Abstract
BACKGROUND
Epidemiological and laboratory studies support the hypothesis that several plant components influence prostate carcinogenesis and holds promise for disease prevention. Previously we reported that Nexrutine® (bark extract from Phellodendron amurense) inhibits proliferation of prostate cancer cells and prostate tumor development in the transgenic adenocarcinoma of mouse prostate (TRAMP) model through modulation of Akt signaling pathway. In the present investigation we conducted studies to further define the mechanism of action of Nexrutine® and to identify the active component associated with its biological activity.
METHODS
Androgen-responsive, androgen-independent human prostate cancer cell lines and tissues from TRAMP mice fed Nexrutine® were used in these studies. Activity guided fractionation identified butanol fraction recapitulating the activities of Nexrutine® assessed by proliferation assays, apoptotic assays (DAPI and TUNEL staining), transient transfections, gel shift assays and Western blotting. In addition ultra-performance liquid chromatography (UPLC) of butanol fraction was used to identify active component of Nexrutine®.
RESULTS
Butanol fraction recapitulated the activities of Nexrutine® in (i) inhibiting proliferation; (ii) inducing apoptosis; and (iii) modulating transcriptional activity of NFκB in prostate cancer cells. Our data also indicates that both Nexrutine® and butanol fraction modulates NFκB transcriptional activity by inhibiting IκBα phosphorylation. Expression of p65 and phosphorylated IκBα are high in tumors from TRAMP mice. In contrast dietary administration of Nexrutine® reduced expression of p65 and phosphorylated IκBα in prostate from TRAMP mice. In addition using UPLC, we have identified berberine or closely related compound in the butanol fraction.
CONCLUSION
The results suggest that berberine or closely related component of butanol fraction may be responsible for the observed biological activities and induce apoptosis in prostate cancer cells by targeting critical cell survival signaling pathways both in vitro and in vivo.
Keywords: nexrutine, apoptosis, fractionation
INTRODUCTION
Prostate cancer is one of the most commonly diagnosed cancers in the United States and is the second leading cause of cancer related deaths [1]. In contrast to localized prostate cancer, which can be treated effectively with surgery or radiation therapy, advanced hormone independent prostate cancer is unresponsive to conventional hormone therapy and it accounts for the majority of the deaths [2]. Novel treatment modalities are therefore essential to treat hormone-resistant tumors and to prevent progression of hormone-sensitive to hormone-refractory stage.
Epidemiological studies suggest that the incidence of prostate cancer is much lower in Asian than Western population. Moreover, migration data show that the incidence increases in men migrating from low incidence to areas of higher incidence. These results suggest that the etiology of prostate cancer may involve dietary, lifestyle and environmental factors [3]. Along with these studies, laboratory studies suggest a link between high-fat diet and increased risk of metastatic prostate cancer. On the other hand, consumption of low-fat diet along with high intake of dark green leafy vegetables, fruits, and soy products has been reported to reduce the incidence prostate cancer [4].
Previously we reported that Nexrutine® (bark extract from Phellodendron amurense) inhibits proliferation of prostate cancer cells through inhibition of Akt mediated activation of CREB [5]. Subsequently we have shown administration of Nexrutine® through diet prevents prostate tumor development in the transgenic adenocarcinoma of mouse prostate (TRAMP) model. Inhibition of prostate tumor development in the TRAMP model is associated with reduced expression of pAkt, pCREB and Cyclin D1 [6]. We have also shown that inactivation of either Akt (using kinase dead mutant) or CREB (using dominant negative CREB) or inactivation of both Akt and CREB by Nexrutine® treatment reduced Cyclin D1 promoter activity in PC-3 cells [6]. In addition to phosphorylating CREB (on ser 133), Akt has been reported to activate transcription factor NFκB that has been implicated with prostate tumor progression [7,8]. NFκB that has been shown to be is constitutively activated in human prostate tumors, androgen-independent prostate cancer cell lines and human prostate tumor xenografts [9-17]. Therefore, agents capable of suppressing constitutive activation of NFκB may potentially be useful in the prevention and management of prostate cancer. Although we have shown Nexrutine® inhibits prostate cell proliferation and tumor development through Akt/CREB/Cyclin D1 signaling, it is not known whether it also affects NFκB signaling. Indeed it is now evident that agents targeting multiple signal transduction pathways have tremendous translational potential due to multifactorial nature of cancer progression [18]. In order to develop Nexrutine® for prostate cancer management it is critical to understand not only the detailed mechanism of action but also the active components associated with its activity. In the present study we conducted studies to identify the active component of Nexrutine® associated with its biological activity. Using activity-guided fractionation we have identified butanol fraction (F3) recapitulating the antiproliferative activity of Nexrutine®. Ultra performance liquid chromatography (UPLC) analysis identified that F3 contains berberine or berberine-like compounds and that pure berberine inhibited the proliferation of prostate cancer cells. Both Nexrutine® and F3 inhibited constitutive NFκB activation through decreased phosphorylation of IκBα in androgen independent prostate cancer cells. Further Nexrutine® administration decreased the levels of phosphorylated IκBα and p65 in tumors generated from transgenic adenocarcinoma of mouse prostate (TRAMP).
MATERIALS AND METHODS
Preparation of Nexrutine® and Fractionation
Nexrutine® was provided by Next Pharmaceuticals (Irvine, CA). Stock solution of Nexrutine® was prepared by dissolving 10 mg of Nexrutine® in 10 ml DMSO to obtain a concentration of 1 mg/ml. This was diluted in growth medium to obtain concentrations of 1-10 μg/ml. Ten gram of Nexrutine® was extracted with 70% ethanol (1:10, w/v) at room temperature three times. After each extraction supernatant was saved. After the third extraction all the supernatants were pooled and concentrated in a rotary evaporator under reduced pressure at room temperature until the ethanol completely evaporated (1.97 g). The residue was resuspended in 20 ml of double distilled water and extracted with different solvents of increasing polarity (1:10, v/v) starting with hexane, followed by ethyl acetate and n-butanol. After each extraction the fraction was evaporated to dryness under reduced pressure at room temperature and yield was calculated. The hexane fraction represented 0.13% (F1); ethyl acetate fraction represented 0.489% (F2) and butanol fraction represented 2.41% (F3). Fractionation was conducted using two different batches of Nexrutine®.
Prostate Cancer Cell Lines
Human PCA cell lines androgen responsive LNCaP and androgen-independent PC-3 cells were grown and maintained as described earlier [19].
Cell Proliferation Assays
Cancer cells were plated in 96-well plates at a density of 4,000 cells per well in triplicate. After attachment (after 24 hr), cells were treated with different concentrations of Nexrutine® and fraction F1, F2, and F3 (1, 2.5, 5, and 10 μg/ml). Control cells received only the solvent (DMSO). Cell proliferation was detected after 72 hr of incubation with Nexrutine® and fractions using Cell Titer 96 Aqueous One (Promega Corporation, Madison, WI) solution assay as described by the manufacturer [20,21].
