Abstract
Meprin metalloproteases are implicated in inflammatory bowel disease, which involves dysfunction of immune cells. However, the roles of meprins in the immune and hematological system remain uncharacterized. In this report, we demonstrate that meprins were expressed in the hematological system, and meprin alpha/beta null (alpha-/-/beta-/-) mice had decreased prevalence of resident monocytes (R-MC) and natural killer (NK) cells in blood, with a concomitant accumulation of inflammatory monocytes (I-MC) and NK cells in bone marrow. In contrast, T and B lymphocytes were not affected by meprin deficiency. In response to acute inflammation induced by intraperitoneal injection of thioglycollate, meprin-deficient mice exhibited higher body temperature than the wild-type mice, which was correlated with accumulation of I-MC but persistent low prevalence of NK cells in blood. These results indicate that meprin metalloproteases play important roles in the homeostasis of monocytes and NK cells, and possibly are involved in egress of these two type cells from bone marrow and homing to the periphery. Our findings are the first report to demonstrate that metalloproteases affect homeostasis of leukocytes, which have important implications for understanding physiology of and pathogenesis in the hematological system.
Keywords: meprin, metalloprotease, monocytes, NK cells, homeostasis, inflammation
Introduction
Meprins were first discovered in the apical brush-border membranes of the epithelia lining renal proximal tubules and subsequently in the intestinal epithelium [1-3]. These metalloproteases have been implicated in several pathological conditions including inflammatory bowel disease (IBD) [4;5].
Meprins are members of the “astacin” family of metalloproteases [3;6]. In mammals, there are 2 meprin subunits, alpha and beta [7], both of which are synthesized as multi-domain trans-membrane proteins [8;9]. In mice, meprin expression is localized to the apical (brush-border) membrane of polarized epithelial cells lining the intestine and proximal tubules in kidney [10]. Meprins have also been detected in macrophages of mesenteric lymph nodes [5]. In humans, in addition to expression in the brush-border membrane of intestines, meprins are also found in epidermal keratinocytes [4;11]. Meprin alpha and beta RNA have been detected in leukocytes of undetermined lineage from lamina propria of human inflammatory bowel tissue [4].
Meprins are capable of hydrolyzing many proteins and peptides [12-14], including extracellular matrix (ECM) [13-15] and cytokines/chemokines [12;16]. While the former suggests roles of meprins in tissue repair and cell migration, the latter implies meprins' involvement in modulation of the immune system. The expression of meprins in leukocytes from inflammatory bowel tissue further points to a possibility that meprins are involved in pathogenesis of dysregulated immune cells [4]. Consistently, in murine models, meprins are detected in macrophages from lymph nodes draining intestine, and leukocytes with null meprin beta are deficient in migration through artificial ECM [5]. These two findings are intriguing and call for further evaluation of meprins' roles in the hematological system in general, and immune cells in particular.
In this report, we examined meprins' roles in the hematological system using mice carrying germline null mutations of both meprin alpha and beta genes. In consideration that meprin alpha and beta possess unique as well as overlapping biochemical activities, we reasoned that disruption of both genes may produce more readily discernable phenotypes. We demonstrate here that deficiency in both meprin subunits resulted in decreased prevalence of monocytes and natural killer (NK) cells in blood, with concomitant accumulation of these two cell types in bone marrow. In response to peritoneal inflammation, meprin-deficient mice manifested a febrile reaction in correlation with retention of monocytes in blood. These studies indicate that meprins are involved in physiological functions of monocytes and NK lymphocytes.
Methods
Mice
Meprin alpha-/-/beta-/- mice (meprin double knockout, dKO) were generated in the laboratory of Judith S. Bond by crossing meprin alpha+/+/beta-/- mice and meprin alpha-/-/beta+/+ mice. The establishment of meprin beta null mice carrying disrupted MEP1B genes has been reported [17]. Characterization of the meprin alpha null mice is discussed in Ph.D. Dissertation by S. Banerjee, Pennsylvania State University, 2008 [18]. In brief, a 129X1/SvJ-based targeting vector was used to insert a neomycin resistant cassette into the MEP1A gene on chromosome 17 by homologous recombination. The inserted PGK-neo cassette disrupted the site encoding the catalytic center in exon 7 of the Mep1a gene. R1 ES clones confirmed with targeted disruption of meprin alpha gene were micro-injected into C57BL/6 blastocysts. Chimeras were crossed with C57BL/6 mice (Jackson Laboratories, Bar Harbor, MN) to generate meprin alpha null mice. Inbred meprin beta null mice and mixed meprin alpha null mice were crossed to generate dKO mice, and the littermates were screened by Southern blotting analysis to confirm genotypes. dKO mice used in this study were 3-5 month old males in a 129XC57BL/6 mixed background, and the wild-type control mice were age- and gender-matched meprin alpha+/+/beta+/+ littermates (dWT) of the dKO mice. All the mice were housed in conventional animal care facility at 23 ± 1°C under a 12/12 h light-dark cycle (lights on at 06:00h) with free access to food chow and tap water, in full compliance with animal use and care regulations. Experimental protocols were approved by Pennsylvania State University College of Medicine Institutional Animal Care and Use Committee.
Cells and cell fractionation
Peripheral blood was obtained by cardiac puncture from anesthetized mice. Bone marrow from femur was harvested as routine. Peripheral blood mononuclear cells (PBMC) and bone marrow mononuclear cells (BMMC) were prepared from peripheral blood and bone marrow cells by centrifugation on a Histopaque 1083 (Sigma, St Louis, MO) gradient. Primary human dermal fibroblast cell lines were established from skin biopsies from forearms and grown in F12/DMEM (Media-tech) as described before [19].
