Abstract
In this review we focus on the mechanisms, regulation, and cellular consequences of nuclear migration in the developing retina. In the nervous system, nuclear migration is prominent during both proliferative and post-mitotic phases of development. Interkinetic nuclear migration is the process where the nucleus oscillates from the apical to basal surfaces in proliferative neuroepithelia. Proliferative nuclear movement occurs in step with the cell cycle, with M-phase being confined to the apical surface and G1-, S-, and G2-phases occurring at more basal locations. Later, following cell cycle exit, some neuron precursors migrate by nuclear translocation. In this mode of cellular migration, nuclear movement is the driving force for motility. Following discussion of the key components and important regulators for each of these processes, we present an emerging model where interkinetic nuclear migration functions to distinguish cell fates among retinal neuroepithelia.
Keywords: interkinetic nuclear migration, nuclear translocation, nucleokinesis, neurogenesis, dynein, cell cycle, cell behavior
1. Overview
Neuronal development is regulated by a complex array of intrinsic and extrinsic signals that act on progenitor neuroepithelial cells to ensure the correct cell type is generated in the right numbers and at the appropriate time of development (Donovan et al., 2005; Cayouette et al., 2006). In addition, multiple signaling cues are integrated in post-mitotic neuronal precursors to facilitate directed cell migration and correct laminar positioning (Malicki et al., 2004). This spatial and temporal regulation of neuronal cell-type fate commitment and histogenesis is dependent on the regulation of the mitotic cell cycle, and in particular at the level of cell cycle exit (Ohnuma and Harris, 2003). Recent data suggest that nuclear migration is a critical process for several aspects of neuronal development, including that of the neural retina. The focus of this review is on interkinetic nuclear migration and nuclear translocation during retinogenesis, although much of what we know is from observations in other regions of the developing nervous system.
1.1 Interkinetic nuclear migration
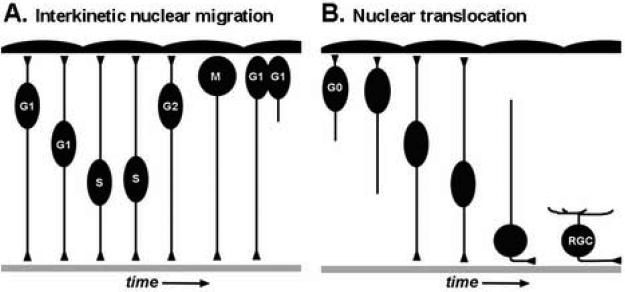
Interkinetic nuclear migration is the proliferative cell behavior where the nuclei of neuroepithelial cells migrate in an apical-basal manner and in phase with the cell cycle (Frade 2002). Neuroepithelial cell divisions are confined to apical locations while S-phase occurs in more basal areas (Figure 1A). The term interkinetic nuclear migration was given in 1935, when F.C. Sauer reported histological observations of the developing vertebrate neural tube. He proposed that the psuedostratification of neuroepithelial cells is facilitated by nuclear movements (Sauer, 1935). Sauer's work built on earlier results and models from Alfred Schaper (Schaper, 1897). The process of interkinetic nuclear migration was confirmed by two critical experimental observations. First, treatment of neuroepithelial tissue with mitotic inhibitors resulted in arrested metaphase cells only at the apical surface. Second, 3H-thymidine pulse-labeling experiments showed differential labeling of progenitor cell nuclei throughout the thickness of the neuroepithelium (Langman et al., 1966; Sauer and Chittenden, 1959; Sauer and Walker, 1959). These and subsequent studies using 3H-thymidine or thymidine analogs like BrdU, have shown that interkinetic nuclear migration occurs in the majority of neuroepithelia of the central nervous system (CNS) and some non-neuronal polarized cells (Sauer, 1936; Bhide, 1996; Takahashi et al., 1999; Nowakowski, 2002; Bort et al., 2006).
Figure 1. Types of nuclear migration during retinal development.
(A) Model of interkinetic nuclear migration. As cells progress through the cell cycle, nuclei of neuroepithelial cells move in an apical-to-basal and then basal-to-apical fashion. M-phase is restricted to the apical surface near the RPE. Note the maintenance of both apical and basal processes throughout the cell cycle. With cytokinesis, typically one daughter cell inherits the basal process and the sibling cell initiates a new basal process. (B) Model of nuclear translocation. Following cell cycle exit, progenitors that migrate via nuclear translocation maintain their apical and basal processes. These processes retract as the soma reaches the appropriate laminar position. Apical RPE is up and basal basement membrane is down.
1.2 Nuclear translocation
Nuclear translocation, on the other hand, occurs following terminal mitosis and is a mode of cellular migration in which the soma of the neuroepithial cell moves by way of nuclear migration while maintaining apically and basally directed processes (Ghashghaei et al., 2007; Figure 1B). In the retina, nuclear or somal translocation is contrasted by other modes of post-mitotic cell migration such as unconstrained migration and guided cell migration (Godinho and Link, 2006). Various cells in the CNS, and in particular those of the cerebral cortex, utilize nuclear translocation (Morest, 1970; Brittis et al., 1995; Nadarajah et al., 2001). Within the retina, nuclear translocation has been demonstrated for retinal ganglion cell and bipolar cell precursors (Hinds and Hinds, 1974; Prada et al., 1981; Snow and Robson, 1994, 1995; Poggi et al., 2005; Zollessi et al., 2006; Morgan et al., 2006). Other retinal cell types may also use nuclear translocation to achieve their appropriate laminar position.