Detection of Apoptosis
PC-3, were plated at a density of 1 × 105 in 60-mm dishes. At 70-80% confluency, cells were treated with different concentrations of Nexrutine® and fractions (F1, F2, and F3). After incubation, both adherent and floating cells were collected by trypsinization for detection of apoptosis by morphologic analysis; TUNEL assay and 4′-6-diamidino-2-phenylindole (DAPI) staining. DAPI staining can be used to distinguish live from apoptotic cells based on the morphology of their nuclei. DAPI forms fluorescent complexes with double-stranded DNA, and DAPI-stained nuclei show a bright blue fluorescence when observed under a fluorescent microscope with DAPI filter.
Transient Expression Assays
Transient transfections were performed using a Lipofectin reagent (Invitrogen, Carlsbad, CA), in accordance with the manufacturer's recommendations. Briefly NFκB reporter plasmid (1 μg/well) and pRLTK plasmid (20 ng/well; Renilla luciferase for normalization) were incubated with the Lipofectin reagent for 30 min at room temperature. The DNA-Lipofectin mixture was then added to the cells and incubated for 24 hr. Twenty-four hours after transfection, the cells were treated with solvent control or 2.5 μg/ml Nexrutine® and fractions for 2 hr. Following treatments, cell extracts were prepared and assayed for luciferase activity, as described earlier [21]. Renilla luciferase activity was used to normalize transfection efficiency. Results are expressed as the ratio of firefly luciferase to Renilla luciferase at equal amounts of protein.
Preparation of Nuclear Extracts and Western Blotting
Cells were treated with Nexrutine® and fractions (2.5 μg/ml for 24 hr time points as indicated in the figures) were harvested and homogenized in a buffer containing 10 mM Hepes, pH 7.9, 1.5 mM MgCl2, 10 mM KCl, 1.0 mM dithiothreitol, 1.0 mM phenylmethylsulfonyl fluoride, and protease inhibitors including 1 mM phenylmethylsulfonyl fluoride, 25 μg/ml leupeptin, 25 μg/ml aprotinin, 25 μg/ml pepstatin. Nuclei were separated from cell debris and lysed in a buffer containing 20 mM Hepes, pH 7.9, 25% (v/v) glycerol, 0.42 M NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 1.0 mM dithiothreitol, and the protease inhibitors. Nuclear protein was collected by centrifugation at 15,000g for 45 min. Protein content in the supernatant was determined by the method of Bradford [20,21]. Equal amounts of extracts were fractionated on a 10% sodium dodecyl sulfate (SDS)- polyacrylamide gel. After electrophoresis, proteins were electrophoretically transferred to a nitrocellulose membrane. The blotted membrane was blocked with 5% nonfat dry milk in TBS containing 0.1% Tween 20 (blocking solution), and incubated with indicated polyclonal antibodies (Santa Cruz Biotechnology, Santa Cruz, CA; Cell Signaling Technology, Inc., Beverly, MA; and Upstate Cell Signaling Solutions, Lake Placid, NY) followed by incubation with HRP-conjugated anti-rabbit IgG antibody (Sigma, St. Louis, MO) in blocking solution. Bound antibody was detected by enhanced chemiluminescence using Western lightning Western chemiluminescence reagent plus (enhanced luminol) following the manufacturer's directions (PerkinElmer Life and Analytical Sciences, Shelton, CT). All the blots were stripped and reprobed with β-actin to ensure equal loading of protein. Each experiment was repeated thrice using different sets of extracts.
In order to determine the degradation of IκBα, LNCaP and PC-3 cells were transfected with pIκB EGFP vector and after 48 hr of transfection cells were stimulated with TNFα (20 ng/ml for 30 min as a positive control for NFκB activation). One group of cells were preincubated with Nexrutine (2.5 μg/ml) prior to stimulation with TNFα and observed under fluorescence microscope.
Immunolocalization of NFκB p65, pIκBα, pSTAT3 inTumor Samples
Expression of p65, pSTAT3 and pIκBα was examined in tumor samples from TRAMP mice administered Nexrutine® using an immunohistochemical method described previously [22]. Briefly, prostate tumor samples were embedded in paraffin and fixed with paraformaldehyde. After being washed in PBS, the slides were blocked with protein block solution (The liquid DAB+ Substrate Chromogen System-HRP used for immunocytochemistry was obtained from DakoCytomation, Carpentaria, CA) for 20 min and then incubated overnight with rabbit polyclonal anti-human p65 (Abcam Inc., Cambridge, MA) mouse monoclonal anti pSTAT3 (Santa Cruz biotechnology) and anti pIκBα (Cell Signaling Technology, Inc.) antibodies (1:200, 1:200, and 1:100, respectively). After the incubation, the slides were washed and incubated with biotinylated link universal antiserum followed by horseradish peroxidase-streptavidin conjugate (LSAB+ kit). Color was developed using 3,3-diaminobenzidine hydrochloride as a chromogen. Sections were rinsed in distilled water, counterstained with Mayer's hematoxylin, and mounted with DPX mounting medium for evaluation. Pictures were captured with a Photometric Cool SNAP CF color camera (Nikon, Lewisville, TX) and MetaMorph version 4.6.5 software (Universal Imaging, Downing-town, PA).
Electrophoretic Mobility Shift Assay (EMSA)
Gel shift assays were done as described elsewhere [19]. Double-stranded NFκB oligonucleotide was end-labeled with γ-p32-ATP using T4 polynucleotide kinase. Nuclear extracts were incubated with the radiolabeled probe in binding buffer (containing 4 mM Tris-HCl, 12 mM Hepes, pH 7.9, 60 mM KCl, 0.5 mM EDTA, 1 mM DTT, and 12% glycerol) for 20 min at room temperature in a final volume of 20 μl. After incubation, samples were fractionated on a 4% polyacrylamide gel in 0.25× TBE at 4°C. Following electrophoresis, the gel was dried and autoradiographed.
UPLC-MS/MS
Multi reactions monitoring (MRM) method was used for identification of compounds in fractions. An API 3200 Qtrap triple quadrupole mass spectrometer (Applied Biosystems/MDS SCIEX, Foster City, CA) equipped with a TurboIonSpray™ source was operated in positive ion mode to perform the analysis. The main working parameters for the mass spectrometers were set as follows: ion spray voltage, 1.5 kV; ion source temperature, 750°C; the nebulizer gas (gas1), nitrogen, 50 psi; turbo gas (gas2), nitrogen, 60 psi; curtain gas, nitrogen, 25 psi. The transition for MRM was 336→320 for berberine. Acquity UPLC BEH C18 column (50 mm × 2.1 mm ID, 1.7 μm, Waters, Milford, MA) with waters Acquity™ DAD detector was used in UPLC. Fractions were separated using 0.1% formic acid (mobile phase A) and 100% acetonitrile (mobile phase B) and a flow rate of 0.5 ml/min.