Reverse Transcription (RT)-PCR
Total RNA was isolated with RNA-Bee reagent (AMS Biotechnology, UK). cDNA was synthesized with the RETROscript kit (Ambian, Austin, TX). The primers used for meprin alpha and beta detection were 5′-CGC CTC AAG TCT TGT GTG GAT TTC-3′/5′-TCA TGT TCA ATG GTG GCC TTA AAG TC-3′ (mouse meprin alpha), 5′-ACA AGG TGT CCT CAC CAT ATC TGG C-3′/5′- GCA CTC AGC TCT GCC ATC TTG G-3′ (mouse meprin beta), 5′-CAG GTG GAC GTT CCC CAT TC-3′/5′-AGC ACC CAT CAA ACT GTT GAA AT-5′ (human meprin alpha), and 5′-AAG GTT TGG GAC TGG ATC TTT TT-3′/5′-GCA TTG AGG ATA ACT CCC TTA GC-3′ (human meprin beta).
Genotyping PCR
Bone marrow cells were boiled and digested overnight with 6U of proteinase K at 42°C in a buffer containing 50 mM Tris-HCL, 100 mM EDTA, 125 mM NaCl, and 1% sodium dodecyl sulfate, followed by ethanol precipitation of genomic DNA. Primer sets used for PCR were 5′-CCC TCT CTT GGG GTA CAA C -3′/5′-GTG CTG GTC TTC AGT GGG A-3′ (mouse meprin beta) and 5′-CCC CTG GAG TCT GTC TAG TAG CCA TCA TC -3′/5′-GCG AAG GAC CTC CCA TGA TAA ACT TAG G-3′ (mouse meprin alpha), which flanked the sites of vector integration.
Western Blotting
Tissue homogenates were prepared from kidney and skeletal muscle in the lysis buffer (100 mM NaCl, 50 1% Triton X-100, 0.5% deoxycholate, 0.2% SDS, 0.2 mM EDTA, 1mM benzamidine, 10 mM HEPES, pH7.5) containing protease Inhibitor (Roche, Indianapolis, IN) with a tissue homogenizer. Protein concentration was determined by the DC Protein Assay kit (Bio-Rad, Hercules, CA), and 200 μg proteins were run on 8%-sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), followed by electro- transfer onto nitrocellulose membranes (Bio-Rad). The membranes were blocked for 1 hour with a solution containing Tris buffer saline, 0.1% Tween-20 and 5% milk powder, and incubated for 1 hour with rabbit derived antisera against mouse meprin alpha or beta (1:10000) and rabbit anti-GAPDH(Santa Cruz, Santa Cruz, CA) (1:1000 dilution). Subsequently, blots were incubated with a horseradish peroxidase-labeled anti-rabbit IgG antibody (IMGENEX, San Diego, CA) for 1 hour (1:10000). Enhanced chemiluminescence (ECL) detection was performed with ECL detection agents (Amersham, Piscataway, NJ) and recorded on Hyperfilm-ECL film (Amersham).
Flow cytometry
Cells were stained with fluorochrome-conjugated antibodies as previously described [20]. In brief, 50 micro-liters of peripheral blood, 0.5 million PBMC, or 0.5 million bone marrow cells were first blocked with anti-mouse CD16/CD32 (10μg/ml, mouse FC Block, BD-Biosciences, San Diego, CA) and 20μg/ml Rat IgG (Sigma, St Louis, MO) for 15 min on ice, followed by incubation on ice for 30 min with fluorochrome-conjugated antibodies against the following markers at concentrations suggested by the providers: monocyte markers CD11b (clone M1/70, BD-Biosciences), CD14 (clone sa2-8, eBioscience, San Diego, CA), Ly6c (clone HK1.4, Southern Biotech, Birmingham, AL); granulocyte marker Ly6g (clone 1A8, BD-Bioscience); B cell marker CD19 (clone 6D5, Miltenyi Biotech, Auburn, CA); NK markers CD49b (clone DX5, Miltenyi Biotech), NK1.1 (clone PK136, eBioscience) and CD122 (clone TM-b1, eBioscience); T cell markers CD90 (clone 30-H12, Miltenyi Biotech) and CD3e (clone 500A2, eBioscience). After washing with a FACS buffer (3% FCS, 0.01% NaN3 in PBS), the samples were fixed with the Lysing Solution (BD-Biosciences) to lyse red blood cells, followed by acquisition on a FACSCalibur flow cytometer (BD-Bioscience). To enumerate the absolute number of leukocytes, Fluorospheres (Beckman-Coulter, Miami, FL) were added to samples prior to acquisition as internal references following instruction from the manufacturer. Isotype matched antibodies conjugated with the same fluorochrome as the test antibodies were used to control non-specific staining.
Induction of acute peritonealitis by thioglycollate (TG) and measurement of body temperature
Mice were injected intraperitoneally (i.p.) with 1 ml of 3% Brewer's TG broth (Sigma-Aldrich) or sterile pyrogen-free saline. Twenty hours later, anal temperature was measured by gently inserting a small Vaseline-coated thermometer probe (model 46 telethermometer, Yellow Springs Instruments, Ohio). Peritoneal exudates were collected with 6 ml cold RPMI.
Statistical analysis and data presentation
Data are presented as mean±SEM and analyzed with Student's t tests for comparison between the meprin null and control mice. P values of <0.05 at a confidence interval of 95% were considered significant. Dot plots shown are representative data from each group to illustrate cell populations analyzed and compared. The bar graphs show compilation of several analyses.
Results
Expression of meprin mRNA in leukocytes, and disruption of meprin genes in dKO mice
To determine whether meprins are expressed in the hematological system, mononuclear leukocytes were isolated by Ficoll gradient centrifugation from peripheral blood and bone marrow of dWT mice, and assayed for meprin mRNA by RT-PCR. The PCR primer pairs spanned intron sequences, allowing detection of only cDNA but not genomic DNA. RT-PCR detected meprin alpha- and beta-specific amplification products from mouse blood and bone marrow leukocytes and kidney, but not skeletal muscle (Fig. 1A). Meprin alpha and beta mRNA were also expressed in human PBMC, but not fibroblasts (Fig. 1B), indicating the human relevance of this study. Direct sequencing of the PCR products confirmed that the amplified products were 100% homologous to the established meprin sequences (data not shown). The expression of meprin in both peripheral blood and hematopoietic tissue indicated a possibility that meprins play roles in the development and function of the hematological system.