2. Cytoskeletal Motors and Adaptor Proteins for Nuclear Migration
2.1 Proteins for moving
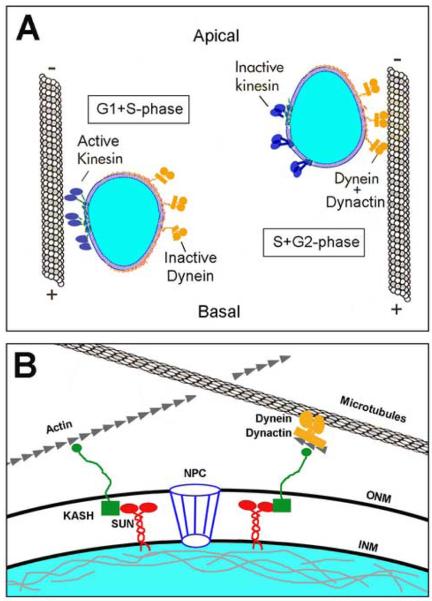
Much of our understanding of the specific molecules involved in nuclear migration comes from genetic studies conducted with fungi and invertebrates. Specifically, mutational screens in yeast (Saccharomyces cervisiae and Schizoscharomyces pombe), filamentous fungi (Aspergillus nidulins and Neurospora crassa), nematodes (Caenorhadbitis elegans), and fruit flies (Drosophila melanogaster) have all implicated microtubule-based motors and specific classes of nuclear envelope proteins as essential for nuclear migration and positioning (Morris, 2003; Xiang and Fischer, 2004; Starr and Fischer, 2005; Wilhelmsen et al., 2006). With regard to cytoskeletal motors, cytoplasmic dynein and dynactin are essential for normal nuclear migration in multiple contexts. Cytoplasmic dynein is a multi-protein minus-end directed microtubule-based motor. Dynactin is also a multi-protein complex that interacts with and activates cytoplasmic dynein. Dynactin is required for the binding of cargo to cytopasmic dynein and also promotes processivity between the motor and microtubules (Schroer, 2004). Mutations in dynactin-1/p150, a key subunit of dynactin, cause nuclear migration and positioning defects in filamentous fungi (Tinsley et al., 1996; Zhang et al., 2003). In flies, truncating mutations in dynactin-1/p150 are responsible for glued phenotypes. In p150glued mutants, M-phase of eye disk epithelial progenitor cells is extended, photoreceptor cell fates are affected, and photoreceptor morphogenesis is disrupted (Fan and Ready, 1997). Moreover, in photoreceptor cells the nuclei fail to move apically toward the minus-end of microtubules, and instead remain at more basal locations. Informatively, this nuclear positioning phenotype can be suppressed by mutations in subunits of Kinesin, the plus-end directed microtubule motor (Whited et al., 2004). Finally, in green alga, post-mitotic nuclear migration is mediated in part by a Kinesin-1 protein (Holzinger and Lutz-Meindl, 2002). Overall, studies in multiple organisms have strongly implicated the cytoplasmic dynein-dynactin motor system in nuclear migration. Kinesin family members likely play pivotal roles in balancing the minus-end directed forces of dynein-dynactin (Figure 2A).
Figure 2. Motor and adaptor proteins for nuclear migration.
(A) Model of interkinetic nuclear migration and coordination with the cell cycle. In some contexts, directed nuclear migration is a balance between the relative activity of plus-end (dark blue) and minus-end (orange) oriented microtubule motor proteins. (B) Model showing proteins known to function during nuclear anchoring and nuclear migration in various contexts. SUN-domain proteins (red); KASH-domain proteins (green); Dynein-dynactin (orange) with associated Arp proteins (grey). Nucleus (blue) is shown with the nuclear lamina (grey). ONM, outer nuclear membrane; INM, inner nuclear membrane; NPC, Nucleopore complex. See text for details regarding each cartoon.
2.2 Linking the nucleus to the cytoskeleton
In Drosophila, the glued phenotype of photoreceptor nuclei is shared by klarsicht mutants (Mosley-Bishop et al., 1999). The klarsicht gene was found to encode a protein with a C-terminal domain shared with ANC-1 of C. elegans and Syne/Nesprin proteins of vertebrates (Starr and Fischer, 2005; Wilhelmsen et al., 2006). This motif was termed the KASH domain for Klarsicht, ANC-1, and Syne homology. Mutations in anc-1 disrupt nuclear positioning in nematodes (Starr and Han, 2002). Targeted mutations to syne-1 in mice result in nuclear positioning defects within skeletal muscle, where mutant cell nuclei localize ectopically away from the neuromuscular junction (Grady et al., 2005). Previously, Syne-1 was shown to bind and tether the MuSK (Muscle, Skeletal, Receptor Tyrosine Kinase) signaling complex to synaptic nuclei (Apel et al., 2000). These and additional studies suggest that KASH-domain proteins link the nuclear envelope to cytoskeletal components and can also localize signaling complexes to perinuclear locations (Figure 2B).
2.3 Nuclear envelope proteins
A third group of nuclear migration and positioning mutants isolated from invertebrates provide information on the nuclear envelope receptors for KASH-domain proteins. These proteins contain a SUN domain. SUN-domain proteins were discovered in yeast (Sad1p mutants) and nematodes (UNC-84) from mutants that displayed nuclear migration phenotypes. SUN-domain proteins have now been found in all eukaryotes examined. Recent biochemical data has demonstrated that SUN-domain proteins are constituents of the inner nuclear membrane and bind KASH-domain proteins (Worman and Gundersen, 2006). SUN-domain proteins are also important for nuclear envelope morphology and link the outer and inner nuclear membranes to the nuclear lamina (Crisp et al., 2006; Figure 2B).
Within the vertebrate nervous system, characterization of KASH- and SUN-domain proteins has not been reported. Furthermore, direct evidence for KASH-domain proteins and interactions with cytoskeletal motors has not been published. However, protein interaction and genetic screens have demonstrated that KASH-domain proteins do contain actin- and microtubule-binding sites, providing the potential for interactions with complexes involving dynactin – which binds both microtubles and actin-related proteins such as Arp-1 and Arp-11 (Figure 2B). Time-lapse imaging in the cortex and retina have shown proliferative neuroepithelial cells have elongated, tear-drop shaped nuclei during interkinetic nuclear migration and round nuclei when the nucleus stops at the apical surface for division (Chenn and McConnell, 1995; Das et al., 2003; Pearson et al., 2005). These observations are consistent with the nucleus being pulled by the cytoskeletal motors attached to the nuclear envelope. Because of the pleiotropy and cell-essential nature of components responsible for nuclear migration and positioning in other contexts, the specific functions of motor proteins and their interacting partners within retinal neuroepithelial cells will require very targeted and conditional experimentation. In the mouse cerebral cortex, a few targeted genetic studies have been conducted and these analyses have confirmed the importance of microtubule motor components and interacting proteins for both interkinetic nuclear migration and nuclear translocation (Feng et al., 2000; Gambello et al., 2003; Tanaka et al., 2004; Tsai et al., 2005). However, within the retina, unlike the cortex, centrioles and the microtubule organizing center (MTOC) do not lead nuclear translocation (Zolessi et al., 2006).
