Abstract
We have hypothesized that in the prenegative selection TCR repertoire, many somatically generated complementary-determining region (CDR) 3 loops combine with evolutionarily selected germline Vα/Vβ CDR1/CDR2 loops to create highly MHC/peptide cross-reactive T cells that are subsequently deleted by negative selection. Here, we present a mutational analysis of the Vβ CDR3 of such a cross-reactive T-cell receptor (TCR), YAe62. Most YAe62 TCRs with the mutant CDR3s became less MHC promiscuous. However, others with CDR3s unrelated in sequence to the original recognized even more MHC alleles than the original TCR. Most importantly, this recognition was still dependent on the conserved CDR1/CDR2 residues. These results bolster the idea that germline TCR V elements are inherently reactive to MHC but that this reactivity is fine-tuned by the somatically generated CDR3 loops.
Keywords: major histocompatibility complex, specificity, T cell
The more than 3 dozen solved structures of T-cell receptor (TCR)/MHC complexes have shown that most TCRs bind to their MHC/peptide ligands, regardless of the MHC allele or class, in a similar diagonal mode, although there is some variation in the angle and pitch of engagement (1). We (2–5) and others (6, 7) have used this structural information and mutational analyses to tackle the question of whether TCRs have been evolutionarily selected to bind to MHC molecules. We focused on the TCR complementary-determining region (CDR) 1 and CDR2 loops, because, unlike the somatically generated CDR3 loops, CDR1s and CDR2s are fully encoded in the germline, and therefore susceptible to evolutionary selection. Also, in many TCR/MHC structures, these loops, especially CDR2s, are often the main source of contact with MHC (1, 8, 9).
For MHCII, the best-documented example of this conserved interaction involves Vβ elements related to mouse Vβ8. Analysis of 9 structures with multiple MHC alleles, different bound peptides, different Vαs, and different Vβ CDR3s has found that in the Vβ8-like Vβs, 46Y, 48Y, and 54E (all in βCDR2) nearly always interact with the same conserved amino acids (α1 39K, 57Q, 60L, 61Q, and 64A) on MHCII (3). The data suggest a similar situation for some Vαs (2, 3). For example, in a number of structures involving Vα elements related to mouse Vα4, Y29 in the Vα CDR1 loop interacts with a conserved site composed of MHCII β 76D, 77T, and 81H (3). As more TCR/MHC structures appear, it is likely that additional conserved interactions will be found (3).
In a series of functional and mutational studies, we examined T cells that develop in “single peptide” mice (i.e., mice whose MHCII is occupied by a single covalently attached peptide) (2, 4, 10–12). These mice contain many T cells that are highly peptide and MHC allele cross-reactive. We explained this by proposing that TCR V regions are biased to react with MHC; thus, the thymus produces a very high frequency of thymocytes bearing TCRs in which conserved V region CDR1/CDR2 interactions with MHCII are very strong, leading to highly degenerate MHC and peptide reactivity. These cells are destroyed by negative selection in normal mice, leaving only T cells in whose TCRs the somatically generated TCR CDR3 loops have subdued the conserved CDR1/CDR2 TCR/MHC interactions sufficiently to allow escape from negative selection. These very cross-reactive thymocytes are not completely negatively selected in single-peptide mice. Thus, in such animals, many peripheral T cells bear these very MHC/peptide cross-reactive TCRs. This idea was supported by analyses of the structures of 2 of these promiscuous TCRs bound to an MHCII/peptide complex (2). In these structures, the conserved Vα and Vβ CDR1/CDR2 interactions discussed previously were even more dominant than they were in complexes involving TCRs from conventional T cells bearing the same or related Vα or Vβ elements. Furthermore, an initial mutational analysis showed that these conserved interactions were generally also required for the TCR cross-reactions.
In the present study, we tested the idea that the cross-reactivities of these promiscuous T cells are not only driven by conserved CDR1/CDR2 interactions but require CDR3 loops that support or at least do not interfere with the cross-reactivity. We therefore examined the role of CDR3 in the reactivity of the T cell, YAe62, typical of CD4 T cells that develop in single-peptide mice (4). YAe62 was produced in IAb single-peptide mice by immunization with IAb bearing the 3K peptide (IAb-3K) (4). It responds to IAb-3K but is highly peptide promiscuous (13) and cross-reactive with WT IAb and many other IA alleles.
Using an extensive mutational analysis, we examined how changes in Vβ CDR3 might influence the reactivity of YAe62. Not unexpectedly, we found mutant CDR3s that lessened or destroyed both the IAb-3K reactivity and the MHC cross-reactivities of YAe62. However, we also found numerous mutant CDR3s, many with no sequence similarity to the original, which not only preserved some of the promiscuity of the TCR but even extended its reactivity to different MHC alleles. Most importantly, TCRs with these mutant CDR3s still depended on some or all of the conserved CDR1 and CDR2 amino acids for their reactivities. These data support our hypotheses that the propensity of TCRs to react with MHC ligands is inherent in their CDR1 and CDR2 loops and that this reactivity can be dialed up or down by CDR3. We propose that this process is required to fine-tune the affinity and specificity of the TCR to allow positive but not negative selection to create a collection of mature T cells bearing TCRs that are specific for self-MHC plus foreign peptide.