Fig. 1. Expression of meprin mRNA in the hematological system.
RNA from blood and bone marrow mononuclear leukocytes was examined by RT-PCR for meprin expression. (A) Meprin alpha and beta mRNA were detected in mouse peripheral blood mononuclear cells (PBMC) and bone marrow mononuclear cells (BMMC), but not in skeletal muscle. (B) Human PBMC but not fibroblasts (SF) expressed meprin alpha and beta mRNA. N.Ctrl, no template control.
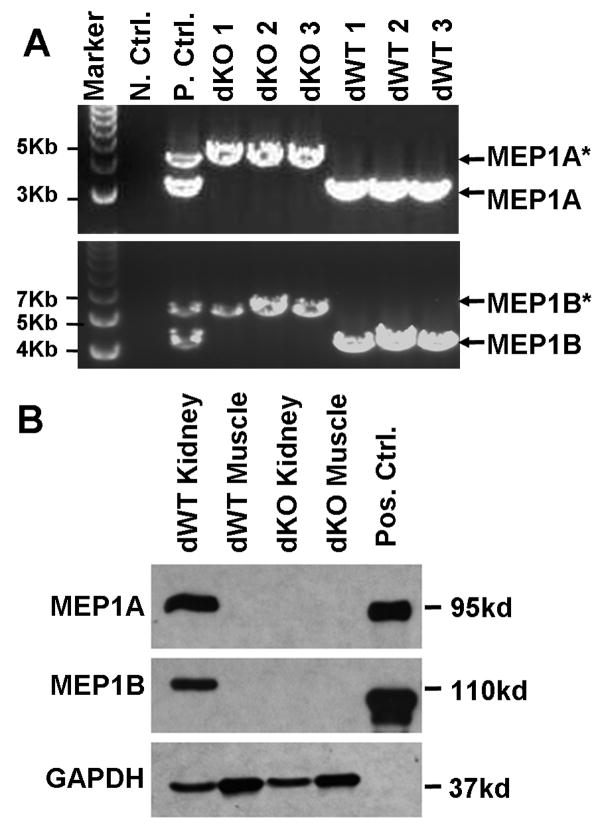
Successful disruption of both meprin genes in dKO mice was verified by PCR genotyping with DNA from mice used in the experiments (Fig.2A). The disrupted meprin genes yielded larger fragments than the WT counterparts, 4.3kb vs. 3.1kb for alpha and 6.3kb vs. 4.8kb for beta, respectively, consistent with the expected patterns for targeted vector integration. Western blots confirmed that meprin alpha and beta proteins were not expressed in kidneys of dKO mice (Fig. 2B). dKO mice had birth weights and growth rates comparable to the dWT mice, exhibited no gross abnormality, and showed no overt susceptibility to diseases in the conventional animal care facility.
Fig. 2. Disruption of meprin genes in dKO mice.
(A). DNA was extracted from bone marrow of the experimental mice and examined by PCR with primers flanking the insertion site of the neo gene in MEP1A (top) and MEP1B (bottom) genes. (*) indicates disrupted meprin gene fragments. N.Ctrl: no template negative control. P.Ctrl: positive control with DNA from meprin heterozygous (alpha+/-/beta+/-) mice. (B). Homogenates of kidney and skeletal muscle from dWT and dKO mice were examined by Western blot analysis for meprin expression. Pos. Ctrl: purified recombinant meprin proteins as positive controls.
dKO mice manifested higher body temperature than their WT counterparts in response to acute local inflammation
Peritoneal injection of TG is a well established method to study acute inflammation [21;22]. This model was used to investigate the impacts of meprin deficiency on immune response to peripheral nonbacterial acute inflammation. Without TG treatment, dKO and dWT mice had comparable body temperatures (36.5 vs. 36.6 °C, p=0.82, Fig. 3A), consistent with reported by others [23]. Twenty-four hours after intraperitoneal injection of TG, the body temperature of dKO mice was one degree higher than that of their dWT counterparts (37.6 vs. 36.5 °C, p<0.01, Fig. 3B). The observed febrile response in dKO mice suggested that meprin deficiency is associated with altered response of the innate immunity to acute inflammation [24].
Fig. 3. dKO, but not dWT, mice manifested a febrile reaction in response to acute peritoneal inflammation induced by TG.
Anal temperature was measured for mice non-treated (A) or 24 hours after i.p. injection of TG (B). Data shown are from 2 independent experiments with a total of 12 pairs of mice.
Meprin deficiency was associated with low frequencies of resident monocytes in blood, but high frequencies of inflammatory monocytes in bone marrow
As the first screen for hematological abnormality in meprin deficient mice, the relative prevalence of blood and bone marrow monocytes was compared in dKO mice and their dWT controls without TG challenge. The expression of CD11b and Ly6c was used to define monocytes. To ensure inclusion of all the hematological components in the analysis, flow cytometry staining was performed using total cells, without prior centrifugation to remove red blood cells and granulocytes.