3. Regulation of Nuclear Migration within Neuroepithelia
3.1 Kinases in nuclear migration
Along with the nuts and bolts of nuclear migration, candidate regulatory factors for nuclear migration have been identified. For example, genetic and biochemical analyses in Drosophila have shown an essential role for the protein kinase encoded by misshapen during apical migration of photoreceptor nuclei (Houalla et al., 2005). Misshapen can increase the phosphorylation of Bicaudal-D, a dynein-interacting protein that is also essential for nuclear migration in flies (Suter and Steward, 1991; Swan et al., 1999). The vertebrate homologue of Misshapen is a GCK (Germinal Center Kinase) termed Mink-1 (for Misshapen/Nik-related Kinase-1). Mutational analysis of Mink-1 in vertebrates has not been reported; however, mink-1 gene expression is upregulated in cortical progenitors when cell migration initiates during development (Dan et al., 2000). In addition, two other kinases, Fak (Focal Adhesion Kinase) and Cdk5 (Cyclin-Dependent Kinase-5), have been shown to work together to regulate nucleokinesis. Cdk5 can phosphorylate Fak at S732. In cortical progenitor cells, S732Fak localizes to microtubules that bridge the nucleus to the centrosomes. Importantly, over-expression of a S732-non-phosphorylatable Fak disrupts these microtubules and impairs nuclear translocation. Furthermore, Ckd5 can directly phosphorylate the microtubule-binding protein Doublecortin to mediate nuclear translocation during post-mitotic cell migration (Tanaka et al., 2004). Consistent with these results, deletion of cdk5 in mice results in lamination defects in the CNS (Ohshima et al., 1996). However, the in vivo role for Fak in nuclear migration, appears to be more complicated. Conditional deletion of Fak in cortical progenitor cells results in cell positioning defects, but this effect is cell-non-autonomous (Beggs et al., 2003). The lamination defects with loss of Fak appear to be caused by defects in basement membrane maintenance by non-neuronal cells. In that study, potential cell-autonomous function of Fak in nucleokinesis may have been compensated for by the closely related kinase, Fak2. Functional analysis of Cdk5 and Fak have not been reported for the retina. In addition to kinases that regulate microtubule-associate proteins, the Cdc42-binding kinase has been shown in cell culture to regulate nuclear movement and polarized cell migration via actin dynamics (Gomes et al., 2005). This kinase, also known as MRCK (Myotonic Dystrophy Related Cdc42-binding Kinase), stimulates a myosin II motor to promote rearward movement of the nucleus. Consistent with these observations, deletion of non-muscle myosin IIB heavy chain in mice results in neuronal lamination defects (Tullio et al., 2001).
3.2 Extrinsic regulation
In addition to these kinases, several secreted factors have been shown to regulate aspects of nuclear migration. As alluded to above, proper secretion and maintenance of basement membrane proteins may be important for nuclear translocation during post-mitotic neuronal migration. Within the developing retina, transient disruptions to the entire basement membrane or specifically to laminin-1 have been shown to regulate retinal cell positioning (Halfter et al. 2001; Semina et al., 2006). However, defects in basement membrane integrity in the proliferating cortex does not affect the location of M-phase, suggesting that interkinetic nuclear migration occurs normally in that region of the CNS (Haubst et al., 2006).
Soluble factors have also been shown to regulate nuclear migration. In a series of papers, Pearson, Mobbs, and colleagues have demonstrated an important role for extracellular ATP in modulating interkinetic nuclear migration (Pearson et al., 2002; Pearson et al., 2004; Pearson et al. 2005a; Pearson et al., 2005b). In those studies, it was found that ATP is released through apical hemichannels on retinal pigment epithelium (RPE) cells, where it binds to purinergic P2Y receptors on adjacent retinal neuroepithelia. Pharmacological experiments suggested that activation of P2Y receptors evoke Ca2+ transients that affect the kinetics of nuclear migration in proliferative retinal progenitors. Consistent with these experiments, observations in explanted chick retina showed that the saltitory movements of interkinetic nuclear migration correlate with elevated bursts of Ca2+ within the retinal neuroepithelial cells. For cortical progenitors in culture, exogenous lysophosphatidic acid (LPA) was found to promote nuclear movements and retraction of lamellipodia, resulting in a cell-rounding phenotype reminiscent of G2/M-phase cells of the ventricular zone (Fukushima et al., 2000). In vivo, the LPA receptor EDG2 (Endothelial Differentiation Gene 2) is highly enriched within the ventricular zone (Hecht et al., 1996). Bioassays of conditioned medium suggested that post-mitotic neurons are the source of secreted LPA. Analysis of the LPA-EDG2 pathway has not been investigated in the retina.
3.3 Regulation with the cell cycle
More broadly with regard to regulation of nuclear migration, interkinetic nuclear migration is coordinated with the cell cycle. This relationship extends beyond simple correlation. When the cell cycle of retinal neuroepithelia is extended by genetic mutations or pharmacological manipulations, interkinetic nuclear migration is slowed proportionately (Pearson et al., 2005; Willer et al., 2005; Baye and Link, 2007). Furthermore, when cell cycle progression is completely inhibited in neuroepithelia by 5-azacytidine and cyclophosphamide, interkinetic nuclear migration is stopped (Ueno et al., 2006). However, treatment with the reversible cell cycle inhibitor hydroxyurea did not have this dramatic effect on preventing interkinetic nuclear migration (Murciano et al. 2002). Conversely, blocking interkinetic nuclear migration shows that the cell cycle and nuclear migration can be uncoupled. Inhibiting interkinetic nuclear migration by cytochalasin B or by disrupting Lis1 function does not block cell cycle progression; rather, mitosis occurs throughout the neuroepithelium (Messier, 1978; Murciano et al., 2002; Gambello et al., 2003). Cytochalasin B prevents F-actin polymerization, while Lis1 is a dynein-interacting protein and underlies a sub-set of lisencephaly-type cortical neuron migration disorders. These results suggest that global regulators of the core cell cycle machinery also regulate the molecular machinery responsible for nuclear migration.