Results
Contribution of the CDR3 Loops of the YAe62 TCR to Its Broad Cross-Reactivity.
TCR CDR3s might contribute to MHC cross-reactivity either by being short and/or containing amino acids with short side chains, thus helping them to stay out of the way of conserved CDR1/CDR2 interactions with MHC, or by contributing to cross-reactivity by also interacting with a conserved area of the MHC ligand (14).
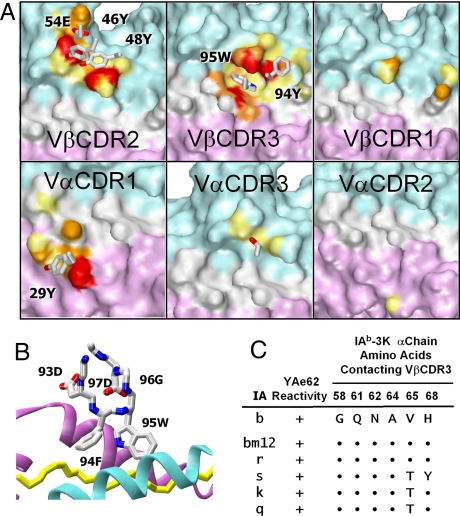
The structure of the cross-reactive YAe62 TCR bound to its ligand, IAb-3K (2), suggests that both of these mechanisms might play a role. Fig. 1A shows the footprints of each of the YAe62 TCR CDR loops on IAb-3K. As discussed previously, Vα CDR1 and Vβ CDR2 make the extensive conserved contacts with the IAb portion of the ligand via Vα 29Y and Vβ 46Y, 48Y, and 54E, whereas Vα CDR2 and Vβ CDR1 make very little contact with the ligand. The YAe62 Vα CDR3 has the shortest CDR3 loop among the TCRs whose structures have been solved. It contacts the IAb molecule only minimally via Vα 94T and has no contact with the 3K peptide. This lack of Vα CDR3 interaction is associated with tipping of the TCR toward the peptide N-terminus, enhancing both Vβ CDR2 and Vα CDR1 contact with the IAb helices.
Fig. 1.
Interaction of YAe62 CDRs with IAb-3K. (A) Footprint of the YAe62 TCR CDRs on the surface of IAb-3K. In each panel, the solvent-accessible surface of the IAb α1 and β1 domains and the 3K peptide is shown calculated from the structure of the complex [Dai S, et al. (2008) Cross-reactive T cells spotlight the germline rules for alphabeta T cell-receptor interactions with MHC molecules. Immunity 28:324–334]. The surface contributed by IAb-3K atoms making Van der Waal's contact with the indicated CDR region of the YAe62 TCR is colored, based on the number of TCR atoms contacted as follows: yellow, 1 or 2 atoms; orange, 3–5 atoms; red, greater than 5 atoms. The rest of the surface is colored as follows: IAb α1, cyan; IAb β1, magenta; peptide, white. The side chains of the CDR amino acids involved in the CDR contacts are shown with Corey–Pauling–Koltun (CPK) coloring. (B) Detail of the YAe62 Vβ CDR3 loop in complex with IAb-3K. A wire-frame representation of the 5 amino acids at the tip of the YAe62 Vβ CDR3 is shown with CPK coloring. Also shown are ribbon representations of the IAb α (cyan) and β (magenta) chain helices and a tube representation (yellow) of the 3K peptide in the region of contact with the Vβ CDR3. (C) Conservation of the IAb amino acids contacting YAe62 Vβ CDR3 in other IA molecules that stimulate the YAe62. On the top line, the 6 IAb α chain amino acids that contact the YAe62 TCR Vβ CDR3 are listed. Below that line, the amino acids at these positions in the 5 other IA alleles that stimulate the YAe62 T cell are shown. ●, identity to IAb.
On the other hand, the Vβ CDR3 loop, via Vβ 94F and 95W, has extensive contact with the IAb α chain helix and, to some extent, with the 3K peptide. In particular, the side chain of Vβ 95W is tucked between the peptide backbone and the MHCII α1 helix (Fig. 1 A and B). The MHC amino acids contacted by Vβ CDR3 are very conserved among the IA alleles with which the YAe62 TCR cross-reacts (15) (Fig. 1C). As previously described (2), these contacts combine with Vα CDR1 and Vβ CDR2 to create the overall compact hydrophobic footprint of this TCR on the ligand.
Therefore, we propose that on one side of the YAe62 TCR, the Vα CDR3 loop contributes to its broad cross-reactivity by avoiding steric interference with the conserved Vα and Vβ CDR1 and CDR2 interactions with MHC. In contrast, on the other side of the TCR, the Vβ CDR3 loop contacts the MHC in such a way that it enhances the CDR1/CDR2 conserved interactions, creating a stable “3-legged stool” structure. To test this idea, we performed several types of mutational analyses of the Vβ CDR3 loop.
Effects of Alanine Substitutions in the YAe62 Vβ CDR Loops.