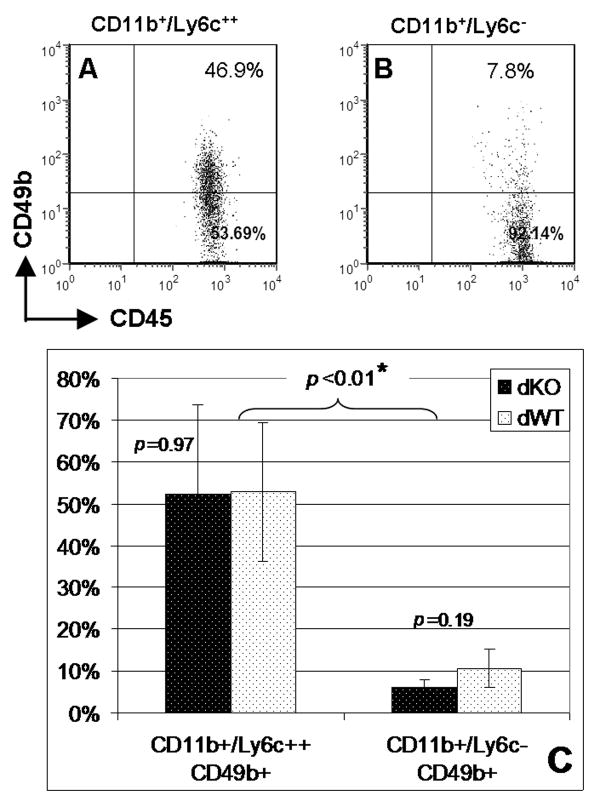
With granulocytes excluded from analysis by gating in FSC/SSC plots, CD11b+ cells can be divided into two distinct populations, Ly6c++ (Fig. 4A) and Ly6c- (Fig. 4D). The CD11b+/Ly6c++ cells stained dimmer for CD45 than their CD11b+/Ly6c- counterparts (Fig. 4B, E), and were greater in size and more granular (Fig. 4C, F). These patterns are consistent with the two subtypes of monocytes well established in mice, “inflammatory” (I-MC, CD11b+/Ly6c++) and “resident” (R-MC, CD11b+/Ly6c-) [22;25;26]. Interestingly, while frequencies of CD11b+/Ly6c- cells were relatively low in blood from dKO (5.2% vs. 11.7%, p<0.05, Fig. 4M), the prevalence of CD11b+/Ly6c++ cells was not significantly different between dKO and dWT mice (3.3% vs. 3.1%, p>0.05). In bone marrow, CD11b+ cells can also be divided into 2 populations based on Ly6c expression. While the CD11b+/Ly6c++ cells formed a well definable population (Fig. 4G), CD11b+/Ly6c- and CD11b+/Ly6c+ populations cannot be clearly separated (Fig. 4J). Interestingly, while dKO mice exhibited a frequency of CD11b+/Ly6c-/+ cells comparable to the one in dWT mice (6.5% vs. 5.5%, p>0.05, Fig. 4M), frequencies of CD11b+/Ly6c++ cells in bone marrow were significantly higher in dKO mice than the dWT controls (17.2% vs. 13.6%, p<0.01). In both the dKO and dWT mice, approximately 50% of the blood I-MC were positive for CD49b, while R-MC contained less than 10% CD49b+ cells (52.6% vs. 8.4%, p<0.01, Fig. 5). However, meprin deficiency did not significantly affect the CD11b+/CD49b+ monocytes, neither Ly6c++ (52.3% vs. 52.8%, p=0.97) nor Ly6c- (6.1% vs. 10.6%, p=0.19). Similar results were obtained from 2 independent experiments with a total of 7 pairs of matched mice. The above findings indicate that meprin deficiency differentially affected CD11b+ monocytes, dependent on their expression of Ly6c and location in the hematological compartments.
Fig. 4. In comparison to dWT mice, dKO mice had low R-MC (CD11b+/Ly6c-) in blood, but high I-MC (CD11b+/Ly6c++) in bone marrow.
Peripheral blood and bone marrow cells from dKO and dWT mice were stained with CD11b-FITC, Ly6c-PE and CD45-PerCP, followed by flow cytometry. Monocytes with the phenotype CD11b+/Ly6c- are defined as resident monocytes (R-MC), and those of CD11b+/Ly6c++ as inflammatory monocytes (I-MC). Representative dot plots (A-L) are to show cell populations compared in M. Darker events are the gated cells in CD11b/Ly6c plots. Granulocytes were excluded by gating in FSC/SSC plots. Data were from 2 independent experiments with a total of 7 mice in each group.
Fig. 5. Blood CD11b+/Ly6c++ I-MC were enriched with CD49b+ cells.
Peripheral blood from dKO and dWT mice was stained with CD11b-FITC, Ly6c-PE, CD45-PerCP and CD49b-APC, followed by flow cytometry. Representative dot plots (A, B) are to show cell populations compared in C. Events in A and B are CD11b+/Ly6c++ and CD11b+/Ly6c- cells, respectively. Granulocytes were excluded by gating in FSC/SSC plots (not shown). Data were from 2 independent experiments with a total of 7 mice in each group.
To characterize monocyte subtypes further, which were differentially affected by meprin deficiency, monocytes were defined by the expression of CD14, a well established marker for human circulating monocytes [28], with a mouse CD14-specific antibody sa2-8 [29]. sa2-8 stained mouse blood and bone marrow leukocytes with patterns very similar to those by the CD11b-specific antibody (Fig.6). In blood, based on Ly6c staining, CD14+ mononuclear leukocytes were also dividable into 2 populations, CD14+/Ly6c++ and CD14+/Ly6c- (Fig. 6A, C), with the former dimmer for CD45 staining (Fig. 6B, D). More significantly, while CD14+/Ly6c++ monocytes were at similar levels in dKO and dWT mice (2.3% vs. 2.4%, p=0.84), dKO mice had a lower frequency of blood CD14+/Ly6c- monocytes than the dWT controls (2.7% vs. 6.2%, p<0.01, Fig. 6G). In bone marrow, similar to CD11b+ I-MC, CD14+/Ly6c++ cells were more prevalent in dKO mice than the dWT controls (21.3% vs. 17.9%, p<0.05). However, unlike CD11b staining (Fig. 4J), both the dKO and dWT mice did not have a clearly definable population for CD14+/Ly6c- cells (Fig. 6E). Co-staining for CD11b with CD14 showed that nearly all the blood CD14+ cells were CD11b+, accounting for approximately 60% of the CD11b+ cells (data not shown). Thus, the above data indicate that the CD14-specific antibody sa2-8 defined a subset of CD11b+ monocytes in blood, and meprin deficiency differentially affected this CD14+ monocyte subset with a pattern similar to the one for CD11b+ monocytes, dependent on Ly6c expression and anatomical compartments.