As an important corollary, nuclear position is not essential for cell cycle progression. Consistent with these postulations, the well-known cell cycle regulator Cdc2 (cell division cycle) protein kinase has been shown to phosphorylate the Kinesin Kif11. Furthermore, Cdc2-dependent phosphorylation modulates Kif11 interaction with dynactin-1 (Blangy et al., 1997). Kif11 is known to facilitate spindle pole formation during M-phase and perhaps regulate nuclear migration during interphase (Miki et al. 2005). Genetic studies in yeast have also shown a critical role for the cyclin dependent kinase, cdk1p, in regulating nuclear migration to the bud neck prior to bud formation. Cdk1p acts on multiple microtubule plus-end binding proteins to facilitate microtubule-based capture of the late G1-phase nucleus. In yeast, regulated microtubule depolymerization at cortical attachment sites pulls the nucleus toward the bud site (Huisman and Segal, 2005). While these observations are provocative, the molecular details of the relationship between cell cycle progression and interkinetic nuclear migration in the vertebrate retina, and elsewhere in the CNS, have yet to be fully explored.
4. What is the function of interkinetic nuclear migration?
While the function of nuclear translocation in post-mitotic cells underlies a strategy for cellular motility, the function of interkinetic nuclear migration is less apparent. Some have proposed that during neuronal development, interkinetic nuclear migration may facilitate the invagination of the neuroepithelium during neural plate development (Langman et al, 1966 and Messier, 1978). Others have suggested that interkinetic nuclear migration may serve as a mechanism for increasing the density of the proliferative pool while still maintaining adherens junctions during mitosis (Frade 2002). In addition, we suggest the following functions of interkinetic nuclear migration based on recent observations.
4.1 Maintenance of apical-basal polarity
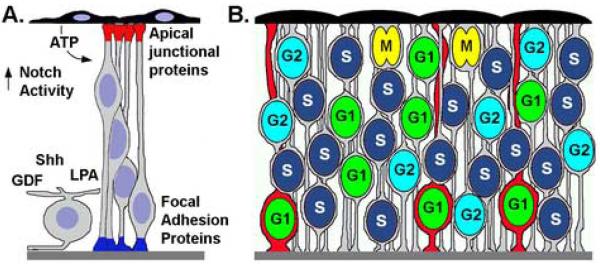
In general, the movement of interphase nuclei away from the apical ventricular zone provides space for M-phase cells to divide while still permitting cells to maintain their apical and basal attachments. The preservation of apical and basal processes allow for maintenance of localized protein signaling complexes. At the apical end of neuroepithelial cells, adherens- and tight junction-associated protein complexes are present (Chenn et al., 1998). These have been shown to have essential roles in cell fate decisions related to cell cycle exit, cell-type choice, and laminar positioning (Malicki, 2004; Gotz and Huttner, 2005). Furthermore, apical junction-associated signaling molecules such as atypical protein kinase C (aPKC) and Cdc42, via their affects on overall cell polarity, are essential for normal interkinetic nuclear migration (Cappello et al., 2006; Cui et al., 2007). At the basal surface, distinct signaling complexes exist including those associated with focal adhesions (Li and Sakaguchi, 2002; Wozniak et al., 2004). As mentioned above, focal adhesion components are critical for maintenance of the extracellular matrix and have been shown to have roles in cell proliferation and morphogenesis. Therefore, the displacement of interphase nuclei throughout the neuroepithelium allows for apical-basal localized signaling complexes to be maintained throughout the cell cycle in the densely packed proliferative neuroepithelium.
4.2 A model for interkinetic nuclear migration and cell fate diversification
While interkinetic nuclear migration may help maintain apical-basal polarity during cell proliferation, this does not explain why neuroepithelial cells undergo interkinetic nuclear migration. One potential role for nuclear oscillation may be to modulate cell fate differences between neuroepithelia (Figure 3). With regard to this function, components of the Notch-Delta signaling pathway are expressed in an apical-basal localized fashion within chick retinal neuroepithelial cells (Murciano et al., 2002). Because a cell's competence to respond to extrinsic cues varies with progression through the cell cycle, spatial differences in the nucleus and cell body position – as mediated by interkinetic nuclear migration – may establish heterogeneity among progenitor cells. Consistent with this model, both the depth of interkinetic nuclear migration, as well as the length of the cell cycle, vary significantly between retinal neuroepithelial cells (Baye and Link, 2005). Asymmetries in the parameters of interkinetic nuclear migration may facilitate differential responses between neuroepithelial cells to intrinsically polarized signaling complexes or to localized extrinsic cues. This could be accomplished by differential activation of cell surface receptors associated with the cell body and/or differential effects on gene regulation within the nucleus. Potential neurogenic-modulating factors include retinal ganglion cell secreted Sonic hedgehog (Shh) and Glia-derived Factor 11 (Gdf11) (Locker et al., 2006; Wang et al., 2005; Kim et al., 2005). Alternatively, differences in nuclear position and the organelles associated with the nucleus may enable differential shuttling/post-translational modifications of fate-determinants, which then affect neurogenesis and mediate differentiation among progenitors cells. An intriguing element with this hypothesis is the requirement for endocytic modification to components of the Notch-Delta pathway (Chitnis 2006; Fischer et al., 2006). In this context, differential modification to fate-determinants may be affected by differential trafficking to nuclear-associated organelles.
Figure 3. Interkinetic nuclear migration and cell fate diversification.
(A) Model showing localized extrinsic (right side) and intrinsic signals (left). Evidence exists for ATP released by the RPE, Notch activating components at the apical side of the neuroepithelium, and various soluble factors released from post-mitotic retinal ganglion cells. Within retinal neuroepithelial cells, distinct signaling complexes reside at the apical and basal poles (Apical junctional complex, red; Focal adhesion complex, blue). (B) Heterogeneity in the position of G1-phase (green nuclei), S-phase (dark blue), and G2-phase (light blue) during interkinetic nuclear migration. M-phase is always restricted to the apical surface (yellow nuclei). In this example, cells in G1-phase are competent to respond to localized intrinsic and/or extrinsic signals that promote a red-cell fate. Because of differential nuclear positions, subsets of neuroepithelia (red cells) diverge in fate from others (grey cells). Note in this example, only G1 cells with more basal nuclei undergo the red cell fate commitment. Apical RPE is up and basal basement membrane is down.
