Abstract
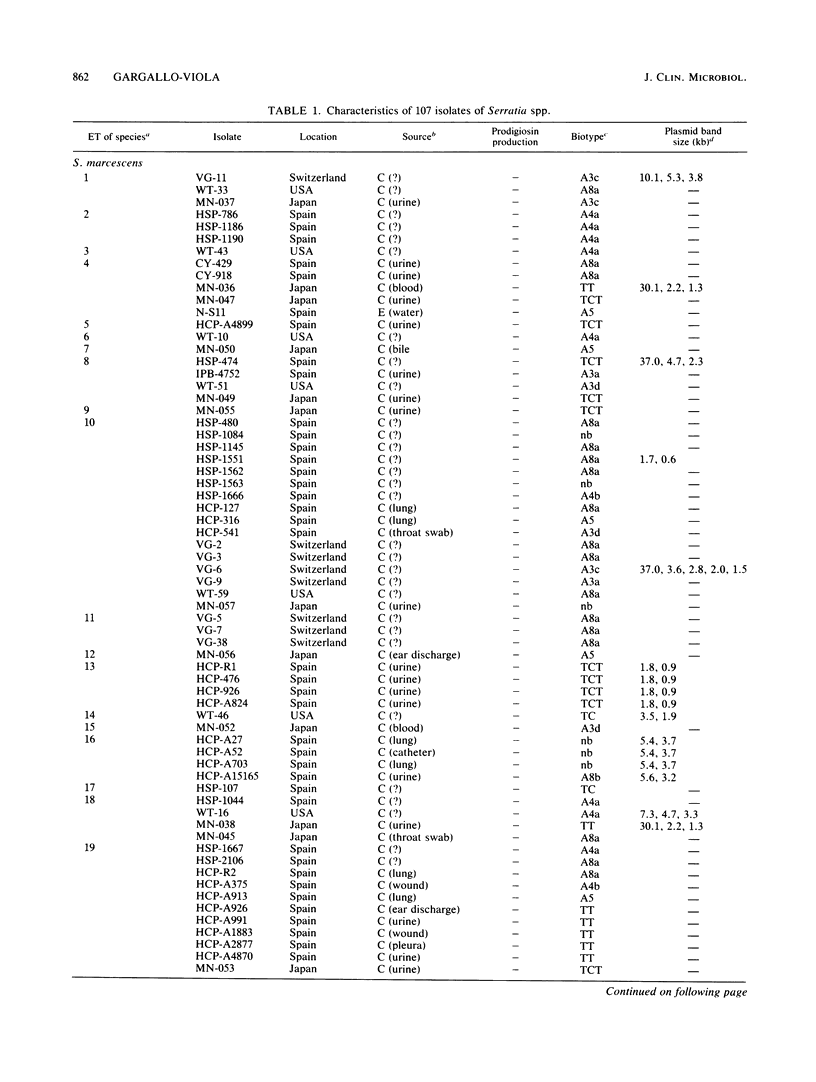
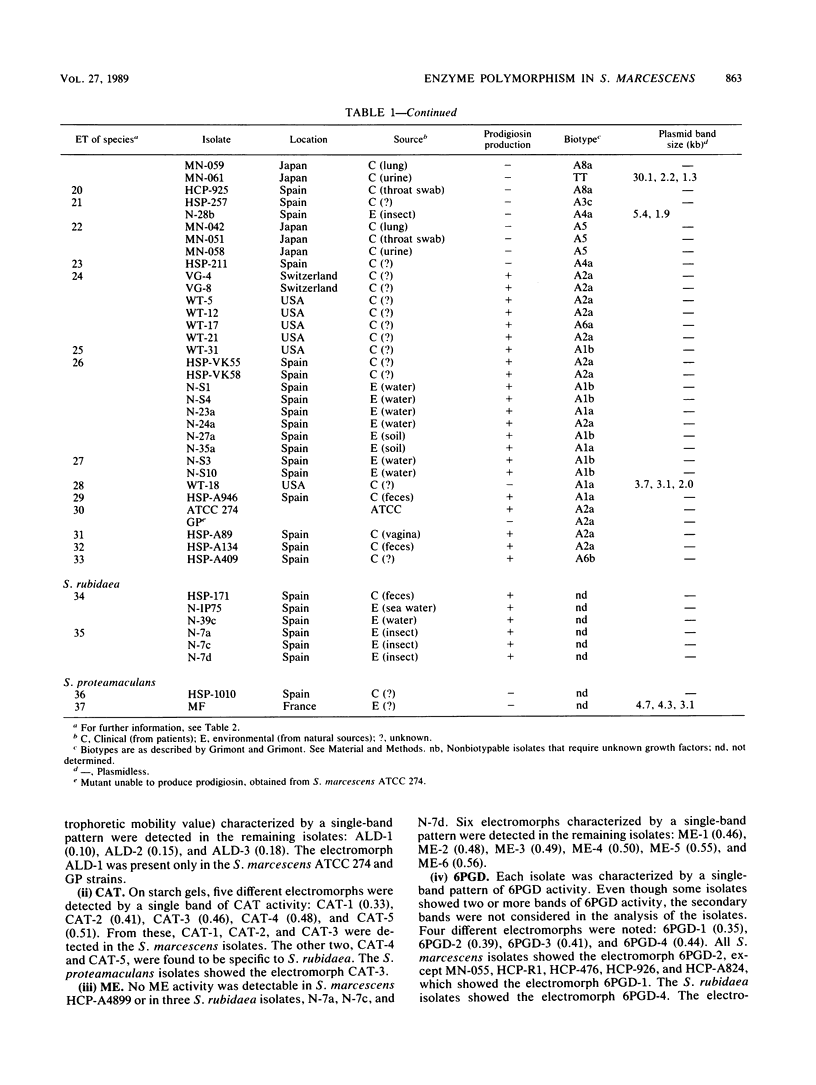
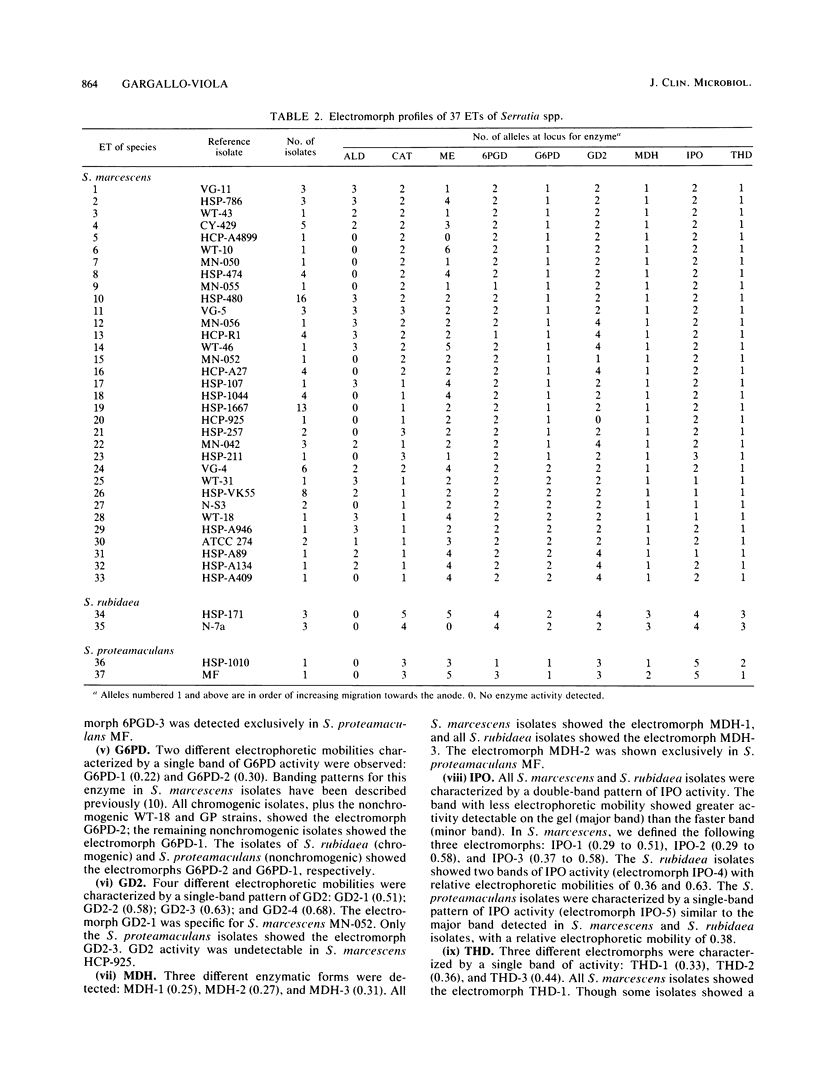
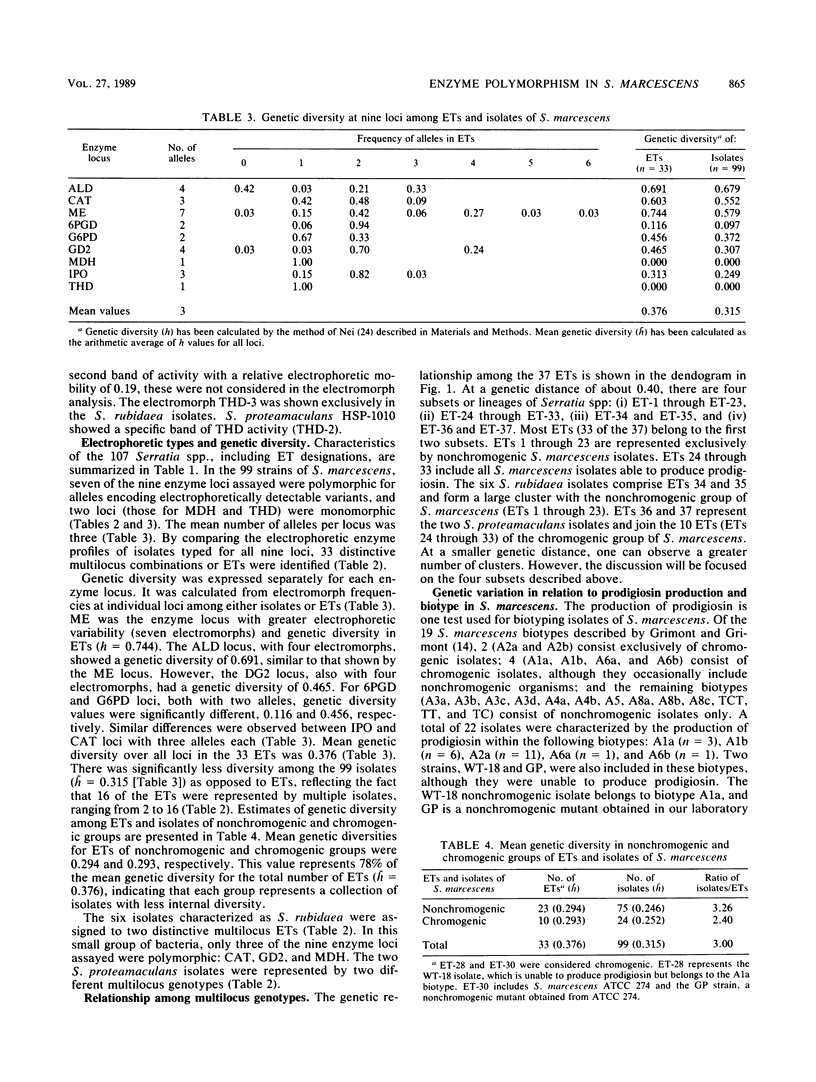
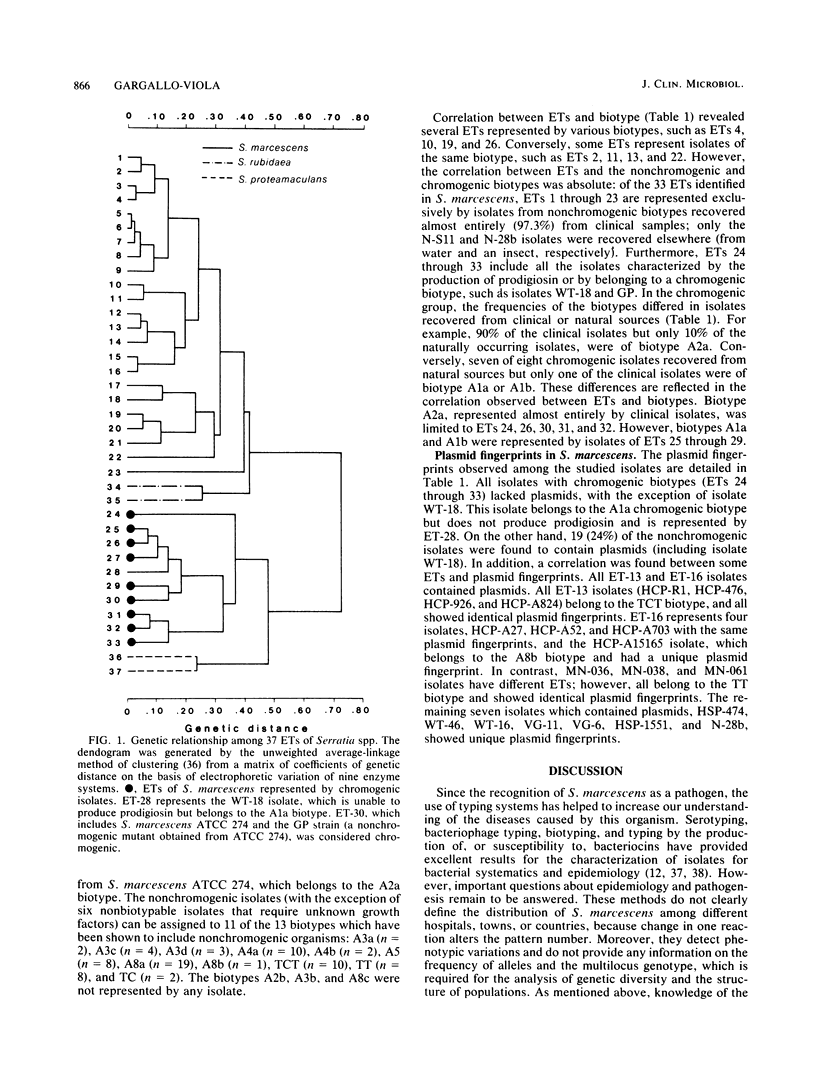
Enzyme polymorphism and genetic relationship among 99 Serratia marcescens isolates obtained from clinical and environmental sources were determined by analysis of electromorphs in nine enzyme loci encoded by chromosomal genes. Seven of the loci were polymorphic, and 33 distinctive electrophoretic types (ETs) representing multilocus genotypes were identified. Cluster analysis, based on the proportion of mismatches between multilocus genotypes, revealed two clearly differentiated groups of ETs in S. marcescens. One was represented exclusively by isolates with nonchromogenic biotypes recovered almost entirely (97.3%) from clinical samples. The other group comprised all isolates characterized by the production of prodigiosin or by belonging to a chromogenic biotype. Absolute correlation was found between the ability to produce prodigiosin and the absence of plasmids. In contrast, 24% of the nonchromogenic isolates contained plasmids. Results obtained by analysis of multilocus genotypes were related to those obtained by biotyping and plasmid fingerprinting. However, more groups could be distinguished by analysis of ETs than by biotyping. Plasmid fingerprinting was a limited typing system because many isolates lacked plasmids. Although the results of this study did not permit a definitive correlation between ETs and pathogenicity of the isolates, more detailed studies of these groups will help to understand the different clinical significances of the nonchromogenic and chromogenic isolates of S. marcescens.