Abstract
Why do some exotic plant species become invasive? Two common hypotheses, increased resource availability and enemy release, may more effectively explain invasion if they favor the same species, and therefore act in concert. This would be expected if plant species adapted to high levels of available resources in their native range are particularly susceptible to enemies, and therefore benefit most from a paucity of enemies in their new range. We tested this possibility by examining how resource adaptations influence pathogen richness and release among 243 European plant species naturalized in the United States. Plant species adapted to higher resource availability hosted more pathogen species in their native range. Plants from mesic environments hosted more fungi than plants from xeric environments, and plants from nitrogen-rich environments hosted more viruses than plants from nitrogen-poor environments. Furthermore, plants classified as competitors hosted more than 4 times as many fungi and viruses as did stress tolerators. Patterns of enemy release mirrored those of pathogen richness: competitors and species from mesic and nitrogen-rich environments were released from many pathogen species, while stress tolerators and species from xeric and nitrogen-poor environments were released from relatively few pathogen species. These results suggest that enemy release contributes most to invasion by fast-growing species adapted to resource-rich environments. Consequently, enemy release and increases in resource availability may act synergistically to favor exotic over native species.
Keywords: enemy release, fluctuating resource hypothesis, global change, introduced plant species, resource-enemy release hypothesis
Understanding the mechanisms by which exotic plant species invade plant communities is key to limiting their impact on agricultural production and biological diversity (1). Unfortunately, despite the existence of many possible explanations for plant invasion, our ability to predict the cause of any particular invasion remains limited. One relatively well-studied explanation for invasion is an increase in the availability of plant resources (2, 3). Increases in resource availability are correlated with invasions at large scales and often increase invasion in experimental settings (4–7; see also ref. 8). Factors that decrease resource uptake (e.g., disturbances that remove plant biomass or reductions in plant diversity) increase resource availability and tend to increase plant invasion (9, 10). Common traits of invasive species, such as high fecundity, specific leaf area, photosynthesis, and growth, also suggest a positive relationship between resource availability and invasion (11–14; but see ref. 15).
However, despite considerable evidence that increases in resource availability are related to plant invasion, these explanations are insufficient to explain invasion. In particular, they do not explain the extraordinary success of some exotic plants. Resource availability would not be expected to differ consistently among geographic ranges, and both native and exotic species should be able to take advantage of high resource availability where it occurs. Nevertheless, exotic species can be exceptionally successful, relative to either the performance of similar species in the same community (16) or their own performance in their native range (17, 18). Such differences are thought to stem from biotic factors that do differ consistently between plants' native and introduced ranges, including herbivores, pathogens, mutualists, and competitors (19, 20).
The most prominent of these biogeographic hypotheses is the enemy-release hypothesis, which suggests that exotic species succeed because they escape important enemies upon moving to a new range (21, 22). Several reviews and meta-analyses have concluded that there is strong evidence of enemy release from intraspecific comparisons among ranges (23–25). Moreover, in a number of studies the degree of enemy release is related to invasiveness (25–27). Evidence for enemy release from interspecific comparisons among native and exotic congeners, however, has been inconsistent (23–25, 28). Perhaps more importantly, there is uncertainty about the importance of enemies, and specialist enemies in particular, in structuring plant communities (29–32).
Broad explanations for invasion, therefore, lead to a paradox. Resource availability has strong effects on plants but cannot explain the exceptional success of exotic species. Enemy release, and other biotic factors that differ predictably among ranges, could explain the exceptional success of exotic species, but it is not clear whether their effects on plants are strong enough to cause the dramatic invasions we often observe.
One possible solution to that paradox would be an interaction between resource availability and enemy release. Global studies of plant traits suggest that plants face a fundamental evolutionary trade-off between resource acquisition and resource conservation, one dimension of which is a trade-off between resource acquisition and defense (33–35). Fast-growing plant species adapted to high resource availability are thought to have few constitutive defenses against enemies (36–38), and therefore to incur relatively large costs when enemies are present, either because of tissue loss (39) or induced defenses (40). Consequently, these species stand to benefit if introduction to a new continent leads to the loss of those enemies (the resource-enemy release hypothesis, or R-ERH) (41, 42). If the same fast-growing species that benefit from high resource availability also benefit most from enemy release, then the two mechanisms may act in concert to cause invasion, which could explain both the strong effects of resource availability on invasion and the extraordinary success of some exotic species.
Resource availability has been found to influence enemy release at the level of individual species. For example, nitrogen addition to 10 taxonomically paired native and exotic species increased herbivore damage more on native than exotic species, and decreased the performance of native relative to exotic species (43). Similarly, both nitrogen fertilization and burning led to increased rust-fungus damage on native, but not exotic, Andropogon species in tall-grass prairie (44). These studies show that the plastic responses of individual species to high resource availability can influence enemy release. The R-ERH predicts that community responses to high resource availability—the replacement of slow-growing by fast-growing species—will further increase enemy release of exotic species and the susceptibility of native species to enemies (42).