Statistical Analysis
Data were presented as average±SD and significance was determined by using Student's t-test. Differences between the experimental groups were considered significant at P < 0.05.
RESULTS AND DISCUSSION
We examined the effects of Nexrutine® and its fractions (F1, F2, and F3) on proliferation of androgen independent PC-3 human prostate cancer cells using the Cell Titer 96 Aqueous One solution assay as described earlier [5]. As shown in Figure 1, consistent with our published results a concentration of 2.5 μg/ml Nexrutine® was sufficient to inhibit the proliferation of androgen independent PC-3 cells by 50%. Similarly 2.5 μg/ml butanol fraction (F3) also inhibited proliferation of PC-3 cells by more than 50%. However other fractions (F1 and F2) or residue of alcoholic extract had no significant effect on proliferation of PC-3 cells. These results were also confirmed by measuring the cell viability using the trypan blue dye exclusion method (data not shown). Similar results were obtained in androgen responsive LNCaP cells (data not shown). We tested these fractions in different combinations (F1 + F3; F2 + F3, and F1 + F2) for their ability to inhibit cell proliferation and found differential ability of the combination to inhibit proliferation (data not shown). However F3 alone exhibited the highest efficiency in proliferation inhibition. These data show that butanol fraction (F3) is as effective as that of the parent compound Nexrutine®.
Fig. 1.
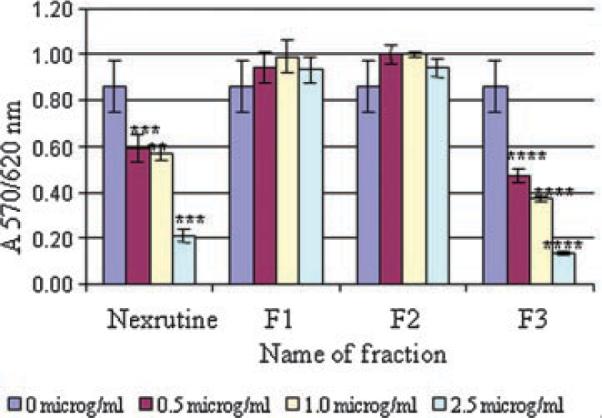
Butanol fraction (F3) recapitulates Nexrutine® in inhibiting proliferation of androgen independent PC-3 prostate cancer cells. Androgen independent (PC-3 cells) were plated in 96-well plates as described in the Materials and Methods Section and treated with indicated concentration of compounds in microgram per mlor solvent control. Cell proliferation was determined using cell Titer 96 Aqueous one solution assay at 72 hr. Absorbance at 570 nm was determined using Spectramax plus plate reader (Molecular devices, Sunnyvale, CA) is shown. The data shown are average ± SD of three replicate well and is representative of three independent experiments. Similar results were obtained in androgen responsive LNCaP cells (data not shown). [Color figure can be viewed in the onlineissue, whichis available at www.interscience.wiley.com.]
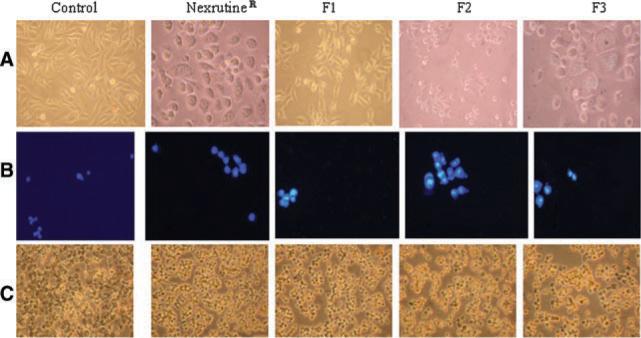
Previously we have shown that Nexrutine® treatment was associated with induction of apoptosis in prostate cancer cells [5,6]. We examined whether butanol fraction (F3) recapitulates the apoptosis-inducing activity of Nexrutine®. PC-3 cells treated with Nexrutine® and fractions (2.5 μg/ml for 24 hr) were examined for apoptotic features by using a combination of methods (i) light microscopy, (ii) DAPI staining, and (iii) TUNEL assay. Changes in the morphology of the cells with granular appearance were evidenced after treatment with Nexrutine® and F3. This resulted in detachment of cells from culture dishes and a significant proportion of the cells started to float by 24 hr (Fig. 2A). Under identical conditions, the vehicle or F1 or F2 did not induce any morphological changes. Presence of apoptosis in treated cells was detected using the DAPI staining, which distinguishes live and apoptotic cells based on the morphology of the nuclei. DAPI forms fluorescent complexes with double-stranded DNA, and DAPI stained nuclei show a bright fluorescence with DAPI filter (Fig. 2B). As shown in Figure 2B, cells treated with Nexrutine®, F1, F2, and F3 showed bright fluorescence. The TUNEL assay used here was a colorimetric assay that involves incorporation of a biotinylated deoxyuridine 5-triphosphate (dUTP) into 3′-hydroxyl groups that were generated due to cleave of apoptotic DNA. Incorporation of dUTP is detected using streptavidin-HRP conjugate and the chromogen (diaminobenzidine) is detected as a dark brown precipitate. The nuclei of Nexrutine®, F1, F2, and F3 treated cells showed characteristic brown staining indicative of DNA fragmentation (Fig. 2C). These data demonstrate that all fractions induce apoptosis in PC-3 cells as that of the parent compound Nexrutine®. However only F3 recapitulated the ability of Nexrutine® to inhibit proliferation and induce apoptosis.
Fig. 2.