Fig. 6. In comparison to dWT mice, dKO mice had low CD14+/Ly6c- R-MC in blood, but high CD14+/Ly6c++ I-MC in bone marrow.
Peripheral blood and bone marrow cells were stained with CD14-FITC, Ly6c-PE and CD45-PerCP, followed by flow cytometry. Representative dot plots (A-F) are to show cell populations compared in G. Darker events are the gated cells in CD14/Ly6c plots. Granulocytes were excluded by gating in the FSC/SSC plots (not shown). Data were from 2 independent experiments with a total of 7 mice in each group.
In comparison to dWT mice, frequencies of blood NK cells but not T and B lymphocytes were low in dKO mice, with simultaneous accumulation of NK cells in the bone marrow
To define the impacts of meprin deficiency on lymphocytes, the relative prevalence of NK, T and B cells was evaluated by staining CD49b+/NK1.1+ (NK), CD90+/CD3e+ (T) and CD19+ (B) cells in the peripheral blood and bone marrow of unchallenged mice. Data from 2 independent experiments with a total of 7 pairs of mice showed that dKO mice had a significantly lower frequency of NK cells than the dWT controls in blood (7.6% vs. 13.4%, p<0.05, Fig. 7A). Inversely correlated to the low prevalence of NK cells in blood, frequencies of NK cells in bone marrow were higher in dKO mice than the dWT controls (4.8% vs. 3.3%, p<0.05). Similar results were obtained when CD11b+ cells were excluded by gating to ensure that the CD49b+ NK cells analyzed did not include monocytes. In contrast to NK cells, T and B lymphocytes had similar prevalence in peripheral blood as well as in bone marrow (Fig. 7B, C).
Fig. 7. In comparison to dWT mice, blood NK cells but not T and B lymphocytes were low in dKO mice, with simultaneous accumulation of NK cells in bone marrow.
Blood and bone marrow cells from dKO and dWT mice were stained with antibodies against CD49b, NK1.1, CD11b and CD45 (A), or CD90 (blood only) or CD3e, CD19 and CD45 (B, C), followed by flow cytometry. Granulocytes were excluded by gating in FSC/SSC plots (not shown). Data were from 2 independent experiments with a total of 7 mice in each group.
dKO mice exhibited distorted monocyte response to acute peritoneal inflammation
The impacts of meprin deficiency on the prevalence of immune cells in response to TG-induced acute peritoneal inflammation were also studied. In correlation with the fever (Fig.3), frequencies of blood I-MC were higher in dKO than dWT mice (5.5% vs. 3.3%, p<0.01, Fig. 8A). This is in contrast to the unchallenged dKO mice, which had comparable frequencies of blood I-MC with their dWT counterparts (Fig. 4). Concurrent with the elevated I-MC, frequencies of blood R-MC in TG-challenged dKO mice increased to a level comparable to the one in dWT mice (15.3% vs. 18.9%, p=0.24, Fig. 8B), in contrast to the unchallenged mice which had lower frequencies of blood R-MC than dWT mice (Fig. 4). This increase in the frequency of blood R-MC in TG-challenged mice could be due to accumulation of I-MC, which have been reported to convert to R-MC in blood [25].
Fig. 8. Meprin deficiency was correlated with distorted monocyte response to acute inflammation.
Flow cytometry analysis of blood and bone marrow cells from dKO and dWT mice 24 hours after i.p. injection of TG for the relative prevalence of monocytes, NK cells, B cells and T cells. Note that dKO mice exhibited a higher level of blood I-MC and lower level of bone marrow R-MC than dWT mice. Data were from 2 independent experiments with a total of 12 mice in each group.
The relative prevalence of bone marrow monocytes in the TG-challenged mice was also distinct from that in unchallenged mice. In contrast to the unchallenged mice where dKO mice had accumulation of I-MC in the bone marrow (Fig. 4M), TG-challenged dWT and dKO mice had comparable frequencies of bone marrow I-MC (17.3% vs. 16.9%, p=0.85, Fig. 8F), suggesting a more robust bone marrow monocytosis in the dWT mice. Consistent with conversion of I-MC to R-MC in bone marrow [26], TG-challenged dWT mice exhibited a higher frequency of bone marrow R-MC than their dKO counterparts (2.4% vs. 1.9%, p<0.05, Fig. 8G), in contrast to the unchallenged mice where bone marrow R-MC in dWT and dKO mice were at comparable levels (Fig. 4M).
Peritoneal inflammation did not significantly affect the skewed homeostasis of NK cells in dKO mice. As in unchallenged mice (Fig.7A), the frequency of NK cells in dKO mice remained lower in blood (Fig. 8C) and higher in bone marrow (Fig. 8H) than their dWT counterparts. In addition, there were no significant differences between dKO and dWT mice in B (Fig. 8D, I) and T (Fig. 8E, J) lymphocytes. These results were consistent with the fact that acute inflammation induced by TG is mostly mediated by macrophages, one of the descendents of circulating monocytes.
The impacts of meprin deficiency on leukocyte homeostasis were also evident from the absolute numbers of blood leukocytes in TG-challenged mice (Fig. 9). The numbers of blood I-MC in the TG-challenged dKO mice were 1.8-fold higher than those in their dWT counterparts (p<0.05). The average number of NK cells in dKO was approximately half of that in dWT mice, with a p value of 0.07. In contrast, TG-challenged dKO and dWT mice had comparable absolute numbers for R-MC, B cells, T cells and granulocytes in blood (Fig.9), with p values of 0.74, 0.81, 0.39 and 0.26, respectively. Thus, the impacts of meprin deficiency on the absolute numbers of blood leukocytes had a trend consistent with the one for relative prevalence (Fig. 8).