We tested the R-ERH by examining the number of pathogen species hosted by 243 European plant species, in both their native European range and their introduced range in the United States (26), as a function of the plant species' resource adaptations. We used 2 independently derived but related measures of resource adaptation (45). Ellenberg indicator values categorize species by the resource availability in their primary habitats (46). C-S-R (competitive-stress tolerant-ruderal) strategy categorizes species by their adaptations to stress and disturbance, both of which are related to resource availability (47). We used data on rust, smut, and powdery mildew fungi (obligate pathogens of above-ground plant tissues), and on viruses (26). Our objective was to test whether fast-growing plant species adapted to high resource availability host more pathogens in Europe and are released from more pathogens in the United States than are slow-growing plant species adapted to low resource availability. Specifically, we predicted that pathogen richness in Europe would (i) increase with increasing Ellenberg values and (ii) be highest for competitors, intermediate for ruderals, and lowest for stress tolerators, as has been predicted with respect to herbivory (48, 49). We further predicted that plant species would lose pathogens in proportion to the number of pathogens in their native range (assuming pathogen loss to be a stochastic process) (50), and therefore that patterns of pathogen release would be similar to patterns of pathogen richness.
Results
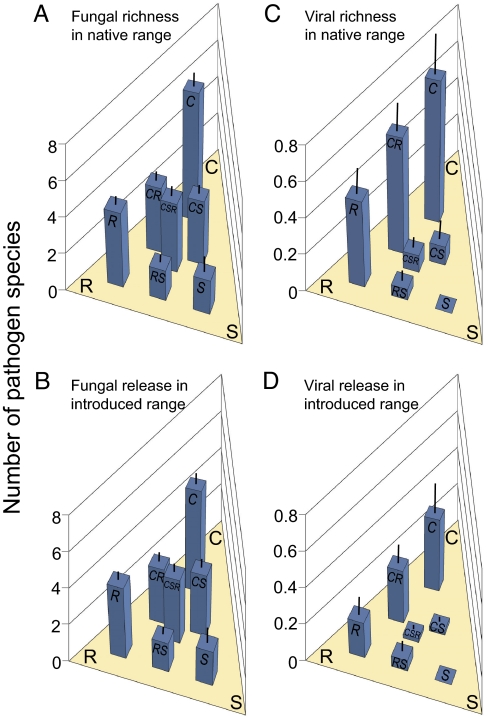
On average, the 243 plant species in the dataset hosted 4.2 (± 0.27 SE) fungal species in their native European range, and experienced a net release from 3.5 (± 0.23) fungal species in their exotic United States range. Based on the Akaike information criterion (AIC), the best model for explaining both fungal richness and fungal release contained C-S-R strategy (henceforth “CSR”), the Ellenberg indicator value for habitat water availability (henceforth “water”), and geographic range size (henceforth “range”) (Table 1). Effects of CSR and water on fungal richness were similar to their effects on fungal release. Stress-tolerant species both hosted and lost few fungi (Fig. 1 A and B). Stress-tolerant species are defined as slow-growing species adapted to “conditions of limited productivity” (47), and in Europe they tend to be associated with xeric habitats (45). In contrast, competitive species are defined as fast-growing species adapted to productive, infrequently disturbed environments (47), and they hosted and lost far more fungi than any other group. Ruderal species, defined as fast-growing species adapted to disturbed, productive environments (47), and species intermediate between competitors and other types, both hosted and lost an intermediate number of pathogens. Species of xeric habitats (water indicator values of 2–3) and wetland habitats (values of 9–10) also hosted and lost few fungi (Fig. 2).
Table 1.
Model selection statistics for the models that most effectively predicted pathogen richness and release
| Model description (by response variable)* | K | Log-likelihood | Q-AICc | Δ Q-AICc | Akaike weight† |
|---|---|---|---|---|---|
| European fungal richness | |||||
| CSR-Water-Range | 12 | −344.7 | 269.9 | 0 | 0.41 |
| CSR-N-Range | 12 | −345.3 | 270.3 | 0.3939 | 0.34 |
| CSR-Light-Water-N-Range | 16 | −335.7 | 272.5 | 2.650 | 0.11 |
| Range | 10 | −355.0 | 272.4 | 2.870 | 0.10 |
| CSR-Light-Range | 12 | −351.0 | 274.4 | 4.475 | 0.044 |
| Fungal release | |||||
| CSR-Water-Range | 12 | −308.4 | 263.4 | 0 | 0.41 |
| CSR-Light-Range | 12 | −310.2 | 264.8 | 1.371 | 0.21 |
| CSR-Range | 10 | −316.7 | 265.4 | 1.999 | 0.15 |
| CSR-N-Range | 12 | −312.0 | 266.1 | 2.730 | 0.10 |
| CSR-Light-Water-N-Range | 16 | −300.8 | 266.6 | 3.217 | 0.082 |
| European viral richness | |||||
| N-Range | 6 | −156.0 | 162.1 | 0 | 0.40 |
| CSR-N-Range | 12 | −143.2 | 162.8 | 0.7426 | 0.28 |
| CSR-Light-Range | 12 | −145.6 | 165.0 | 2.964 | 0.091 |
| CSR-Range | 10 | −150.4 | 165.3 | 3.224 | 0.080 |
| Light-Water-N-Range | 10 | −150.8 | 165.6 | 3.541 | 0.069 |
| Viral release | |||||
| CSR-Range | 10 | −76.7 | 133.7 | 0 | 0.26 |
| CSR-N-Range | 12 | −73.7 | 133.8 | 0.1203 | 0.25 |
| N-Range | 6 | −82.8 | 134.0 | 0.3718 | 0.22 |
| CSR-Water-Range | 12 | −75.6 | 136.4 | 2.759 | 0.066 |
| CSR-Light-Range | 12 | −75.7 | 136.6 | 2.947 | 0.060 |
| CSR-N | 10 | −79.1 | 137.1 | 3.432 | 0.047 |
*Sample sizes (number of plant species) were 243 for European fungal richness, 235 for fungal release, 242 for European viral richness, and 234 for viral release.