5. Conclusions and Future Prospects
In summary, nuclear migration is an evolutionarily conserved phenomenon that underlies both proliferative and post-mitotic phases of neuronal development. While many of the critical proteins that facilitate and regulate nuclear migration have been identified, primarily through genetic efforts in fungi and invertebrates, exploration of the specific activities and functions in the vertebrate nervous system has only just begun. Many significant questions remain regarding nuclear migration in the nervous system and in particular the neural retina. For example, how is the cell cycle co-regulated with interkinetic nuclear migration? Does interkinetic nuclear migration function in signal-integration and cell-fate decisions? If so, what are the mechanisms? Similarly, how is nuclear translocation regulated with laminar positioning? Continued and focused experimentation in fungi, invertebrates and vertebrates will no doubt shed light on these and other important questions.
Acknowledgements
We would like acknowledge the following grants for funding research related to this topic: NIH Training Fellowship in Vision Sciences 5T32EY014536 (LB), NIH grant R01EY01467 (BL) and a March of Dimes Basil O'Connor Fellowship (BL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Apel ED, Lewis RM, Grady RM, Sanes JR. Syne-1, a dystrophin- and Klarsicht-related protein associated with synaptic nuclei at the neuromuscular junction. J Biol Chem. 2000;275:31986–95. doi: 10.1074/jbc.M004775200. [DOI] [PubMed] [Google Scholar]
- Baye LM, Link BA. Examining interkinetic nuclear migration during retinal development. FASEB JOURNAL. 2005;19:A1367. abstract. [Google Scholar]
- Baye LM, Link BA. The disarrayed mutation results in cell cycle and neurogenesis defects during retinal development in zebrafish. BMC Dev Biol. 2007;7:28. doi: 10.1186/1471-213X-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beggs HE, Schahin-Reed D, Zang K, Goebbels S, Nave KA, Gorski J, Jones KR, Sretavan D, Reichardt LF. FAK deficiency in cells contributing to the basal lamina results in cortical abnormalities resembling congenital muscular dystrophies. Neuron. 2003;40:501–14. doi: 10.1016/s0896-6273(03)00666-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhide PG. Cell cycle kinetics in the embryonic mouse corpus striatum. J Comp Neurol. 1996;374:506–22. doi: 10.1002/(SICI)1096-9861(19961028)374:4<506::AID-CNE3>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Blangy A, Arnaud L, Nigg EA. Phosphorylation by p34cdc2 protein kinase regulates binding of the kinesin-related motor HsEg5 to the dynactin subunit p150. J Biol Chem. 1997;272:19418–24. doi: 10.1074/jbc.272.31.19418. [DOI] [PubMed] [Google Scholar]
- Bort R, Signore M, Tremblay K, Martinez Barbera JP, Zaret KS. Hex homeobox gene controls the transition of the endoderm to a pseudostratified, cell emergent epithelium for liver bud development. Dev Biol. 2006;290:44–56. doi: 10.1016/j.ydbio.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Brittis PA, Meiri K, Dent E, Silver J. The earliest patterns of neuronal differentiation and migration in the mammalian central nervous system. Exp Neurol. 1995;134:1–12. doi: 10.1006/exnr.1995.1031. [DOI] [PubMed] [Google Scholar]
- Cappello S, Attardo A, Wu X, Iwasato T, Itohara S, Wilsch-Brauninger M, Eilken HM, Rieger MA, Schroeder TT, Huttner WB, Brakebusch C, Gotz M. The Rho-GTPase cdc42 regulates neural progenitor fate at the apical surface. Nat Neurosci. 2006;9:1099–107. doi: 10.1038/nn1744. [DOI] [PubMed] [Google Scholar]
- Cayouette M, Poggi L, Harris WA. Lineage in the vertebrate retina. Trends Neurosci. 2006;29:563–70. doi: 10.1016/j.tins.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Chenn A, McConnell SK. Cleavage orientation and the asymmetric inheritance of Notch1 immunoreactivity in mammalian neurogenesis. Cell. 1995;82:631–41. doi: 10.1016/0092-8674(95)90035-7. [DOI] [PubMed] [Google Scholar]
- Chenn A, Zhang YA, Chang BT, McConnell SK. Intrinsic polarity of mammalian neuroepithelial cells. Mol Cell Neurosci. 1998;11:183–93. doi: 10.1006/mcne.1998.0680. [DOI] [PubMed] [Google Scholar]
- Chitnis A. Why is delta endocytosis required for effective activation of notch? Dev Dyn. 2006;235:886–94. doi: 10.1002/dvdy.20683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisp M, Liu Q, Roux K, Rattner JB, Shanahan C, Burke B, Stahl PD, Hodzic D. Coupling of the nucleus and cytoplasm: role of the LINC complex. J Cell Biol. 2006;172:41–53. doi: 10.1083/jcb.200509124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui S, Otten C, Rohr S, Abdelilah-Seyfried S, Link BA. Analysis of aPKClambda and aPKCzeta reveals multiple and redundant functions during vertebrate retinogenesis. Mol Cell Neurosci. 2007 doi: 10.1016/j.mcn.2006.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan I, Watanabe NM, Kobayashi T, Yamashita-Suzuki K, Fukagaya Y, Kajikawa E, Kimura WK, Nakashima TM, Matsumoto K, Ninomiya-Tsuji J, Kusumi A. Molecular cloning of MINK, a novel member of mammalian GCK family kinases, which is up-regulated during postnatal mouse cerebral development. FEBS Lett. 2000;469:19–23. doi: 10.1016/s0014-5793(00)01247-3. [DOI] [PubMed] [Google Scholar]
