Abstract
Functional rehabilitation of the cortex following peripheral or central nervous system damage is likely to be improved by a combination of behavioural training and natural or therapeutically enhanced synaptic plasticity mechanisms. Experience-dependent plasticity studies in the somatosensory cortex have begun to reveal those synaptic plasticity mechanisms that are driven by sensory experience and might therefore be active during behavioural training. In this review the anatomical pathways, synaptic plasticity mechanisms and structural plasticity substrates involved in cortical plasticity are explored, focusing on work in the somatosensory cortex and the barrel cortex in particular.
Keywords: barrel cortex, synaptic plasticity, stroke
1. Introduction
The somatosensory cortex serves several key functions in the brain. By integrating and analysing somatosensory information, it leads to perception of somatosensory stimuli and by projecting to motor areas such as striatum, motor cortex, pontine nuclei (and hence cerebellum) and ventral horn it enables planning, execution and dynamic modulation of coordinated movement (Johansson & Cole 1992; Ferezou et al. 2007). In concert, with the right posterior insular cortex and visual cortex, the somatosensory cortex also governs the sense of body ownership and leads to a sense of self (Tsakiris et al. 2007). Consequently, the loss of function in the somatosensory cortex due to head trauma or stroke can produce profound physical and cognitive deficits (Dobkin 1991; Perennou 2006; Committeri et al. 2007; Castillo et al. 2008; Gallace & Spence 2008). Somatosensory cortex is vulnerable to injury because the middle cerebral artery is often affected during stroke and is the major artery that supplies the somatomotor cortex in general.
A good deal of cortical reorganization occurs naturally during recovery or partial recovery from stroke (Nelles et al. 1999), but there are limits to what the brain can achieve without therapy. Treatments aimed at improving recovery from stroke can be envisaged as comprising three stages, namely, neuroprotection, neuronal repair and functional rehabilitation. Neuroprotection is aimed at minimizing the damage immediately following injury. Neuronal repair involves replacing damaged neurons with new ones, creating new axonal pathways and sprouting connections within the cortex in order that areas unaffected by the stroke can compensate for the loss of function in the damaged areas (Dobkin 2000). Functional rehabilitation might involve both selecting the useful functional connections from the exuberant growth of connections created in the neuronal repair phase and also retraining pathways and synapses spared from any damage in order to compensate for the loss of other connections. The majority of work in the field has so far focused on neuroprotection. Work on neuronal repair and functional rehabilitation is very much in its infancy at the time of writing.
Studies aimed at understanding basic mechanisms of neuronal growth and plasticity are vital to creating successful treatments for cortical damage (Dobkin 2007). Undertaking neuronal repair involves applying an understanding of neuronal differentiation, axon guidance, neurotrophins and developmental neuroscience to increasing the sprouting and growth of new connections. Undertaking functional rehabilitation involves applying an understanding of experience-dependent plasticity mechanisms to increasing plasticity and improving the selection of new connections in the sprouted pathways. The basic synaptic plasticity mechanisms involved in this third phase of treatment form the subject of this review.
It needs to be kept in mind that increasing plasticity is not a solution to rehabilitation on its own; behavioural training plays a major role (Dobkin 2008). Not only must the synapses in question be plastic, but they must also be provided with the right sensory information to guide the formation of useful connections. It is already known that deficits in stroke can be stimulated to recovery more completely by forcing increased use of the affected neurological system. The most common example of this principle is in ‘constraint-induced therapy’ (CIT) as a treatment for a weak arm, where the unaffected arm is constrained and the patient is forced to use the affected arm (Cramer & Riley 2008). Overuse of the affected arm improves functional use of that arm. Functional brain mapping studies indicate that CIT causes an increase in remapping of arm function in peri-infarct cortex (Sutcliffe et al. 2007). The assumption from these studies is that there is an interaction between behaviourally induced activity in the affected limb and neuronal activity in the cortex that promotes recovery. It is for this reason that it is particularly important to understand synaptic plasticity mechanisms that are activated by natural sensory stimuli, a process referred to here as experience-dependent plasticity.
In this paper, some of the basic synaptic plasticity mechanisms discovered in the somatosensory cortex are reviewed. Some of the anatomical pathways involved are considered first (§2), before going on to deal with neocortical synaptic plasticity mechanisms (§3), with an emphasis on mechanisms that can be shown to be driven by natural sensory stimuli in vivo. Longer term plasticity mechanisms are then considered in the sections on structural plasticity (§4) and the role of gene expression in plasticity and stability (§5). Much of our knowledge in this area comes from studies in the barrel cortex, which is that part of the rodent cortex receiving input from the vibrissae (Woolsey & Van der Loos 1970). Coincidentally, barrel cortex also provides a useful model for stroke research both because the area affected by the infarct can be defined quite accurately with reference to the barrels (Carmichael et al. 2001) and because the ability to stimulate the whiskers provides an easy method for checking whether stimulus-induced increases in blood flow are affected by the stroke (Berwick et al. 2002; Whitaker et al. 2007).
2. Anatomical pathways mediating plasticity
(a) Pathways for potentiation and horizontal transmission
One of the characteristic features of plasticity in the somatosensory system is the horizontal spread of the body representation spared the injury or deprivation that subsequently causes a rearrangement in the cortical map of the body surface (figure 1). For example, patients with arm amputations show expansion of the face representation into the hand region of the somatosensory cortex (Flor et al. 1995; Borsook et al. 1998; Ramachandran 2005), and similarly, digit amputation will cause expansion of the representations of the neighbouring intact digits to fill the space occupied by the amputated digit (Kaas et al. 1983). Some of the expansion can be attributed to unmasking of pre-existing connections. Anaesthesia of a digit will cause the expansion of the representation for the unanaesthetized neighbouring digits (Calford & Tweedale 1991). However, over a longer time period, newly extended connections can also reinforce map changes. In monkeys with arm or digit amputation, corticocortical connections increase their horizontally span by over 3 mm on average, both within the same cortical area and across cytoarchitectonic subdivisions (Florence et al. 1998).
Figure 1.
Cortical map plasticity. (a–c) 2-Deoxyglucose mapping in barrel cortex shows expansion of the cortical domain activated by a single whisker in horizontal sections through barrel cortex. (a) Control condition showing the effect of stimulating the C3 whisker. (b) Vibrissectomy condition (follicle lesion) showing that the C3 whisker domain has expanded two months after ablating all but the C3 follicle (Kossut et al. 1988). (c) A similar expansion occurs after two-month whisker deprivation of all but the C3 whisker (Hand 1982). (d) Activation of layer II/II produced by stimulating the D1 whisker is largely confined to the D1 barrel in a normal animal (the D1 barrel is darker grey). Each circle represents the response in an electrode penetration in mouse barrel cortex grey-coded for average intensity of response (white greater than or equal to 1 spike per stimulus; light grey greater than or equal to 0.5 spikes per stimulus; black less than 0.5 spikes per stimulus). (e) The D1 domain in layer II/III after 18 days single whisker experience, i.e. the D1 whisker is spared and all the other whiskers deprived (adapted with permission from Barrel cortex, 2008, Cambridge University Press (Fox 2008)). (f) Plasticity in human somatosensory cortex following amputation of the right arm below the elbow is revealed using magnetic encephalography. In the left hemisphere the hand representation is missing and only present in the right hemisphere (green). The left hemisphere hand cortex has remodelled to respond to face (red) and upper arm (blue). (Adapted from Ramachandran & Hirstein (1998) by permission of Oxford University Press.)
Horizontal expansions of the spared sensory surface also occur due to the loss of sensory drive rather than peripheral nerve injury. For example, removing all but one whisker will cause expansion of its representation within the cortex (Hand 1982; Fox 1992; figure 1). While peripheral nerve and dorsal column injury causes subcortical as well as cortical plasticity (Sengelaub et al. 1997; Kaas et al. 2008), early studies in this field showed that the experience-dependent aspect of plasticity is cortical in origin (Fox 1994). In fact, it is difficult to detect any experience-dependent plasticity subcortically in rat ventroposteriormedial thalamic nucleus (VPm; Fox et al. 2002). Therefore, anatomical pathways subserving functional expansion of a spared sensory input should run between cortical columns to link horizontally neighbouring areas of cortex. One way of determining which pathways are involved is to see whether lesions of particular horizontal pathways prevent the expression of plasticity. In the barrel cortex, lesions of the septal region lying between the edges of two neighbouring barrels have been found to be sufficient to prevent horizontal transmission of information between the two barrels (Fox 1994). The specificity of this effect is clear from the finding that recordings made from the same animal in a neighbouring barrel that had not been cut off by a septal region, still receive input from that barrel (figure 2). In these examples, the animal had been reared with just the D1 vibrissa intact, which leads to potentiated responses in surrounding barrels (Fox 1992). The septal lesions are therefore able to eliminate the potentiated responses in surrounding barrels and in fact reduce the D1 vibrissa representation to less than that found in a normally reared animal (Fox 1994). These data suggest that a horizontal pathway exists between barrels that can, under certain rearing conditions, be potentiated. It is likely that longer range cortical pathways, such as those running between S1 and M1, may also benefit from functional rehabilitation in addition to the intercolumnar connections considered here. However, it is also likely that by understanding experience-dependent plasticity in intercolumnar pathways one can gain insight into plasticity of other cortical connections.
