Abstract
Regulation of age-related changes in gene expression underlies many diseases. We previously discovered the first puberty-onset gene switch, the age-related stability element (ASE)/age-related increase element (AIE)-mediated genetic mechanism for age-related gene regulation. Here, we report that this mechanism underlies the mysterious puberty-onset amelioration of abnormal bleeding seen in hemophilia B Leyden. Transgenic mice robustly mimicking the Leyden phenotype were constructed. Analysis of these animals indicated that ASE plays a central role in the puberty-onset amelioration of the disease. Human factor IX expression in these animals was reproducibly nullified by hypophysectomy, but nearly fully restored by administration of growth hormone, being consistent with the observed sex-independent recovery of factor IX expression. Ets1 was identified as the specific liver nuclear protein binding only to the functional ASE, G/CAGGAAG, and not to other Ets consensus elements. This study demonstrates the clinical relevance of the first discovered puberty-onset gene switch, the ASE/AIE-mediated regulatory mechanism.
Keywords: factor IX, growth hormone, sex hormone, puberty, gene switch
Although the importance of aging on human health is well known, only recently has the first specific molecular mechanism for age-related gene regulation been discovered (1–3). This mechanism, the age-related stability element (ASE)/age-related increase element (AIE)-mediated genetic mechanism for age-related gene regulation, was originally discovered through extensive studies of transgenic mice carrying minigenes of human blood coagulation factor IX (hFIX) (1) and antiblood coagulation factor protein C (hPC) (2). In this mechanism, 2 genetic elements, ASE and AIE, play critical roles in generating patterns of stable puberty-onset gene expression and age-related increases in gene expression, respectively. The ASEs, GAGGAAG and CAGGAAG identified in the hFIX and hPC genes, respectively, contain an Ets consensus sequence, GGAA/T, and are present in the 5′ upstream region of regulated genes. AIE is present in the 3′ UTR and potentially functions as a RNA stem-loop forming structure after being transcribed. The clinical relevance of this mechanism was previously unknown.
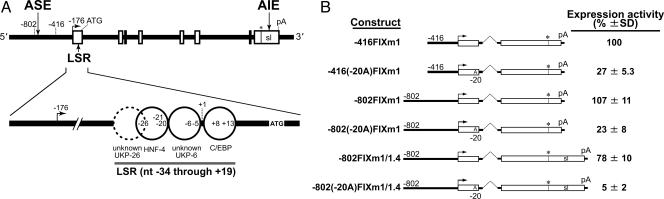
Hemophilia B is a male-dominant human bleeding disorder caused by reduced or absent levels of circulating hFIX (4). Expression of the FIX gene in humans normally rapidly rises during the perinatal stage of life (5), reaches a near young adult level at around the age of weaning, and gradually increases throughout adulthood (6, 7). A similar age-related pattern is seen in mice (8, 9). Hemophilia B Leyden is a unique subset of hemophilia B in which abnormal bleeding is present soon after birth but spontaneously ameliorates beginning at puberty. This amelioration corresponds to a puberty-onset natural recovery of hFIX expression, leading to a clinically normal state by around the third to fourth decade (10). To date, 81 hemophilia B Leyden families with at least 14 unique single base mutations or deletions in their FIX genes have been identified world-wide (11). As shown in Fig. 1A, all of these mutations are clustered in an approximately 50 base pair (bp) region, nucleotide (nt) -34 through nt +19 (see ref. 12 for nt numbering), known as the Leyden-specific region (LSR) in the 5′ UTR (13, 14). The wild-type gene LSR is known to bind 3 nuclear proteins, HNF-4 (15), an unidentified protein binding to the region spanning the nt -6 site (hereafter called UKP-6) (13) and C/EBP (16). Single base mutations disrupting binding of HNF-4 or C/EBP to the LSR result in severe prepubertal hemophilia B Leyden, while disruption of UKP-6 binding results in milder prepubertal hemophilia B Leyden. The second unknown protein (hereafter called UKP-26), shown by the dotted circle in Fig. 1A, can bind to a region partially overlapped with the HNF-4 binding region only when HNF-4 binding is abrogated by specific mutations, including a T→A mutation at nt -20 (T-20A) (14). Disruption of the binding of both UKP-26 and HNF-4 due to a mutation G-26C (known as the Brandenberg mutation) results in severe hemophilia B with no expression of hFIX either before or after puberty (17, 18).
Fig. 1.
The hFIX gene and its minigene constructs with transient expression activities. (A) A schematic drawing of the hFIX gene and LSR. The hFIX gene is shown at the top with its 5′ end at left. Exons are shown by rectangles. Relative positions of ASE in the 5′ upstream, AIE in the 3′ UTR, and LSR in the 5′ UTR are shown. Right-angled arrow, asterisk with vertical thin line, and pA indicate the transcription start site, translation stop site, and polyadenylation site, respectively. The symbol sl (potential stem loop forming structure) in the last exon rectangle represents AIE. Solid line circles represent proteins binding to the wild-type LSR sequence while the dotted circle indicates the binding of UKP-26. Locations of representative mutations are shown with nucleotide (nt) numbering (12). (B) Human FIX minigene constructs and transient expression activities in vitro. Human FIX minigenes containing a representative Leyden phenotype mutation T-20A and their normal counterparts relevant to the present study are shown. These constructs extend 5′ end to nt -802 or nt -416, and have the middle portion of the 3′ UTR deleted (m1) or not deleted (m1/1.4). Transient expression activity levels relative to that of -416FIXm1 (approximately 50 ng/106 HepG2 cells/48 h) are shown at the right with SDs (averages of 5 independent assays).