- Das T, Payer B, Cayouette M, Harris WA. In vivo time-lapse imaging of cell divisions during neurogenesis in the developing zebrafish retina. Neuron. 2003;37:597–609. doi: 10.1016/s0896-6273(03)00066-7. [DOI] [PubMed] [Google Scholar]
- Donovan SL, Dyer MA. Regulation of proliferation during central nervous system development. Semin Cell Dev Biol. 2005;16:407–21. doi: 10.1016/j.semcdb.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Fan SS, Ready DF. Glued participates in distinct microtubule-based activities in Drosophila eye development. Development. 1997;124:1497–507. doi: 10.1242/dev.124.8.1497. [DOI] [PubMed] [Google Scholar]
- Feng Y, Olson EC, Stukenberg PT, Flanagan LA, Kirschner MW, Walsh CA. LIS1 regulates CNS lamination by interacting with mNudE, a central component of the centrosome. Neuron. 2000;28:665–79. doi: 10.1016/s0896-6273(00)00145-8. [DOI] [PubMed] [Google Scholar]
- Fischer JA, Eun SH, Doolan BT. Endocytosis, endosome trafficking, and the regulation of Drosophila development. Annu Rev Cell Dev Biol. 2006;22:181–206. doi: 10.1146/annurev.cellbio.22.010605.093205. [DOI] [PubMed] [Google Scholar]
- Frade JM. Interkinetic nuclear movement in the vertebrate neuroepithelium: encounters with an old acquaintance. Prog Brain Res. 2002;136:67–71. doi: 10.1016/s0079-6123(02)36007-2. [DOI] [PubMed] [Google Scholar]
- Fukushima N, Weiner JA, Chun J. Lysophosphatidic acid (LPA) is a novel extracellular regulator of cortical neuroblast morphology. Dev Biol. 2000;228:6–18. doi: 10.1006/dbio.2000.9930. [DOI] [PubMed] [Google Scholar]
- Gambello MJ, Darling DL, Yingling J, Tanaka T, Gleeson JG, Wynshaw-Boris A. Multiple dose-dependent effects of Lis1 on cerebral cortical development. J Neurosci. 2003;23:1719–29. doi: 10.1523/JNEUROSCI.23-05-01719.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghashghaei HT, Lai C, Anton ES. Neuronal migration in the adult brain: are we there yet? Nat Rev Neurosci. 2007;8:141–151. doi: 10.1038/nrn2074. [DOI] [PubMed] [Google Scholar]
- Godinho L, Link BA. Cell Migration. In: Sernagor E, Eglen S, Harris WA, Wong RO, editors. Retinal Development. Cambridge University Press; Cambridge, UK: 2006. [Google Scholar]
- Gomes ER, Jani S, Gundersen GG. Nuclear movement regulated by Cdc42, MRCK, myosin, and actin flow establishes MTOC polarization in migrating cells. Cell. 2005;121:451–63. doi: 10.1016/j.cell.2005.02.022. [DOI] [PubMed] [Google Scholar]
- Gotz M, Huttner WB. The cell biology of neurogenesis. Nat Rev Mol Cell Biol. 2005;6:777–88. doi: 10.1038/nrm1739. [DOI] [PubMed] [Google Scholar]
- Grady RM, Starr DA, Ackerman GL, Sanes JR, Han M. Syne proteins anchor muscle nuclei at the neuromuscular junction. Proc Natl Acad Sci U S A. 2005;102:4359–64. doi: 10.1073/pnas.0500711102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfter W, Dong S, Balasubramani M, Bier ME. Temporary disruption of the retinal basal lamina and its effect on retinal histogenesis. Dev Biol. 2001;238:79–96. doi: 10.1006/dbio.2001.0396. [DOI] [PubMed] [Google Scholar]
- Haubst N, Georges-Labouesse E, De Arcangelis A, Mayer U, Gotz M. Basement membrane attachment is dispensable for radial glial cell fate and for proliferation, but affects positioning of neuronal subtypes. Development. 2006;133:3245–54. doi: 10.1242/dev.02486. [DOI] [PubMed] [Google Scholar]
- Hecht JH, Weiner JA, Post SR, Chun J. Ventricular zone gene-1 (vzg-1) encodes a lysophosphatidic acid receptor expressed in neurogenic regions of the developing cerebral cortex. J Cell Biol. 1996;135:1071–83. doi: 10.1083/jcb.135.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinds JW, Hinds PL. Early ganglion cell differentiation in the mouse retina: an electron microscopic analysis utilizing serial sections. Dev Biol. 1974;37:381–416. doi: 10.1016/0012-1606(74)90156-0. [DOI] [PubMed] [Google Scholar]
- Holzinger A, Lutz-Meindl U. Kinesin-like proteins are involved in postmitotic nuclear migration of the unicellular green alga Micrasterias denticulata. Cell Biol Int. 2002;26:689–97. doi: 10.1006/cbir.2002.0920. [DOI] [PubMed] [Google Scholar]
- Houalla T, Hien Vuong D, Ruan W, Suter B, Rao Y. The Ste20-like kinase misshapen functions together with Bicaudal-D and dynein in driving nuclear migration in the developing drosophila eye. Mech Dev. 2005;122:97–108. doi: 10.1016/j.mod.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Huisman SM, Segal M. Cortical capture of microtubules and spindle polarity in budding yeast - where's the catch? J Cell Sci. 2005;118:463–71. doi: 10.1242/jcs.01650. [DOI] [PubMed] [Google Scholar]
- Kim J, Wu HH, Lander AD, Lyons KM, Matzuk MM, Calof AL. GDF11 controls the timing of progenitor cell competence in developing retina. Science. 2005;308:1927–30. doi: 10.1126/science.1110175. [DOI] [PubMed] [Google Scholar]
- Langman J, Guerrant RL, Freeman BG. Behavior of neuro-epithelial cells during closure of the neural tube. J Comp Neurol. 1966;127:399–411. doi: 10.1002/cne.901270308. [DOI] [PubMed] [Google Scholar]
- Li M, Sakaguchi DS. Expression patterns of focal adhesion associated proteins in the developing retina. Dev Dyn. 2002;225:544–53. doi: 10.1002/dvdy.10195. [DOI] [PubMed] [Google Scholar]