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achtman M., Heuzenroeder M., Kusecek B., Ochman H., Caugant D., Selander R. K., Väisanen-Rhen V., Korhonen T. K., Stuart S., Orskov F. Clonal analysis of Escherichia coli O2:K1 isolated from diseased humans and animals. Infect Immun. 1986 Jan;51(1):268–276. doi: 10.1128/iai.51.1.268-276.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baptist J. N., Shaw C. R., Mandel M. Zone electrophoresis of enzymes in bacterial taxonomy. J Bacteriol. 1969 Jul;99(1):180–188. doi: 10.1128/jb.99.1.180-188.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caugant D. A., Bøvre K., Gaustad P., Bryn K., Holten E., Høiby E. A., Frøholm L. O. Multilocus genotypes determined by enzyme electrophoresis of Neisseria meningitidis isolated from patients with systemic disease and from healthy carriers. J Gen Microbiol. 1986 Mar;132(3):641–652. doi: 10.1099/00221287-132-3-641. [DOI] [PubMed] [Google Scholar]
- Chun P. K., Sensabaugh G. F., Vedros N. A. Genetic relationships among Neisseria species assessed by comparative enzyme electrophoresis. J Gen Microbiol. 1985 Nov;131(11):3105–3115. doi: 10.1099/00221287-131-11-3105. [DOI] [PubMed] [Google Scholar]