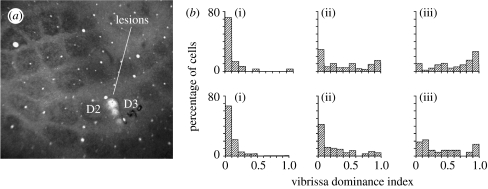
Figure 2.
Cortical pathways for horizontal transmission of information between barrels. (a) An example of three microlesions placed in between the D2 and D3 barrels. The lesions are not only shown here in layer IV, but also extend vertically into layer III. The lesions are designed to disconnect D2 from D3. In this case, the D2 whisker was spared, the D3 barrel was severed and the other barrels including, for example, D1 were connected. (b) Vibrissae dominance is affected by the type of lesion shown in (a), where the experienced barrel (D2 in that case) is severed from the deprived barrel (D3). Upper three graphs, layer II/III cells; lower three graphs, layer IV. (i) Severed: almost all the cells respond to the principal whisker and hardly any to the spared ‘experienced whisker’. (ii) Connected: this is similar to the case of the animals with no lesion shown to the right. (iii) No lesion: in a normal animal with single whisker experience most cells respond to the spared whisker (1) compared with the deprived principal whisker (0). A vibrissa dominance of 1 indicates only responses to the spared whisker, 0.5 equals response to spared and principal whisker and 0 only responses to the principal whisker. (Adapted from Fox (1994) by permission of the Society for Neuroscience.)
Considerable evidence exists for horizontal pathways in the cortex. Bernado et al. (1990a) have shown that tracer injections within barrels leads to a radiating pattern of horizontally projecting fibres in the cortex, which stretch furthest in extragranular layers (Bernardo et al. 1990a,b). Horizontal pathways have been shown to sprout in extragranular layers of cortex following stroke (Carmichael et al. 2001) and following peripheral amputation (Florence et al. 1998). Other experiments in rats have shown that septal locations contain a pattern of linked horizontal pathways within layer IV (Hoeflinger et al. 1995; Kim & Ebner 1999). It is noticeable that there is little projection from the septal layer IV cells back into the neighbouring barrel column, however, which suggests horizontal spread via the septal regions probably links back into the neighbouring barrels via their connections with extragranular layers. While the barrel and septal subdivision are peculiar to barrel cortex, they do form a useful experimental tool for distinguishing between parallel anatomical pathways that are normally indistinguishable in other species. The VPm projection is centred on the barrels while the posteriormedial thalamic nucleus projection is concentrated on the septal subdivision. In addition, projections to motor cortex tend to emanate from septal columns rather than barrel columns (Alloway et al. 2004).
Single cell fills with biocytin show that while layer IV cells principally project vertically within a column, they also send axons diagonally into neighbouring barrels (Lubke et al. 2000). Stimulation of layer IV cells can cause the activation of monosynaptic excitatory post-synaptic potentials (EPSPs) in layer II/III cells in neighbouring barrels (Hardingham et al. 2003), which implies that such axons form functional connections. Finally on this point, layer II/III cells have a large dendritic spread spanning approximately the same horizontal dimensions as the width of a barrel. Therefore, a cell would need to lie with its cell body near the centre of the barrel to avoid sampling vertically projecting input from neighbouring columns, a situation that is probabilistically unlikely. In conclusion then, the anatomical substrate clearly exists for horizontal spread of excitation between columns in supragranular layers of somatosensory cortex.
Layer V cells also show high levels of horizontal interconnectivity that might account for horizontal spread of excitation. In addition, layer V to V synapses show potentiation or depression when tested with spike pairing protocols (Markram & Tsodyks 1996; Sjostrom et al. 2003). Layer V cells also show experience-dependent plasticity similar to that of the layer II/III cells in that they exhibit depression of their centre receptive fields and potentiation of their surrounds when subject to chessboard deprivation of the whiskers (Wright et al. 2008). However, a difference exists between the intrinsic burster and regular spiking layer V cells, with the intrinsic bursters preferentially showing potentiation (Petreanu et al. 2005). Given that layer V cells are highly connected between columns, one pathway for lateral spread of excitation could be achieved via multisynaptic layer V connections and these may potentiate during experience-dependent plasticity. In conclusion, a number of cortical pathways exist that could be responsible for horizontal spread of information between columns that are also capable of synaptic plasticity.
(b) Pathways for depression and vertical transmission
Early studies on plasticity in the cortex showed that not only do the spared sensory representations expand, but also the deprived or missing sensory pathways contract. The cells in the corresponding cortical columns show weaker responses to stimulation even if deprivation is discontinued (Glazewski & Fox 1996; Glazewski et al. 1998). Depression mechanisms are likely to be valuable for rehabilitation as some reorganization that occurs after stroke can be maladaptive and may need to be downregulated to achieve a therapeutic benefit (Cheetham & Finnerty 2007). Because layer IV does not show much plasticity in older animals, the depression is likely to occur in vertical pathways projecting out of layer IV to layer II/III above and to layer V below (Glazewski & Fox 1996). This view is supported by studies that show that long-term depression (LTD) can be produced quite readily in vertical connections from layer IV to layer II/III in normal animals, and that LTD is occluded in the same pathway in whisker-deprived animals (Allen et al. 2003). It has also been shown that the probability of finding connected layer II/III cells in whisker-deprived animals decreases in the deprived barrel–column almost to zero (Cheetham et al. 2007). Since layer II/III excitatory feedback interconnections are likely to play a role in amplifying input from layer IV, this loss of interconnectivity could also result in depression of layer II/III responses.
Layer V cells in deprived columns also show centre receptive field (principal whisker) depression following whisker deprivation. It has been shown that a projection from layer II/III to layer Vb is also depressed after 10 days of whisker deprivation (Petreanu et al. 2005). In conclusion, principal whisker pathways travel within the column and can depress in at least three connections: layer II/III to II/III, layer IV to II/III and layer II/III to Vb. Other pathways are likely to be involved in depression too, but have not yet been studied at the cellular level.
3. Molecular pathways mediating plasticity
Cortical plasticity involves potentiation and depression of synapses. In the previous section, anatomical pathways were highlighted that might be affected at the circuit level by sensory deprivation. Vertical columnar pathways are subject to depression of synaptic responses following sensory deprivation while intercolumnar horizontal pathways undergo potentiation. Some of the mechanisms that might be involved in the two forms of plasticity are reviewed below.
(a) Mechanisms for potentiation
(i) N-methyl d-aspartate (NMDA) and metabotropic glutamate receptors (mGluRs)
NMDA receptors are involved in long-term potentiation (LTP) and some forms of LTD and so may also be involved in both potentiation and depression mechanisms in vivo. Unfortunately, there are limits to what can be determined in vivo about the role of post-synaptic NMDA receptors in cortical plasticity. While it is clear that cortical activity per se is important as the instigator of cortical plasticity (Wallace et al. 2001), it is not easy to demonstrate that the pathway by which this is achieved in vitro, i.e. via the NMDA receptor, is also the critical pathway in vivo. The problem arises due to post-synaptic NMDA receptors being involved in normal synaptic transmission in the neocortex (Fox et al. 1989; Armstrong-James et al. 1993). This means that blocking NMDA receptors with an NMDA antagonist can cause a decrease in sensory responses in the same way as (say) a small dose of gamma-amino butyric acid agonist would also decrease sensory responses. This problem is avoided in vitro because under the condition in which cells operate in the slice, NMDA receptors are not activated substantially, except during high-frequency stimulation or direct membrane depolarization. Therefore, blocking NMDA receptors in vitro does not affect the baseline synaptic responses. In vivo, baseline sensory responses are definitely reduced by NMDA receptor antagonists and since any factor decreasing transmission of sensory information would be likely to affect plasticity, NMDA receptors are no exception; but unfortunately owing to this fact, neither can they be shown to have a special role in plasticity in this way.
Although NMDA receptors have not been shown to play a special role in experience-dependent plasticity, they do play a role in synaptic plasticity of the layer IV to II/III pathway. Studies of LTP in mice show that NMDA receptors are required for LTP in this pathway (Hardingham et al. 2003). In addition, whisker deprivation in young mice (post-natal day (P) 12–14) alters the action of NMDA receptors and reveals a role for metabotropic glutamate receptors (mGluRs; Clem et al. 2008). Single whisker experience causes occlusion of LTP in the spared barrel's principal or ‘home’ column in the layer IV to II/III pathway. This is the opposite of what happens in the deprived column where LTP is enhanced (Allen et al. 2003). Curiously, LTP is restored in this pathway if NMDA receptors are antagonized, which could indicate that NMDA-dependent LTD is enhanced in spared columns and overwrites any LTP that might occur. The novel LTP in the spared column is prevented by antagonists for mGluRs, suggesting that they play a role in metaplastic LTP (Clem et al. 2008). Sensory deprivation has also been shown to produce an increase in strength of connections in spared columns in slightly older animals (P19) and may therefore be involved in plasticity in adult cortex (Cheetham et al. 2007).