Fig. 9. Meprin deficiency differentially affected the absolute prevalence of blood leukocytes.
Flow cytometry analysis of blood from dKO and dWT mice 24 hours after i.p. injection of TG for the absolute prevalence of monocytes, NK cells, B cells, T cells and granulocytes. The absolute leukocyte numbers were corrected with Fluorospheres as internal reference. Note that dKO mice exhibited a higher level of blood I-MC than their WT counterparts. Data were from 2 independent experiments with a total of 12 mice in each group.
Discussion
We demonstrate in this report that meprin deficiency altered homeostasis of monocytes, with decreased prevalence of the R-MC subset in blood. Inversely correlated with this decrease was the increase of the I-MC subset in bone marrow. This correlation is consistent with the interpretation that decreased monocytes in blood resulted from accumulation of monocytes in bone marrow, based on the current thinking that homeostasis of blood monocytes requires continuous replenishment from bone marrow [30]. It is unexpected that blood R-MC, rather than I-MC, were affected most profoundly by meprin deficiency in the unchallenged state (Fig. 4), considering a model suggesting that bone marrow I-MC disseminate into blood first, which then either develop into R-MC [25], or shuffle back to bone marrow in the absence of peripheral inflammation [26]. However, if the transition of I-MC between bone marrow and blood, and the conversion of I-MC to R-MC are highly efficient, the net outcome of the complex kinetics of monocyte development may present impacts of meprin deficiency mainly on the relatively stationary R-MC, not the highly dynamic I-MC.
Similar to monocytes, NK cells in meprin-deficient mice were low in blood compared to the wild-type controls, but high in bone marrow (Fig. 7A), again in an inverse correlation suggesting that the decrease in blood is a consequence of accumulation in bone marrow. It is noteworthy that the inversely correlated prevalence of monocytes in blood and bone marrow is very similar to the findings in a recent study, which showed that CCR2/MCP play critical roles in monocyte mobilization from bone marrow [31]. In contrast to the meprin null mice, CCR2 and MCP deficiency only impairs bone marrow egress of monocytes but not NK cells.
In contrast to monocytes and NK cells, the prevalence of T and B lymphocytes in blood were not significantly affected by meprin deficiency. The differential impacts of meprin deficiency on leukocyte lineages may be related to the distinct homeostasis mechanisms for individual leukocyte lineages. To exercise their functions, leukocytes have to egress from bone marrow to blood, followed by further dissemination to the periphery and recirculation among tissues. In physiological conditions, blood monocytes and NK cells maintain their homeostasis mostly by receiving direct continuous replenishment from bone marrow, although extra-bone marrow sites have also been suggested for NK cell maturation [30;32]. In contrast, T and B cells egress from bone marrow at an early stage of development, and homeostasis of blood T and B cells is largely independent of bone marrow [33;34].
This work also demonstrates that the involvement of meprins in the hematological system is pathologically significant. In a disease model mimicking peripheral infection by non-bacterial inflammation induced by TG, meprin-deficient mice exhibited retention of monocytes in the circulation and manifested a febrile response (Fig. 8, 9, 3). One interpretation for this retention of meprin-deficient monocytes in blood is that these cells were defective in homing to inflammation cues, which resulted in a suboptimal local inflammation response and subsequently triggered the febrile reaction. Fever activates innate immune system [35], and heat stress has been shown to enhance the function of monocytes-derived dendritic cells and NK cells [36-38]. Bone marrow monocytes in dKO mice also responded to the peripheral inflammation aberrantly compared to dWT mice. In response to TG challenge, bone marrow I-MC and R-MC in dWT mice increased to the level comparable to, or higher than that in dKO mice (Fig. 8F, G), suggesting that monocytosis in dKO bone marrow were not as robust as in dWT bone marrow monocytosis.
In summary, from this study a scenario emerges in which meprins play important roles in leukocyte function. In the steady state, meprin deficiency may adversely affect egress of bone marrow I-MC to blood, resulting in accumulation of I-MC in the bone marrow and decreased prevalence of R-MC in blood (Fig.4), possibly due to the quick conversion of I-MC to R-MC. During active peripheral inflammation, meprin deficiency may also affect emigration of blood I-MC in dKO mice, manifesting as retention of I-MC in the blood (Fig.8A). The incurring inadequate monocyte recruitment to inflammation site may have the consequence of a defective inflammation response, which elicited a suboptimal bone marrow monocytosis (Fig. 8F, G) and triggered a febrile response (Fig. 3) possibly in an attempt to mobilize the innate immunity at the systemic level.
The exact mechanisms underlying the dysfunctional monocytes and NK cells as a result of meprin deficiency remain to be determined. Leukocyte egress from bone marrow and homing to the peripheral tissue involve multiple distinct molecular processes, including chemotaxis driven by chemokines, degradation of ECM, and interaction between adhesion molecules [39]. The proteolytic activities of meprin metalloproteases imply that meprins have the potential to be involved in at least 2 of these processes, modulation of cytokines/chemokines and degradation of ECM [3]. To understand the molecular and biochemical mechanisms for the hematological disorders associated with meprin deficiency, active research is ongoing in our laboratory to compare functional capacity of leukocytes between meprin deficient and wild-type mice, and to determine the contribution of each meprin subunits to the functions of leukocytes.
The findings reported in this study have significance in understanding the physiopathology of the hematological and immune system. Monocytes and their descendents, macrophages and dendritic cells, play critical roles in the innate immunity and serve as the bridge leading to successful adaptive immune responses. NK cells are components of the innate immunity participating in the first line of immune defense against viral infection and tumor. Based on the above, it is reasonable to predict that meprin deficiency is associated with compromised immunity in human pathological conditions yet to be recognized. The meprin null mice are useful animal models in this area of research which has the potential to elucidate mechanisms of the hematological system in health and disease.