- Ding M. J., Williams R. P. Biosynthesis of prodigiosin by white strains of Serratia marcescens isolated from patients. J Clin Microbiol. 1983 Mar;17(3):476–480. doi: 10.1128/jcm.17.3.476-480.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardes M., Bousquet J., Lalonde M. Isozyme Variation among 40 Frankia Strains. Appl Environ Microbiol. 1987 Jul;53(7):1596–1603. doi: 10.1128/aem.53.7.1596-1603.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargallo D., Lorén J. G., Guinea J., Viñas M. Glucose-6-phosphate dehydrogenase alloenzymes and their relationship to pigmentation in Serratia marcescens. Appl Environ Microbiol. 1987 Aug;53(8):1983–1986. doi: 10.1128/aem.53.8.1983-1986.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimont P. A., Grimont F. Biotyping of Serratia marcescens and its use in epidemiological studies. J Clin Microbiol. 1978 Jul;8(1):73–83. doi: 10.1128/jcm.8.1.73-83.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimont P. A., Grimont F. The genus Serratia. Annu Rev Microbiol. 1978;32:221–248. doi: 10.1146/annurev.mi.32.100178.001253. [DOI] [PubMed] [Google Scholar]
- Katz D. S., Sobieski R. J. Detection of pigment precursors in white clinical strains of Serratia marcescens. J Clin Microbiol. 1979 Feb;9(2):301–303. doi: 10.1128/jcm.9.2.301-303.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusecek B., Wloch H., Mercer A., Vaisänen V., Pluschke G., Korhonen T., Achtman M. Lipopolysaccharide, capsule, and fimbriae as virulence factors among O1, O7, O16, O18, or O75 and K1, K5, or K100 Escherichia coli. Infect Immun. 1984 Jan;43(1):368–379. doi: 10.1128/iai.43.1.368-379.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Minor L., Chippaux M., Pichinoty F., Coynault C., Piéchaud M. Méthodes simples permettant de rechercher la tétrathionate-réductase en cultures liquides ou sur colonies isolées. Ann Inst Pasteur (Paris) 1970 Dec;119(6):733–737. [PubMed] [Google Scholar]