The mechanism underlying the puberty-onset amelioration seen in hemophilia B Leyden has remained controversial. Some groups proposed that binding of androgen receptor (17) and other transcription factors were responsible (19). All of those studies were performed in vitro, with the implicit assumption that the puberty-related male sex hormone surge is directly involved. In contrast, our earlier works in vitro did not support a direct role for either testosterone or androgen receptors (13, 14). One transgenic mouse model study was previously reported for a hemophilia B Leyden mutation, A + 13G (20). That study, however, was seriously limited by examination of only one test transgenic animal with the Leyden mutation and the use of the 5′ end sequence of the hFIX gene only up to nucleotide (nt) -189, thus lacking critical regulatory elements later shown to be necessary for basal promoter activity (1).
In the present study, we report successful constructions of transgenic mice robustly recapitulating the phenotype of hemophilia B Leyden and Brandenberg mutation, and demonstrate that ASE of the ASE/AIE-mediated genetic mechanism plays an essential role in puberty-onset amelioration of hemophilia B Leyden. We also show that ASE specifically binds Ets1 and that growth hormone is directly responsible for puberty-onset recovery of FIX production. These findings have helped establish the long elusive molecular mechanisms underlying hemophilia B Leyden, and show for the first time the critical role of the ASE/AIE-mediated genetic mechanism in clinical disease.
Results and Discussion
Transgenic Mouse Models Recapitulating Hemophilia B Leyden.
Hemophilia B patients carrying a representative Leyden mutation T-20A show no detectable serum hFIX before puberty, consequently manifesting severe abnormal bleeding (21). Through and after the puberty period, their abnormal bleeding gradually ameliorates. This mutation disrupts HNF-4 binding to a LSR subsite nt -27 through nt -17 (14) (Fig. 1A). In Fig. 1B, newly constructed hFIX minigenes -416(-20A)FIXm1, -802(-20A)FIXm1, and -802(-20A)FIXm1/1.4, all containing the T-20A mutation, are shown along with their wild-type counterparts. Minigenes -416(-20A)FIXm1 and -802(-20A)FIXm1 lacked or contained an ASE, respectively. The functional differences between their wild-type counterparts, -416FIXm1 and -802FIXm1, which show age-related unstable and stable expressions, respectively, were previously scrutinized in relation to their structures and demonstrated to be solely due to the absence and presence of an ASE in the 5′ upstream region (1). Both -416(-20A)FIXm1 and -802(-20A)-FIXm1 had a shortened 3′ UTR sequence with no AIE, while -802(-20A)FIXm1/1.4 contained the full wild-type 3′ UTR sequence of 1.4 kilobase (kb) pairs with AIE in the middle. Expression activities of these minigene constructs were first tested in vitro in HepG2 cells by assaying secreted hFIX protein using hFIX-specific enzyme linked immunosorbent assay (ELISA). Minigenes -416(-20A)FIXm1 and -802(-20A)FIXm1 showed transient expression activities 27% and 23%, respectively, of that of the wild-type reference minigene -416FIXm1. Minigene -802(-20A)FIXm1/1.4 gave even less activity than -802(-20A)FIXm1, confirming the unique and moderate in vitro inhibitory effect of AIE shown in previous observations with the wild-type minigene -802FIXm1/1.4 containing AIE (1).
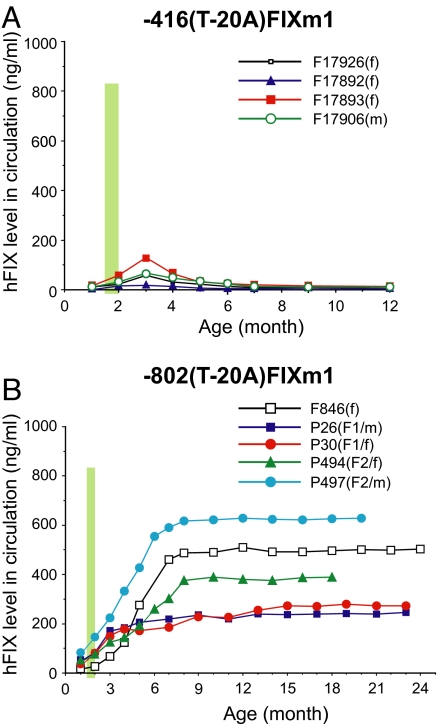
As shown in Fig. 2A, both male and female transgenic animals carrying minigene -416(-20A)FIXm1 failed to recapitulate the Leyden phenotype. Serum hFIX levels assayed by ELISA among founder animals were at nondetectable background levels at 1 month of age (before puberty) and increased through puberty, peaking at around 3 months of age. Subsequently, however, hFIX expression rapidly declined and returned to background levels by 5–6 months of age, resulting in only transient puberty-onset expression of hFIX and an ultimate failure to recapitulate the Leyden phenotype. This result contradicts the mechanistic models previously proposed (17, 18) since, according to those models, -416(-20A)FIXm1 should contain all genetic components required for recapitulation of puberty-onset recovery of hFIX expression.
Fig. 2.
Age-related expression profiles of hFIX minigenes with a hemophilia B Leyden mutation in transgenic mice. (A) Age-related hFIX expression profiles of -416(-20A)FIXm1 in transgenic mice. Serum hFIX concentrations (vertical axis) are plotted against age (horizontal axis). Animals are identified as previously defined by the format [(F or P for founder or progeny, respectively)(identification number)(progeny generation)/(sex)] (1). For founder animals, no progeny generation number is given. Vertical thick green lines represent the relative puberty period. Age 0 represents the birth time point. (B) Age-related hFIX expression profiles of -802(-20A)FIXm1 in transgenic mice. All definitions are as described in A.