Morphological features indicative of apoptosisis also recapitulated by butanol fraction from Nexrutine® in PC-3 cells. PC-3 cell swere treated with solvent DMSO, Nexrutine® andits Fractions (2.5 mg/ml for 24 hr). Morphological alterations were evaluated microscopically and photomicrographs of cells were taken by phase contrast microscope using a Nikon microscope with a digital camera systemcoolpix 995 (Nikon Corporation, Tokyo, Japan) with magnification of 20 × (A); Apoptosis deter mined by DAPI staining showing intact DNA in control with less staining. On the other hand cell treated with Nexrutine® and fractions show intense staining in the nucleus (B); Evidence for induction of apoptosis asdetermined by TUNEL staining. Untreated cells showedno brown staining in dicative of apoptosis, whereas cells treated with Nexrutine® and butanol fraction showed brown stained nuclei. Other fractions showed moderate number of brown stained nuclei (C). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
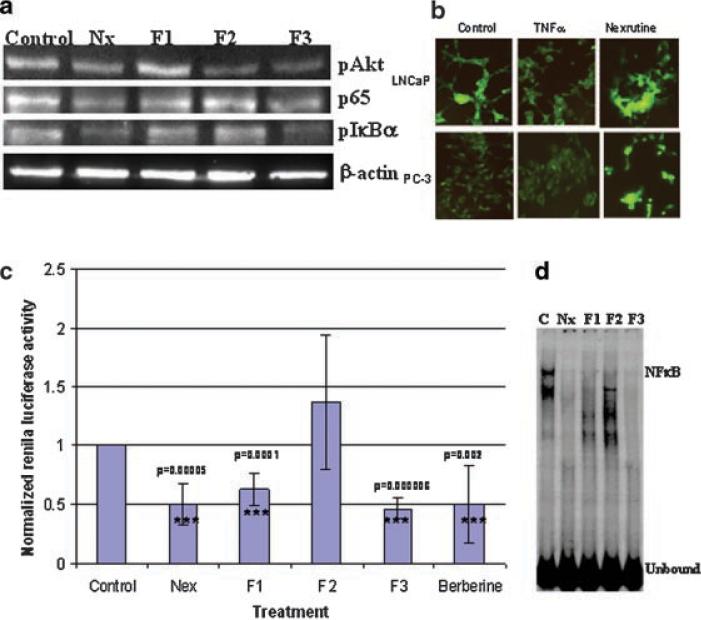
Previously we have shown that Nexrutine® treatment inhibits cell survival through Akt, which regulates cell survival through inhibition of apoptosis [5]. In addition to blocking apoptosis directly, Akt also signals activation of NFκB through phosphorylation and activation of IKKα or by phosphorylating RelA [23,24]. Under normal conditions, NFκB is sequestered in the cytoplasm as a heterodimer composed of Rel proteins p50 and p65 by the inhibitory protein IκBα [25,26]. In response to cell stimulation, IκBα is rapidly phosphorylated and targeted to be degraded allowing nuclear translocation of NFκB where it regulates the expression of target genes. In order to investigate whether Nexrutine® treatment also affects NFκB, we analyzed expression of pAkt, p65 and pIκBa in PC-3 cells treated with Nexrutine® and the fractions using immunoblot analysis. As shown in Figure 3a treatment of PC-3 cells with Nexrutine® reduced the expression of pAkt and p65 by more than 50%. It is known that NFκB is activated by phosphorylation and subsequent degradation of IκBα; we measured the levels of pIκBα in these extracts. As shown in Figure 3A, untreated PC-3 cells showed higher levels of pIκBa indicating activation of NFκB. Treatment with Nexrutine® reduced the levels of pIκBa indicating inactivation of NFκB signaling. We confirmed these observations by an independent approach using pIκBEGFP reporter plasmid. This NFκB reporter contains destabilized enhanced green fluorescent protein (d2EGFP) that allows real time detection of signaling using fluorescence microscopy. As shown in Figure 3B, LNCaP cells showed fluorescence that was decreased in response to TNF-α stimulation indicating activation of NFκB signaling. On the other hand, PC-3 cells showed low levels of fluorescence indicating constitutively high levels of NFκB, which is consistent with the published literature. However, preincubation with Nexrutine® increased the fluorescence indicating inactivation of NFκB signaling. These data collectively indicate that butanol fraction (F3) recapitulated the NFκB inhibitory activity of Nexrutine®.
Fig. 3.
Butanol fraction recapitulates Nexrutine® in modulating NFκB signalingin PC-3 cells. A:Both Nexrutine® and butanol fraction treatment reduces protein levels of pAkt, p65 and pIκbα in PC-3 cells. Immunoblot analysis ofnuclear and cytoplasmic extracts prepared from PC-3 cells treated with Nexrutine® and fractions(2.5mg/ml for 24 hr)using pAkt, p65 and pIκBα. Equal amounts of the extracts were fractionatedona 10% SDS-polyacrylamide gel transferred onto a nitrocellulose membrane. The blotted membrane was blocked with 5% nonfat dry milk in TBS containing 1% Tween-20 (blocking solution) and incubated with indicated antibodies followed by incubation with HRP conjugated anti-rabbit IgG antibody (sigma) in blocking solution. Bound antibody was detected by enhanced chemiluminescence using Western lightning Western chemiluminescence reagent plus (enhancedluminol) following the manufacturer's directions (Perkin Elmer Lifeand Analytical Sciences, Shelton, CT). All the blots were stripped and reprobed withβ-actin to ensure equal loading of protein. Each experiment was repeated thrice using differentsets of extracts. The blot shownis a representative three different experiments. B: Nexrutine® prevents IκBα degradation. LNCa Pand PC-3 cells were transfected with pIκB EGFP vector and after 48 hr of transfection cells were stimulated with TNFα (20ng/ml for 30 min asapositive control for NFκB activation). One group of cells were preincubated with Nexrutine (2.5 μg/ml) prior to stimulation withT NFα and observed under fluorescence microscope. C: Inhibition of NFκB transcriptional activity by Nexrutine® and its fractions. Transient transfections were performed with NFκB reporter plasmid (1 μg/well) and pRLTK plasmid (20 ng/well) as described in the materials and methods using Lipofectamine reagent. Twenty-four hours after transfection, the cell swere treated with Nexrutine® and fractions(2.5 μg/ml) for 2hr. Luciferaseactivity was measured using the Dual-luciferase Reporter assay system (Promega Corporation Inc., Madison,WI) in triplicate samples containing equal amounts of protein. Renilla luciferase activity was used to normalize transfection efficiency. Results are expressed as the ratio of firefly luciferase to Renilla luciferase at equal amounts of protein. The data shown here are representative of three independent experiments conducted with two different preparations of plasmids. D: Inhibition of NFκB DNA binding by Nexrutine® and its fractions. EMSA of nuclear extracts prepared from PC-3 cells treated with Nexrutine® and its fractions (2.5 μg/ml for 2 hr). Five microgram extract was incubated with NFκB consensus oligonucleotide as radiolabeled probe and the DNA-protein complexes were resolved on a 4% nondenaturing gel by electrophoresis and subject to autoradiography. The blot shown here is representative of two independent experiments.[Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Since NFκB has been shown to regulate genes involved in various processes including apoptosis, Nexrutine® and fractions may induce apoptosis through modulation of NFκB-regulated gene products. In order to investigate this, transient transfections were performed using NFκB luciferase reporter containing three NFκB sites and luciferase activity was measured as described previously by us [20]. As shown in Figure 3C, Nexrutine® inhibited NFκB promoter activity in PC-3 cells. Subsequently we tested if F3 recapitulates the NFκB inhibitory activity of Nexrutine® in PC-3 cells. As shown in Figure 3c, like Nexrutine®, F3 inhibited the promoter activity of NFκB significantly (P = 0.00006). Although F2 had no inhibitory effect, F1 inhibited NFκB promoter activity. It is possible that F1 may contain compound(s) that may act as NFκB inhibitors without being cytotoxic. It is noteworthy to mention that both F1 and F2 induced apoptosis. Nonetheless inhibition of NFκB activation without exhibiting antiproliferative activity by F1 requires further investigations.