- McLellan T. Molecular charge and electrophoretic mobility in cetacean myoglobins of known sequence. Biochem Genet. 1984 Feb;22(1-2):181–200. doi: 10.1007/BF00499297. [DOI] [PubMed] [Google Scholar]
- Musser J. M., Barenkamp S. J., Granoff D. M., Selander R. K. Genetic relationships of serologically nontypable and serotype b strains of Haemophilus influenzae. Infect Immun. 1986 Apr;52(1):183–191. doi: 10.1128/iai.52.1.183-191.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei M. Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics. 1978 Jul;89(3):583–590. doi: 10.1093/genetics/89.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochman H., Selander R. K. Evidence for clonal population structure in Escherichia coli. Proc Natl Acad Sci U S A. 1984 Jan;81(1):198–201. doi: 10.1073/pnas.81.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochman H., Whittam T. S., Caugant D. A., Selander R. K. Enzyme polymorphism and genetic population structure in Escherichia coli and Shigella. J Gen Microbiol. 1983 Sep;129(9):2715–2726. doi: 10.1099/00221287-129-9-2715. [DOI] [PubMed] [Google Scholar]
- Porras O., Caugant D. A., Lagergård T., Svanborg-Edén C. Application of multilocus enzyme gel electrophoresis to Haemophilus influenzae. Infect Immun. 1986 Jul;53(1):71–78. doi: 10.1128/iai.53.1.71-78.