In a previous study, we examined the effects of mutating Vα 29Y and Vβ 48Y and 54E on the responses of YAe62 to its various ligands (2). Here, we have extended that analysis to many other amino acids on all 3 of the CDR loops of the YAe62 Vβ region to compare the relative importance of the Vβ CDR1/CDR2 loops with that of the Vβ CDR3 loop in the reactivities of the T cell. The mutated β chains and the WT YAe62 α chain were introduced into a TCR-negative hybridoma by retroviral transduction. The resultant transductants were tested for both IAb-3K reactivity and alloreactivity to various H-2 haplotypes. The results are shown in Fig. 2. For each TCR amino acid, this figure shows the number of contacts to the MHC and peptide portion of the IAb-3K ligand in the crystal structure of the WT TCR and IL-2 production by the mutant transductants in response to stimulation with either IAb-3K or antigen-presenting cells bearing various H-2 haplotypes.
Fig. 2.
Alanine scanning of Vβ CDR1, 2, and 3 of YAe62. The number of atom-to-atom contacts between each amino acid in the Vβ CDR1, CDR2, and CDR3 of the YAe62 TCR and IAb or p3K in the crystal structure was calculated, as described elsewhere [Dai S, et al. (2008) Cross-reactive T cells spotlight the germline rules for alphabeta T cell-receptor interactions with MHC molecules. Immunity 28:324–334]. Indicated amino acids were individually mutated to alanine. The resultant mutant transductants were stimulated with antigen-presenting cells (APCs) presenting different TCR ligands. IL-2 production was assayed. The data are presented as boxes filled with a sliding color scale from white to red (see color code). Data represent the mean of IL-2 produced in 3 independent experiments. All mutants were able to respond to anti-CD3 equally and did not produce IL-2 in response to media alone (data not shown).
As previously shown (2), Vβ 48Y and 54E were critical for the reactivity of YAe62 to IAb-3K. In addition, Vβ 46Y and 95W were essential for the response. These results agree well with the crystallographic data, because these 4 amino acids contribute more than 60% of the total contacts between the YAe62 TCR and the IAb-3K ligand (2). There was little effect of mutating any of the amino acids in Vβ CDR1, consistent with the minimal contact between Vβ CDR1 and IAb-3K in the complex. Mutation of Vβ CDR3 93D, 94F, or 98T did not eliminate the response to IAb-3K but resulted in a greater than 10-fold loss in IL-2 production. This was not surprising for 94F, because this amino acid makes extensive contact with IAb-3K in the structure. However, neither 93D nor 98T made any contact with IAb-3K in the structure. These amino acids are on the beginning of the β strands that support the Vβ CDR3 loop, and their mutation might affect the packing of these strands against the other β strands of Vβ and influence the conformation of the tip of the CDR3 loop indirectly (16).
In general, similar results were obtained when the mutant transductants were tested with the MHC haplotypes with which YAe62 cross-reacts. Although the patterns of reactivity were not identical, some or all of the Vβ amino acids (46Y, 48Y, 54E, and 95W) played a major role in the allo-MHC cross-reactivities as well. The 3 Vβ CDR2 amino acids interact very similarly with a number of different IA alleles in numerous published TCR/MHC structures, such that our results suggest that the YAe62 TCR most likely interacts with these other MHC alleles in a manner similar to that seen with IAb-3K (i.e., by contacting the same conserved sites on MHC).
Many Different Vβ CDR3 Loops Support the Cross-Reactivity of YAe62.
Our mutational data suggested that the YAe62 Vβ CDR3 synergized with conserved interactions mediated by Vα 29Y and Vβ 46Y, 48Y, and 54E to produce its many cross-reactivities. The major contribution from 95W in Vβ CDR3 might indicate that only a very restricted set of CDR3s would allow such extensive cross-reactivity. This raised the possibility that it was the special nature of Vβ CDR3 rather than the CDR1/CDR2 amino acids of YAe62 that drove its promiscuous reactivity. To test this idea, we used a retroviral approach similar to that described previously to prepare a library of viruses encoding the YAe62 β chain with the 5 amino acids (amino acids 93–97) at the tip of the loop randomized, which were used to transduce the cell already expressing the WT TCR-α chain.
The initial mixed library of transductants had a weak reactivity with MHC when tested with the H-2d– and H-2b–bearing B-cell lymphoma hybridoma, LB-27.4 (17, 18) [Fig. S1A]. As described in Materials and Methods (18), the library was enriched for reactivity to LB-27.4 using the T cell recognition by protein transfer (TRAP) method (19). Over 8 cycles, there was a gradual increase both in the response of the bulk-sorted population and in the percentage of clones from the sorted populations that responded to LB-27.4 (Fig. S1 A and B).
Because many of the clones contained more than 1 virus, individual CDR3s were sequenced, recloned, and reintroduced in the α chain containing recipient. A final set of 19 transductant clones was selected for characterization. All clones had similar levels of surface TCR and responded well to the Vβ8-specific superantigen, Staphylococcal enterotoxin B (SEB) (data not shown). The transductant clones were compared for their responses to either IAb-3K, different H-2 haplotypes, or cells bearing the MHCI molecules, Kb and Db, in the absence of MHCII. We hypothesized that if the unique properties of the original YAe62 Vβ CDR3 were responsible for its MHC cross-reactivity, the enriched library should contain Vβ CDR3s very similar to the original. On the other hand, if the role of Vβ CDR3 was to provide the third leg to the 3-legged stool enhancing the TCR CDR1/CDR2-conserved interactions, there should be many other additional unrelated CDR3s that could provide this function. The results showed that the latter prediction was correct.