Acknowledgments
The authors thank Ge Jin for excellent technical support, and Drs. Gaylen Bradley and Barbara Miller for critical reading of the manuscript.
This work was supported by the Children's Miracle Network Research Funds of Penn State University Children's Hospital (to Q.Sun), and by National Institutes of Health Grants DK-54625 and DK-19691 (to J. S. Bond).
Footnotes
Conflict-of-interest statement: The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Beynon RJ, Shannon JD, Bond JS. Purification and characterization of a metallo-endoproteinase from mouse kidney. Biochem J. 1981;199:591–598. doi: 10.1042/bj1990591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sterchi EE, Naim HY, Lentze MJ, Hauri HP, Fransen JA. N-benzoyl-L-tyrosyl-p-aminobenzoic acid hydrolase: a metalloendopeptidase of the human intestinal microvillus membrane which degrades biologically active peptides. Arch Biochem Biophys. 1988;265:105–118. doi: 10.1016/0003-9861(88)90376-1. [DOI] [PubMed] [Google Scholar]
- 3.Bond JS, Matters GL, Banerjee S, Dusheck RE. Meprin metalloprotease expression and regulation in kidney, intestine, urinary tract infections and cancer. FEBS Lett. 2005;579:3317–3322. doi: 10.1016/j.febslet.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 4.Lottaz D, Hahn D, Muller S, Muller C, Sterchi EE. Secretion of human meprin from intestinal epithelial cells depends on differential expression of the alpha and beta subunits. Eur J Biochem. 1999;259:496–504. doi: 10.1046/j.1432-1327.1999.00071.x. [DOI] [PubMed] [Google Scholar]
- 5.Crisman JM, Zhang B, Norman LP, Bond JS. Deletion of the Mouse Meprin β Metalloprotease Gene Diminishes the Ability of Leukocytes to Disseminate through Extracellular Matrix. J Immunol. 2004;172:4510–4519. doi: 10.4049/jimmunol.172.7.4510. [DOI] [PubMed] [Google Scholar]
- 6.Stocker W, Zwilling R. Astacin. Methods Enzymol. 1995;248:305–325. doi: 10.1016/0076-6879(95)48021-8. [DOI] [PubMed] [Google Scholar]
- 7.Bertenshaw GP, Norcum MT, Bond JS. Structure of homo- and hetero-oligomeric meprin metalloproteases. Dimers, tetramers, and high molecular mass multimers. J Biol Chem. 2003;278:2522–2532. doi: 10.1074/jbc.M208808200. [DOI] [PubMed] [Google Scholar]
- 8.Marchand P, Tang J, Bond JS. Membrane association and oligomeric organization of the alpha and beta subunits of mouse meprin A. J Biol Chem. 1994;269:15388–15393. [PubMed] [Google Scholar]
- 9.Hahn D, Pischitzis A, Roesmann S, Hansen MK, Leuenberger B, Luginbuehl U, Sterchi EE. Phorbol 12-myristate 13-acetate-induced ectodomain shedding and phosphorylation of the human meprinbeta metalloprotease. J Biol Chem. 2003;278:42829–42839. doi: 10.1074/jbc.M211169200. [DOI] [PubMed] [Google Scholar]
- 10.Kumar JM, Bond JS. Developmental expression of meprin metalloprotease subunits in ICR and C3H/He mouse kidney and intestine in the embryo, postnatally and after weaning. Biochim Biophys Acta. 2001;1518:106–114. doi: 10.1016/s0167-4781(01)00188-9. [DOI] [PubMed] [Google Scholar]
- 11.Becker-Pauly C, Howel M, Walker T, Vlad A, Aufenvenne K, Oji V, Lottaz D, Sterchi EE, Debela M, Magdolen V, Traupe H, Stocker W. The alpha and beta subunits of the metalloprotease meprin are expressed in separate layers of human epidermis, revealing different functions in keratinocyte proliferation and differentiation. J Invest Dermatol. 2007;127:1115–1125. doi: 10.1038/sj.jid.5700675. [DOI] [PubMed] [Google Scholar]
- 12.Bertenshaw GP, Turk BE, Hubbard SJ, Matters GL, Bylander JE, Crisman JM, Cantley LC, Bond JS. Marked Differences between Metalloproteases Meprin A and B in Substrate and Peptide Bond Specificity. J Biol Chem. 2001;276:13248–13255. doi: 10.1074/jbc.M011414200. [DOI] [PubMed] [Google Scholar]
- 13.Kruse MN, Becker C, Lottaz D, Kohler D, Yiallouros I, Krell HW, Sterchi EE, Stocker W. Human meprin alpha and beta homo-oligomers: cleavage of basement membrane proteins and sensitivity to metalloprotease inhibitors. Biochem J. 2004;378:383–389. doi: 10.1042/BJ20031163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bylander JE, Bertenshaw GP, Matters GL, Hubbard SJ, Bond JS. Human and mouse homo-oligomeric meprin A metalloendopeptidase: substrate and inhibitor specificities. Biol Chem. 2007;388:1163–1172. doi: 10.1515/BC.2007.156. [DOI] [PubMed] [Google Scholar]
- 15.Kaushal GP, Walker PD, Shah SV. An old enzyme with a new function: purification and characterization of a distinct matrix-degrading metalloproteinase in rat kidney cortex and its identification as meprin. J Cell Biol. 1994;126:1319–1327. doi: 10.1083/jcb.126.5.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herzog C, Kaushal GP, Haun RS. Generation of biologically active interleukin-1beta by meprin B. Cytokine. 2005;31:394–403. doi: 10.1016/j.cyto.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 17.Norman LP, Jiang W, Han X, Saunders TL, Bond JS. Targeted Disruption of the Meprin β Gene in Mice Leads to Underrepresentation of Knockout Mice and Changes in Renal Gene Expression Profiles. Mol Cell Biol. 2003;23:1221–1230. doi: 10.1128/MCB.23.4.1221-1230.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Banerjee S. PhD Thesis. Pennsylvania State University; 2008. Meprin metalloproteases modulate the intestinal host response. http://etda.libraries.psu.edu/theses/approved/WorldWideIndex/ETD-2541/index.html. [Google Scholar]