Similar to animals with -416(-20A)FIXm1, serum hFIX levels of animals with -802(-20A)FIXm1 were at background levels before puberty but followed by puberty-onset steady increases (Fig. 2B). The hFIX levels in these animals continued to rise, in contrast to the animals carrying -416(-20A)FIXm1, and stabilized by around 6 months of age at levels as high as approximately 650 ng/ml. Thus, all animals with -802(-20A)FIXm1 fully reproduced the puberty-onset recovery and subsequent age-associated maintenance of hFIX expression clinically observed in the human Leyden phenotype. These age-related hFIX expression patterns were observed in both male and female transgenic animals. Variations in plateaued maximum hFIX expression level observed among animals are likely transgene positional effects. The dramatic differences observed between animals carrying -416(-20A)FIXm1 and -802(-20A)FIXm1 correlated with the absence and presence of ASE (GAGGAAG) in these minigenes, respectively, demonstrating the critical role of ASE in puberty-onset amelioration of the Leyden phenotype. These findings are consistent with the previous observations obtained from wild-type hFIX minigenes, -416FIXm1 harboring no ASE and -802FIXm1 harboring a unit of ASE, showing a puberty-onset loss of hFIX expression and an age-related stable hFIX expression, respectively (1, 3). In this study, -802(-20A)-FIXm1/1.4 was not used in animals since, as previously shown with -416FIXm1/1.4 (1), AIE failed to produce an age-stable expression pattern, a critical condition required for amelioration of the Leyden phenotype.
The fact that both male and female transgenic mice with -802(-20A)FIXm1 showed similar age-related hFIX gene expression patterns clearly suggested that puberty-onset amelioration is not specifically dependent on the male sex hormone surge during puberty. This agreed with our previous observations in vitro that there is neither a direct role of testosterone (dihydrotestosterone, DHT) in inducing hFIX expression from Leyden phenotype minigenes nor any appreciable binding of androgen receptor to the LSR (13, 14). These observations are again in contrast with the mechanisms proposed by others for the hemophilia B Leyden phenotype (17, 18).
We also successfully constructed transgenic mouse models carrying -802(+13G) FIXm1 [see supporting information (SI) Fig. S1], a hFIX minigene with a second hemophilia B Leyden mutation A + 13G (16). These animals, 2 males and 1 female, exhibited the typical puberty-onset recovery of hFIX expression as seen in human patients with the mutation (Fig. S1).
Transgenic mice with -802(-26C)FIXm1, a minigene -802FIXm1 containing G-26C mutation (the Brandenburg mutation) (Fig. 1A), were also constructed. In contrast to hemophilia B Leyden patients, human patients with this mutation do not express hFIX before, during, or after puberty. Similarly, animals with -802(-26C)FIXm1 failed to show any detectable hFIX expression in all male and female animals generated (n = 12 founder lines) at least up to 8 months of age (data not shown).
We now have successfully constructed a series of transgenic mouse models for hemophilia B Leyden and a non-Leyden Brandenburg mutation, robustly recapitulating their human phenotypes. These studies demonstrated the clinical relevance of the ASE/AIE-mediated regulatory mechanism in human disease.
Identification of Ets1 as the ASE Binding Protein.
As shown by EMSAs in Fig. 3A, binding of nuclear proteins from mouse liver nuclear protein extracts (NEs) to the ASE greatly increased from a very low level at 1 month (prepubertal) through puberty, and plateaued at maximal levels by 6–12 months of age. This age-related pattern of nuclear protein binding to the ASE approximately paralleled the puberty-onset increase in hFIX expression in animals with the Leyden mutant minigene -802(-20A)FIXm1 (Fig. 2B). Oligonucleotide probes with core heptamer sequences of the functional ASEs, G/CAGGAAG, bind an identical mouse liver nuclear protein (1, 2). Similar to the ASEs, but subtly different heptamer sequences, GAGGAAA and GAGGATG, which do not function as ASEs (2) (see Table S1), also bound liver nuclear proteins (Table S2). Competitive EMSAs, however, showed that these proteins are different from those binding to the ASEs. As summarized in Table S2, further systematic EMSAs of all other combinations of the core heptamer sequence with mouse liver NEs showed no appreciable protein binding. When HepG2 cell NEs were used, besides those that bind liver nuclear proteins, 3 additional probes harboring GACGAAG, GAGCAAG, or GAGGAAC were found to bind proteins at appreciable levels (Table S2). Competitive EMSAs with these probes again showed that those proteins are different from that binding to the ASE sequences. Together, these results indicated that the nuclear protein binding to the functional ASEs (G/CAGGAAG) is unique, specific, and different from those binding to any other pseudo-ASE oligonucleotides.
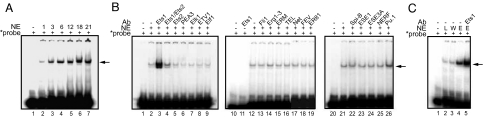
Fig. 3.