Since NFκB regulates plethora of genes, inhibition of NFκB activation by Nexrutine® or F3 may prevent prostate tumorigenesis through modulation of down stream gene expression. We measured NFκB DNA binding activity using Electrophoretic mobility shift assay (EMSA) in nuclear extracts prepared from PC-3 cells treated with Nexrutine® and fractions. As shown in Figure 3d, nuclear extracts from untreated PC-3 cells formed two complexes with NFκB probe. These data is consistent with the published reports showing constitutively active NFκB DNA binding activity in PC-3 cells [26]. In contrast such DNA-protein complexes were undetectable when nuclear extracts from PC-3 cells treated with Nexrutine®, F1 and F3 were used. Although the significance of these observations is not clear at present, these data is consistent with the above observations demonstrating inhibition of NFκB promoter activity by F2. However nuclear extracts from F2 formed one complex. These findings showing inhibition of NFκB activation by Nexrutine® and F3 corroborates with the studies carried out by us and other investigators with various phytochemicals and their analogues [5,6,19-21,27]. In our laboratory, we have shown that curcumin analogue, HMBME targets Akt/NFκB cell survival signaling pathway and has potential for prostate cancer management [20]. Recently, Deeb et al. [28] have reported that curcumin sensitizes human prostate cancer cells to TRAIL by suppressing NFκB via inhibition of the Akt signaling. Sarkar and Li have [29] suggested that genistein inhibits cell growth, induces apoptosis through NFκB mediated, in part, via inhibition of Akt phosphorylation in PC-3 prostate cancer cells but not in non tumorigenic CRL-2221 human prostate epithelial.
Recently we demonstrated that dietary administration of Nexrutine® prevented tumor development in TRAMP mice [6]. We have also shown that inhibition of prostate tumor development in the TRAMP model is associated with reduced expression of pAkt, pCREB and Cyclin D1 [6]. Published evidence suggests that Akt also signals activation of NFκB through phosphorylation and activation of IKKα or by phosphor-ylating RelA and that Cyclin D1 is one of the downstream targets of NFκB [23,24]. However it is not known whether the observed inhibition of tumor development is also through modulation of NFκB signaling in vivo. In order to investigate this, we tested expression of p65 and pIκBα in the prostate from TRAMP mice administered Nexrutine® for 20 weeks and age-matched control TRAMP mice receiving normal diet using immunohistochemistry. As shown in Figure 4 both p65 and pIκBα are expressed in the prostate from control mice. These data showing increased expression of p65 is consistent with the published data [22]. Expression of both p65 and pIκBα was reduced by more than 50% in the prostate from animals receiving Nexrutine® Interestingly we did not observe any change in the expression of pStat3 in these samples indicating the specificity of the staining. These data implicate a potential role for inhibition of NFκB activation as a mechanism for Nexrutine® induced biological effects in vivo.
Fig. 4.
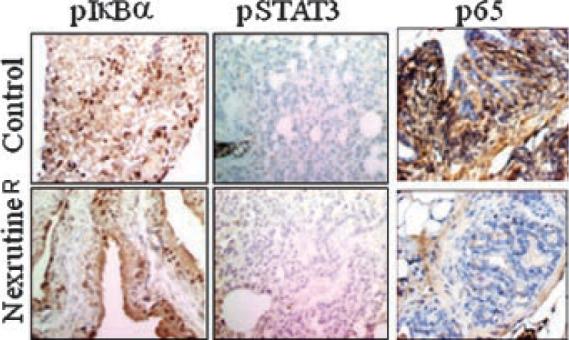
Dietary administration of Nexrutine® reduces expression of p65 and pIkBa but not pStat3 in the prostate fromTRAMP mice. IHC of representative tumors or prostate tissue from 28 -week-old control (C) or treated TRAMP miceis shownin figure. Paraffin-embedded tissues sections were stained overnight with rabbit polyclonal anti-human p65 (Abcam Inc., Cambridge, MA) mouse monoclonal anti pSTAT3 (Santa Cruz Biotechnology,, Santa Cruz, CA) and anti pIκBα (Cell SignalingTechnology, Inc., Beverly, MA) antibodies (1:200,1:200, and1:100, respectively). Immune complexes were revealed with abiotinylated link universal antiserum followed by horseradish peroxidase-streptavidin conjugate (LSAB+ kit) and color was developed using 3,3-diaminobenzidine hydrochloride as a chromogen. Finally, sections were rinsed in distilled water, counterstained with Mayer's hematoxylin, andmounted with DPX mounting medium for evaluation. Pictures were capturedwith a Photometric Cool SNAP CF color camera (Nikon,Lewisville,TX) and MetaMorph version 4.6.5 software (Universal Imaging, Down-ingtown, PA). Negative controls were included by omitting the primary antibody. [Color figure can be viewed in the online issue, whichis available at www.interscience.wiley.com.]
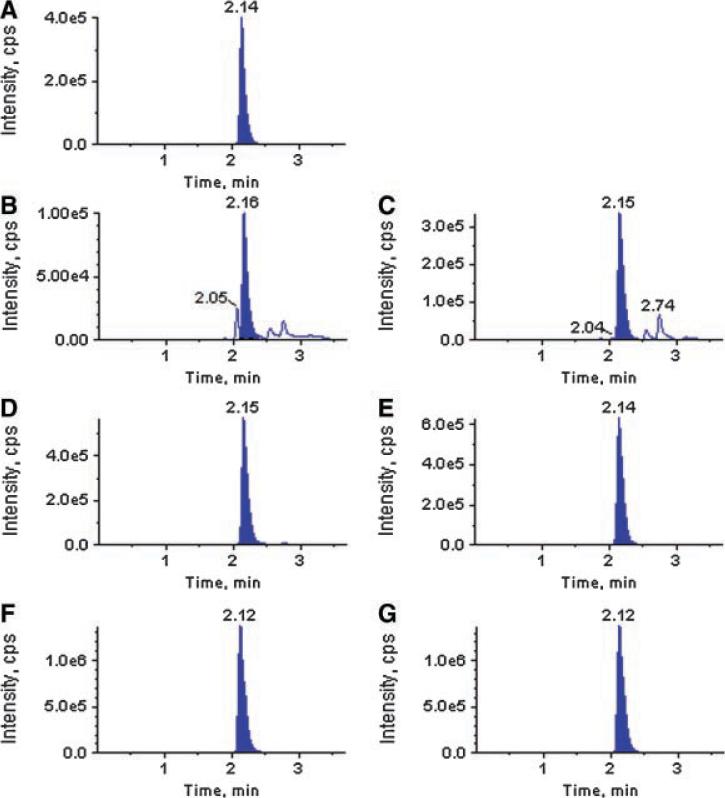
Data presented so far suggests that butanol fraction (F3) recapitulates Nexrutine® in inhibiting (i) proliferation; (ii) inducing apoptosis; (iii) NFκB promoter activity; (iv) NFκB DNA binding activity; and (v) protein expression of pAkt, p65, and pIκBα in PC-3 cells. However the biologically active component associated with F3 is not known. We performed ultraperformance liquid chromatographic (UPLC) analysis using two different methods. A representative UPLC profile is shown in Figure 5A. These data show the presence of several peaks in the extract. However fraction 3 showed a prominent peak with retention time of 1.6 min that was not seen in fractions 1 and 2. The presence of this dominant peak correlated with % proliferation inhibition. As shown in Figure 1 proliferation inhibition observed with F1 and F2 were about 10% whereas approximately 80% inhibition was observed with F3. According to Chinese literature Phellodendron extract contain different compounds including berberine and palmatine. We have used pure compounds as standards in our UPLC analysis to determine if the major peak appearing with retention time of 1.6 min could be one of these compounds. As shown in Figure 5A berberine and palmatine appeared with a major peak at 1.6 min. Since berberine and palmatine showed strong structural similarity, we could not separate them well using UPLC alone. So taking advantage of their molecular weight difference, we tried to use UPLC-MS/MS with multi reaction monitoring (MRM) method to identify these compounds in the fractions prepared from two different batches of Nexrutine® as explained before. MRM chromatograms shown in Figure 6, fractions 1, 2, and 3 showed a major peak eluting with a retention time of 2.1 min. Pure berberine eluted with the same retention.