- 19.Sun Q, Burton RL, Dai LJ, Lucas KG. B-lymphoblastoid cell lines expressing CMV pp65 elicited ex vivo polyclonal expansion of CD8+cytotoxic T-lymphocytes against Epstein-Barr virus and cytomegalovirus. Blood. 1999;94:556A. [PubMed] [Google Scholar]
- 20.Sun Q, Brewer N, Dunham K, Chen L, Bao L, Burton R, Lucas KG. Interferon-gamma expressing EBV LMP2A-specific T cells for cellular immunotherapy. Cell Immunol. 2007;246:81–91. doi: 10.1016/j.cellimm.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Lagasse E, Weissman IL. Flow cytometric identification of murine neutrophils and monocytes. J Immunol Methods. 1996;197:139–150. doi: 10.1016/0022-1759(96)00138-x. [DOI] [PubMed] [Google Scholar]
- 22.Geissmann F, Jung S, Littman DR. Blood Monocytes Consist of Two Principal Subsets with Distinct Migratory Properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 23.Benamar K, McMenamin M, Geller EB, Chung YG, Pintar JE, Adler MW. Unresponsiveness of mu-opioid receptor knockout mice to lipopolysaccharide-induced fever. Br J Pharmacol. 2005;144:1029–1031. doi: 10.1038/sj.bjp.0706145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanson DF. Fever, temperature, and the immune response. Ann N Y Acad Sci. 1997;813:453–464. doi: 10.1111/j.1749-6632.1997.tb51733.x. [DOI] [PubMed] [Google Scholar]
- 25.Sunderkotter C, Nikolic T, Dillon MJ, van Rooijen N, Stehling M, Drevets DA, Leenen PJM. Subpopulations of Mouse Blood Monocytes Differ in Maturation Stage and Inflammatory Response. J Immunol. 2004;172:4410–4417. doi: 10.4049/jimmunol.172.7.4410. [DOI] [PubMed] [Google Scholar]
- 26.Varol C, Landsman L, Fogg DK, Greenshtein L, Gildor B, Margalit R, Kalchenko V, Geissmann F, Jung S. Monocytes give rise to mucosal, but not splenic, conventional dendritic cells. J Exp Med. 2007;204:171–180. doi: 10.1084/jem.20061011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leon B, Martinez del Hoyo G, Parrillas V, Vargas HH, Sanchez-Mateos P, Longo N, Lopez-Bravo M, Ardavin C. Dendritic cell differentiation potential of mouse monocytes: monocytes represent immediate precursors of CD8- and CD8+ splenic dendritic cells. Blood. 2004;103:2668–2676. doi: 10.1182/blood-2003-01-0286. [DOI] [PubMed] [Google Scholar]
- 28.Passlick B, Flieger D, Ziegler-Heitbrock HW. Identification and characterization of a novel monocyte subpopulation in human peripheral blood. Blood. 1989;74:2527–2534. [PubMed] [Google Scholar]
- 29.Akashi S, Saitoh Si, Wakabayashi Y, Kikuchi T, Takamura N, Nagai Y, Kusumoto Y, Fukase K, Kusumoto S, Adachi Y, Kosugi A, Miyake K. Lipopolysaccharide Interaction with Cell Surface Toll-like Receptor 4-MD-2: Higher Affinity than That with MD-2 or CD14. The Journal of Experimental Medicine. 2003;198:1035–1042. doi: 10.1084/jem.20031076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 31.Tsou CL, Peters W, Si Y, Slaymaker S, Aslanian AM, Weisberg SP, Mack M, Charo IF. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J Clin Invest. 2007;117:902–909. doi: 10.1172/JCI29919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yokoyama WM, Kim S, French AR. The dynamic life of natural killer cells. Annu Rev Immunol. 2004;22:405–429. doi: 10.1146/annurev.immunol.22.012703.104711. [DOI] [PubMed] [Google Scholar]
- 33.Jameson SC. Maintaining the norm: T-cell homeostasis. Nat Rev Immunol. 2002;2:547–556. doi: 10.1038/nri853. [DOI] [PubMed] [Google Scholar]
- 34.Mackay F, Schneider P, Rennert P, Browning J. BAFF AND APRIL: A Tutorial on B Cell Survival. Annu Rev Immunol. 2003;21:231–264. doi: 10.1146/annurev.immunol.21.120601.141152. [DOI] [PubMed] [Google Scholar]
- 35.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 36.Ostberg JR, Repasky EA. Emerging evidence indicates that physiologically relevant thermal stress regulates dendritic cell function. Cancer Immunol Immunother. 2006;55:292–298. doi: 10.1007/s00262-005-0689-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ostberg JR, Dayanc BE, Yuan M, Oflazoglu E, Repasky EA. Enhancement of natural killer (NK) cell cytotoxicity by fever-range thermal stress is dependent on NKG2D function and is associated with plasma membrane NKG2D clustering and increased expression of MICA on target cells. J Leukoc Biol. 2007;82:1322–1331. doi: 10.1189/jlb.1106699. [DOI] [PubMed] [Google Scholar]
- 38.Hatzfeld-Charbonnier AS, Lasek A, Castera L, Gosset P, Velu T, Formstecher P, Mortier L, Marchetti P. Influence of heat stress on human monocyte-derived dendritic cell functions with immunotherapeutic potential for antitumor vaccines. J Leukoc Biol. 2007;81:1179–1187. doi: 10.1189/jlb.0506347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ebnet K, Vestweber D. Molecular mechanisms that control leukocyte extravasation: the selectins and the chemokines, Histochem. Cell Biol. 1999;112:1–23. doi: 10.1007/s004180050387. [DOI] [PubMed] [Google Scholar]