Identification of the ASE binding nuclear protein. (A) EMSAs with liver NEs prepared from mice at various ages. All lanes contain the 32P-labeled ASE probe (approximately 40,000 cpm). Lane 1, without NEs; lanes 2–7, with NEs (80 μg) prepared from the liver tissues of 1-, 3-, 6-, 12-, 18-, and 21-month-old animals, respectively. Arrow: shifted band position. (B) Supershift EMSAs with antibodies specific to various Ets family proteins. All lanes contain the 32P-labeled ASE probe (approximately 20,000 cpm). Lanes 1, 10, and 20, with no NEs; lanes 2–9, 12–19, and 21–26 with liver NEs (40 μg) prepared from 6-month-old mice. Lane 11, anti-Ets1 antibody with the 32P-labeled probe with no NEs. Lanes 3–9, 13–19, and 22–26 are supershift EMSAs with various antibodies to specific proteins labeled at the top of lanes. Lanes without antibody are noted with (-) as shown at top. Arrow: shifted band position. (C) EMSAs and supershift EMSA with NEs prepared from 293T cells overexpressing mEts1. 32P-labeled ASE probe and procedures are as described above differing only in NEs used. Lane 1, without NEs; lane 2, with 10 μg NEs from 6-month-old mouse liver; lane 3, with 10 μg NEs from wild-type 293T cells; lane 4, with 10 μg NEs from 293T cells overexpressing mEts1; lane 5, same as lane 4 with anti-Ets1 antibody. Arrow: shifted band position.
To identify the nuclear protein specifically binding to the functional ASEs, we then carried out supershift EMSA analyses with an oligonucleotide probe with a GAGGAAG sequence and mouse liver NEs in combination with an array of antibodies specific to various known Ets family proteins (22), including Ets1, Ets1/Ets2, Ets2, PEA3, Elk1, ETV1, Elf1, NERF, PU.1, ERM, FEV, ER8l, Fli1, Erg1–3, TEL, Spi-B, ESE1, ESE3A, and Net. Among these antibodies, only antibodies to Ets1 reproducibly gave a response, a dramatic increase in the shifted band intensity with no obvious shift in the band position (Fig. 3B, lane 3). The observed response does uniquely differ from a typical supershift pattern, which shows a further retardation in band migration without no significant increase in shifted band intensity (14). Anti-Ets1/Ets2 antibody, which recognizes both Ets1 and Ets2, gave a weaker, but still significant enhancement of the shifted band intensity (Fig. 3B, lane 4). The antibody to Pu.1, a transcription factor with a high degree of similarity to Ets1 in DNA binding characteristics (23), also showed a similar, less dramatic supershift pattern than that of Ets1 (Fig. 3B, lane 26). No other Ets family protein antibodies, including those specific to Ets2, gave any significant response.
The specific binding of Ets1 to the ASE was then tested by 2 different experimental methods. In one approach, 293T cells overexpressing murine Ets1 (mEts1) were constructed by transfecting a mEts1 expression vector. NEs prepared from these Ets1 overexpressing cells or from nontransformed control 293T cells were used in EMSA and supershift EMSA with ASE probe (Fig. 3C). In comparison to NEs from nontransfected control cells (Fig. 3C, lane 3), NEs of the Ets1 overexpressing cells gave a significant increase in the shifted band intensity (Fig. 3C, lane 4), consistent with a significant increase in ASE:Ets1 complex formation due to Ets1 overexpression. With anti-Ets1 antibody then added to EMSA (supershift EMSA), the shifted band intensity was further dramatically increased without any appreciable shifting in the band position (Fig. 3C, lane 5). These results confirmed the findings obtained from EMSAs with the liver NEs, supporting that the specific ASE binding protein is Ets1. Atypical supershift EMSA patterns reproducibly observed for the ASE probe:Ets1:anti-Ets1 antibody complex may be due to the unique behavior of Ets1, known to undergo substantial conformational changes under various conditions including phosphorylation (24). The underlying precise mechanism remains to be investigated.
The second approach for verification of the specific binding of Ets1 to ASE took a combination of procedures, including preparative EMSAs with ASE and non-ASE probes, 2D gel electrophoresis (2DE) of the proteins contained in the shifted band, and finally Western blot analysis of the 2DE gel with anti-Ets1 antibodies (Fig. S2). Protein extracts prepared from dried gel pieces of preparative EMSAs containing ASE probe:nuclear protein complexes were subjected to matched parallel 2DE analyses. This generated a pair of closely similar 2DE gels for each experimental condition. One gel was stained with SYPRO Ruby dye for visualizing protein spots (Fig. S2 Left), while the other gel was subjected to Western blot analysis with anti-Ets1 antibody (Fig. S2 Right). As shown in Fig. S2 (Top) anti-Ets1 antibody visualized positive protein spots horizontally lined up along the pH axis with a main spot of apparent size of 53 kDa and pI at around 5.5 (solid arrow). This reasonably matched with values predicted for Ets1 (50.4 kDa and pI 5.03) based on amino acid composition. Anti-Ets1 antibody reproducibly detected specific protein spots in the final Western blot analysis only when the ASE probe was used in the initial preparative EMSAs (Fig. S2 Top). No such spots were observed when a non-ASE oligonucleotide probe (GAGGAAA) or no probe oligonucleotide was used, further confirming Ets1 binding to the ASE (Fig. S2 Middle and Bottom respectively). MALDI-TOF/MS analyses of anti-Ets1-detected protein spots in the Western blot of 2DE further confirmed Ets1 binding to the ASE (data not shown). Thus, 3 different lines of experiments established that Ets1 is the nuclear protein binding to the ASE.
Ets1 is a transcriptional factor previously known for its proto-oncogenic properties (25), enhancing invasive behavior of cancers by regulating MMPs, uPA, VEGF, and VEGF receptor genes, among others (26). In addition, the present work now assigns Ets1 a novel role in physiologic regulation of age-related gene expression. Since ASE has a unique role in tissue-specific regulation (27), Ets1 is a unique multifunctional transcription factor.