(ii) Calcium/calmodulin-dependent kinase type II (CaMKII)
αCAMKII appears to be necessary for LTP in the hippocampus (Silva et al. 1992) and neocortex (Kirkwood et al. 1997). This kinase is able to phosphorylate α amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPA receptors; Barria et al. 1997) and is well situated to affect plasticity as it forms some 16 per cent of the post-synaptic density (Miller & Kennedy 1985). Experience-dependent potentiation in the barrel cortex has also been found to be dependent on αCAMKII, raising the possibility that experience-dependent potentiation mechanisms may be related to LTP mechanisms. Potentiation of spared vibrissae responses occurs in neurons located in layers II/III of wild-type adult mice but not in null mutants lacking the functional αCAMKII gene (Glazewski et al. 1996). An important characteristic of CAMKII is that it retains a ‘memory’ of past synaptic activity by phosphorylating itself. The threonine-286 site is where autophosphorylation normally occurs and prolongs the activity of the kinase. Point mutant mice lacking only the autophosphorylation site of αCaMKII do not express experience-dependent potentiation in the barrel cortex. (Glazewski et al. 2000).
(iii) Nitric oxide synthase (NOS)
LTP and experience-dependent plasticity in the cortex appear to be strongly dependent on the neuronal form of nitric oxide synthase (NOS1; Dachtler et al. 2008). NOS is the source of the retrograde messenger nitric oxide (NO), implicated in LTP in the hippocampus by several studies (see Son et al. 1996). NOS is activated by calmodulin and is also a substrate for phosphorylation by CaMKII (Bredt et al. 1992; Watanabe et al. 2003). Studies in the neocortex show that post-synaptically located NOS is required for plasticity in the layer IV to II/III pathway (Hardingham & Fox 2006) as shown in figure 3, and this may also be the reason why NOS is also involved in experience-dependent plasticity (Dachtler et al. 2008). Blocking post-synaptic NOS prevents presynaptic potentiation in wild-type mice while leaving the post-synaptic component intact. Blocking NOS in GluR-1 knockout mice blocks all LTP by abolishing the only component of plasticity present in these animals, i.e. the presynaptic component of LTP (Hardingham & Fox 2006).
Figure 3.
Dependence of cortical LTP on NO. (a) In wild-type mice, stimulation of the IV to II/III pathway diagonally between barrels causes LTP when a presynaptic spike is timed to occur 10 ms before a post-synaptic spike (the arrow indicates the time at which the forward pairing (FP) protocol was applied). However, plasticity is reduced if a NOS antagonist (L-NG-nitroarginine, L-NNA) is included post-synaptically via the electrode solution. Open circles, L-NNA, n=22; filled circles, control, n=24. (b) The same data in (a) are shown in 10 min averages. Note that only time points beyond 30 min are significantly different between drug treated and control, indicating the slower onset of NOS-dependent LTP in cortical neurons. (c) In GluR1 knockout mice, early LTP (less than 10 min) is reduced compared with wild types, but slowly developing LTP is intact (filled circles). However, the residual LTP present in GluR1 knockouts is completely prevented by post-synaptic NOS antagonism with intracellular L-NNA (open circles). Open circles, L-NNA, n=18; filled circles, control, n=18. (d) The 60 min average values (± s.e.m.) for the four cases shown in (a) and (c) are plotted for comparison. Filled square, control; open square, L-NNA. (Adapted from Hardingham & Fox (2006) by permission of the Society for Neuroscience.)
(b) Mechanisms for depression
Two main depression mechanisms have been found in the somatosensory cortex so far, one acting post-synaptically via the GluR1 subunit of the AMPA receptor and the other presynaptically via cannabinoid receptors. Much of the evidence gathered so far relies on in vitro studies of plasticity. The findings in vitro can be linked to findings in vivo by the observation that whisker deprivation, which itself causes depression in vivo, occludes depression in vitro (Allen et al. 2003).
(i) Protein kinase A (PKA) and GluR1
PKA is not involved in cortical LTP nor experience-dependent potentiation in the same way as CaMKII. Inhibition of PKA does not affect the magnitude of LTP in the columnar IV to II/III pathway in mouse barrel cortex (Hardingham et al. in revision). However, following whisker deprivation, LTP is larger in magnitude and sensitive to inhibition of PKA (Hardingham et al. in revision). Because whisker deprivation causes depression of synapses, the extra ability to undergo LTP may be created by depressing synapses that would otherwise be too potentiated to potentiate any further. In other words, whisker deprivation may cause desaturation. The further inference is that potentiation from the depressed state requires PKA while potentiation from the basal state does not. The mechanisms involved in PKA-dependent potentiation could also act via the GluR-1 subunit of the AMPA channel. It is possible that such depression causes dephosphorylation of the AMPA channel subunit GluR-1 at the Ser 845 site. Phosphorylation at the Ser 845 site then causes an increase in open time through the AMPA channel (Banke et al. 2000) as well as increasing surface expression of GluR1 (Man et al. 2007). Dephosphorylation of this site could therefore explain the LTP unmasked by whisker deprivation. In further support of this theory, it has been found that the depression produced by whisker deprivation does not occur in GluR1 knockouts (Wright et al. 2008).
(ii) Cannabinoid receptor-dependent depression mechanisms
The role played by GluR1 in depression of vertical layer IV to II/III pathway responses has so far only been demonstrated in adolescent animals (P 28 and older). Another depression mechanism exists in barrel cortex, which is dependent on cannabinoid receptors and has so far only been demonstrated in immature animals (P13–19; Bender et al. 2005) and (P13–15; Nevian & Sakmann 2006). Blocking cannabinoid receptors with AM251 prevents LTD induction by a spike pairing protocol. Interestingly, this form of plasticity requires presynaptic NMDA receptors. Because presynaptic receptors only occur in cortex up to approximately P23 (Corlew et al. 2007), it is possible that cannabinoid-dependent depression is limited to early life, though this has not so far been tested explicitly.
4. Structural plasticity in somatosensory cortex
Structural plasticity can involve a number of different cellular elements in the cortex including changes in dendritic spines, presynaptic terminals and axons and on a larger scale whole dendrites. In practice, the behaviours of these structural elements are coordinated, but so far they have tended to be studied separately and are therefore reviewed separately here.
(a) Dendritic spine plasticity
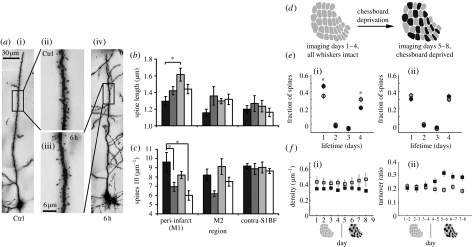
Most excitatory synapses onto excitatory cells are located at dendritic spines making them a major site for plasticity in the cortex. Spine plasticity can be gauged by how rapidly new spines appear and disappear. This process is age dependent, with greater turnover in younger animals, but is also modulated by sensory experience (Trachtenberg et al. 2002). Recent studies have suggested that spines also turnover faster in the peri-infarct area (figure 4), suggesting that they may be a natural substrate for increased plasticity during recovery of function (Brown et al. 2008).
Figure 4.
Spine plasticity is affected by stroke and somatosensory experience. (a) Golgi-Cox labelled layer V pyramidal cells; (i) and (ii), control (Ctrl); (iii) and (iv), cells in the peri-infarct region 6 hours following stroke. (b) Quantification of spine length and (c) spine density measured 0, 2, 6 and 24 hours after stroke in the peri-infarct region (peri-infarct (M1)), the ipsilateral secondary motor cortex (M2) and the contralateral barrel field (contra-S1BF). (Adapted from Brown et al. (2008) by permission of Lippincott & Williams.) (d) The pattern of barrel deprived in chessboard deprivation (black, deprived; grey, spared). (e) Spine lifetimes are plotted over 4 days for dendrites located (i) inside and (ii) outside the barrel cortex. The black circles are data means for deprived cortex and open circles for undeprived cortex. Note that transient (1 day) spines increase and persistent (4 days) spines decrease following deprivation (asterisk). (f)(i) Spine density is relatively unaffected by deprivation while (ii) spine turnover increases immediately following deprivation (note that first 4 days are undeprived). (Reprinted by permission from Macmillan Publishers Ltd: Nature, Trachtenberg et al. (2002), © 2002.)
Spines can broadly be divided into two classes on the basis of their shape and motility. In young adult mouse barrel cortex, the highly motile filopodia are long thin spines that tend to come and go within a 24 hour period and comprise approximately 20 per cent of the total population (Trachtenberg et al. 2002). At the other end of the spectrum, approximately 60 per cent tend to be bulbous-headed or mushroom spines that last at least 8 days at P42–70 (Trachtenberg et al. 2002). In even more mature mice, three to six months of age, the stable population can comprise approximately 75 per cent of the total (Holtmaat et al. 2005). These estimates of relative stability contrast with the situation in developing animals where approximately 75 per cent of the spines may turnover within 4 days at P8–12 in layer II/III (Lendvai et al. 2000).
Sensory experience can alter this background level of spontaneous change. Chessboard whisker deprivation has been found to increase the rate at which filopodia turnover and simultaneously increase the turnover of spines that have the morphology normally attributable to stable spines (figure 4). Spine turnover increases as a result of whisker deprivation, but obviously the degree of plasticity is partly a function of age, because baseline levels of spine turnover are lower in older animals. At P11–13, which is the height of the critical period for layer II/III forming connections, total whisker deprivation increases spine turnover by 37 per cent (Lendvai et al. 2000). Chessboard deprivation in animals imaged between P34–74 causes an increase in spine turnover of approximately 10 per cent (daily turnover) in the same neurons (Trachtenberg et al. 2002). This is what might be expected if cells were forming new synapses as a result of whisker deprivation. An alternative explanation might be that synapses are being formed and eliminated all the time and that whisker deprivation prevents their natural elimination with age. Deprivation at four to six weeks coincides with the period when spine dynamics are still naturally decreasing (Zuo et al. 2005). Therefore, deprivation between four and six weeks might be expected to prevent approximately 12 per cent of the spine loss that would normally occur during that period (Zuo et al. 2005). At present, the issue of whether deprivation increases the number of spines or prevents a decrease in the number of spines at these ages remains unresolved.
