Chicago Press; Chicago: 2005. [Google Scholar]
- Clemens Z, Fabo D, Halasz P. Overnight verbal memory retention correlates with the number of sleep spindles. Neuroscience. 2005;132:529–535. doi: 10.1016/j.neuroscience.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Efron B, Tibshirani RJ. An introduction to the bootstrap. Chapman and Hall; London: 1993. [Google Scholar]
- Eisenberg JF. An analysis of trends in evolution, adaptation and behavior. The Univ. of Chicago Press; Chicago: 1981. The mammalian radiations. [Google Scholar]
- Elgar MA, Pagel MD, Harvey PH. Sleep in mammals. Anim Behav. 1988;36:1407–1419. [Google Scholar]
- Elgar MA, Pagel MD, Harvey PH. Sources of variation in mammalian sleep. Anim Behav. 1990;40:991–995. [Google Scholar]
- Felsenstein J. Phylogenies and the comparative method. Am Nat. 1985;125:1–15. [Google Scholar]
- Freckleton RP, Harvey PH, Pagel M. Phylogenetic analysis and comparative data: a test and review of evidence. Am Nat. 2002;160:712–726. doi: 10.1086/343873. [DOI] [PubMed] [Google Scholar]
- Garland T, Harvey PA, Ives AR. Procedures for the analysis of comparative data using phylogenetically independent contrasts. Syst Biol. 1992;41:18–32. [Google Scholar]
- Garland T, Bennett AF, Rezende EL. Phylogenetic approaches in comparative physiology. J Exp Biol. 2005;208:3015–3035. doi: 10.1242/jeb.01745. [DOI] [PubMed] [Google Scholar]
- Harvey PA, Pagel M. The comparative method in evolutionary biology. Oxford Univ. Press; Oxford: 1991. [Google Scholar]
- Hayssen V, van Tienhoven A, van Tienhoven A. Asdell’s patterns of mammalian reproduction. Cornell Univ. Press; Ithaca, New York: 1993. [Google Scholar]
- Hobson JA. Sleep is of the brain, by the brain and for the brain. Nature. 2005;437:1254–1256. doi: 10.1038/nature04283. [DOI] [PubMed] [Google Scholar]
- Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature. 2004;430:78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- Jouvert-Monier D, Astic L, Lacote D. Ontogenesis of the states of sleep in the rat, cat and guinea pig during the first postnatal month. Dev Psychobiol. 1970;2:216–239. doi: 10.1002/dev.420020407. [DOI] [PubMed] [Google Scholar]
- Lesku JA, Roth TC, Amlaner CJ, Lima SL. A phylogenetic analysis of sleep architecture in mammals: the integration of anatomy, physiology and ecology. Am Nat. 2006;168:441–453. doi: 10.1086/506973. [DOI] [PubMed] [Google Scholar]
- Mangold-Wirz K. Cerebralisation und ontogenesemodus bei eutherien. Acta Anat. 1966;63:449–508. [PubMed] [Google Scholar]
- Maquet P, Phillips C. Rapid eye movement sleep: cerebral metabolism to functional brain mapping. In: Inoue S, editor. Rapid eye movement sleep. Marcel Dekker; New York: 1999. pp. 276–285. [Google Scholar]
- Marks GA, Shaffery JP, Oksenberg A, Speciale SG, Roffwarg HP. A functional role for REM sleep in brain maturation. Behav Brain Res. 1995;69:1–11. doi: 10.1016/0166-4328(95)00018-o. [DOI] [PubMed] [Google Scholar]
- Martins E, Garland T. Phylogenetic analyses of the correlated evolution of continuous characters: a simulation study. Evolution. 1991;45:534–557. doi: 10.1111/j.1558-5646.1991.tb04328.x. [DOI] [PubMed] [Google Scholar]
- McNab BK. On the utility of uniformity in the definition of basal rate of metabolism. Physiol Zool. 1997;70:718–720. doi: 10.1086/515881. [DOI] [PubMed] [Google Scholar]
- McNab BK. On the comparative ecological and evolutionary significance of total and mass-specific rates of metabolism. Physiol Biochem Zool. 1999;72:642–644. doi: 10.1086/316701. [DOI] [PubMed] [Google Scholar]
- McNamara P, Capellini I, Harris E, Nunn CL, Barton RA, Preston B. The Phylogeny of Sleep Database: a new resource for sleep scientists. The New Sleep Journal. 2008;1:11–14. doi: 10.2174/1874620900801010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunn CN, Barton RA. Comparative methods for studying primate adaptation and allometry. Evol Anthropol. 2001;100:81–98. [Google Scholar]
- Pagel M. Inferring evolutionary processes from phylogenies. Zool Scripta. 1997;26:331–348. [Google Scholar]
- Pagel M. Inferring the historical patterns of biological evolution. Nature. 1999;401:877–884. doi: 10.1038/44766. [DOI] [PubMed] [Google Scholar]
- Pagel M, Harvey PH. Diversity in the brain sizes of newborn mammals. Allometry, energetics or life history tactics? BioScience. 1990;40:116–122. [Google Scholar]
- Petraitis PS, Dunham AE, Niewiarowski PH. Inferring multiple causality: the limitations of path analysis. Funct Ecol. 1996;10:421–431. [Google Scholar]