Determination of the Essential Role of Growth Hormone for Amelioration of Hemophilia B Leyden.
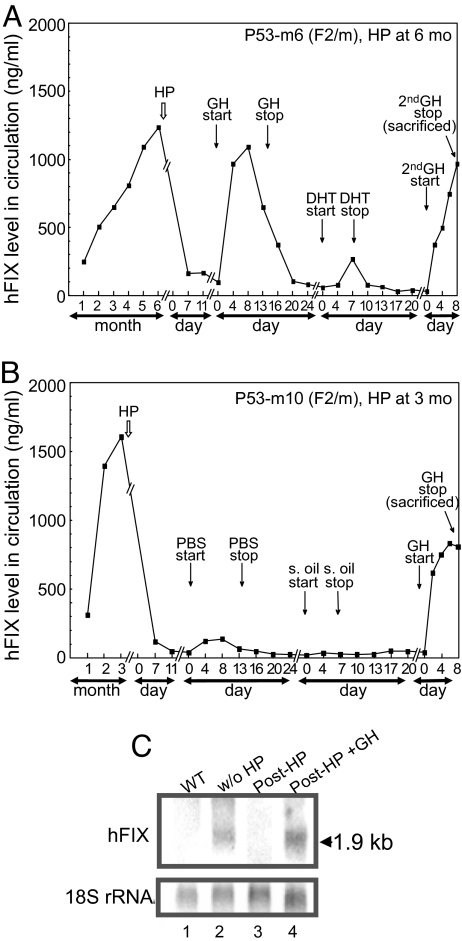
We next explored the physiological mechanism underlying the sex-independent, puberty-onset spontaneous amelioration of the Leyden phenotype. Previously, Georgatsou et al. (28) elegantly showed that sex-limited liver protein C4-Slp is controlled directly by GH produced by the pituitary and only indirectly by male hormone. We, therefore, hypothesized that the growth hormone (GH) surge in both males and females during puberty, a physiological effect in part influenced by sex-steroid surges, plays a direct role in the amelioration mechanism. To test this hypothesis, hypophysectomy (HP), an established surgical procedure for removing the pituitary organ (29), was performed on transgenic mice carrying -802(-20A)FIXm1. Successful HP was confirmed by loss of major urinary protein (MUP) (30). As expected, following HP, levels of MUP in hypophysectomized animals sharply dropped to nondetectable levels, followed by a recovery upon GH administration (see Fig. S3A, lane 4 and 6, respectively). Similarly, HP treatments were performed to the Leyden phenotype transgenic animals carrying -802(-20A)FIXm1 (total n = 18; 8 males and 10 females). Upon hypophysectomy at 6 months of age, both male and female animals, P53-m6(F2/m and P53-f6(F2/f), respectively (representatives of n = 11; 5 males and 6 females), showed a rapid decrease in hFIX expression to background levels (Fig. 4A and Fig. S3B, respectively). Subsequent intraperitoneal administrations of GH (20 μg every 12 h) for a duration up to 13 days fully restored hFIX expression with a peak at around day 4–8, followed by rapid lowering to background levels upon termination of GH administration (Fig. 4A and Fig. S3B, respectively). In contrast, subsequent administration of a course of DHT in male animals or estrogen (17β-estradiol: E2) in female animals resulted in much lower levels of hFIX induction (less than 10% the prehypophysectomy level) (Fig. 4A and Fig. S3B, respectively). Subsequent second course of GH administration to these animals again robustly restored the circulating hFIX level to nearly 80% of prehypophysectomy levels (Fig. 4A and Fig. S3B, respectively). When male and female control animals, P53-m10(F2/m) and P53-f12(F2/f), received PBS without GH and sesame oil without DHT or E2, respectively, hFIX expression stayed at the background levels (Fig. 4B and Fig. S3C, respectively). However, GH administration after completing courses of PBS and sesame oil administration again induced hFIX production to approximately 80% the prehypophysectomy level, reproducing the observations with the last GH administration obtained with animals P53-m6(F2/m) and P53-f6(F2/f) subjected to a full course of DHT or E2 administration, respectively (Fig. 4B and Fig. S3C). These protein level changes agreed with a total loss of hFIX mRNA in similarly treated hFIX-transgenic animals killed 30 days posthypophysectomy and its reappearance after GH administration posthypophysectomy up to 8 days (Fig. 4C).
Fig. 4.
Effects of HP and hormone treatments on hFIX expression in transgenic mice carrying a hemophilia B Leyden phenotype minigene, -802(-20A) FIXm1. (A) Effects of HP and a course of administration of GH and DHT, followed by the second course of GH administration, on hFIX expression in a male mouse P53-m6(F2/m) carrying -802(-20A)FIXm1. HP was done at 6 months of age. Vertical arrows show time points for HP, initiation and termination of GH, DHT, and the 2nd GH administration. Vertical axis: serum hFIX concentration level; horizontal axis: age and duration of various treatments in month or day. (B) Effects of HP and subsequent administrations of PBS, sesame oil, and GH, on hFIX expression in a male animal P53-m10(F2/m) carrying -802(-20A) FIXm1. The rest are the same as in (A). (C) Northern blot analyses of mouse liver hFIX mRNA preparations. Total liver RNA samples (15 μg per lane) were used for analyses. Lane 1, wild-type animal; lane 2–4, Leyden phenotype animals carrying -802(-20A)FIXm1. Lane 2, animal P127(F1/f) without HP; lane 3, animal P126(F1/f) after HP; lane 4, P108(F1/f) animal sacrificed immediately after the course of GH injection at eighth day. (Upper) Filter probed with the SspI/BamHI fragment of hFIX cDNA; (Lower) filter re-hybridized with RNR18 probe (1). A horizontal arrow on the right indicates the hFIX mRNA band position with size information.