- Locker M, Agathocleous M, Amato MA, Parain K, Harris WA, Perron M. Hedgehog signaling and the retina: insights into the mechanisms controlling the proliferative properties of neural precursors. Genes Dev. 2006;20:3036–3048. doi: 10.1101/gad.391106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malicki J. Cell fate decisions and patterning in the vertebrate retina: the importance of timing, asymmetry, polarity and waves. Curr Opin Neurobiol. 2004;14:15–21. doi: 10.1016/j.conb.2004.01.015. [DOI] [PubMed] [Google Scholar]
- Messier PE. Microtubules, interkinetic nuclear migration and neurulation. Experientia. 1978;34:289–96. doi: 10.1007/BF01922992. [DOI] [PubMed] [Google Scholar]
- Miki H, Okada Y, Hirokawa N. Analysis of the kinesin superfamily: insights into structure and function. Trends Cell Biol. 2005;15:467–76. doi: 10.1016/j.tcb.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Morest DK. A study of neurogenesis in the forebrain of opossum pouch young. Z Anat Entwicklungsgesch. 1970;130:265–305. doi: 10.1007/BF00520999. [DOI] [PubMed] [Google Scholar]
- Morgan JE, Datta AV, Erichsen JT, Albon J, Boulton ME. Retinal ganglion cell remodelling in experimental glaucoma. Adv Exp Med Biol. 2006;572:397–402. doi: 10.1007/0-387-32442-9_56. [DOI] [PubMed] [Google Scholar]
- Morris NR. Nuclear positioning: the means is at the ends. Curr Opin Cell Biol. 2003;15:54–9. doi: 10.1016/s0955-0674(02)00004-2. [DOI] [PubMed] [Google Scholar]
- Mosley-Bishop KL, Li Q, Patterson L, Fischer JA. Molecular analysis of the klarsicht gene and its role in nuclear migration within differentiating cells of the Drosophila eye. Curr Biol. 1999;9:1211–20. doi: 10.1016/s0960-9822(99)80501-6. [DOI] [PubMed] [Google Scholar]
- Murciano A, Zamora J, Lopez-Sanchez J, Frade JM. Interkinetic nuclear movement may provide spatial clues to the regulation of neurogenesis. Mol Cell Neurosci. 2002;21:285–300. doi: 10.1006/mcne.2002.1174. [DOI] [PubMed] [Google Scholar]
- Nadarajah B, Brunstrom JE, Grutzendler J, Wong RO, Pearlman AL. Two modes of radial migration in early development of the cerebral cortex. Nat Neurosci. 2001;4:143–50. doi: 10.1038/83967. [DOI] [PubMed] [Google Scholar]
- Nowakowski RS, Caviness VS, Jr., Takahashi T, Hayes NL. Population dynamics during cell proliferation and neuronogenesis in the developing murine neocortex. Results Probl Cell Differ. 2002;39:1–25. doi: 10.1007/978-3-540-46006-0_1. [DOI] [PubMed] [Google Scholar]
- Ohnuma S, Harris WA. Neurogenesis and the cell cycle. Neuron. 2003;40:199–208. doi: 10.1016/s0896-6273(03)00632-9. [DOI] [PubMed] [Google Scholar]
- Ohshima T, Ward JM, Huh CG, Longenecker G, Veeranna, Pant HC, Brady RO, Martin LJ, Kulkarni AB. Targeted disruption of the cyclin-dependent kinase 5 gene results in abnormal corticogenesis, neuronal pathology and perinatal death. Proc Natl Acad Sci U S A. 1996;93:11173–8. doi: 10.1073/pnas.93.20.11173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson R, Catsicas M, Becker D, Mobbs P. Purinergic and muscarinic modulation of the cell cycle and calcium signaling in the chick retinal ventricular zone. J Neurosci. 2002;22:7569–79. doi: 10.1523/JNEUROSCI.22-17-07569.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson RA, Catsicas M, Becker DL, Bayley P, Luneborg NL, Mobbs P. Ca(2+) signalling and gap junction coupling within and between pigment epithelium and neural retina in the developing chick. Eur J Neurosci. 2004;19:2435–45. doi: 10.1111/j.0953-816X.2004.03338.x. [DOI] [PubMed] [Google Scholar]
- Pearson RA, Dale N, Llaudet E, Mobbs P. ATP released via gap junction hemichannels from the pigment epithelium regulates neural retinal progenitor proliferation. Neuron. 2005a;46:731–44. doi: 10.1016/j.neuron.2005.04.024. [DOI] [PubMed] [Google Scholar]
- Pearson RA, Luneborg NL, Becker DL, Mobbs P. Gap junctions modulate interkinetic nuclear movement in retinal progenitor cells. J Neurosci. 2005b;25:10803–14. doi: 10.1523/JNEUROSCI.2312-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poggi L, Vitorino M, Masai I, Harris WA. Influences on neural lineage and mode of division in the zebrafish retina in vivo. J Cell Biol. 2005;171:991–9. doi: 10.1083/jcb.200509098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prada C, Puelles L, Genis-Galvez JM. A golgi study on the early sequence of differentiation of ganglion cells in the chick embryo retina. Anat Embryol (Berl) 1981;161:305–17. doi: 10.1007/BF00301828. [DOI] [PubMed] [Google Scholar]
- Sauer FC. Mitosis in the neural tube. J. Comp. Neurol. 1935;62:377–397. [Google Scholar]
- Sauer FC. The interkinetic migration of embryonic epithelia nuclei. J. Morphol. 1936;60:1–11. [Google Scholar]
- Sauer ME, Chittenden AC. Deoxyribonucleic acid content of cell nuclei in the neural tube of the chick embryo: evidence for intermitotic migration of nuclei. Exp Cell Res. 1959;16:1–6. doi: 10.1016/0014-4827(59)90189-2. [DOI] [PubMed] [Google Scholar]
- Sauer ME, Walker BE. Radioautographic study of interkinetic nuclear migration in the neural tube. Proc Soc Exp Biol Med. 1959;101:557–60. doi: 10.3181/00379727-101-25014. [DOI] [PubMed] [Google Scholar]
- Schaper A. The earliest differentiation in the central nervous system. Science. 1897;5:430–431. [Google Scholar]
- Schroer TA. Dynactin. Annu Rev Cell Dev Biol. 2004;20:759–79. doi: 10.1146/annurev.cellbio.20.012103.094623. [DOI] [PubMed] [Google Scholar]