Fig. 5.
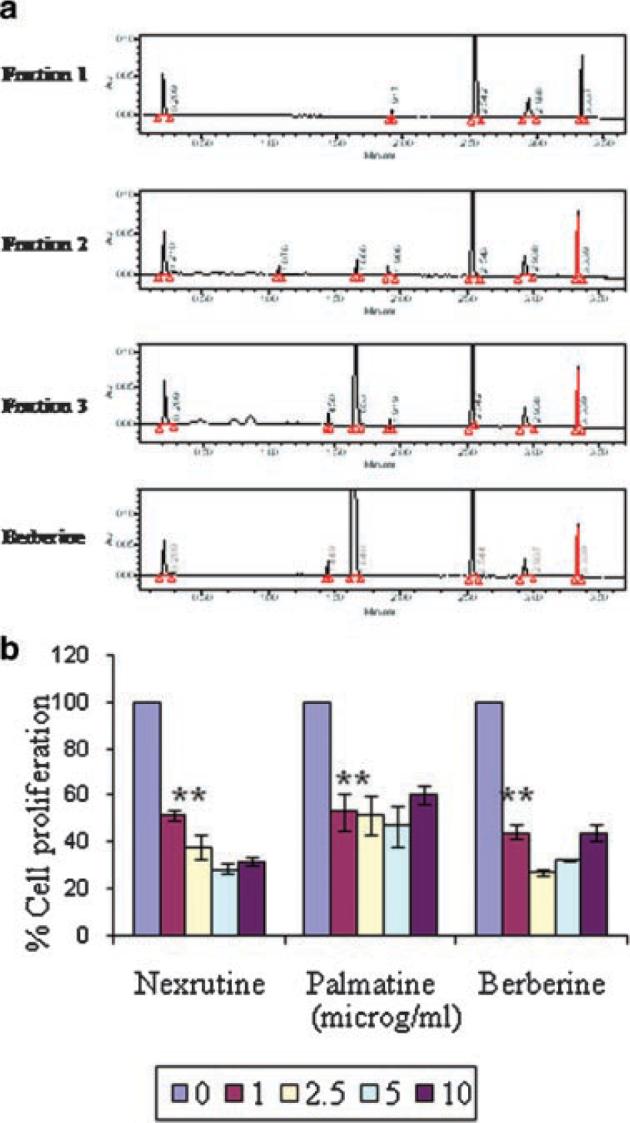
Ultraperformance liquid chromatographic (UPLC) analysis of fractions identified berberine or a related compound in the butanol fraction. A representative UPLC profile is shown in figure A. Inhibition of proliferation of PC-3 cells by Nexrutine®, butanol fraction and berberine is shown in figure B. PC-3 cells were plated in 96-well plates and treated with 2.5 μg/ml of fractions1, 2, 3, Nexrutine®, berberine, palmatine or solvent control. Cell proliferation was determined using cell Titer 96 Aqueous one solution assay at 72 hr. The data shown are average ± SD of three replicate wells and normalized to the proliferation obtained in the absence of compounds. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Fig. 6.
Identification of berberine or closely related compound in the butanol fraction. Fractions prepared from two different batches of Nexrutine® were used in analysis by UPLC-MS/MS (ABI 3200 QTrap). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
We compared the growth inhibitory activity of these compounds with that of the fractions using cell proliferation assay. Both berberine and palmatine inhibited the proliferation of PC-3 cells significantly (Fig. 5B and 1). Berberine is an isoquinoline alkaloid that exists in Hydrastis canadensis (golden seal), P. amurense, Coptis chinensis, Berberis vulgaris (barberry), Berberis aquifolium (Oregon grape), and Berberis aristata [30]. Berberine possesses wide spectrum of biochemical and pharmacological activities and has been used as a tonic remedy for liver and heart problems. For example berberine has been shown to induce apoptosis in HeLa, leukemia, colon, melanoma and lung cancer cells. Further berberine has recently been shown to inhibit proliferation of prostate cancer cells through induction of apoptosis involving deregulation of cell cycle checkpoint proteins [31,32]. It is well established that NFκB is constitutively activated in prostate cancer cell lines as well as in patients with prostate cancer [11-18]. Further inhibitors of NFκB have been shown to suppress various cellular processes including angiogenesis, invasion and metastasis in prostate cancer cells [11-18]. Results presented in this manuscript demonstrate butanol fraction containing berberine inhibits NFκB activity (both promoter and DNA binding) in PC-3 cells that was not reported before. Although Mantena et al. reported berberine induced inhibition of proliferation of prostate cancer cells through induction of apoptosis they did not investigated if berberine inhibits NFκB activity [31,32]. Additionally berberine has also been reported to suppress acetaldehyde induced NFκB activity by inhibiting IκBα phosphorylation and degradation [33]. Our results reporting the inhibition of NFκB signaling by F3 with retention time similar to berberine is consistent with the published findings. These studies implicate berberine or closely related compound as an active component associated with the observed growth inhibitory activities with Nexrutine®.