(b) Effect of experience on cortical presynaptic structure
Changes in spines are presumed to occur in concert with changes in presynaptic terminals. It has been shown that newly formed spines make contact with presynaptic boutons and form synapses (Trachtenberg et al. 2002). Therefore, one might expect axons to show plasticity as well as spines. In fact, similar to spines, axonal arbours are largely stable in barrel cortex over long periods of time, especially those corresponding to thalamic inputs to cortex (De Paola et al. 2006). It has been estimated that 94 per cent of the axonal arbour remains stable over a period of 24 days. However, some classes of axons reshape relatively often, and branches can elongate and retract by tens of microns over a period of one month. En passant boutons seem to be more stable than terminal end branch boutons. For example, 60 per cent of terminal boutons of layer VI recurrent projections to layer I are dynamic, while only 15 per cent of en passant boutons show losses or gains in thalamic or intracortical connections over a period of one month (De Paola et al. 2006). The most dynamic axons found to date are those of layer VI cells that project to layer I.
In developing animals, sensory experience is well known to affect the morphology of intracortical axons. Normally in the barrel cortex, intracortical axons tend to project along the rows rather than along the arcs of barrels, but complete whisker trimming from birth disrupts this bias and the connections form symmetrically around any single barrel (Keller & Carlson 1999). A similar effect can be produced in developing barrel cortex by blocking cortical NMDA receptors (Dagnew et al. 2003) suggesting that the connections require neuronal activity to form correctly. Cutting the infraorbital nerve at p7 in the mouse also prevents the proper maturation of horizontally directed transcolumnar projections from layer II/III and V cells (McCasland et al. 1992).
Peripheral nerve lesion also affects intracortical axon trajectories in mature animals. In experiments where the innervation of the vibrissae follicles is cut, except that innervating the C-row of vibrissae, axons projecting horizontally from spared barrels extend over greater distances than either control axons or axons projecting from deprived barrel–columns (Kossut & Juliano 1999). The axonal density is 70 per cent greater for the spared barrel–column projections, which suggests that the complexity of the projections also increases. These studies are in concert with the findings in monkey somatosensory cortex, that long-term digit amputation, arm amputation or even wrist fracture can alter the distribution of intracortical axons (Florence et al. 1998).
(c) Dendritic plasticity
Lesion-induced plasticity also affects dendrites in the cortex. Denervation of the vibrissae follicles causes a reorientation of dendritic arbours in layer III and IV of adult rats (Tailby et al. 2005). Layer IV stellate cells normally have their dendrites oriented towards a particular barrel (Harris & Woolsey 1981) but after denervation they lose their orientation bias and grow into surrounding septal areas. This is similar to the rat forepaw representation of somatosensory cortex where dendrites reorient relative to the boundary of the forepaw representation following denervation of the limb (Hickmott & Steen 2005). Whisker trimming does not affect the dendritic structure in layer II/III cells beyond P15 (Maravall et al. 2004) and yet follicle damage can induce new dendritic growth in layer IV in adults (Hickmott & Steen 2005; Tailby et al. 2005). This suggests that dendritic plasticity may be a possible target for functional rehabilitation where peripheral damage is involved.
5. Plasticity, stability and the role of gene expression
Stability is an important property of a system as well as plasticity, particularly in a neuronal system like the somatosensory cortex that processes similar information throughout life. Stability might involve relatively short periods of absolute stability or a level of dynamic stability where the synaptic population remains constant while individual synapses change. Of course, the question of how form is maintained despite continual turnover of molecular and cellular components is one of the central questions in biology. In the context of synaptic plasticity, it is almost more remarkable that synapses can maintain a given connection at a particular synaptic strength than it is that synapses can change state, given that the receptors, channels, kinases and other enzymes mentioned above (§3) turnover with a lifetime of hours. While one can imagine that larger assemblies of molecules might be able to maintain greater stability than single molecules or small aggregates of molecules, even entire synapses continually turnover in the cortex (§4). These observations lead on to the view that any stability present in the cortex is a dynamic stability, involving the continual replacement of molecular assemblies that either self-organize locally at a subcellular level, or are kept in equilibrium by interactions with other cells with which they make contact, including both neurons and glia.
Gene expression can play a role in organizing stable structures in at least two ways. The first is by epigenetic mechanisms, where the baseline level of production of molecules by the nucleus is able to provide the replacements for the removed and metabolized molecules. The second mechanism is by the production of proteins important for stabilizing synaptic changes that have been initiated by the early phase mechanisms described above (§3). This idea was originally introduced as the protein synthesis-dependent stage of plasticity (Dash et al. 1990) and elaborated as the synaptic-tagging hypothesis in order to explain how a single nucleus might supply necessary proteins to synapses and yet have LTP maintain synapse specificity in order to code the memory (Frey & Morris 1997). The field of gene expression in synaptic plasticity is in its infancy at present and here we only explore two examples of the type of mechanism described above. The first is the effect of the HDAC (histone deacetylase) inhibitor trichostatin on plasticity as evidence that epigenetic mechanisms play a powerful role and the other is CREB (cyclic-AMP response element binding protein) as an example of gene expression that could lead to cementing changes in synaptic transmission.
(a) Epigenetic mechanisms for providing synaptic stability
Epigenetic mechanisms are able to control gene expression at a very basic level by affecting chromatin structure and hence exposure of DNA for transcription. The exposure of particular regions of DNA is controlled by histone methylation, phosphorylation and acetylation (Levenson & Sweatt 2005). In so far as the state of DNA exposure is stable in differentiated cells, it could confer stability on the cell by maintaining relatively constant levels of production of molecules that turn over at a high rate. Many molecules present at the synapse that are directly involved in synaptic transmission are continuously turned over and recycled. For example, GluR2 recycles at the synapse at a rate determined locally by such factors as GRIP, PICK, stargazin and PSD-95 and by as yet unidentified ‘slot’ proteins that hold places in the synapse for insertion of AMPA receptors. In order to maintain this level of GluR2 turnover, GluR2 is also continuously produced at a certain rate by the nucleus and trafficked to the synapse (Shi et al. 2001). In fact, in barrel cortex, GluR2 mRNA levels have been shown to be twice that of any other GluR subunit (Wright et al. 2008), and this level of production is presumably related to its high rate of recycling.
Epigenetic mechanisms might also affect synapse function by controlling the rate of production of PKA and the relative production of catalytic and regulatory subunits. PKA is a holoenzyme that comprises two regulatory and two catalytic subunits. The regulatory subunits prevent the catalytic subunits from becoming active until they bind cyclic adenosine monophosphate (cAMP), at which point they dissociate. However, the baseline ratio of catalytic to regulatory subunit production will affect the overall level of PKA activity and the threshold needed to activate it (Chain et al. 1999). Given that PKA controls phosphorylation of AMPA receptor subunits, it could thereby control the basal state of synaptic transmission.
Histone acetylation has recently been discovered to play a role in synaptic plasticity, experience-dependent plasticity, learning and memory and neuroprotection. The HDAC inhibitor trichostatin is able to increase hippocampal LTP and long-term memory (Levenson et al. 2004). Studies on plasticity in the visual cortex have shown that trichostatin is able to increase ocular dominance plasticity, most likely via a pathway involving extracellular signal-regulated kinase and CREB (Putignano et al. 2007). HDAC inhibitors are also broadly neuroprotective in stroke (Langley et al. 2005) and might therefore not only be useful tools for increasing rehabilitation in the days and months following a stroke, but also in the protection of neuronal populations immediately following the stroke.
(b) Experience-dependent gene expression
CREB is a cyclic AMP-dependent transcription factor that regulates transcription when it is caused to dimerize, following phosphorylation by PKA. Since PKA can be activated by calcium-dependent adenylate cyclase I, gene regulation can in principle be activated by post-synaptic calcium and therefore by synaptic activity. CREB appears to be required for late-phase protein-dependent aspects of plasticity because it is required for hippocampal LTP to last longer than 2 hours (Bourtchuladze et al. 1994), for long-term plasticity in Aplysia (Alberini et al. 1994), and long-term memory in flies and mice (Bourtchuladze et al. 1994; Yin et al. 1994). Whisker deprivation in barrel cortex also produces a form of plasticity that lasts longer than 2 hours. Changes in spines associated with whisker deprivation occur over periods of days (Trachtenberg et al. 2002) and changes in receptive fields take several days to accumulate (Glazewski & Fox 1996). Because changes cannot accumulate in this way if plasticity decays rapidly, plasticity must last at least several days in this system. Consistent with this view, knockouts lacking the alpha and delta isoforms of CREB show approximately 50 per cent potentiation of the spared whisker response that is normally seen in wild-type littermates (Glazewski et al. 1999). The fact that not all plasticity is abolished in the alpha/delta knockouts may be due to a beta isoform upregulated in the barrel cortex causing a partial rescue of the mutation (Glazewski et al. 1999).