- Semina EV, Bosenko DV, Zinkevich NS, Soules K, Hyde DR, Vihtelic TS, Willer GB, Gregg RG, Link BA. Mutations in laminin alpha 1 result in complex, lens-independent ocular phenotypes in zebrafish. Dev Biol. 2006;299:63–77. doi: 10.1016/j.ydbio.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Shkumatava A, Neumann CJ. Shh directs cell-cycle exit by activating p57Kip2 in the zebrafish retina. EMBO Rep. 2005;6:563–9. doi: 10.1038/sj.embor.7400416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow RL, Robson JA. Ganglion cell neurogenesis, migration and early differentiation in the chick retina. Neuroscience. 1994;58:399–409. doi: 10.1016/0306-4522(94)90046-9. [DOI] [PubMed] [Google Scholar]
- Snow RL, Robson JA. Migration and differentiation of neurons in the retina and optic tectum of the chick. Experimental Neurology. 1995;134:13–24. doi: 10.1006/exnr.1995.1032. [DOI] [PubMed] [Google Scholar]
- Starr DA, Fischer JA. KASH ′n Karry: the KASH domain family of cargo-specific cytoskeletal adaptor proteins. Bioessays. 2005;27:1136–46. doi: 10.1002/bies.20312. [DOI] [PubMed] [Google Scholar]
- Starr DA, Han M. Role of ANC-1 in tethering nuclei to the actin cytoskeleton. Science. 2002;298:406–9. doi: 10.1126/science.1075119. [DOI] [PubMed] [Google Scholar]
- Suter B, Steward R. Requirement for phosphorylation and localization of the Bicaudal-D protein in Drosophila oocyte differentiation. Cell. 1991;67:917–26. doi: 10.1016/0092-8674(91)90365-6. [DOI] [PubMed] [Google Scholar]
- Swan A, Nguyen T, Suter B. Drosophila Lissencephaly-1 functions with Bic-D and dynein in oocyte determination and nuclear positioning. Nat Cell Biol. 1999;1:444–9. doi: 10.1038/15680. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Goto T, Miyama S, Nowakowski RS, Caviness VS., Jr. Sequence of neuron origin and neocortical laminar fate: relation to cell cycle of origin in the developing murine cerebral wall. J Neurosci. 1999;19:10357–71. doi: 10.1523/JNEUROSCI.19-23-10357.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Serneo FF, Tseng HC, Kulkarni AB, Tsai LH, Gleeson JG. Cdk5 phosphorylation of doublecortin ser297 regulates its effect on neuronal migration. Neuron. 2004;41:215–27. doi: 10.1016/s0896-6273(03)00852-3. [DOI] [PubMed] [Google Scholar]
- Tinsley JH, Minke PF, Bruno KS, Plamann M. p150Glued, the largest subunit of the dynactin complex, is nonessential in Neurospora but required for nuclear distribution. Mol Biol Cell. 1996;7:731–42. doi: 10.1091/mbc.7.5.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai JW, Chen Y, Kriegstein AR, Vallee RB. LIS1 RNA interference blocks neural stem cell division, morphogenesis, and motility at multiple stages. J Cell Biol. 2005;170:935–45. doi: 10.1083/jcb.200505166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tullio AN, Bridgman PC, Tresser NJ, Chan CC, Conti MA, Adelstein RS, Hara Y. Structural abnormalities develop in the brain after ablation of the gene encoding nonmuscle myosin II-B heavy chain. J Comp Neurol. 2001;433:62–74. doi: 10.1002/cne.1125. [DOI] [PubMed] [Google Scholar]
- Ueno M, Katayama K, Yamauchi H, Nakayama H, Doi K. Cell cycle progression is required for nuclear migration of neural progenitor cells. Brain Res. 2006;1088:57–67. doi: 10.1016/j.brainres.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Wang Y, Dakubo GD, Thurig S, Mazerolle CJ, Wallace VA. Retinal ganglion cell-derived sonic hedgehog locally controls proliferation and the timing of RGC development in the embryonic mouse retina. Development. 2005;132:5103–13. doi: 10.1242/dev.02096. [DOI] [PubMed] [Google Scholar]
- Whited JL, Cassell A, Brouillette M, Garrity PA. Dynactin is required to maintain nuclear position within postmitotic Drosophila photoreceptor neurons. Development. 2004;131:4677–86. doi: 10.1242/dev.01366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelmsen K, Ketema M, Truong H, Sonnenberg A. KASH-domain proteins in nuclear migration, anchorage and other processes. J Cell Sci. 2006;119:5021–9. doi: 10.1242/jcs.03295. [DOI] [PubMed] [Google Scholar]
- Willer GB, Lee VM, Gregg RG, Link BA. Analysis of the zebrafish perplexed mutation reveals tissue-specific roles for de novo pyrimidine synthesis during development. Genetics. 2005;170:1827–37. doi: 10.1534/genetics.105.041608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worman HJ, Gundersen GG. Here come the SUNs: a nucleocytoskeletal missing link. Trends Cell Biol. 2006;16:67–9. doi: 10.1016/j.tcb.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Wozniak MA, Modzelewska K, Kwong L, Keely PJ. Focal adhesion regulation of cell behavior. Biochim Biophys Acta. 2004;1692:103–19. doi: 10.1016/j.bbamcr.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Xiang X, Fischer R. Nuclear migration and positioning in filamentous fungi. Fungal Genet Biol. 2004;41:411–9. doi: 10.1016/j.fgb.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Zhang J, Li S, Fischer R, Xiang X. Accumulation of cytoplasmic dynein and dynactin at microtubule plus ends in Aspergillus nidulans is kinesin dependent. Mol Biol Cell. 2003;14:1479–88. doi: 10.1091/mbc.E02-08-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolessi FR, Poggi L, Wilkinson CJ, Chien CB, Harris WA. Polarization and orientation of retinal ganglion cells in vivo. Neural Develop. 2006;1:2. doi: 10.1186/1749-8104-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]