Although less dramatic than these observations with adult animals, similar sex-independent GH inductions of hFIX expression were reproducibly observed in animals hypophysectomized before puberty at 1 month of age (n = 3, 2 males and 1 female) (see Fig. S4 A–C). After hypophysectomy before the completion of puberty, these animals neither increased their body weights nor recovered hFIX expression. While DHT administration to animals, P139(F2/m) and P140(F2/m), gave only very low inductions in hFIX expression, much larger inductions were obtained by GH administration. These results indicated that even if some GH may be produced by organs other than the pituitary through puberty, such extrapituitary GH does not play a significant role in inducing hFIX expression in the liver. Signal transduction pathways linking GH signaling to expression of Ets1 and FIX have yet to be determined.
These results indicate a critical role of GH signal transduction in regulating hFIX gene expression in a sex-independent manner, and are consistent with the puberty-onset and sex-independent induction of hFIX expression observed in animals carrying -802(-20A)FIXm1 (Fig. 2B). Pituitary GH production is a downstream event of both pubertal male or female sex hormone surges. Therefore, hFIX inductions observed with DHT and E2 administrations are presumably due to this pathway (Fig. 4A and Fig. S3B). Further study is required to determine the mechanisms underlying low inductions obtained with DHT as well as E2.
Recently, Wong et al. (31) reported that substantial sex-related differences in thrombosis in mice are mediated by sex-specific pulsatile or continuous secretion pattern of growth hormone. Similar to their observations, our animal study with GH given in a pulsatile manner showed no obvious sex differences in induction of hFIX expression from -802(-20A)FIXm1, while continuous GH infusion still remains to be tested. Our hemophilia B Leyden animal model may be used for scrutinizing the relationship among different manners of GH administration, gene expression, thrombosis, and age.
Significance of Regulatory Mechanisms of Age Related Homeostasis in Human Diseases.
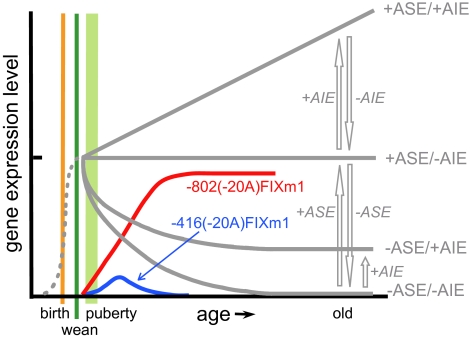
We now have established the long elusive molecular mechanisms underlying the puberty-onset spontaneous amelioration of hemophilia B Leyden (Fig. 5). At puberty, the ASE/AIE-mediated mechanism begins to replace the LSR-related regulatory mechanism, which is essential for FIX gene expression from the perinatal stage through weaning and to the stage just before puberty. Importantly, there exists a reciprocal relationship between the puberty-onset up- and down-regulations in hFIX expression obtained with -802(-20A)FIXm1 (red curved line) and with -416FIXm1 (1) (gray curved line depicted by -ASE/-AIE), respectively (Fig. 5). These concurrent up and down regulatory patterns appear to complement each other, thus generating puberty-onset age-stable expression pattern as depicted by +ASE/-AIE. This pattern corresponds to that of -802FIXm1 (1). As shown in Fig. 3A, the age-related increase in Ets1 binding to ASE approximately parallels the age-related up-regulation of hFIX expression from -802(-20A)FIXm1 and further supports the pivotal role of ASE in the puberty-onset recovery in hFIX gene expression, a hallmark of amelioration of hemophilia B Leyden. These findings also agree with the observations that animals carrying -416(-20A)FIXm1 only end up with a premature termination of the puberty-onset recovery in hFIX expression (Fig. 2A; blue line curve in Fig. 5). This termination is due to a strong age-related down-regulation seen with -416FIXm1 (depicted with -ASE/-AIE in Fig. 5) (1).
Fig. 5.
Molecular mechanisms underlying the puberty-onset amelioration of hemophilia B Leyden in relation to the ASE/AIE-mediated regulatory mechanism. Vertical and horizontal axes are for relative gene expression levels and age, respectively. Relative positions along the age axis for birth, weaning, and puberty are shown with vertical thin orange, green, and a fat light green lines, respectively. A thin gray dotted line represents hFIX expression from the perinatal stage through 1 month of age. Open gray arrows indicate directions of up or down changes in hFIX expression in the presence (+) or absence (−) of ASE and/or AIE. Four age-related patterns of hFIX expression generated by the ASE/AIE-mediated genetic regulation are shown by solid gray lines depicted with specific combinations of ASE and AIE shown with + or − on the right. Age-related patterns of hFIX expression from minigenes -802(-20A)FIXm1 and -416(-20A)FIXm1 carrying a representative Leyden mutation (T-20A) are shown by red and blue curved lines, respectively.
This is the first demonstration of the clinical relevance of an age-associated genetic regulatory mechanism in human disease.
Materials and Methods
See SI Text for the details of experimental procedures.
Construction of hFIX Minigenes with Leyden Mutations.
Human FIX minigene expression vectors with a representative hemophilia B Leyden mutation T-20A were created by site-directed mutagenesis (1, 32).
Construction of Transgenic Mice and Longitudinal in Vivo Assays for hFIX Expression.