†Models are presented in order of decreasing Akaike weights, and only models with at least 10% of the Akaike weight of the best model are presented.
Fig. 1.
Pathogen richness per plant species in Europe and net decrease in pathogen richness per plant species in the United States (+/– 1 SE) as a function of CSR strategy: competitive (C), ruderal (R), stress tolerant (S), and intermediate. The axes from C/R to S describe increasing adaptation to stress, including slower growth. The axes from C/S to R represent increasing adaptation to disturbance (47). Sample sizes (number of species) for C, CR, CS, CSR, R, SR and S, respectively, were 57, 37, 42, 45, 39, 18, and 5 for fungal richness (A), 54, 34, 41, 45, 39, 17, and 5 for fungal release (B), 57, 37, 41, 45, 39, 18, and 5 for viral richness (C), and 53, 35, 41, 45, 37, 18, and 5 for viral release (D).
Fig. 2.
Fungal richness per plant species in Europe and net decrease in fungal richness per plant species in the United States (+/– 1 SE) as a function of Ellenberg indicator value for water, which categorizes species by the water availability in their primary habitat. Solid lines describe poisson regression models including only linear and quadratic effects of water. Sample sizes (number of species) for levels 2 to 10, respectively, were 11, 33, 70, 59, 24, 22, 16, 3, and 5 for richness (A), and 11, 33, 68, 58, 21, 21, 15, 3, 5 for release (B).
Other resources may also influence fungal richness and release, but were consistently less important than water (see Table 1). The Ellenberg indicator value for habitat nitrogen availability (henceforth “nitrogen”) was present in the second-best model for fungal richness, which received almost as much support as the best model (based on the ratios of Akaike weights among models), and the Ellenberg indicator value for habitat light availability (henceforth “light”) was present in the second-best model for fungal release (see Table 1). Based on the sum of their Akaike weights across the full set of models analyzed, water was 1.3 to 2.6 times more important than nitrogen, and 1.7 to 4.1 times more important than light in explaining fungal richness and release. The same comparison could not be made between Ellenberg values and CSR, as they were present in different numbers of models.
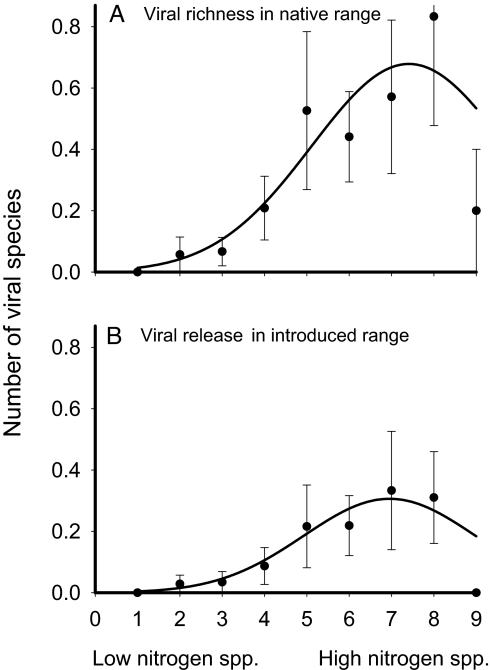
Plants hosted an average of 0.44 (± 0.1 SE) viruses in their native range and were released from 0.11 (± 0.046) viruses in their introduced range. The model that best explained viral richness contained nitrogen and range, while the model that best explained viral release contained CSR and range (see Table 1). In both cases, the second-best model contained both nitrogen and CSR in addition to range (see Table 1). Patterns of viral release mirrored patterns of richness, as in the analyses of fungi, despite the fact that plants were released from a much smaller proportion of viruses than of fungi. Stress-tolerant species and species with intermediate CSR strategies that included stress tolerance hosted and were released from few viruses relative to ruderals and, particularly, competitors (Fig. 1 C and D). Both viral richness and release increased with increasing habitat nitrogen availability (Fig. 3). The exception to this pattern was low viral richness and release at the highest level of nitrogen availability, comprised of 5 plant species (many fewer than other levels) adapted to heavily fertilized or polluted habitats. There was little evidence that resources other than nitrogen influenced viral richness and release. Across the full set of models, nitrogen was 4 to 6 times more important than water or light in explaining viral richness and release, and models including water or light received, at most, 25% as much support as the best models.
Fig. 3.