An example pattern of response for the WT YAe62 and one of the T-cell transductants from the Vβ CDR3 library is shown in Fig. 3. As expected, cells expressing WT YAe62 TCR responded to H-2b, bm12, r, k, q, s but not to H-2d, f, nod or to Kb/Db (Fig. 3A). The Vβ CDR3 of clone 1 differs from WT YAe62 by 1 amino acid, 97D>I. Despite this single change, this clone had a somewhat different pattern of reactivity (Fig. 3B). It still responded to IAb-3K, but less strongly than WT YAe62. More strikingly, this mutant lost the ability to respond to H-2k but acquired responsiveness to H-2d and H-2f.
Fig. 3.
TCRs composed of the WT YAe62 β chain or Vβ CDR3 mutants have different patterns of allo-MHC reactivity. IL-2 production by WT YAe62 (A) or mutant transductant 1 (B) in response to the different antigen-presenting cells is shown. Black bars indicate stimuli that produced a response in WT YAe62. White bars indicate stimuli that did not produce a response with WT YAe62. CDR3 sequences of the WT and mutant TCRs are shown. Data are represented as mean ± SEM.
The Vβ CDR3 sequences and patterns of reactivity of all the clones are summarized in Fig. 4. The mutants can be divided into 3 categories. Group A (clones 1–4) have Vβ CDR3s that contain 95W, the same as WT YAe62 TCR. The individual Vβ sequences ranged from clone 1, described previously, whose CDR3 shared 4 of 5 amino acids with WT YAe62 Vβ CDR3, to clone 4, in which only 95W was shared with the YAe62 Vβ CDR3. Clones 1–3 all had a detectable response to IAb-3K and multiple other MHC reactivities. By contrast, clone 4 did not respond to IAb-3K but still responded to 2 of the MHC haplotypes.
Fig. 4.
Vβ CDR3 mutants have different patterns of MHC reactivities. Nineteen transductants from the Vβ CDR3 library enriched for H-2b/H-2d reactive cells were tested for response to different antigen-presenting cells. The figure shows their Vβ CDR3 sequences and the mean of IL-2 produced in 3 separate experiments. The data are presented as boxes filled with a sliding color scale (see color code). The transductants are presented in 3 groups: group A, mutants with Vβ 95W (similar to the YAe62 WT CDR3); group B, mutants without 95W that responded to at least 1 of the MHC stimuli; and group C, mutants that did not respond to any of the MHC stimuli. All clones produced similar amounts of IL-2 in response to SEB presented by A20 lymphoma B cells [Kim KJ, Kanellopoulos-Langevin C, Merwin RM, Sachs DH, Asofsky R (1979) Establishment and characterization of BALB/c lymphoma lines with B cell properties. J Immunol 122:549–554], and none responded to media alone (data not shown).
Group B (clones 5–15) Vβ CDR3 lacked 95W and were not obviously related to the WT CDR3, but these clones still responded to 1 or more of the tested MHCs and a few continued to respond to IAb-3K. None of the clones in group C (clones 16–19) responded to any of the stimuli. These clones were primarily identified in the enriched transductants that carried more than 1 mutant CDR3, only 1 of which had IAb-3K or allo-MHC reactivity. The results are consistent with our prediction that although the CDR3 of the WT YAe62 participates in its multiple MHC reactivities (especially via 95W), other unrelated CDR3 sequences can be substituted without the loss of its MHC cross-reactivity or, in some cases, even its IAb-3K reactivity.
An unexpected finding was that 3 of the 4 Vβ CDR3 mutant T cells in group A (including mutant 1 described previously) and 8 of 11 in group B had gained a response to 1 or more of the H-2 haplotypes not recognized by the WT YAe62 T cell. Mutant 5 was particularly striking. It reacted to all stimuli tested, including cells bearing only H-2b MHCI molecules. The WT YAe62 T cell does not respond to H-2b MHCI-expressing cells directly but can be positively selected to become a CD8 T cell in MHCII-negative mice expressing H-2b MHCI (4). The Vβ CDR3 region in mutant 5 may have increased the affinity of its TCR for H-2b MHCI plus an unknown self-peptide enough to change a positively selecting ligand into an overtly stimulating one.
These results support the idea that depending on the particulars of the CDR3 regions, a given receptor can be broadly cross-reactive not only to different alleles of MHCII molecule but also to MHCI.
Vα 29Y and Vβ 46Y, 48Y, and 54E Are Important in the MHC Reactivity of TCRs with the Mutant Vβ CDR3s.
The results in Fig. 4 show that the broad MHC cross-reactivity of the YAe62 TCR can be dialed up or down by the composition of Vβ CDR3. This result led us to the central question of this study. Regardless of the CDR3 composition, are the cross-reactivities of the mutant TCRs still dependent on the conserved interaction of Vα 29Y and Vβ 46Y, 48Y, and 54E with the MHC portion of the ligand? If so, this would indicate that these amino acids are the main drivers of MHC recognition, with CDR3 playing a supporting modulating role. To test this idea, we combined the TCR-β chain expressing 2 of the most MHC cross-reactive mutant Vβ CDR3 loops (clones 5 and 7), not related to the original CDR3, with mutations of either Vα 29Y or Vβ 46Y, 48Y, or 54E and tested the effect of these combined mutations on reactivities to the different MHCs. The results are summarized in Fig. 5.