Transgenic mice were constructed according to the standard methods (33) at the Bio-medical Research Animal Model Core facility at the University of Michigan as previously described (1, 2). Serum hFIX concentrations of mice were determined by duplicated hFIX-specific ELISA, and average values were plotted. Duplicated ELISA values varied less than 11% from averages. All animal experiments were carried out in accordance with the institutional guidelines of the University of Michigan and National Institute of Advanced Industrial Science and Technology.
Electrophoretic Mobility Shift Assay (EMSA).
EMSAs and supershift EMSAs were performed as previously described with minor modifications (14). Supershift EMSAs were performed with antibodies to various Ets family proteins (Santa Cruz Biotechnology) according to the manufacturer's instructions. For competitive EMSA, probes without 32P-lableing were used (2). Preparative EMSAs were carried out with larger amounts of liver NEs (160 μg aliquots) with 120,000 cpm of 32P-labeled oligonucleotide probe using 18 × 18 cm electrophoresis glass plates.
Preparation of NEs of 293T Cells Overexpressing Murine Ets1.
Murine Ets1 expression vector pCMV-SPORT6 and 293T cell line MGC-18571 were purchased from ATCC, and cell transfection was performed using FuGENE 6 (Roche). After two days of posttransfection incubation, cells were harvested and used for preparing NEs according to Dignam et al. (34).
Hypophysectomy of Mice.
Hypophysectomy procedures were performed according to the methods of Davey et al. with some modifications (29). Successful hypophysectomy was confirmed by electrophoretic analysis of urine samples for major urinary protein as previously described (30).
Hormone Treatments of Hypophysectomized Mice.
Hypophysectomized transgenic mice were i.p. given with murine GH (20 μg/injection) at 12 h intervals (29, 30) for 13 or 14 days before stopping. This was then followed by intra-peritoneal administration of DHT (200 μg/injection) or E2 (500 μg/injection) for male or female animals, respectively (35).
Supplementary Material
Acknowledgments.
We thank Dr. A. F. Pavlow at the National Hormone and Pituitary Program, Harbor- University of California, Los Angeles Medical Center, for providing us murine GH. We thank E. Kasama, T. Tanaka, J.B. Hoff for their technical helps. This work was supported by research funds from the National Institutes of Advanced Industrial Science and Technology and National Institutes of Health Grants HL64522 and HL38644 (to K.K.). J.S.H. was supported by a Howard Hughes Medical Institute Medical Student Research Training Fellowship and a Cellular and Molecular Biology Training Grant (National Institutes of Health T32-GM07315).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0902191106/DCSupplemental.
References
- 1.Kurachi S, Deyashiki Y, Takeshita J, Kurachi K. Genetic mechanisms of age regulation of human blood coagulation factor IX. Science. 1999;285:739–743. doi: 10.1126/science.285.5428.739. [DOI] [PubMed] [Google Scholar]
- 2.Zhang K, Kurachi S, Kurachi K. Genetic mechanisms of age regulation of protein C and blood coagulation. J Biol Chem. 2002;277:4532–4540. doi: 10.1074/jbc.M109524200. [DOI] [PubMed] [Google Scholar]
- 3.Kurachi K, Kurachi S. Molecular mechanisms of age-related regulation of genes. J Thromb Haemost. 2005;3:909–914. doi: 10.1111/j.1538-7836.2005.01123.x. [DOI] [PubMed] [Google Scholar]
- 4.Bolton-Maggs PH, Pasi KJ. Haemophilias A and B. Lancet. 2003;361:1801–1809. doi: 10.1016/S0140-6736(03)13405-8. [DOI] [PubMed] [Google Scholar]
- 5.Terwiel JP, Veltkamp JJ, Bertina RM, van der Linden IK, van Tilburg NH. Coagulation factors in the premature infant born after about 32 weeks of gestation. Biol Neonate. 1985;47:9–18. doi: 10.1159/000242085. [DOI] [PubMed] [Google Scholar]
- 6.Sweeney JD, Hoernig LA. Age-dependent effect on the level of factor IX. Am J Clin Pathol. 1993;99:687–688. doi: 10.1093/ajcp/99.6.687. [DOI] [PubMed] [Google Scholar]
- 7.Lowe GD, et al. Epidemiology of coagulation factors, inhibitors and activation markers: The Third Glasgow MONICA Survey. I. Illustrative reference ranges by age, sex and hormone use. Br J Haematol. 1997;97:775–784. doi: 10.1046/j.1365-2141.1997.1222936.x. [DOI] [PubMed] [Google Scholar]
- 8.Yao SN, DeSilva AH, Kurachi S, Samuelson LC, Kurachi K. Characterization of a mouse factor IX cDNA and developmental regulation of the factor IX gene expression in liver. Thromb Haemostasis. 1991;65:52–58. [PubMed] [Google Scholar]
- 9.Kurachi S, Hitomi E, Kurachi K. Age and sex dependent regulation of the factor IX gene in mice. Thromb Haemostasis. 1996;76:965–969. [PubMed] [Google Scholar]
- 10.Briet E, Bertina RM, van Tilburg NH, Veltkamp JJ. Hemophilia B Leyden: A sex-linked hereditary disorder that improves after puberty. N Engl J Med. 1982;306:788–790. doi: 10.1056/NEJM198204013061306. [DOI] [PubMed] [Google Scholar]