Viral richness per plant species in Europe and net decrease in viral richness per plant species in the United States (+/– 1 SE) as a function of Ellenberg indicator value for nitrogen, which categorizes species by the nitrogen availability in their primary habitat. Solid lines describe poisson regression models including only linear and quadratic effects of nitrogen. Sample sizes (number of species) for levels 1 to 9, respectively, were 11, 35, 30, 24, 38, 34, 35, 30, and 5 for richness (A), and 11, 35, 29, 23, 37, 32, 33, 29, and 5 for release (B).
Results were similar when we also controlled for variation in sampling effort (the intensity with which individual species have been studied). The identity and order of the best models were largely unchanged [supporting information (SI) Table S1]. The primary difference was that nitrogen replaced water in the best model of fungal richness, which also included range and CSR, and became 2.7 times as important as water across models. In addition, model-selection uncertainty increased in analyses of fungal and viral release, with 7 and 11 models, respectively, attaining 10% of the Akaike weight of the best models.
Discussion
Plant species classified as competitors, adapted to high nitrogen availability or adapted to moderate-to-high water availability hosted the most pathogen species in Europe and lost the most pathogen species upon being introduced to the United States. These patterns were unexpectedly consistent, given our relatively coarse measures of plant resource adaptation. They were also fairly robust: both CSR strategy and Ellenberg values remained in the best statistical models when potential confounding factors, geographic range size and sampling effort, were included. Together, the results suggest that plants face trade-offs between growth and defense with respect to pathogens. They also suggest that this trade-off may be important to plant invasion, demonstrating that plant resource adaptations can influence enemy release (the R-ERH).
To date, the growth-rate hypothesis of plant defense, which proposes that fast-growing plants from high resource habitats will be more susceptible to enemies (36), has been studied largely with respect to herbivores (42, 51). This study demonstrates that pathogens are also influenced by plant resource adaptations across a broad range of species. The most striking pattern we observed was that competitors hosted more than 4 times as many fungi and more than 7 times as many viruses as stress tolerators or stress-tolerant ruderals (see Fig. 1). Similarly, the one other study to examine the relationship between growth rate and pathogens found that fast-growing populations of radish, Raphanus sativus L., were most susceptible to Fusarium oxysporum (52). The patterns we observed also match previous predictions with respect to CSR strategy and herbivores: competitors should be most consumed, followed by ruderals, which should be less consumed because they are unapparent (difficult to locate in space or time), and then stress tolerators, which should be least consumed because they are well defended (48, 49). Previous tests of these predictions found that herbivores inhibited competitors and ruderals more than stress tolerators, but were not designed to examine lack of apparency (49, 53). Our observation of lower pathogen richness for ruderals than competitors supports the idea that apparency, as well as growth rate, influences attack by enemies.
The relationships between Ellenberg indicator values and pathogen richness were partially in accord with the growth-rate hypothesis. The number of fungi hosted increased between low and high water availability for upland plants (Ellenberg water indicator values 2–8), but was quite low for wetland plants (values 9–10) (see Fig. 2). Similarly, plant species adapted to moderately high-nitrogen environments hosted more viruses than those adapted to low-nitrogen environments, but plants of very heavily fertilized sites hosted few pathogens (see Fig. 3). Nitrogen may influence viruses because most known plant viruses are transmitted by herbivorous arthropods (54), which are commonly nitrogen-limited (55).
Resource adaptations also influenced pathogen release, as predicted by the R-ERH. Because most fungi and many viruses were lost when plants were introduced to the United States (26), plants that had more pathogens lost more pathogens. As with pathogen richness, the most striking patterns were observed with respect to CSR strategy. Fast-growing competitive plant species were released from the most fungi and viruses, while slow-growing stress-tolerant plant species were released from few fungi and very few viruses (see Fig. 1). With respect to ruderals, however, our results do not support the R-ERH, which would have predicted fast-growing ruderals to have high pathogen release. The fact that ruderals had intermediate pathogen release, together with the suggestion that ruderals should escape enemies because of a lack of apparency (49, 56), indicates that plant apparency, as well as resource adaptations, may be important to pathogen release. Fungal and viral release also increased with increasing Ellenberg values, as predicted by the R-ERH, but for different resources. As with pathogen richness, release of plants from fungi depended on plant affinity for water, while release of plants from viruses depended on plant affinity for nitrogen. In both cases, release was greater for plants from habitats with moderate-to-high resource availability than for plants from habitats with low resource availability.
To gauge the importance of these results to plant invasion, it is necessary to consider how patterns of pathogen release, as measured by decreases in pathogen richness, are likely to be related to patterns of enemy release, including population-level effects of both herbivores and pathogens. Differences in pathogen richness might overestimate the strength of resource-enemy release relationships if some pathogens have little effect on their hosts (57), if herbivores are less sensitive than pathogens to resource adaptations, or if herbivore release does not correlate with pathogen release (28). Conversely, if the plant traits hypothesized to increase pathogen richness—fast growth, low defense investment and high nutrient content—also increase susceptibility to other enemies, or effects of individual enemies (49, 58–60), pathogen richness might underestimate the strength of resource-enemy release relationships. For example, given that most viruses are transmitted by arthropod herbivores (54), the relatively high viral richness and release observed among competitors and species adapted to high-nitrogen environments could reflect relatively strong herbivory and herbivore release for competitors and species adapted to high-nitrogen environments. Determining how enemy release per se relates to resource adaptations will require community or biogeographic studies (23) that include multiple species, control resource strategy, and directly measure effects of enemies.