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramshaw J. A., Coyne J. A., Lewontin R. C. The sensitivity of gel electrophoresis as a detector of genetic variation. Genetics. 1979 Dec;93(4):1019–1037. doi: 10.1093/genetics/93.4.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaberg D. R., Alford R. H., Anderson R., Farmer J. J., 3rd, Melly M. A., Schaffner W. An outbreak of nosocomial infection due to multiply resistant Serratia marcescens: evidence of interhospital spread. J Infect Dis. 1976 Aug;134(2):181–188. doi: 10.1093/infdis/134.2.181. [DOI] [PubMed] [Google Scholar]
- Schill W. B., Phelps S. R., Pyle S. W. Multilocus Electrophoretic Assessment of the Genetic Structure and Diversity of Yersinia ruckeri. Appl Environ Microbiol. 1984 Nov;48(5):975–979. doi: 10.1128/aem.48.5.975-979.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selander R. K., Caugant D. A., Ochman H., Musser J. M., Gilmour M. N., Whittam T. S. Methods of multilocus enzyme electrophoresis for bacterial population genetics and systematics. Appl Environ Microbiol. 1986 May;51(5):873–884. doi: 10.1128/aem.51.5.873-884.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selander R. K., Korhonen T. K., Väisänen-Rhen V., Williams P. H., Pattison P. E., Caugant D. A. Genetic relationships and clonal structure of strains of Escherichia coli causing neonatal septicemia and meningitis. Infect Immun. 1986 Apr;52(1):213–222. doi: 10.1128/iai.52.1.213-222.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selander R. K., Levin B. R. Genetic diversity and structure in Escherichia coli populations. Science. 1980 Oct 31;210(4469):545–547. doi: 10.1126/science.6999623. [DOI] [PubMed] [Google Scholar]