- 11.Giannelli F, et al. Haemophilia B: Database of point mutations and short additions and deletions–fourth edition. Nucleic Acids Res. 1993;21:3075–3087. doi: 10.1093/nar/21.13.3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshitake S, Schach BG, Foster DC, Davie EW, Kurachi K. Nucleotide sequence of the gene for human factor IX (antihemophilic factor B) Biochemistry. 1985;24:3736–3750. doi: 10.1021/bi00335a049. [DOI] [PubMed] [Google Scholar]
- 13.Hirosawa S, et al. Structural and functional basis of the developmental regulation of human coagulation factor IX gene: Factor IX Leyden. Proc Natl Acad Sci USA. 1990;87:4421–4425. doi: 10.1073/pnas.87.12.4421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurachi S, et al. Regulatory mechanism of human factor IX gene: Protein binding at the Leyden-specific region. Biochemistry. 1994;33:1580–1591. doi: 10.1021/bi00172a039. [DOI] [PubMed] [Google Scholar]
- 15.Reijnen MJ, Sladek FM, Bertina RM, Reitsma PH. Disruption of a binding site for hepatocyte nuclear factor 4 results in hemophilia B Leyden. Proc Natl Acad Sci USA. 1992;89:6300–6303. doi: 10.1073/pnas.89.14.6300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crossley M, Brownlee GG. Disruption of a C/EBP binding site in the factor IX promoter is associated with haemophilia B. Nature. 1990;345:444–446. doi: 10.1038/345444a0. [DOI] [PubMed] [Google Scholar]
- 17.Crossley M, et al. Recovery from hemophilia B Leyden: An androgen-responsive element in the factor IX promoter. Science. 1992;257:377–379. doi: 10.1126/science.1631558. [DOI] [PubMed] [Google Scholar]
- 18.Heit JA, et al. Haemophilia B Brandenberg-type promoter mutation. Haemophilia. 1999;5:73–75. doi: 10.1046/j.1365-2516.1999.00193.x. [DOI] [PubMed] [Google Scholar]
- 19.Picketts DJ, Lillicrap DP, Mueller CR. Synergy between transcription factors DBP and C/EBP compensates for a haemophilia B Leyden factor IX mutation. Nat Genet. 1993;3:175–179. doi: 10.1038/ng0293-175. [DOI] [PubMed] [Google Scholar]
- 20.Boland EJ, et al. Age-specific regulation of clotting factor IX gene expression in normal and transgenic mice. Blood. 1995;86:2198–2205. [PubMed] [Google Scholar]
- 21.Reitsma PH, Bertina RM, Ploos van Amstel JK, Riemens A, Briet E. The putative factor IX gene promoter in hemophilia B Leyden. Blood. 1988;72:1074–1076. [PubMed] [Google Scholar]
- 22.Sementchenko VI, Watson DK. Ets target genes: Past, present and future. Oncogene. 2000;19:6533–6548. doi: 10.1038/sj.onc.1204034. [DOI] [PubMed] [Google Scholar]
- 23.Graves BJ, Gillespie ME, McIntosh LP. DNA binding by the ETS domain. Nature. 1996;384:322. doi: 10.1038/384322a0. [DOI] [PubMed] [Google Scholar]
- 24.Pufall MA, et al. Variable control of Ets-1 DNA binding by multiple phosphates in an unstructured region. Science. 2005;309:142–145. doi: 10.1126/science.1111915. [DOI] [PubMed] [Google Scholar]
- 25.Wasylyk B, Hahn SL, Giovane A. The Ets family of transcription factors. Eur J Biochem. 1993;211:7–18. doi: 10.1007/978-3-642-78757-7_2. [DOI] [PubMed] [Google Scholar]
- 26.Dittmer J. The biology of the Ets1 proto-oncogene. Mol Cancer. 2003;2:29. doi: 10.1186/1476-4598-2-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang K, Kurachi S, Kurachi K. New function for age-related stability element in conferring strict tissue-specific expression of human factor IX and protein C genes. Thromb Haemostasis. 2002;88:537–538. [PubMed] [Google Scholar]
- 28.Georgatsou E, Bourgarel P, Meo T. Male-specific expression of mouse sex-limited protein requires growth hormone, not testosterone. Proc Natl Acad Sci USA. 1993;90:3626–3630. doi: 10.1073/pnas.90.8.3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davey HW, Park SH, Grattan DR, McLachlan MJ, Waxman DJ. STAT5b-deficient mice are growth hormone pulse-resistant. Role of STAT5b in sex-specific liver p450 expression. J Biol Chem. 1999;274:35331–35336. doi: 10.1074/jbc.274.50.35331. [DOI] [PubMed] [Google Scholar]
- 30.Norstedt G, Palmiter R. Secretory rhythm of growth hormone regulates sexual differentiation of mouse liver. Cell. 1984;36:805–812. doi: 10.1016/0092-8674(84)90030-8. [DOI] [PubMed] [Google Scholar]
- 31.Wong JH, et al. Sex differences in thrombosis in mice are mediated by sex-specific growth hormone secretion patterns. J Clin Invest. 2008;118:2969–2978. doi: 10.1172/JCI34957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kurachi S, Pantazatos DP, Kurachi K. The carboxyl-terminal region of factor IX is essential for its secretion. Biochemistry. 1997;36:4337–4344. doi: 10.1021/bi962002v. [DOI] [PubMed] [Google Scholar]
- 33.Hogan B, Constantini F, Lacy E. A Laboratory Manual. New York: Cold Spring Harbor Press; 1994. Manipulating the Mouse Embryo. [Google Scholar]
- 34.Dignam JD, Lebovitz RM, Roeder RG. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983;11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Svechnikov K, Ritzen EM, Holst M. Androgen and estrogen stimulation of ornithine decarboxylase activity in mouse kidney. J Steroid Biochem Mol Biol. 2000;75:329–333. doi: 10.1016/s0960-0760(00)00184-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.