CONCLUSIONS
Chemoprevention by edible phytochemicals is now considered to be an inexpensive, readily applicable approach to cancer control and management. Healthcare cost is the key issue today. It would be costeffective to promote the awareness and consumption of phytochemicals as a cancer preventive strategy for the general public. Previously, we have documented that Nexrutine® treatment inhibits the proliferation of human PCA cells prevents prostate tumor development in TRAMP mice [5,6].
In this report we have demonstrated that butanol fraction (F3) of Nexrutine® has the same potential as that of Nexrutine® to induce apoptosis in PC-3 cells than the other two fractions (F1 and F2). In addition inhibition of proliferation of PC-3 cells by Nexrutine® is correlated with reduced levels of pAkt, p65, NFκB activation and pIκBα. However, additional experiments are needed to establish the precise molecular mechanism to delineate the cause and effect relationships between Akt and NFκB during Nexrutine® and berberine induced apoptosis in prostate cancer cells. It is noteworthy to mention that oral intervention with 0.5 g of berberine twice a day for 3 months significantly lowered the serum levels of cholesterol, triglycerides and LDL-cholesterol by 29%, 35%, and 25%, respectively [34]. Similar results have been obtained recently in patients with type 2 diabetes mellitus [35]. Although these studies did not measure the serum levels of berberine, since intervention with berberine resulted in biological effect we expect that inhibitory levels of berberine can be achieved in the serum. However much preclinical work needs to be done before concluding that berberine would be effective for prostate cancer.
ACKNOWLEDGMENTS
Supported in part by ACS RSG-04-169, CA 98744 (APK) and San Antonio Cancer Institute, Cancer Center Support Grant (P30 CA54174) is acknowledged. We thank Dr. Velagapudi Chakradhar for technical assistance with fractionation.
Grant sponsor: San Antonio Cancer Institute, Cancer Center Support; Grant numbers: P30 CA54174, ACS RSG-04-169, CA 98744.
REFERENCES
- 1.Albano JD, Ward E, Jemal A, Anderson R, Cokkinides VE, Murray T, Henley J, Liff J, Thun MJ. Cancer mortality in the United States by education level and race. J Natl Cancer Inst. 2007;18:1384–1394. doi: 10.1093/jnci/djm127. [DOI] [PubMed] [Google Scholar]
- 2.Crawford ED, Rosenblum M, Ziada AM, Lange PH. Hormone refractory prostate cancer. Urology. 1999;54:1–7. doi: 10.1016/s0090-4295(99)00447-1. [DOI] [PubMed] [Google Scholar]
- 3.Kurahashi N, Sasazuki S, Iwasaki M, Inoue M, Tsugane S, JPHC Study Group Green tea consumption and prostate cancer risk in Japanese men: A prospective study. Am J Epidemiol. 2008;1:71–77. doi: 10.1093/aje/kwm249. [DOI] [PubMed] [Google Scholar]
- 4.Kurahashi N, Iwasaki M, Sasazuki S, Otani T, Inoue M, Tsugane S, Japan Public Health Center-Based Prospective Study Group Soy product and isoflavone consumption in relation to prostate cancer in Japanese men. Cancer Epidemiol Biomarkers Prev. 2007;3:538–545. doi: 10.1158/1055-9965.EPI-06-0517. [DOI] [PubMed] [Google Scholar]
- 5.Garcia GE, Nicole A, Bhaskaran S, Gupta A, Kyprianou N, Kumar AP. Akt-and CREB-mediated prostate cancer cell proliferation inhibition by Nexrutine, a Phellodendron amurense extract. Neoplasia. 2006;6:523–533. doi: 10.1593/neo.05745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar AP, Bhaskaran S, Ganapathy M, Crosby K, Davis MD, Kochunov P, Schoolfield J, Yeh IT, Troyer DA, Ghosh R. Akt/cAMP-responsive element binding protein/cyclin D1 network: A novel target for prostate cancer inhibition in transgenic adenocarcinoma of mouse prostate model mediated by Nexrutine, a Phellodendron amurense bark extract. Clin Cancer Res. 2007;9:2784–2794. doi: 10.1158/1078-0432.CCR-06-2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suh J, Payvandi F, Edelstein LC, Amenta PS, Zong WX, Gélinas C, Rabson AB. Mechanisms of constitutive NF-kappaB activation in human prostate cancer cells. Prostate. 2002;3:183–200. doi: 10.1002/pros.10082. [DOI] [PubMed] [Google Scholar]
- 8.Mitsiades CS, Mitsiades N, Koutsilieris M. The Akt pathway: Molecular targets for anti-cancer drug development. Curr Cancer Drug Targets. 2004;3:235–256. doi: 10.2174/1568009043333032. [DOI] [PubMed] [Google Scholar]
- 9.Vessella RL, Corey E. Targeting factors involved in bone remodeling as treatment strategies in prostate cancer bone metastasis. Clin Cancer Res. 2006;12:6285–6290. doi: 10.1158/1078-0432.CCR-06-0813. [DOI] [PubMed] [Google Scholar]
- 10.Shukla S, Maclennan GT, Marengo SR, Resnick MI, Gupta S. Constitutive activation of P I3 K-Akt and NF-kappaB during prostate cancer progression in autochthonous transgenic mouse model. Prostate. 2005;3:224–239. doi: 10.1002/pros.20217. [DOI] [PubMed] [Google Scholar]
- 11.Sweeney C, Li L, Shanmugam R, Bhat-Nakshatri P, Jayaprakasan V, Baldridge LA, Gardner T, Smith M, Nakshatri H, Cheng L. Nuclear factor-kappaB is constitutively activated in prostate cancer in vitro and is overexpressed in prostatic intraepithelial neoplasia and adenocarcinoma of the prostate. Clin Cancer Res. 2004;16:5501–5507. doi: 10.1158/1078-0432.CCR-0571-03. [DOI] [PubMed] [Google Scholar]
- 12.Dutta J, Fan Y, Gupta N, Fan G, Gélinas C. Current insights into the regulation of programmed cell death by NF-kappaB. Oncogene. 2006;51:6800–6816. doi: 10.1038/sj.onc.1209938. [DOI] [PubMed] [Google Scholar]
- 13.Mukhopadhyay A, Bueso-Ramos C, Chatterjee D, Pantazis P, Aggarwal BB. Curcumin downregulates cell survival mechanisms in human prostate cancer cell lines. Oncogene. 2001;52:7597–7609. doi: 10.1038/sj.onc.1204997. [DOI] [PubMed] [Google Scholar]