The question of whether enemy release contributes meaningfully to invasion remains controversial (23–25, 50). Our results suggest that the answer may be predicted by the type of exotic plant and the type of invaded environment. Enemy release may be strongest for fast-growing, apparent species (competitors) adapted to mesic, nitrogen-rich environments. Consequently, enemy release may be most likely to contribute to invasion in the resource-rich, periodically disturbed ecosystems that select for fast-growing, apparent species. Conversely, enemy release might be an unlikely explanation for invasion by slow-growing or unapparent species, or for invasion of resource-poor or frequently disturbed ecosystems. Such differential enemy release may help to explain why plants with rapid growth rates, and other traits associated with rapid resource acquisition, tend be most invasive (11–14; but see ref. 15), and why resource-rich environments tend to be relatively invasible (4–6, 61).
These results also indicate that increases in plant resource availability and enemy release may act in concert to cause plant invasion. Increases in resource availability may lead to invasion not just by providing colonization opportunities for fast-growing species (2), but also, in so doing, by selecting for species that are strongly released from enemies. Enemy release should, in turn, favor exotic fast-growing species over similar native species. To take nitrogen as an example, both competitors and species with high Ellenberg nitrogen values are particularly responsive to, and favored by, high nitrogen availability (49, 62–64). By favoring competitors and species with high Ellenberg nitrogen values, the same species we found to be most strongly released (see Figs. 1 and 3), increases in nitrogen availability may also indirectly increase enemy release. Such dual effects of available resources could explain both the extent of plant invasion, as large-scale increases in resource availability facilitate fast-growing species (7), and the biogeographic nature of plant invasion, as strong enemy release favors fast-growing exotic species over fast-growing native species.
Methods
We studied the growth-rate hypothesis of plant defense by analyzing the individual and combined effects of CSR strategy and Ellenberg indicator values for light, water, and nitrogen on pathogen richness (the number of pathogens hosted by a species in its native range). We then studied the R-ERH by analyzing effects of the same explanatory variables on pathogen release (the net loss of pathogen species between the native range and introduced range). Note that the R-ERH predicts that species adapted to high resource availability will lose more pathogens because they have more pathogens in their native range, not because they will lose a larger proportion of their pathogens. Consequently, we use absolute net pathogen loss to test the R-ERH. Because resources may have direct effects on fungal pathogens and on virus vectors, in addition to effects mediated by host plants, we conducted separate analyses for fungi and viruses. In all sets of analyses, we controlled for host geographic-range size, which is positively related to pathogen species richness (26, 50). We also conducted tests that included an estimate of sampling effort, to control for differences in the intensity with which individual species have been studied.
Data Collection.
We examined the 243 plant species from Mitchell and Power (26), for which we were able to obtain data on geographic range size, CSR strategy, and light, water, and nitrogen Ellenberg indicator values. In each analysis, the unit of replication was a plant species. We used data on pathogen species richness and release from Mitchell and Power (26). Briefly, pathogen richness values represent the summed number of rust, smut, and powdery mildew fungi, or the number of viruses, reported to occur on a plant species in either Europe and the Mediterranean or the United States. Native geographic-range size is the summed area of European floristic regions in which a plant species occurs (65). Ellenberg indicator values and CSR strategies were obtained from the BIOLFLOR database (66). Sampling effort was estimated separately for Europe and the United States by counting the number of citations of a species or its synonyms (U.S. Department of Agriculture Plants Database, http://plants.usda.gov/) for which the first author's address was in Europe or the United States, respectively, in the ISI Web of Science Database (between 1955 and 2002, up to the date when pathogen data were compiled; http://isiknowledge.com/) (67). The few species (8 species for analyses of both fungi and viruses) with negative release (i.e., more pathogens in the United States than in Europe) were omitted from analyses of release, because negative values are intractable in poisson regression. In addition, one outlier, Beta vulgaris (beet), was omitted from analyses of viruses because the large number of viruses it hosts (more than twice as many as any other species) is likely to be influenced by cultivation.
Data Analysis.
We used an information theoretic approach (68) to design sets of models and identify the models that best represented the data. Identical sets of models were analyzed for pathogen richness and release. Each set of analyses contained 19 models: a global model including light, water, nitrogen, CSR, and range, and 18 subsets of that global model. Because we were primarily interested in the effects of individual Ellenberg variables and CSR, we included models for 5 combinations of the Ellenberg variables: none, light, water, N, and light*water*N, with and without CSR (yielding 9 models). As we had no a priori expectation that the effects of Ellenberg variables and range would be linear, we included both linear and quadratic terms for these variables. To control for effects of range, we also included each of these models with range added (9 additional models), and range by itself. We then controlled for sampling effort by including it in all of the above models (an additional 4 sets of 19 models).