Fig. 5.
Vβ CDR3 mutants use Vα 29Y and Vβ 46Y, 48Y, and 54E to contact MHC. Within group B of Fig. 4, amino acids Vα 29Y and Vβ 46Y, 48Y, and 54E of the TCRs of the clones 5 (light green) and 7 (light blue) were individually mutated to alanine. These mutated clones and unmutated T cells (red, YAe62; dark green, 5; dark blue, 7) were tested for IL-2 production in response to the 8 different MHC haplotypes and MHC class II-deficient spleen cells (Kb/Db). Similar results were obtained for H-2nod (data not shown). Data are represented as mean of normalized data from 3 experiments. SEMs are shown for T-cell/antigen-presenting cell combinations present in all 3 experiments. All mutants were able to respond to anti-CD3 stimulation and did not produce IL-2 in response to media alone (data not shown).
The effects of these mutations were very similar to those seen previously with the WT YAe62 TCR (i.e., the mutation of Vβ 48Y had the strongest effect, followed by that of Vβ 46Y and then that of Vβ 54E and Vα 29Y) (2) (Fig. 2). These data show that even with a completely different Vβ CDR3 sequence, these 2 mutant TCRs still depend on the same germline-encoded CDR1/CDR2 amino acids to recognize different MHC molecules as did the original YAe62 TCR. These results also suggest that for each MHC ligand, the 3 TCRs use a similar docking mode.
Overall, these data support our hypothesis that some germline-encoded CDR1 and CDR2 amino acids (Vβ 46Y, 48Y, and 54E and Vα 29Y) are the chief contributors to the MHC reactivity of TCR containing Vβ8- or Vα4-related V elements. Our results also are consistent with the idea that TCR CDR3 regions can modulate this reactivity to prevent self-reactivity, while imparting peptide and MHC allele specificity.
Discussion
The question of whether TCR genes have been evolutionarily selected to interact with MHC was raised by Jerne (20) in 1971. In modern terms, the question has become what the relative contribution of the germline-encoded Vα and Vβ CDR1 and CDR2 loops is vs. the somatically generated CDR3 loops to the shaping of the MHC-specific T-cell repertoire. Several studies have indicated that the frequency of MHC-reactive T cells in the random Vα/Vβ repertoire is high (21–23), but the early sets of structures of TCRs bound to MHC did not reveal any interaction sites between TCRs and MHC that were common from one structure to another and were germline encoded. However, as more structures have accumulated, repeated patterns of TCR CDR1 and CDR2 interactions with MHC have been observed (2, 6, 7). This pattern has been easier to define with MHCII ligands. For MHCI, although a very conserved MHCI “restriction triad” (18) (α1 65 and 69 and α2 155) repeatedly contacted by TCR has been noted, the TCR CDR1 and CDR2 loops appear to have more flexibility in interacting with these sites.
The accumulated data favor the idea that TCR CDR1 and CDR2 loops have been evolutionarily selected for MHC reactivity. But what about CDR3? Based on the high sequence variability of TCR CDR3 loops, Bjorkman and Davis (24) originally predicted that they would be primarily responsible for peptide contact in TCR/MHC complexes. The subsequent TCR/MHC structural data have shown that, indeed, in nearly all cases, the TCR CDR3 loops sit squarely over the peptide. Moreover, several studies have shown that mutations in CDR3 loops can have different effects on TCR/MHC interaction. Thus, CDR3 mutants occasionally acquire higher affinity for the ligand (25, 26); however, more often, CDR3 mutants lose the ability to bind their ligands (16, 27, 28). These findings raise the question of whether the TCR CDR3 loops play an active or passive role in shaping the T-cell predisposition for MHC. Our results in single-peptide mice have led us to propose that many CDR3 loops are permissive for strong CDR1/CDR2 interaction of TCR with multiple MHC molecules, such that the main function of thymic selection is to sort for those CDR3 loops that reduce the inherent affinity of the TCR for MHC into a range that promotes positive selection but allows escape from negative selection to the periphery.
We showed here that the YAe62 Vβ CDR3 loop played a strong role in MHC cross-reactivity; however, other unrelated CDR3 loops either decrease or increase its range of MHC cross-reactivity. Most importantly, the same 4 conserved CDR1/CDR2 amino acids were involved in the cross-reactivities of TCRs with different unrelated CDR3 loops. These findings are consistent with the idea that MHC reactivity is inherent in random combinations of Vα and Vβ via particular amino acids in CDR1 and CDR2. However, TCR CDR3 loops determine the strength and specificity of this reactivity. This idea is also supported by the recent work of Jones et al. (26), who examined the MHCI-reactive 2C TCR that is cross-reactive between Kb and Ld bearing different peptides. They selected different Vα CDR3s for this TCR based on much higher affinity of the different TCR for the Ld ligand. They showed that the mutant Vα CDR3s improved affinity without disturbing the conserved CDR1/CDR2 interactions with Ld seen with the WT 2C TCR.