Further evidence for the role of CREB in experience-dependent plasticity comes from studying genes that are expressed due to CREB. Inducible cAMP early repressor (ICER) is a negative feedback gene that requires CREB for activation, but then acts to reduce any further CREB expression. Whisker deprivation leads to the expression of ICER, supporting the idea that CREB is activated by sensory deprivation (Bisler et al. 2002). Studies using a beta-galactosidase reporter gene (lacZ) downstream of a CREB-activated promoter also show that CREB-mediated gene transcription occurs in layer IV of the spared whisker barrel following whisker deprivation (Barth et al. 2000).
It is not presently known which genes activated by CREB might be important for plasticity in the barrel cortex. However, a number of genes associated with plasticity have promoters that bind CREB, including BDNF (brain-derived neurotrophic factor) and CPG15 (also known as neuritin-1). CPG15 is a small membrane-bound protein that regulates growth of apposing axonal and dendritic arbours (Nedivi et al. 1998; Cantallops et al. 2000) and is upregulated in barrel cortex following single whisker experience (Harwell et al. 2005). BDNF is a trophic factor that affects maturation of silent synapses (Itami et al. 2003) and LTP (Zakharenko et al. 2003; Barco et al. 2005) and is upregulated in barrel cortex during increased whisker stimulation (Rocamora et al. 1996). It is possible that these factors and other CREB-dependent genes play a role in plasticity and their relative contribution to experience-dependent plasticity will need to be determined in the future.
6. Remaining questions
There are a large number of questions that remain to be answered in this field, partly owing to the complexity of the cortex and partly due to the limitations of current methodologies. First, it is clear that plasticity mechanisms in the cortex differ from one synaptic pathway to the next, and while there are some commonalities between hippocampal CA1 and neocortical layer II/III plasticity, there are sufficient differences to warrant individual study of plasticity in individual pathways. In particular, molecular mechanisms of plasticity have not been studied in layer II/III to II/III pathways, nor between layer II/III and V, and only to a limited extent between layer V and layer V. In addition, longer-range pathways linking somatosensory cortex with motor cortex and the second somatosensory cortex have not yet been studied. All of these pathways are prime targets for functional rehabilitation. Second, the plasticity of inhibitory pathways remains an understudied area, complicated by the diversity of inhibitory cell types. Third, even though it has become clear that synapses turnover in the adult brain and that spine motility increases in the brain after a stroke, the molecular mechanisms that control spine growth and retraction are not well understood. One can imagine that direct activation of existing synapses might control homosynaptic spine retraction and perhaps initiation of spine protrusions locally on the dendrite, but the signalling pathways involved are not yet delineated. In addition, it is not known how a prospective spine finds a presynaptic terminal in order to form a synapse or vice versa. Fourth, the field's studies on plasticity mechanisms are at present very biased towards younger animals. This is partly owing to the greater technical ease with which cells can be recorded intracellularly in brain slices approximately 14 days post-natal and partly owing to the range of manipulations and measurements that can be performed in cell culture experiments. However, it is not at all clear at present that the developmental mechanisms discovered in these preparations are available to adult animals. In fact, it is known that developmental mechanisms such as unsilencing silent synapses are prevalent in young brains but undetected at present in animals older than about P24 (Rumpel et al. 2004; Hardingham & Fox 2006). This raises the question of whether plasticity in older animals might operate via different mechanisms or whether development is recapitulated in extreme circumstances such as those that occur during stroke. There is, therefore, a need to know the answer to these questions in order to develop therapies to aid recovery of function in adults where the prevalence of stroke and other debilitating injuries and diseases is the greatest.
Footnotes
One contribution of 12 to a Theme Issue ‘Sensory learning: from neural mechanisms to rehabilitation’.
References
- Alberini C.M., Ghirardi M., Metz R., Kandel E.R. C/EBP is an immediate-early gene required for the consolidation of long-term facilitation in Aplysia. Cell. 1994;76:1099–1114. doi: 10.1016/0092-8674(94)90386-7. doi:10.1016/0092-8674(94)90386-7 [DOI] [PubMed] [Google Scholar]
- Allen C.B., Celikel T., Feldman D.E. Long-term depression induced by sensory deprivation during cortical map plasticity in vivo. Nat. Neurosci. 2003;6:291–299. doi: 10.1038/nn1012. doi:10.1038/nn1012 [DOI] [PubMed] [Google Scholar]
- Alloway K.D., Zhang M., Chakrabarti S. Septal columns in rodent barrel cortex: functional circuits for modulating whisking behavior. J. Comp. Neurol. 2004;480:299–309. doi: 10.1002/cne.20339. doi:10.1002/cne.20339 [DOI] [PubMed] [Google Scholar]
- Armstrong-James M., Welker E., Callahan C.A. The contribution of NMDA and non-NMDA receptors to fast and slow transmission of sensory information in the rat SI barrel cortex. J. Neurosci. 1993;13:2149–2160. doi: 10.1523/JNEUROSCI.13-05-02149.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banke T.G., Bowie D., Lee H., Huganir R.L., Schousboe A., Traynelis S.F. Control of GluR1 AMPA receptor function by cAMP-dependent protein kinase. J. Neurosci. 2000;20:89–102. doi: 10.1523/JNEUROSCI.20-01-00089.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barco A., Patterson S., Alarcon J.M., Gromova P., Mata-Roig M., Morozov A., Kandel E.R. Gene expression profiling of facilitated L-LTP in VP16-CREB mice reveals that BDNF is critical for the maintenance of LTP and its synaptic capture. Neuron. 2005;48:123–137. doi: 10.1016/j.neuron.2005.09.005. doi:10.1016/j.neuron.2005.09.005 [DOI] [PubMed] [Google Scholar]
- Barria A., Muller D., Derkach V., Griffith L.C., Soderling T.R. Regulatory phosphorylation of AMPA-type glutamate receptors by CaM-KII during long-term potentiation. Science. 1997;276:2042–2045. doi: 10.1126/science.276.5321.2042. doi:10.1126/science.276.5321.2042 [DOI] [PubMed] [Google Scholar]
- Barth A.L., McKenna M., Glazewski S., Hill P., Impey S., Storm D., Fox K. Upregulation of cAMP response element-mediated gene expression during experience-dependent plasticity in adult neocortex. J. Neurosci. 2000;20:4206–4216. doi: 10.1523/JNEUROSCI.20-11-04206.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender, K. J., Allen, C. B. & Feldman, D. E. 2005 Characterization of whisker deprivation-induced synaptic depression in rat somatosensory cortex. In Society for Neuroscience Annual Meeting Abstract Viewer/Itinerary Planner, no. 26.3. Washington, DC: Society for Neuroscience. [DOI] [PMC free article] [PubMed]
- Bernardo K.L., McCasland J.S., Woolsey T.A. Local axonal trajectories in mouse barrel cortex. Exp. Brain Res. 1990a;82:247–253. doi: 10.1007/BF00231244. doi:10.1007/BF00231244 [DOI] [PubMed] [Google Scholar]
- Bernardo K.L., McCasland J.S., Woolsey T.A., Strominger R.N. Local intra- and interlaminar connections in mouse barrel cortex. J. Comp. Neurol. 1990b;291:231–255. doi: 10.1002/cne.902910207. doi:10.1002/cne.902910207 [DOI] [PubMed] [Google Scholar]
- Berwick J., Martin C., Martindale J., Jones M., Johnston D., Zheng Y., Redgrave P., Mayhew J. Hemodynamic response in the unanesthetized rat: intrinsic optical imaging and spectroscopy of the barrel cortex. J. Cereb. Blood Flow Metab. 2002;22:670–679. doi: 10.1097/00004647-200206000-00005. doi:10.1097/00004647-200206000-00005 [DOI] [PubMed] [Google Scholar]
- Bisler S., Schleicher A., Gass P., Stehle J.H., Zilles K., Staiger J.F. Expression of c-Fos, ICER, Krox-24 and JunB in the whisker-to-barrel pathway of rats: time course of induction upon whisker stimulation by tactile exploration of an enriched environment. J. Chem. Neuroanat. 2002;23:187–198. doi: 10.1016/s0891-0618(01)00155-7. doi:10.1016/S0891-0618(01)00155-7 [DOI] [PubMed] [Google Scholar]
- Borsook D., et al. Acute plasticity in the human somatosensory cortex following amputation. Neuroreport. 1998;9:1013–1017. doi: 10.1097/00001756-199804200-00011. doi:10.1097/00001756-199804200-00011 [DOI] [PubMed] [Google Scholar]
- Bourtchuladze R., Frenguelli B., Blendy J., Cioffi D., Schutz G., Silva A.J. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell. 1994;79:59–68. doi: 10.1016/0092-8674(94)90400-6. doi:10.1016/0092-8674(94)90400-6 [DOI] [PubMed] [Google Scholar]