Because the data were counts, we analyzed each model using poisson regression, using a log-link function. We used x2 goodness-of-fit tests to check for over-dispersion in the global model for each response variable. Because over-dispersion was detected in each global model (P < 0.15), the small-sample quasi-likelihood information criterion (QAICc) was used (68). All models were fit using SAS/Insight 9.1. Information criteria were calculated by hand. Akaike weights were summed across all models in each set to quantitatively compare the relative importance of the light, water, and nitrogen Ellenberg indicator variables.
Supplementary Material
Acknowledgments.
We thank Z. Sixtová for technical assistance, C. Brooks and J. Regier for compilation and interpretation of preliminary data, and E. Hardy, L. Perry, and L. Ziska for comments on earlier drafts of this article. The pathogen data were compiled in collaboration with A.G. Power. This work was supported by the National Science Foundation Grant EF-0525641 (to C.E.M), and the Integrated Project ALARM Grant GOCE-CT-2003–506675 of the FP6 of the European Union, and Institutional Long-Term Research Plans AV0Z60050516 from the Academy of Sciences of the Czech Republic, and MSM0021620828, MSM0021622416, and Grant LC06073 from the Ministry of Education of the Czech Republic (to P.P. and V.J.). Our collaboration was facilitated by the National Science Foundation-funded Research Coordination Network on Integrating the Ecology and Evolution of Invasions (http://www.invasionsrcn.org/).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0812607106/DCSupplemental.
References
- 1.Levine JM, et al. Mechanisms underlying the impacts of exotic plant invasions. Proc R Soc Lond, Ser B: Biol Sci. 2003;270:775–781. doi: 10.1098/rspb.2003.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davis MA, Grime JP, Thompson K. Fluctuating resources in plant communities: a general theory of invasibility. J Ecol. 2000;88:528–534. [Google Scholar]
- 3.Shea K, Chesson P. Community ecology theory as a framework for biological invasions. Trends Ecol Evol. 2002;17:170–176. [Google Scholar]
- 4.Milchunas DG, Lauenroth WK. Inertia in plant community structure: state changes after cessation of nutrient-enrichment stress. Ecol Appl. 1995;5:452–458. [Google Scholar]
- 5.Daehler CC. Performance comparisons of co-occurring native and alien invasive plants: Implications for conservation and restoration. Annu Rev Ecol Evol Syst. 2003;34:183–211. [Google Scholar]
- 6.Davis MA, Pelsor M. Experimental support for a resource-based mechanistic model of invasibility. Ecol Lett. 2001;4:421–428. [Google Scholar]
- 7.Dukes JS, Mooney HA. Does global change increase the success of biological invaders? Trends Ecol Evol. 1999;14:135–139. doi: 10.1016/s0169-5347(98)01554-7. [DOI] [PubMed] [Google Scholar]
- 8.Maron JL, Marler M. Effects of native species diversity and resource additions on invader impact. Am Nat. 2008;172(Suppl):S18–S33. doi: 10.1086/588303. [DOI] [PubMed] [Google Scholar]
- 9.Hobbs RJ, Huenneke LF. Disturbance, diversity, and invasion: implications for conservation. Conserv Biol. 1992;6:324–337. [Google Scholar]
- 10.Levine JM. Species diversity and biological invasions: Relating local process to community pattern. Science. 2000;288:852–854. doi: 10.1126/science.288.5467.852. [DOI] [PubMed] [Google Scholar]
- 11.Pyšek P, Richardson DM. In: Biological Invasion, Ecological Studies. Nentwig W., editor. Vol. 193. Berlin & Heidelberg: Springer-Verlag; 2007. pp. 97–126. [Google Scholar]
- 12.Rejmánek M, Richardson DM. What attributes make some plant species more invasive? Ecology. 1996;77:1655–1661. [Google Scholar]
- 13.Grotkopp E, Rejmánek M, Rost TL. Toward a causal explanation of plant invasiveness: Seedling growth and life-history strategies of 29 pine (Pinus) species. Am Nat. 2002;159:396–419. doi: 10.1086/338995. [DOI] [PubMed] [Google Scholar]
- 14.Leishman MR, Haslehurst T, Ares A, Baruch Z. Leaf trait relationships of native and invasive plants: community- and global-scale comparisons. New Phytol. 2007;176:635–643. doi: 10.1111/j.1469-8137.2007.02189.x. [DOI] [PubMed] [Google Scholar]
- 15.Funk JL, Vitousek PM. Resource-use efficiency and plant invasion in low-resource systems. Nature. 2007;446:1079–1081. doi: 10.1038/nature05719. [DOI] [PubMed] [Google Scholar]
- 16.LeJeune KD, Seastedt TR. Centaurea species: the forb that won the west. Conserv Biol. 2001;15:1568–1574. [Google Scholar]