The paradox of the coexistence of self-MHC restriction of foreign peptide recognition and the relatively high frequency of allo-MHC reactive T cells has been a thorny issue for many years. Several recent articles have reviewed the topic and the current ideas and structural data relevant to the problem (29–31). One important question has been whether the recognition of different MHC molecules by the same TCR involves fundamentally different docking modes of the TCR or simply changes in a few individual TCR-to-MHC contacts without much change in orientation. This issue has not been fully settled. In a few of the published structures directly comparing an individual TCR bound to 2 different MHC ligands, the familiar general diagonal binding mode was seen in both complexes (31, 32). Nevertheless, in some cases, there were significant differences in TCR pitch and rotation, leading to changes in the details of atom-to-atom contacts with MHC. Also some of the TCR CDR loops have somewhat different conformations when bound to different ligands (9, 32–36). This change in CDR loop structure has also been seen in some comparisons of free vs. MHC-bound TCR (9, 35, 37–40). This has led to the concept that the flexibility or plasticity of the TCR may account for its cross-reactivity rather than any conservation of TCR/MHC contacts.
Based on mutational data presented here and previously (2), we argue that highly promiscuous T cells, such as YAe62, developing in single-peptide mice represent a unique population of T cells whose strong interactions with many MHC ligands are driven primarily by the same conserved features of the germline-encoded Vα and Vβ elements. Although this conclusion predicts a very similar docking mode of these TCRs on their various ligands, we have not yet confirmed this with x-ray crystallographic data.
Materials and Methods
Refer to SI Text for detailed materials and methods.
Generation of Vβ CDR3 Library and TCR Mutants.
For detailed methods, refer to SI Text. Briefly, sequences encoding the WT YAe62 TCR-α and -β chains were cloned into murine stem cell virus (MSCV)-based retroviral plasmids (41, 42). DNA fragments encoding the YAe62 TCR-α or -β chain with individual mutations were constructed using PCR with overlapping primers and cloned into MSCV vectors. A Vβ CDR3 library was created using oligonucleotide primers randomized [via the codon NN(G/C)] at positions encoding Vβ 93–97. The mixture of PCR fragments was cloned in the MSCV plasmid encoding the YAe62 β chain, producing a mixture of ≈2 × 105 independent plasmid clones.
Retroviral supernatants were generated in Phoenix cells (18) and used to spinfect T-cell hybridomas (18). Cells were analyzed and sorted 24 h after the second spinfection.
Enrichment of the Vβ CDR3 Library for MHC-Reactive T-Cell Transductants Using the TRAP Method.
For detailed methods, refer to SI Text. Briefly, the TRAP method (19) was used to enrich MHC-reactive T-cell transductants from the Vβ CDR3 library (see SI Text).
IL-2 Assay.
A total of 105 transduced T cells per well (96-well plate) were incubated with 106 spleen cells expressing various MHC alleles (H-2bm12, H-2k, H-2s, H-2r, H-2q, H-2nod, and Kb/Db) or 105 B-cell lymphoma A20 (H-2d) (43), Chb 2.4.4 (H-2b) (44), or 105 fibroblasts transfected with IAb-3K intercellular adhesion molecule/B7 (4) in 250 μL of S-MEM/10% (vol/vol) FCS. Twenty-four hours later, the supernatants were screened for IL-2 content as previously described (11).
Flow Cytometry and Cell Sorting.
Flow cytometry analysis and cell sorting were performed at the Flow Cytometry Facility at National Jewish Health.
Mice.
B10.BR (H-2k), B10M (H-2f), B6.CH2 (H-2bm12), B10.RIII (H-2r), B10.S (H-2s), and B10.D1 (H-2q) mice were obtained from Jackson Laboratory. MHCII−/−, Ii−/− mice have been previously described (10), and nonobese diabetic mice were provided by Kathryn Haskins (National Jewish Health Molecular Resource Facility). All mice were maintained in a pathogen-free environment in accordance with institutional guidelines in the Biological Resource Center at the National Jewish Health.
Supplementary Material
Acknowledgments.
The authors thank Janice White, Rachel Frugé, Ella Kushnir, and Christopher Brown for technical assistance; J. Loomis for assistance with flow cytometry; and Randy Anselment and Amy Marrs in the National Jewish Health Molecular Resource Facility. This work was supported in part by US Public Health Service grants AI-17134, AI-18785, AI-22295, and AI-07405.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0902728106/DCSupplemental.