- 14.Aggarwal BB, Ichikawa H. Molecular targets and anticancer potential of indole-3-carbinol and its derivatives. Cell Cycle. 2005;9:1201–1215. doi: 10.4161/cc.4.9.1993. [DOI] [PubMed] [Google Scholar]
- 15.Kikuchi E, Horiguchi Y, Nakashima J, Kuroda K, Oya M, Ohigashi T, Takahashi N, Shima Y, Umezawa K, Murai M. Suppression of hormone-refractory prostate cancer by a novel nuclear factor kappaB inhibitor in nude mice. Cancer Res. 2003;1:107–110. [PubMed] [Google Scholar]
- 16.Li Y, Sarkar FH. Inhibition of nuclear factor kappaB activation in PC3 cells by genistein is mediated via Akt signaling pathway. Clin Cancer Res. 2002;7:2369–2377. [PubMed] [Google Scholar]
- 17.Catz SD, Babior BM, Johnson JL. JFC1 is transcriptionally activated by nuclear factor-kappaB and up-regulated by tumour necrosis factor alpha in prostate carcinoma cells. Biochem J. 2002;367:791–799. doi: 10.1042/BJ20020345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Madrid LV, Wang CY, Guttridge DC, Schottelius AJ, Baldwin AS, Jr., Mayo MW. Akt suppresses apoptosis by stimulating the transactivation potential of the RelA/p65 subunit of NF-kappaB. Mol Cell Biol. 2000;5:1626–1638. doi: 10.1128/mcb.20.5.1626-1638.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar AP, Garcia GE, Slaga TJ. 2-Methoxyestradiol blocks cell-cycle progression at G(2)/M phase and inhibits growth of human prostate cancer cells. Mol Carcinog. 2001;3:111–124. doi: 10.1002/mc.1046. [DOI] [PubMed] [Google Scholar]
- 20.Kumar AP, Garcia GE, Ghosh R, Rajnarayanan RV, Alworth WL, Slaga TJ. 4-Hydroxy-3-methoxybenzoic acid methyl ester: A curcumin derivative targets Akt/NF kappa B cell survival signaling pathway: Potential for prostate cancer management. Neoplasia. 2003;3:255–266. doi: 10.1016/S1476-5586(03)80057-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia GE, Wisniewski HG, Lucia MS, Arevalo N, Slaga TJ, Kraft SL, Strange R, Kumar AP. 2-Methoxyestradiol inhibits prostate tumor development in transgenic adenocarcinoma of mouse prostate: Role of tumor necrosis factor-alpha-stimulated gene 6. Clin Cancer Res. 2006;12:980–988. doi: 10.1158/1078-0432.CCR-05-2068. [DOI] [PubMed] [Google Scholar]
- 22.Aggarwal BB, Shishodia S, Takada Y, Banerjee S, Newman RA, Bueso-Ramos CE, Price JE. Curcumin suppresses the paclitaxelinduced nuclear factor-kappaB pathway in breast cancer cells and inhibits lung metastasis of human breast cancer in nude mice. Clin Cancer Res. 2005;20:7490–7498. doi: 10.1158/1078-0432.CCR-05-1192. [DOI] [PubMed] [Google Scholar]
- 23.Marte BM, Downward J. PKB/Akt: Connecting phosphoinositide 3-kinase to cell survival and beyond. Trends Biochem Sci. 1997;9:355–358. doi: 10.1016/s0968-0004(97)01097-9. [DOI] [PubMed] [Google Scholar]
- 24.Kane LP, Shapiro VS, Stokoe D, Weiss A. Induction of NF-kappaB by the Akt/PKB kinase. Curr Biol. 1999;11:601–604. doi: 10.1016/s0960-9822(99)80265-6. [DOI] [PubMed] [Google Scholar]
- 25.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;2:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 26.Deeb D, Jiang H, Gao X, Hafner MS, Wong H, Divine G, Chapman RA, Dulchavsky SA, Gautam SC. Curcumin sensitizes prostate cancer cells to tumor necrosis factor-related apoptosisinducing ligand/Apo2L by inhibiting nuclear factor-kappaB through suppression of IkappaBalpha phosphorylation. Mol Cancer Ther. 2004;7:803–812. [PubMed] [Google Scholar]
- 27.Syed DN, Khan N, Afaq F, Mukhtar H. Chemoprevention of prostate cancer through dietary agents: Progress and promise. Cancer Epidemiol Biomarkers Prev. 2007;11:2193–2203. doi: 10.1158/1055-9965.EPI-06-0942. [DOI] [PubMed] [Google Scholar]
- 28.Deeb D, Jiang H, Gao X, Al-Holou S, Danyluk AL, Dulchavsky SA, Gautam SC. Curcumin [1,7-bis(4-hydroxy-3-methoxyphenyl)-1-6-heptadine-3,5-dione; C21H20O6] sensitizes human prostate cancer cells to tumor necrosis factor-related apoptosisinducing ligand/Apo2L-induced apoptosis by suppressing nuclear factor-kappaB via inhibition of the prosurvival Akt signaling pathway. J Pharmacol Exp Ther. 2007;2:616–625. doi: 10.1124/jpet.106.117721. [DOI] [PubMed] [Google Scholar]
- 29.Sarkar FH, Li Y. NF-kappaB: A potential target for cancer chemoprevention and therapy. Front Biosci. 2008;13:2950–2959. doi: 10.2741/2900. [DOI] [PubMed] [Google Scholar]
- 30.Ikram M. A review on the chemical and pharmacological aspects of genus Berberis. Planta Med. 1975;4:353–358. doi: 10.1055/s-0028-1097869. [DOI] [PubMed] [Google Scholar]
- 31.Meeran SM, Katiyar S, Katiyar SK. Berberine-induced apoptosis in human prostate cancer cells is initiated by reactive oxygen species generation. Toxicol Appl Pharmacol. 2008;1:33–43. doi: 10.1016/j.taap.2007.12.027. [DOI] [PubMed] [Google Scholar]
- 32.Mantena SK, Sharma SD, Katiyar SK. Berberine, a natural product, induces G1-phase cell cycle arrest and caspase-3-dependent apoptosis in human prostate carcinoma cells. Mol Cancer Ther. 2006;2:296–308. doi: 10.1158/1535-7163.MCT-05-0448. [DOI] [PubMed] [Google Scholar]
- 33.Hsiang CY, Wu SL, Cheng SE, Ho TY. Acetaldehydeinduced interleukin-1beta and tumor necrosis factor-alpha production is inhibited by berberine through nuclear factor-kappaB signaling pathway in HepG2 cells. J Biomed Sci. 2005;5:791–801. doi: 10.1007/s11373-005-9003-4. [DOI] [PubMed] [Google Scholar]
- 34.Kong W, Wei J, Abidi P, Lin M, Inaba S, Li C, Wang Y, Wang Z, Si S, Pan H, Wang S, Wu J, Wang Y, Li Z, Liu J, Jiang JD. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat Med. 2004;12:1344–1351. doi: 10.1038/nm1135. Epub 2004 Nov 7. [DOI] [PubMed] [Google Scholar]
- 35.Yin J, Xing H, Ye J. Efficacy of berberine in patients with type 2 diabetes mellitus. Metabolism. 2008;5:712–717. doi: 10.1016/j.metabol.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]