- 17.Jakobs G, Weber E, Edwards PJ. Introduced plants of the invasive Solidago gigantea (Asteraceae) are larger and grow denser than conspecifics in the native range. Divers Distrib. 2004;10:11–19. [Google Scholar]
- 18.Bossdorf O, et al. Phenotypic and genetic differentiation between native and introduced plant populations. Oecologia. 2005;144:1–11. doi: 10.1007/s00442-005-0070-z. [DOI] [PubMed] [Google Scholar]
- 19.Hierro JL, Maron JL, Callaway RM. A biogeographical approach to plant invasions: the importance of studying exotics in their introduced and native range. J Ecol. 2005;93:5–15. [Google Scholar]
- 20.Mitchell CE, et al. Biotic interactions and plant invasions. Ecol Lett. 2006;9:726–740. doi: 10.1111/j.1461-0248.2006.00908.x. [DOI] [PubMed] [Google Scholar]
- 21.Maron JL, Vila M. When do herbivores affect plant invasion? Evidence for the natural enemies and biotic resistance hypotheses. Oikos. 2001;95:361–373. [Google Scholar]
- 22.Keane RM, Crawley MJ. Exotic plant invasions and the enemy release hypothesis. Trends Ecol Evol. 2002;17:164–170. [Google Scholar]
- 23.Colautti RI, Ricciardi A, Grigorovich IA, MacIsaac HJ. Is invasion success explained by the enemy release hypothesis? Ecol Lett. 2004;7:721–733. [Google Scholar]
- 24.Liu H, Stiling P. Testing the enemy release hypothesis: a review and meta-analysis. Biol Invasions. 2006;8:1535–1545. [Google Scholar]
- 25.Hawkes CV. Are invaders moving targets? The generality and persistence of advantages in size, reproduction, and enemy release in invasive plant species with time since introduction. Am Nat. 2007;170:832–843. doi: 10.1086/522842. [DOI] [PubMed] [Google Scholar]
- 26.Mitchell CE, Power AG. Release of invasive plants from fungal and viral pathogens. Nature. 2003;421:625–627. doi: 10.1038/nature01317. [DOI] [PubMed] [Google Scholar]
- 27.Dewalt SJ, Denslow JS, Ickes K. Natural-enemy release facilitates habitat expansion of the invasive tropical shrub Clidemia hirta. Ecology. 2004;85:471–483. [Google Scholar]
- 28.Agrawal AA, et al. Enemy release? An experiment with congeneric plant pairs and diverse above- and belowground enemies. Ecology. 2005;86:2979–2989. [Google Scholar]
- 29.Crawley MJ. Insect herbivores and plant population dynamics. Annu Rev Entomol. 1989;34:531–564. [Google Scholar]
- 30.Maron JL, Crone E. Herbivory: effects on plant abundance, distribution and population growth. Proc R Soc Lond, Ser B: Biol Sci. 2006;273:2575–2584. doi: 10.1098/rspb.2006.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bigger DS, Marvier MA. How different would a world without herbivory be?: A search for generality in ecology. Integrative Biol. 1998;1:60–67. [Google Scholar]
- 32.Gilbert GS. Evolutionary ecology of plant diseases in natural ecosystems. Annu Rev Phytopathol. 2002;40:13–43. doi: 10.1146/annurev.phyto.40.021202.110417. [DOI] [PubMed] [Google Scholar]
- 33.Reich PB, Walters MB, Ellsworth DS. From tropics to tundra: Global convergence in plant functioning. Proc Natl Acad Sci USA. 1997;94:13730–13734. doi: 10.1073/pnas.94.25.13730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Diaz S, et al. The plant traits that drive ecosystems: Evidence from three continents. J Veg Sci. 2004;15:295–304. [Google Scholar]
- 35.Wright IJ, et al. The worldwide leaf economics spectrum. Nature. 2004;428:821–827. doi: 10.1038/nature02403. [DOI] [PubMed] [Google Scholar]
- 36.Coley PD, Bryant JP, Chapin FS. Resource availability and plant antiherbivore defense. Science. 1985;230:895–899. doi: 10.1126/science.230.4728.895. [DOI] [PubMed] [Google Scholar]
- 37.Fine PVA, Mesones I, Coley PD. Herbivores promote habitat specialization by trees in Amazonian forests. Science. 2004;305:663–665. doi: 10.1126/science.1098982. [DOI] [PubMed] [Google Scholar]
- 38.Janzen DH. Tropical blackwater rivers, animals, and mast fruiting by the Dipterocarpaceae. Biotropica. 1974:69–103. [Google Scholar]
- 39.Cebrian J, Duarte CM. The dependence of herbivory on growth-rate in natural plant-communities. Funct Ecol. 1994;8:518–525. [Google Scholar]
- 40.Van Zandt PA. Plant defense, growth, and habitat: A comparative assessment of constitutive and induced resistance. Ecology. 2007;88:1984–1993. doi: 10.1890/06-1329.1. [DOI] [PubMed] [Google Scholar]
- 41.Blumenthal D. Interrelated causes of plant invasion. Science. 2005;310:243–244. doi: 10.1126/science.1114851. [DOI] [PubMed] [Google Scholar]
- 42.Blumenthal DM. Interactions between resource availability and enemy release in plant invasion. Ecol Lett. 2006;9:887–895. doi: 10.1111/j.1461-0248.2006.00934.x. [DOI] [PubMed] [Google Scholar]