References
- 1.Rudolph MG, Stanfield RL, Wilson IA. How TCRs bind MHCs, peptides, and coreceptors. Annu Rev Immunol. 2006;24:419–466. doi: 10.1146/annurev.immunol.23.021704.115658. [DOI] [PubMed] [Google Scholar]
- 2.Dai S, et al. Cross-reactive T cells spotlight the germline rules for alphabeta T cell-receptor interactions with MHC molecules. Immunity. 2008;28:324–334. doi: 10.1016/j.immuni.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marrack P, Scott-Browne JP, Dai S, Gapin L, Kappler JW. Evolutionarily conserved amino acids that control TCR-MHC interaction. Annu Rev Immunol. 2008;26:171–203. doi: 10.1146/annurev.immunol.26.021607.090421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huseby ES, et al. How the T cell repertoire becomes peptide and MHC specific. Cell. 2005;122:247–260. doi: 10.1016/j.cell.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 5.Marrack P, Rubtsova K, Scott-Browne J, Kappler JW. T cell receptor specificity for major histocompatibility complex proteins. Curr Opin Immunol. 2008;20:203–207. doi: 10.1016/j.coi.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feng D, Bond CJ, Ely LK, Maynard J, Garcia KC. Structural evidence for a germline-encoded T cell receptor-major histocompatibility complex interaction ‘codon’. Nat Immunol. 2007;8:975–983. doi: 10.1038/ni1502. [DOI] [PubMed] [Google Scholar]
- 7.Maynard J, et al. Structure of an autoimmune T cell receptor complexed with class II peptide-MHC: Insights into MHC bias and antigen specificity. Immunity. 2005;22:81–92. doi: 10.1016/j.immuni.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 8.Garcia KC, Teyton L, Wilson IA. Structural basis of T cell recognition. Annu Rev Immunol. 1999;17:369–397. doi: 10.1146/annurev.immunol.17.1.369. [DOI] [PubMed] [Google Scholar]
- 9.Garcia KC, et al. An alphabeta T cell receptor structure at 2.5 A and its orientation in the TCR-MHC complex. Science. 1996;274:209–219. [PubMed] [Google Scholar]
- 10.Ignatowicz L, Kappler J, Marrack P. The repertoire of T cells shaped by a single MHC/peptide ligand. Cell. 1996;84:521–529. doi: 10.1016/s0092-8674(00)81028-4. [DOI] [PubMed] [Google Scholar]
- 11.Huseby ES, Crawford F, White J, Kappler J, Marrack P. Negative selection imparts peptide specificity to the mature T cell repertoire. Proc Natl Acad Sci USA. 2003;100:11565–11570. doi: 10.1073/pnas.1934636100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huseby ES, Crawford F, White J, Marrack P, Kappler JW. Interface-disrupting amino acids establish specificity between T cell receptors and complexes of major histocompatibility complex and peptide. Nat Immunol. 2006;7:1191–1199. doi: 10.1038/ni1401. [DOI] [PubMed] [Google Scholar]
- 13.Crawford F, Huseby E, White J, Marrack P, Kappler JW. Mimotopes for alloreactive and conventional T cells in a peptide-MHC display library. PLoS Biol. 2004;2:E90. doi: 10.1371/journal.pbio.0020090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Torres-Nagel N, Deutschlander A, Herrmann T, Arden B, Hunig T. Control of TCR V alpha-mediated positive repertoire selection and alloreactivity by differential J alpha usage and CDR3 alpha composition. Int Immunol. 1997;9:1441–1452. doi: 10.1093/intimm/9.10.1441. [DOI] [PubMed] [Google Scholar]
- 15.Bell JI, Denny DW, Jr, McDevitt HO. Structure and polymorphism of murine and human class II major histocompatibility antigens. Immunol Rev. 1985;84:51–71. doi: 10.1111/j.1600-065x.1985.tb01125.x. [DOI] [PubMed] [Google Scholar]
- 16.McBeth C, et al. A new twist in TCR diversity revealed by a forbidden alphabeta TCR. J Mol Biol. 2008;375:1306–1319. doi: 10.1016/j.jmb.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kappler J, White J, Wegmann D, Mustain E, Marrack P. Antigen presentation by Ia+ B cell hybridomas to H-2-restricted T cell hybridomas. Proc Natl Acad Sci USA. 1982;79:3604–3607. doi: 10.1073/pnas.79.11.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tynan FE, et al. T cell receptor recognition of a ‘super-bulged’ major histocompatibility complex class I-bound peptide. Nat Immunol. 2005;6:1114–1122. doi: 10.1038/ni1257. [DOI] [PubMed] [Google Scholar]
- 19.Beadling C, Slifka MK. Quantifying viable virus-specific T cells without a priori knowledge of fine epitope specificity. Nat Med. 2006;12:1208–1212. doi: 10.1038/nm1413. [DOI] [PubMed] [Google Scholar]
- 20.Jerne NK. The somatic generation of immune recognition. Eur J Immunol. 1971;1:1–9. doi: 10.1002/eji.1830010102. [DOI] [PubMed] [Google Scholar]
- 21.Blackman M, et al. The T cell repertoire may be biased in favor of MHC recognition. Cell. 1986;47:349–357. doi: 10.1016/0092-8674(86)90591-x. [DOI] [PubMed] [Google Scholar]
- 22.Merkenschlager M, et al. How many thymocytes audition for selection? J Exp Med. 1997;186:1149–1158. doi: 10.1084/jem.186.7.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zerrahn J, Held W, Raulet DH. The MHC reactivity of the T cell repertoire prior to positive and negative selection. Cell. 1997;88:627–636. doi: 10.1016/s0092-8674(00)81905-4. [DOI] [PubMed] [Google Scholar]