- Bredt D.S., Ferris C.D., Snyder S.H. Nitric oxide synthase regulatory sites. Phosphorylation by cyclic AMP-dependent protein kinase, protein kinase C, and calcium/calmodulin protein kinase; identification of flavin and calmodulin binding sites. J. Biol. Chem. 1992;267:10 976–10 981. [PubMed] [Google Scholar]
- Brown C.E., Wong C., Murphy T.H. Rapid morphologic plasticity of peri-infarct dendritic spines after focal ischemic stroke. Stroke. 2008;39:1286–1291. doi: 10.1161/STROKEAHA.107.498238. doi:10.1161/STROKEAHA.107.498238 [DOI] [PubMed] [Google Scholar]
- Calford M.B., Tweedale R. Immediate expansion of receptive fields of neurons in area 3b of macaque monkeys after digit denervation. Somatosens. Mot. Res. 1991;8:249–260. doi: 10.3109/08990229109144748. [DOI] [PubMed] [Google Scholar]
- Cantallops I., Haas K., Cline H.T. Postsynaptic CPG15 promotes synaptic maturation and presynaptic axon arbor elaboration in vivo. Nat. Neurosci. 2000;3:1004–1011. doi: 10.1038/79823. doi:10.1038/79823 [DOI] [PubMed] [Google Scholar]
- Carmichael S.T., Wei L., Rovainen C.M., Woolsey T.A. New patterns of intracortical projections after focal cortical stroke. Neurobiol. Dis. 2001;8:910–922. doi: 10.1006/nbdi.2001.0425. doi:10.1006/nbdi.2001.0425 [DOI] [PubMed] [Google Scholar]
- Castillo E.M., Boake C., Breier J.I., Men D., Garza H.M., Passaro A., Papanicolaou A.C. Aberrant cortical functionality and somatosensory deficits after stroke. J. Clin. Neurophysiol. 2008;25:132–138. doi: 10.1097/WNP.0b013e318176c0d4. doi:10.1097/WNP.0b013e318176c0d4 [DOI] [PubMed] [Google Scholar]
- Chain D.G., et al. Mechanisms for generating the autonomous cAMP-dependent protein kinase required for long-term facilitation in Aplysia. Neuron. 1999;22:147–156. doi: 10.1016/s0896-6273(00)80686-8. doi:10.1016/S0896-6273(00)80686-8 [DOI] [PubMed] [Google Scholar]
- Cheetham C.E., Finnerty G. Plasticity and its role in neurological diseases of the adult nervous system. Adv. Clin. Neurosci. Rehabil. 2007;7:8–9. [PMC free article] [PubMed] [Google Scholar]
- Cheetham C.E., Hammond M.S., Edwards C.E., Finnerty G.T. Sensory experience alters cortical connectivity and synaptic function site specifically. J. Neurosci. 2007;27:3456–3465. doi: 10.1523/JNEUROSCI.5143-06.2007. doi:10.1523/JNEUROSCI.5143-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clem R.L., Celikel T., Barth A.L. Ongoing in vivo experience triggers synaptic metaplasticity in the neocortex. Science. 2008;319:101–104. doi: 10.1126/science.1143808. doi:10.1126/science.1143808 [DOI] [PubMed] [Google Scholar]
- Committeri G., et al. Neural bases of personal and extrapersonal neglect in humans. Brain. 2007;130:431–441. doi: 10.1093/brain/awl265. doi:10.1093/brain/awl265 [DOI] [PubMed] [Google Scholar]
- Corlew R., Wang Y., Ghermazien H., Erisir A., Philpot B.D. Developmental switch in the contribution of presynaptic and postsynaptic NMDA receptors to long-term depression. J. Neurosci. 2007;27:9835–9845. doi: 10.1523/JNEUROSCI.5494-06.2007. doi:10.1523/JNEUROSCI.5494-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer S.C., Riley J.D. Neuroplasticity and brain repair after stroke. Curr. Opin. Neurol. 2008;21:76–82. doi: 10.1097/WCO.0b013e3282f36cb6. [DOI] [PubMed] [Google Scholar]
- Dachtler, J., Wright, N., Glazewski, S. & Fox, K. 2008 The role of nitric oxide in experience-dependent plasticity in the mouse barrel cortex. In Society for Neuroscience Annual Meeting Neuroscience meeting planner, no. 39.9. Washington, DC: Society for Neuroscience.
- Dagnew E., Latchamsetty K., Erinjeri J.P., Miller B., Fox K., Woolsey T.A. Glutamate receptor blockade alters the development of intracortical connections in rat barrel cortex. Somatosens. Mot. Res. 2003;20:77–84. doi: 10.1080/0899022031000083852. doi:10.1080/0899022031000083852 [DOI] [PubMed] [Google Scholar]
- Dash P.K., Hochner B., Kandel E.R. Injection of the cAMP-responsive element into the nucleus of Aplysia sensory neurons blocks long-term facilitation. Nature. 1990;345:718–721. doi: 10.1038/345718a0. doi:10.1038/345718a0 [DOI] [PubMed] [Google Scholar]
- De Paola V., Holtmaat A., Knott G., Song S., Wilbrecht L., Caroni P., Svoboda K. Cell type-specific structural plasticity of axonal branches and boutons in the adult neocortex. Neuron. 2006;49:861–875. doi: 10.1016/j.neuron.2006.02.017. doi:10.1016/j.neuron.2006.02.017 [DOI] [PubMed] [Google Scholar]
- Dobkin B.H. The rehabilitation of elderly stroke patients. Clin. Geriatr. Med. 1991;7:507–523. [PubMed] [Google Scholar]
- Dobkin B.H. Functional rewiring of brain and spinal cord after injury: the three Rs of neural repair and neurological rehabilitation. Curr. Opin. Neurol. 2000;13:655–659. doi: 10.1097/00019052-200012000-00007. doi:10.1097/00019052-200012000-00007 [DOI] [PubMed] [Google Scholar]
- Dobkin B.H. Curiosity and cure: translational research strategies for neural repair-mediated rehabilitation. Dev. Neurobiol. 2007;67:1133–1147. doi: 10.1002/dneu.20514. doi:10.1002/dneu.20514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobkin B.H. Training and exercise to drive poststroke recovery. Nat. Clin. Pract. Neurol. 2008;4:76–85. doi: 10.1038/ncpneuro0709. doi:10.1038/ncpneuro0709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferezou I., Haiss F., Gentet L.J., Aronoff R., Weber B., Petersen C.C. Spatiotemporal dynamics of cortical sensorimotor integration in behaving mice. Neuron. 2007;56:907–923. doi: 10.1016/j.neuron.2007.10.007. doi:10.1016/j.neuron.2007.10.007 [DOI] [PubMed] [Google Scholar]
- Flor H., Elbert T., Knecht S., Wienbruch C., Pantev C., Birbaumer N., Larbig W., Taub E. Phantom-limb pain as a perceptual correlate of cortical reorganization following arm amputation. Nature. 1995;375:482–484. doi: 10.1038/375482a0. doi:10.1038/375482a0 [DOI] [PubMed] [Google Scholar]
- Florence S.L., Taub H.B., Kaas J.H. Large-scale sprouting of cortical connections after peripheral injury in adult macaque monkeys. Science. 1998;282:1117–1121. doi: 10.1126/science.282.5391.1117. doi:10.1126/science.282.5391.1117 [DOI] [PubMed] [Google Scholar]
- Fox K. A critical period for experience-dependent synaptic plasticity in rat barrel cortex. J. Neurosci. 1992;12:1826–1838. doi: 10.1523/JNEUROSCI.12-05-01826.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox K. The cortical component of experience-dependent synaptic plasticity in the rat barrel cortex. J. Neurosci. 1994;14:7665–7679. doi: 10.1523/JNEUROSCI.14-12-07665.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox K. Cambridge University Press; Cambridge, UK: 2008. Barrel cortex. [Google Scholar]
- Fox K., Sato H., Daw N. The location and function of NMDA receptors in cat and kitten visual cortex. J. Neurosci. 1989;9:2443–2454. doi: 10.1523/JNEUROSCI.09-07-02443.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox K., Wallace H., Glazewski S. Is there a thalamic component to experience-dependent cortical plasticity? Phil. Trans. R. Soc. B. 2002;357:1709–1715. doi: 10.1098/rstb.2002.1169. doi:10.1098/rstb.2002.1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey U., Morris R.G. Synaptic tagging and long-term potentiation. Nature. 1997;385:533–536. doi: 10.1038/385533a0. doi:10.1038/385533a0 [DOI] [PubMed] [Google Scholar]
- Gallace A., Spence C. The cognitive and neural correlates of “tactile consciousness”: a multisensory perspective. Conscious Cogn. 2008;17:370–407. doi: 10.1016/j.concog.2007.01.005. doi:10.1016/j.concog.2007.01.005 [DOI] [PubMed] [Google Scholar]
- Glazewski S., Fox K. Time course of experience-dependent synaptic potentiation and depression in barrel cortex of adolescent rats. J. Neurophysiol. 1996;75:1714–1729. doi: 10.1152/jn.1996.75.4.1714. [DOI] [PubMed] [Google Scholar]
- Glazewski S., Chen C.M., Silva A., Fox K. Requirement for alpha-CaMKII in experience-dependent plasticity of the barrel cortex. Science. 1996;272:421–423. doi: 10.1126/science.272.5260.421. doi:10.1126/science.272.5260.421 [DOI] [PubMed] [Google Scholar]
- Glazewski S., McKenna M., Jacquin M., Fox K. Experience-dependent depression of vibrissae responses in adolescent rat barrel cortex. Eur. J. Neurosci. 1998;10:2107–2116. doi: 10.1046/j.1460-9568.1998.00222.x. doi:10.1046/j.1460-9568.1998.00222.x [DOI] [PubMed] [Google Scholar]
- Glazewski S., Barth A.L., Wallace H., McKenna M., Silva A., Fox K. Impaired experience-dependent plasticity in barrel cortex of mice lacking the alpha and delta isoforms of CREB. Cereb. Cortex. 1999;9:249–256. doi: 10.1093/cercor/9.3.249. doi:10.1093/cercor/9.3.249 [DOI] [PubMed] [Google Scholar]
- Glazewski S., Giese K.P., Silva A., Fox K. The role of alpha-CaMKII autophosphorylation in neocortical experience-dependent plasticity. Nat. Neurosci. 2000;3:911–918. doi: 10.1038/78820. doi:10.1038/78820 [DOI] [PubMed] [Google Scholar]
- Hand P.J. Plasticity of the rat barrel system. In: Morrison A.R., Strick P.L., editors. Changing Concepts of the Nervous System. Academic Press; New York, NY: 1982. pp. 49–68. [Google Scholar]