- Selander R. K., McKinney R. M., Whittam T. S., Bibb W. F., Brenner D. J., Nolte F. S., Pattison P. E. Genetic structure of populations of Legionella pneumophila. J Bacteriol. 1985 Sep;163(3):1021–1037. doi: 10.1128/jb.163.3.1021-1037.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub W. H. Continued surveillance of Serratia marcescens infections by bacteriocin typing: investigation of two outbreaks of cross-infection in an intensive care unit. Appl Microbiol. 1972 May;23(5):982–985. doi: 10.1128/am.23.5.982-985.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub W. H., Kleber I. Continued epidemiological surveillance of Serratia marcescens infections by bacteriocin typing, with particular reference to strains isolated at Erlangen. Zentralbl Bakteriol Orig A. 1974;229(3):372–382. [PubMed] [Google Scholar]
- Véron M. Nutrition et taxonomie des Enterobacteriaceae et bactéries voisines. I. Méthode d'étude des auxanogrammes. Ann Microbiol (Paris) 1975 Apr;126(3):267–274. [PubMed] [Google Scholar]
- Whittam T. S., Ochman H., Selander R. K. Multilocus genetic structure in natural populations of Escherichia coli. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1751–1755. doi: 10.1073/pnas.80.6.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Graevenitz A. The role of opportunistic bacteria in human disease. Annu Rev Microbiol. 1977;31:447–471. doi: 10.1146/annurev.mi.31.100177.002311. [DOI] [PubMed] [Google Scholar]