- 43.Leger EA, et al. The interaction between soil nutrients and leaf loss during early establishment in plant invasion. For Sci. 2007;53:701–709. [Google Scholar]
- 44.Han X, Dendy SP, Garrett KA, Fang L, Smith MD. Comparison of damage to native and exotic tallgrass prairie plants by natural enemies. Plant Ecol. 2008;198:197–210. [Google Scholar]
- 45.Franzaring J, Fangmeier A, Hunt R. On the consistencies between CSR plant strategies and Ellenberg ecological indicator values. J Appl Bot Food Qual-Angewandte Botanik. 2007;81:86–94. [Google Scholar]
- 46.Ellenberg H, et al. Zeigerwerte von Pflanzen in Mitteleuropa. Scripta Geobotanica. 1991;18:1–248. [Google Scholar]
- 47.Grime JP. Evidence for the existence of 3 primary strategies in plants and its relevance to ecological and evolutionary theory. Am Nat. 1977;111:1169–1194. [Google Scholar]
- 48.Coley PD. Interspecific variation in plant anti-herbivore properties—the role of habitat quality and rate of disturbance. New Phytol. 1987;106:251–263. [Google Scholar]
- 49.Fraser LH, Grime JP. Interacting effects of herbivory and fertility on a synthesized plant community. J Ecol. 1999;87:514–525. [Google Scholar]
- 50.Torchin ME, Mitchell CE. Parasites, pathogens, and invasions by plants and animals. Front Ecol Environ. 2004;2:183–190. [Google Scholar]
- 51.Stamp N. Out of the quagmire of plant defense hypotheses. Q Rev Biol. 2003;78:23–55. doi: 10.1086/367580. [DOI] [PubMed] [Google Scholar]
- 52.Hoffland E, et al. Relative growth rate correlates negatively with pathogen resistance in radish: The role of plant chemistry. Plant Cell Env. 1996;19:1281–1290. [Google Scholar]
- 53.Grime JP, Cornelissen JHC, Thompson K, Hodgson JG. Evidence of a causal connection between anti-herbivore defence and the decomposition rate of leaves. Oikos. 1996;77:489–494. [Google Scholar]
- 54.Power A, Flecker A. In: Ecology of Infectious Disease: Effects of Ecosystems on Disease and of Disease on Ecosystems. Ostfeld R, Keesing F, Eviner V, editors. Princeton: Princeton University Press; 2008. pp. 30–47. [Google Scholar]
- 55.Mattson WJ. Herbivory in relation to plant nitrogen content. Annu Rev Ecol Syst. 1980;11:119–161. [Google Scholar]
- 56.Feeny P. In: Biochemical Interactions Between Plants and Insects. Wallace J, Mansell RL, editors. New York, NY: Plenum Press; 1976. pp. 1–40. [Google Scholar]
- 57.Roy BA, Kirchner JW. Evolutionary dynamics of pathogen resistance and tolerance. Evolution. 2000;54:51–63. doi: 10.1111/j.0014-3820.2000.tb00007.x. [DOI] [PubMed] [Google Scholar]
- 58.Bryant JP, Kuropat PJ, Cooper SM, Frisby K, Owensmith N. Resource availability hypothesis of plant antiherbivore defense tested in a South African savanna ecosystem. Nature. 1989;340:227–229. [Google Scholar]
- 59.Pérez-Harguindeguy N, et al. Leaf traits and herbivore selection in the field and in cafeteria experiments. Austral Ecol. 2003;28:642–650. [Google Scholar]
- 60.Sheldon SP. The effects of herbivorous snails on submerged macrophyte communities in Minnesota lakes. Ecology. 1987;68:1920–1931. doi: 10.2307/1939883. [DOI] [PubMed] [Google Scholar]
- 61.Bobbink R, Hornung M, Roelofs JG. The effects of air-borne nitrogen pollutants on species diversity in natural and semi-natural European vegetation. J Ecol. 1998;86:717–738. [Google Scholar]
- 62.Fichner K, Schulze ED. The effect of nitrogen nutrition on growth and biomass partitioning of annual plants originating from habitats of different nitrogen availability. Oecologia. 1992;92:236–241. doi: 10.1007/BF00317370. [DOI] [PubMed] [Google Scholar]
- 63.Grime JP, et al. Integrated screening validates primary axes of specialisation in plants. Oikos. 1997;79:259–281. [Google Scholar]
- 64.Hill MO, Carey PD. Prediction of yield in the Rothamsted Park grass experiment by Ellenberg indicator values. J Veg Sci. 1997;8:579–586. [Google Scholar]
- 65.Tutin TG, et al. Flora Europaea, Vols 1–5. Cambridge: Cambridge Univ Press; 1964–1980. [Google Scholar]
- 66.Klotz S, Kühn I, Durka W. BIOLFLOR - Eine Datenbank mit biologisch-ökologischen Merkmalen zur Flora von Deutschland. Schriftenreihe für Vegetationskunde. 2002;38:1–334. [Google Scholar]
- 67.Lindenfors P, et al. Parasite species richness in carnivores: effects of host body mass, latitude, geographical range and population density. Global Ecol Biogeogr. 2007;16:496–509. [Google Scholar]
- 68.Burnham KP, Anderson DR. Model Selection and Multimodel Inference. 2nd edition. New York: Springer; 2002. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.