- Hardingham N., Fox K. The role of nitric oxide and GluR1 in presynaptic and postsynaptic components of neocortical potentiation. J. Neurosci. 2006;26:7395–7404. doi: 10.1523/JNEUROSCI.0652-06.2006. doi:10.1523/JNEUROSCI.0652-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham N., Glazewski S., Pakhotin P., Mizuno K., Chapman P.F., Giese K.P., Fox K. Neocortical long-term potentiation and experience-dependent synaptic plasticity require alpha-calcium/calmodulin-dependent protein kinase II autophosphorylation. J. Neurosci. 2003;23:4428–4436. doi: 10.1523/JNEUROSCI.23-11-04428.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham, N. R., Wright, N., Dachtler, J. & Fox, K. In revision. Sensory deprivation unmasks a PKA-dependent synaptic plasticity mechanism that operates in parallel with CaMKII. Neuron60 [DOI] [PubMed]
- Harris R.M., Woolsey T.A. Dendritic plasticity in mouse barrel cortex following postnatal vibrissa follicle damage. J. Comp. Neurol. 1981;196:357–376. doi: 10.1002/cne.901960302. doi:10.1002/cne.901960302 [DOI] [PubMed] [Google Scholar]
- Harwell C., Burbach B., Svoboda K., Nedivi E. Regulation of cpg15 expression during single whisker experience in the barrel cortex of adult mice. J. Neurobiol. 2005;65:85–96. doi: 10.1002/neu.20176. doi:10.1002/neu.20176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickmott P.W., Steen P.A. Large-scale changes in dendritic structure during reorganization of adult somatosensory cortex. Nat. Neurosci. 2005;8:140–142. doi: 10.1038/nn1384. doi:10.1038/nn1384 [DOI] [PubMed] [Google Scholar]
- Hoeflinger B.F., Bennett-Clarke C.A., Chiaia N.L., Killackey H.P., Rhoades R.W. Patterning of local intracortical projections within the vibrissae representation of rat primary somatosensory cortex. J. Comp. Neurol. 1995;354:551–563. doi: 10.1002/cne.903540406. doi:10.1002/cne.903540406 [DOI] [PubMed] [Google Scholar]
- Holtmaat A.J., Trachtenberg J.T., Wilbrecht L., Shepherd G.M., Zhang X., Knott G.W., Svoboda K. Transient and persistent dendritic spines in the neocortex in vivo. Neuron. 2005;45:279–291. doi: 10.1016/j.neuron.2005.01.003. doi:10.1016/j.neuron.2005.01.003 [DOI] [PubMed] [Google Scholar]
- Itami C., Kimura F., Kohno T., Matsuoka M., Ichikawa M., Tsumoto T., Nakamura S. Brain-derived neurotrophic factor-dependent unmasking of “silent” synapses in the developing mouse barrel cortex. Proc. Natl Acad. Sci. USA. 2003;100:13 069–13 074. doi: 10.1073/pnas.2131948100. doi:10.1073/pnas.2131948100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson R.S., Cole K.J. Sensory-motor coordination during grasping and manipulative actions. Curr. Opin. Neurobiol. 1992;2:815–823. doi: 10.1016/0959-4388(92)90139-c. doi:10.1016/0959-4388(92)90139-C [DOI] [PubMed] [Google Scholar]
- Kaas J.H., Merzenich M.M., Killackey H.P. The reorganization of somatosensory cortex following peripheral nerve damage in adult and developing mammals. Annu. Rev. Neurosci. 1983;6:325–356. doi: 10.1146/annurev.ne.06.030183.001545. doi:10.1146/annurev.ne.06.030183.001545 [DOI] [PubMed] [Google Scholar]
- Kaas J.H., Qi H.X., Burish M.J., Gharbawie O.A., Onifer S.M., Massey J.M. Cortical and subcortical plasticity in the brains of humans, primates, and rats after damage to sensory afferents in the dorsal columns of the spinal cord. Exp. Neurol. 2008;209:407–416. doi: 10.1016/j.expneurol.2007.06.014. doi:10.1016/j.expneurol.2007.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller A., Carlson G.C. Neonatal whisker clipping alters intracortical, but not thalamocortical projections, in rat barrel cortex. J. Comp. Neurol. 1999;412:83–94. doi: 10.1002/(sici)1096-9861(19990913)412:1<83::aid-cne6>3.0.co;2-7. doi:10.1002/(SICI)1096-9861(19990913)412:1<83::AID-CNE6>3.0.CO;2-7 [DOI] [PubMed] [Google Scholar]
- Kim U., Ebner F.F. Barrels and septa: separate circuits in rat barrels field cortex. J. Comp. Neurol. 1999;408:489–505. doi:10.1002/(SICI)1096-9861(19990614)408:4<489::AID-CNE4>3.0.CO;2-E [PubMed] [Google Scholar]
- Kirkwood A., Silva A., Bear M.F. Age-dependent decrease of synaptic plasticity in the neocortex of alphaCaMKII mutant mice. Proc. Natl Acad. Sci. USA. 1997;94:3380–3383. doi: 10.1073/pnas.94.7.3380. doi:10.1073/pnas.94.7.3380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kossut M., Juliano S.L. Anatomical correlates of representational map reorganization induced by partial vibrissectomy in the barrel cortex of adult mice. Neuroscience. 1999;92:807–817. doi: 10.1016/s0306-4522(98)00722-2. doi:10.1016/S0306-4522(98)00722-2 [DOI] [PubMed] [Google Scholar]
- Kossut M., Hand P.J., Greenberg J., Hand C.L. Single vibrissal cortical column in SI cortex of rat and its alterations in neonatal and adult vibrissa-deafferented animals: a quantitative 2DG study. J. Neurophysiol. 1988;2:829–852. doi: 10.1152/jn.1988.60.2.829. [DOI] [PubMed] [Google Scholar]
- Langley B., Gensert J.M., Beal M.F., Ratan R.R. Remodeling chromatin and stress resistance in the central nervous system: histone deacetylase inhibitors as novel and broadly effective neuroprotective agents. Curr. Drug Targets CNS Neurol. Disord. 2005;4:41–50. doi: 10.2174/1568007053005091. doi:10.2174/1568007053005091 [DOI] [PubMed] [Google Scholar]
- Lendvai B., Stern E.A., Chen B., Svoboda K. Experience-dependent plasticity of dendritic spines in the developing rat barrel cortex in vivo. Nature. 2000;404:876–881. doi: 10.1038/35009107. doi:10.1038/35009107 [DOI] [PubMed] [Google Scholar]
- Levenson J.M., Sweatt J.D. Epigenetic mechanisms in memory formation. Nat. Rev. Neurosci. 2005;6:108–118. doi: 10.1038/nrn1604. doi:10.1038/nrn1604 [DOI] [PubMed] [Google Scholar]
- Levenson J.M., O'Riordan K.J., Brown K.D., Trinh M.A., Molfese D.L., Sweatt J.D. Regulation of histone acetylation during memory formation in the hippocampus. J. Biol. Chem. 2004;279:40 545–40 559. doi: 10.1074/jbc.M402229200. doi:10.1074/jbc.M402229200 [DOI] [PubMed] [Google Scholar]
- Lubke J., Egger V., Sakmann B., Feldmeyer D. Columnar organization of dendrites and axons of single and synaptically coupled excitatory spiny neurons in layer 4 of the rat barrel cortex. J. Neurosci. 2000;20:5300–5311. doi: 10.1523/JNEUROSCI.20-14-05300.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man H.Y., Sekine-Aizawa Y., Huganir R.L. Regulation of {alpha}-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor trafficking through PKA phosphorylation of the Glu receptor 1 subunit. Proc. Natl Acad. Sci USA. 2007;104:3579–3584. doi: 10.1073/pnas.0611698104. doi:10.1073/pnas.0611698104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maravall M., Koh I.Y., Lindquist W.B., Svoboda K. Experience-dependent changes in basal dendritic branching of layer 2/3 pyramidal neurons during a critical period for developmental plasticity in rat barrel cortex. Cereb. Cortex. 2004;14:655–664. doi: 10.1093/cercor/bhh026. doi:10.1093/cercor/bhh026 [DOI] [PubMed] [Google Scholar]
- Markram H., Tsodyks M. Redistribution of synaptic efficacy between neocortical pyramidal neurons. Nature. 1996;382:807–810. doi: 10.1038/382807a0. doi:10.1038/382807a0 [DOI] [PubMed] [Google Scholar]
- McCasland J.S., Bernardo K.L., Probst K.L., Woolsey T.A. Cortical local circuit axons do not mature after early deafferentation. Proc. Natl Acad. Sci. USA. 1992;89:1832–1836. doi: 10.1073/pnas.89.5.1832. doi:10.1073/pnas.89.5.1832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S.G., Kennedy M.B. Distinct forebrain and cerebellar isozymes of type II Ca2+/calmodulin-dependent protein kinase associate differently with the postsynaptic density fraction. J. Biol. Chem. 1985;260:9039–9046. [PubMed] [Google Scholar]
- Nedivi E., Wu G.Y., Cline H.T. Promotion of dendritic growth by CPG15, an activity-induced signaling molecule. Science. 1998;281:1863–1866. doi: 10.1126/science.281.5384.1863. doi:10.1126/science.281.5384.1863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelles G., Spiekermann G., Jueptner M., Leonhardt G., Muller S., Gerhard H., Diener H.C. Reorganization of sensory and motor systems in hemiplegic stroke patients. A positron emission tomography study. Stroke. 1999;30:1510–1516. doi: 10.1161/01.str.30.8.1510. [DOI] [PubMed] [Google Scholar]
- Nevian T., Sakmann B. Spine Ca2+ signaling in spike-timing-dependent plasticity. J. Neurosci. 2006;26:11 001–11 013. doi: 10.1523/JNEUROSCI.1749-06.2006. doi:10.1523/JNEUROSCI.1749-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perennou D. Postural disorders and spatial neglect in stroke patients: a strong association. Restor. Neurol. Neurosci. 2006;24:319–334. [PubMed] [Google Scholar]
- Petreanu, L., Shepherd, G. M. & Svoboda, K. 2005 Laser scanning photostimulation reveals that two classes of layer 5B neurons mediate distinct aspects of experience-dependent plasticity. In Society for Neuroscience Annual Meeting Abstract viewer/Itinerary planner, no. 985.2. Washington, DC: Society for Neuroscience.