- Purvis A, Rambaut A. Comparative analysis by independent contrasts (CAIC): an Apple Macintosh application for analysing comparative data. Comput Appl Biosci. 1995;11:247–251. doi: 10.1093/bioinformatics/11.3.247. [DOI] [PubMed] [Google Scholar]
- Rattenborg NC, Amlaner CJ. Phylogeny of sleep. In: Lee-Chiong TL, Sateia MJ, Carskadon MA, editors. Sleep medicine. Hanley & Belfus, Inc; Philadelphia: 2002. pp. 7–22. [Google Scholar]
- Rechtschaffen A. Current perspectives on the function of sleep. Persp Biol Med. 1998;41:359–391. doi: 10.1353/pbm.1998.0051. [DOI] [PubMed] [Google Scholar]
- Roffwarg HP, Muzio JN, Dement WC. Ontogenetic development of the human sleap-dream cycle. Science. 1966;152:604–619. doi: 10.1126/science.152.3722.604. [DOI] [PubMed] [Google Scholar]
- Ruckebush Y. Etude EEG et comportamentale des alternantes veille-sommeil chez lane. C R Seances Soc Biol Fil. 1963;157:840–844. [PubMed] [Google Scholar]
- Sacher GA, Staffeldt EF. Relation of gestation time to brain weight for placental mammals: implications for the theory of vertebrate growth. Am Nat. 1974;108:593–612. [Google Scholar]
- Savage VM, West GB. A quantitative, theoretical framework for understanding mammalian sleep. Proc Natl Acad Sci. 2007;104:1051–1056. doi: 10.1073/pnas.0610080104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipley B. Cause and correlation in biology. Cambridge Univ. Press; Cambridge: 2000. [Google Scholar]
- Siegel JM. Phylogeny and the function of REM sleep. Behav Brain Res. 1995;69:29–34. doi: 10.1016/0166-4328(95)00023-m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JM. The REM sleep-memory consolidation hypothesis. Science. 2001;294:1058–1063. doi: 10.1126/science.1063049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JM. Why we sleep. Scientific American. 2003;289:92–97. doi: 10.1038/scientificamerican1103-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JM. Sleep phylogeny: clues to the evolution and function of sleep. In: Luppi PH, editor. Sleep: circuits and functions. CRC Press; Boca Ranton, FL: 2004. pp. 163–176. [Google Scholar]
- Siegel JM. Clues to the function of mammalian sleep. Nature. 2005;437:1264–1271. doi: 10.1038/nature04285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiger A. Sleep and endocrinology. J Internal Med. 2003;254:13–22. doi: 10.1046/j.1365-2796.2003.01175.x. [DOI] [PubMed] [Google Scholar]
- Stickgold R. Sleep: off-line memory processing. Trends Cogn Sci. 1998;2:484–492. doi: 10.1016/s1364-6613(98)01258-3. [DOI] [PubMed] [Google Scholar]
- Stickgold R. Sleep-dependent memory consolidation. Nature. 2005;437:1272–1278. doi: 10.1038/nature04286. [DOI] [PubMed] [Google Scholar]
- Trachsel LD, Edgar M, Heller HC. Are ground squirrels sleep deprived during hibernation? Am J Physiol Regul Integr Comp Physiol. 1991;260:R1123–R1129. doi: 10.1152/ajpregu.1991.260.6.R1123. [DOI] [PubMed] [Google Scholar]
- Van Cauter E, Plat L, Copinschi G. Interrlations between sleep and the somatotropic axis. Sleep. 1998;21:553–566. [PubMed] [Google Scholar]
- Verhoeven KJF, Simonsen KL, McIntyre LM. Implementing false discovery rate control: increasing your power. Oikos. 2005;108:643–647. [Google Scholar]
- Vertes RP. Memory consolidation in sleep: dream or reality. Neuron. 2004;44:135–148. doi: 10.1016/j.neuron.2004.08.034. [DOI] [PubMed] [Google Scholar]
- Von Rohrs M, Ebinger P. How is cranial capacity related to brain volume in mammals? Mamm Biol. 2001;66:102–110. [Google Scholar]
- Walker MP, Stickgold R. Sleep, memory and plasticity. Ann Rev Psychol. 2006;57:139–166. doi: 10.1146/annurev.psych.56.091103.070307. [DOI] [PubMed] [Google Scholar]
- White CR, Phillips NF, Seymour RS. The scaling and temperature dependence of vertebrate metabolism. Biol Lett. 2006;2:125–127. doi: 10.1098/rsbl.2005.0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zepelin H. Mammalian sleep. In: Kryger MH, Roth T, Dement WC, editors. Principles and practices of sleep medicine. Saunders; Philadelphia: 1989. pp. 30–49. [Google Scholar]
- Zepelin H, Rechtschaffen A. Mammalian sleep, longevity and energy metabolism. Brain Behav Evol. 1974;10:425–470. doi: 10.1159/000124330. [DOI] [PubMed] [Google Scholar]
- Zepelin H, Siegel JM, Tobler I. Mammalian sleep. In: Kryger MH, Roth T, Dement WC, editors. Principles and practices of sleep medicine. Saunders; New York: 2005. pp. 91–100. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