- 24.Bjorkman PJ, Davis MM. Model for the interaction of T-cell receptors with peptide/MHC complexes. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):365–373. doi: 10.1101/sqb.1989.054.01.045. [DOI] [PubMed] [Google Scholar]
- 25.Chlewicki LK, Holler PD, Monti BC, Clutter MR, Kranz DM. High-affinity, peptide-specific T cell receptors can be generated by mutations in CDR1, CDR2 or CDR3. J Mol Biol. 2005;346:223–239. doi: 10.1016/j.jmb.2004.11.057. [DOI] [PubMed] [Google Scholar]
- 26.Jones LL, Colf LA, Stone JD, Garcia KC, Kranz DM. Distinct CDR3 conformations in TCRs determine the level of cross-reactivity for diverse antigens, but not the docking orientation. J Immunol. 2008;181:6255–6264. doi: 10.4049/jimmunol.181.9.6255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goyarts EC, et al. Point mutations in the beta chain CDR3 can alter the T cell receptor recognition pattern on an MHC class I/peptide complex over a broad interface area. Mol Immunol. 1998;35:593–607. doi: 10.1016/s0161-5890(98)00056-x. [DOI] [PubMed] [Google Scholar]
- 28.Patten PA, et al. Transfer of putative complementarity-determining region loops of T cell receptor V domains confers toxin reactivity but not peptide/MHC specificity. J Immunol. 1993;150:2281–2294. [PubMed] [Google Scholar]
- 29.Godfrey DI, Rossjohn J, McCluskey J. The fidelity, occasional promiscuity, and versatility of T cell receptor recognition. Immunity. 2008;28:304–314. doi: 10.1016/j.immuni.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 30.Archbold JK, Macdonald WA, Burrows SR, Rossjohn J, McCluskey J. T-cell allorecognition: A case of mistaken identity or deja vu? Trends Immunol. 2008;29:220–226. doi: 10.1016/j.it.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 31.Colf LA, et al. How a single T cell receptor recognizes both self and foreign MHC. Cell. 2007;129:135–146. doi: 10.1016/j.cell.2007.01.048. [DOI] [PubMed] [Google Scholar]
- 32.Reiser JB, et al. Crystal structure of a T cell receptor bound to an allogeneic MHC molecule. Nat Immunol. 2000;1:291–297. doi: 10.1038/79728. [DOI] [PubMed] [Google Scholar]
- 33.Ding YH, et al. Two human T cell receptors bind in a similar diagonal mode to the HLA-A2/Tax peptide complex using different TCR amino acids. Immunity. 1998;8:403–411. doi: 10.1016/s1074-7613(00)80546-4. [DOI] [PubMed] [Google Scholar]
- 34.Ding YH, Baker BM, Garboczi DN, Biddison WE, Wiley DC. Four A6-TCR/peptide/HLA-A2 structures that generate very different T cell signals are nearly identical. Immunity. 1999;11:45–56. doi: 10.1016/s1074-7613(00)80080-1. [DOI] [PubMed] [Google Scholar]
- 35.Garcia KC, et al. Structural basis of plasticity in T cell receptor recognition of a self peptide-MHC antigen. Science. 1998;279:1166–1172. doi: 10.1126/science.279.5354.1166. [DOI] [PubMed] [Google Scholar]
- 36.Reiser JB, et al. CDR3 loop flexibility contributes to the degeneracy of TCR recognition. Nat Immunol. 2003;4:241–247. doi: 10.1038/ni891. [DOI] [PubMed] [Google Scholar]
- 37.Kjer-Nielsen L, et al. A structural basis for the selection of dominant alphabeta T cell receptors in antiviral immunity. Immunity. 2003;18:53–64. doi: 10.1016/s1074-7613(02)00513-7. [DOI] [PubMed] [Google Scholar]
- 38.Kjer-Nielsen L, et al. The 1.5 A crystal structure of a highly selected antiviral T cell receptor provides evidence for a structural basis of immunodominance. Structure. 2002;10:1521–1532. doi: 10.1016/s0969-2126(02)00878-x. [DOI] [PubMed] [Google Scholar]
- 39.Housset D, et al. The three-dimensional structure of a T-cell antigen receptor V alpha V beta heterodimer reveals a novel arrangement of the V beta domain. EMBO J. 1997;16:4205–4216. doi: 10.1093/emboj/16.14.4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reiser JB, et al. A T cell receptor CDR3beta loop undergoes conformational changes of unprecedented magnitude upon binding to a peptide/MHC class I complex. Immunity. 2002;16:345–354. doi: 10.1016/s1074-7613(02)00288-1. [DOI] [PubMed] [Google Scholar]
- 41.Persons DA, et al. Retroviral-mediated transfer of the green fluorescent protein gene into murine hematopoietic cells facilitates scoring and selection of transduced progenitors in vitro and identification of genetically modified cells in vivo. Blood. 1997;90:1777–1786. [PubMed] [Google Scholar]
- 42.Pearce EL, et al. Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science. 2003;302:1041–1043. doi: 10.1126/science.1090148. [DOI] [PubMed] [Google Scholar]
- 43.Kim KJ, Kanellopoulos-Langevin C, Merwin RM, Sachs DH, Asofsky R. Establishment and characterization of BALB/c lymphoma lines with B cell properties. J Immunol. 1979;122:549–554. [PubMed] [Google Scholar]
- 44.Haughton G, Arnold LW, Bishop GA, Mercolino TJ. The CH series of murine B cell lymphomas: Neoplastic analogues of Ly-1+ normal B cells. Immunol Rev. 1986;93:35–51. doi: 10.1111/j.1600-065x.1986.tb01501.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.