- Putignano E., Lonetti G., Cancedda L., Ratto G., Costa M., Maffei L., Pizzorusso T. Developmental downregulation of histone posttranslational modifications regulates visual cortical plasticity. Neuron. 2007;53:747–759. doi: 10.1016/j.neuron.2007.02.007. doi:10.1016/j.neuron.2007.02.007 [DOI] [PubMed] [Google Scholar]
- Ramachandran V.S. Plasticity and functional recovery in neurology. Clin. Med. 2005;5:368–373. doi: 10.7861/clinmedicine.5-4-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran V.S., Hirstein W. The perception of phantom limbs. The D.O. Hebb lecture. Brain. 1998;121:1603–1630. doi: 10.1093/brain/121.9.1603. doi:10.1093/brain/121.9.1603 [DOI] [PubMed] [Google Scholar]
- Rocamora N., Welker E., Pascual M., Soriano E. Upregulation of BDNF mRNA expression in the barrel cortex of adult mice after sensory stimulation. J. Neurosci. 1996;16:4411–4419. doi: 10.1523/JNEUROSCI.16-14-04411.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumpel S., Kattenstroth G., Gottmann K. Silent synapses in the immature visual cortex: layer-specific developmental regulation. J. Neurophysiol. 2004;91:1097–1101. doi: 10.1152/jn.00443.2003. doi:10.1152/jn.00443.2003 [DOI] [PubMed] [Google Scholar]
- Sengelaub D.R., Muja N., Mills A.C., Myers W.A., Churchill J.D., Garraghty P.E. Denervation-induced sprouting of intact peripheral afferents into the cuneate nucleus of adult rats. Brain Res. 1997;769:256–262. doi: 10.1016/s0006-8993(97)00708-7. doi:10.1016/S0006-8993(97)00708-7 [DOI] [PubMed] [Google Scholar]
- Shi S., Hayashi Y., Esteban J.A., Malinow R. Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell. 2001;105:331–343. doi: 10.1016/s0092-8674(01)00321-x. doi:10.1016/S0092-8674(01)00321-X [DOI] [PubMed] [Google Scholar]
- Silva A.J., Stevens C.F., Tonegawa S., Wang Y. Deficient hippocampal long-term potentiation in alpha-calcium-calmodulin kinase II mutant mice. Science. 1992;257:201–206. doi: 10.1126/science.1378648. doi:10.1126/science.1378648 [DOI] [PubMed] [Google Scholar]
- Sjostrom P.J., Turrigiano G.G., Nelson S.B. Neocortical LTD via coincident activation of presynaptic NMDA and cannabinoid receptors. Neuron. 2003;39:641–654. doi: 10.1016/s0896-6273(03)00476-8. doi:10.1016/S0896-6273(03)00476-8 [DOI] [PubMed] [Google Scholar]
- Son H., Hawkins R.D., Martin K., Kiebler M., Huang P.L., Fishman M.C., Kandel E.R. Long-term potentiation is reduced in mice that are doubly mutant in endothelial and neuronal nitric oxide synthase. Cell. 1996;87:1015–1023. doi: 10.1016/s0092-8674(00)81796-1. doi:10.1016/S0092-8674(00)81796-1 [DOI] [PubMed] [Google Scholar]
- Sutcliffe T.L., Gaetz W.C., Logan W.J., Cheyne D.O., Fehlings D.L. Cortical reorganization after modified constraint-induced movement therapy in pediatric hemiplegic cerebral palsy. J. Child. Neurol. 2007;22:1281–1287. doi: 10.1177/0883073807307084. doi:10.1177/0883073807307084 [DOI] [PubMed] [Google Scholar]
- Tailby C., Wright L.L., Metha A.B., Calford M.B. Activity-dependent maintenance and growth of dendrites in adult cortex. Proc. Natl Acad. Sci. USA. 2005;102:4631–4636. doi: 10.1073/pnas.0402747102. doi:10.1073/pnas.0402747102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachtenberg J.T., Chen B.E., Knott G.W., Feng G., Sanes J.R., Welker E., Svoboda K. Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature. 2002;420:788–794. doi: 10.1038/nature01273. doi:10.1038/nature01273 [DOI] [PubMed] [Google Scholar]
- Tsakiris M., Hesse M.D., Boy C., Haggard P., Fink G.R. Neural signatures of body ownership: a sensory network for bodily self-consciousness. Cereb. Cortex. 2007;17:2235–2244. doi: 10.1093/cercor/bhl131. doi:10.1093/cercor/bhl131 [DOI] [PubMed] [Google Scholar]
- Wallace H., Glazewski S., Liming K., Fox K. The role of cortical activity in experience-dependent potentiation and depression of sensory responses in rat barrel cortex. J. Neurosci. 2001;21:3881–3894. doi: 10.1523/JNEUROSCI.21-11-03881.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y., Song T., Sugimoto K., Horii M., Araki N., Tokumitsu H., Tezuka T., Yamamoto T., Tokuda M. Post-synaptic density-95 promotes calcium/calmodulin-dependent protein kinase II-mediated Ser847 phosphorylation of neuronal nitric oxide synthase. Biochem. J. 2003;372:465–471. doi: 10.1042/BJ20030380. doi:10.1042/BJ20030380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker V.R., Cui L., Miller S., Yu S.P., Wei L. Whisker stimulation enhances angiogenesis in the barrel cortex following focal ischemia in mice. J. Cereb. Blood Flow Metab. 2007;27:57–68. doi: 10.1038/sj.jcbfm.9600318. doi:10.1038/sj.jcbfm.9600318 [DOI] [PubMed] [Google Scholar]
- Woolsey T.A., Van der Loos H. The structural organization of layer IV in the somatosensory region (SI) of mouse cerebral cortex. The description of a cortical field composed of discrete cytoarchitectonic units. Brain Res. 1970;17:205–242. doi: 10.1016/0006-8993(70)90079-x. doi:10.1016/0006-8993(70)90079-X [DOI] [PubMed] [Google Scholar]
- Wright N., Glazewski S., Hardingham N., Phillips K., Pervolaraki E., Fox K. Laminar analysis of the role of GluR1 in experience-dependent and synaptic depression of sensory responses in barrel cortex. Nat. Neurosci. 2008;11:1140–1142. doi: 10.1038/nn.2188. doi:10.1038/nn.2188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J.C., Wallach J.S., Del Vecchio M., Wilder E.L., Zhou H., Quinn W.G., Tully T. Induction of a dominant negative CREB transgene specifically blocks long-term memory in Drosophila. Cell. 1994;79:49–58. doi: 10.1016/0092-8674(94)90399-9. doi:10.1016/0092-8674(94)90399-9 [DOI] [PubMed] [Google Scholar]
- Zakharenko S.S., Patterson S.L., Dragatsis I., Zeitlin S.O., Siegelbaum S.A., Kandel E.R., Morozov A. Presynaptic BDNF required for a presynaptic but not postsynaptic component of LTP at hippocampal CA1-CA3 synapses. Neuron. 2003;39:975–990. doi: 10.1016/s0896-6273(03)00543-9. doi:10.1016/S0896-6273(03)00543-9 [DOI] [PubMed] [Google Scholar]
- Zuo Y., Yang G., Kwon E., Gan W.B. Long-term sensory deprivation prevents dendritic spine loss in primary somatosensory cortex. Nature. 2005;436:261–265. doi: 10.1038/nature03715. doi:10.1038/nature03715 [DOI] [PubMed] [Google Scholar]