Abstract
The mechanisms underlying successful biological invasions often remain unclear. In the case of the tropical water flea Daphnia lumholtzi, which invaded North America, it has been suggested that this species possesses a high thermal tolerance, which in the course of global climate change promotes its establishment and rapid spread. However, D. lumholtzi has an additional remarkable feature: it is the only water flea that forms rigid head spines in response to chemicals released in the presence of fishes. These morphologically (phenotypically) plastic traits serve as an inducible defence against these predators. Here, we show in controlled mesocosm experiments that the native North American species Daphnia pulicaria is competitively superior to D. lumholtzi in the absence of predators. However, in the presence of fish predation the invasive species formed its defences and became dominant. This observation of a predator-mediated switch in dominance suggests that the inducible defence against fish predation may represent a key adaptation for the invasion success of D. lumholtzi.
Keywords: biological invasion, fish predation, inducible defence, invasion success, key adaptation, phenotypic plasticity
1. Introduction
Biological invasions (i.e. the successful establishment and spread of species outside their native range) have become important topics for ecology, evolutionary biology and biogeography. The frequency and effects of invasions have accelerated due to human activity and increasing globalization (Lodge 1993; Vitousek et al. 1997), and there are now many examples of successful invasive species across diverse habitats, geographical regions and taxonomic groups (e.g. Mack et al. 2000; Sakai et al. 2001; Jeschke & Strayer 2005). Severe economic and ecological consequences (e.g. Mack et al. 2000) demonstrate the urgent need to understand the population biology of invaders and their interactions with the recipient communities (Sakai et al. 2001; Shea & Chesson 2002; Facon et al. 2006). Invasive species have been recognized as a major driver of biodiversity loss (Millennium Ecosystem Assessment 2005). Successful invasions provide interesting field experiments for the analysis of adaptation and competition. If these new habitats are already populated, the exotic species have to compete against native species, which have been long term adapted to the specific environmental conditions.
Previous research in invasion biology has suggested some characteristics that make a community susceptible to invasions (Orians 1986; Lodge 1993), and attributes that distinguish a successful invader (Kolar & Lodge 2001). It has been proposed that invasive species may own particular ecological traits, such as a high competitive ability (Lodge 1993; Sakai et al. 2001). Several studies evaluated competitive effects between introduced and native plant species (e.g. Hager 2004; for a review, see Vilà & Weiner 2004), as well as between alien and indigenous animal species (e.g. Byers 2000; Baker & Levinton 2003). All of these studies attributed a high competitiveness in the non-native species to its invasion success.
Also, phenotypic plasticity has been suggested to play a key role for the range expansion of invading species (Agrawal 2001; Ghalambor et al. 2007). Plasticity in specific traits may allow an invader to rapidly adapt to a new environment. While some studies found morphological, physiological and life-history plasticity in various exotic species to promote invasiveness, these studies were mainly restricted to introduced plants (e.g. Kaufman & Smouse 2001; Sexton et al. 2002; Parker et al. 2003). In recent years, climate change emerged as an important factor allowing warm adapted species to expand their range (e.g. Stachowicz et al. 2002; Walther et al. 2002) into habitats where native species already face their thermal limits (Holzapfel & Vinebrooke 2005).
The limnetic water flea Daphnia lumholtzi (Sars), which is naturally distributed throughout the tropics and subtropics of Africa, Asia and Australia (Benzie 1988), has successfully invaded North America. Daphnia lumholtzi first appeared in Texas and Missouri by 1990/1991 (Sorensen & Sterner 1992; Havel & Hebert 1993). Subsequently, it rapidly spread throughout much of the south-eastern and mid-western United States, and has recently even been found in the Laurentian Great Lakes in the north and in California in the west (Havel & Shurin 2004). The geographical origin of the invading D. lumholtzi populations has not yet been identified. The most likely scenario is an introduction by stocking of lakes with exotic fish species (e.g. Nile perch Lates niloticus) from Africa (Havel & Hebert 1993).
Earlier studies that investigated the reasons behind this successful invasion of D. lumholtzi focused on abiotic conditions (e.g. temperature, salinity, nutrients), primarily based on a correlation of its seasonal abundance with water temperature (Havel et al. 1995; Work & Gophen 1999; Havel & Graham 2006). Daphnia lumholtzi was assumed to fill a ‘vacant’ thermal (seasonal or spatial) niche during summer (Lennon et al. 2001). However, most of these studies were carried out in the field, where multiple (abiotic and biotic) factors are responsible for the composition of the resident community. For example, temperature is also correlated with predation by fishes. Therefore, the occurrence of D. lumholtzi in nature corresponds not only to high temperatures but also to intense fish predation (Lienesch & Gophen 2001).
Many cladoceran species have been shown to form morphological structures, such as helmets, spines and neckteeth, in response to chemical cues (kairomones) from invertebrate predators. These traits reduce the mortality risk exerted by these invertebrate predators (reviewed in Tollrian & Dodson 1999). However, D. lumholtzi is remarkable among Daphnia because it can carry exceptionally long head and tail spines. Green (1967) observed the spatial separation of a helmeted and a non-helmeted form of D. lumholtzi (at that time described as different varieties) in Lake Albert (east Africa) and suggested that the helmets might act as a defence against fishes. Tollrian (1994) and Dzialowski et al. (2003) found that the helmets can be induced by chemicals associated with fishes (figure 1 in the electronic supplementary material). Swaffar & O'Brien (1996) and Kolar & Wahl (1998) reported a benefit of the head spine in D. lumholtzi against fish predation. Thus, the helmet formation in D. lumholtzi represents an inducible defence against fishes, and therefore potentially influences the competitive interaction with native Daphnia species. As D. lumholtzi is the only cladoceran that reacts with distinct helmets to fish kairomones, this morphological (phenotypic) plasticity may be a key adaptation, which—exclusively or in combination with other factors—facilitates the invasion in North America.
Here, we tested the hypothesis that phenotypic plasticity in defensive traits gives D. lumholtzi an advantage over native North American species. We performed controlled laboratory mesocosm experiments with different clones of D. lumholtzi and the most common North American Daphnia species in the presence and the absence of fish predation.
2. Material and methods
(a) Species and experimental conditions
We used three clones of the invasive species D. lumholtzi that differ in their inducibility by predator cues (R. Tollrian 1999, unpublished data). Clone D. lumholtzi AR originated from Canyon Lake, Arizona (kindly provided by J. Elser), and is constitutively morphologically defended. It is characterized by a permanently high helmet and a very long tail spine. The two other clones are inducible ones, i.e. they do not form helmets without predator cues. Daphnia lumholtzi TE was isolated from Fairfield Reservoir, Texas (kindly provided by K. H. Sorensen & R. W. Sterner), and D. lumholtzi LA1 originated from the Atchafalaya River Basin, Louisiana (kindly provided by C. Ramcharan). The body length (BL; from the eye to the base of the tail spine) of our adult D. lumholtzi was on average 1.5–1.6 mm.
Daphnia pulicaria (Forbes) is the most widely distributed Daphnia species native to North America. It occurs in lakes and permanent ponds from Mexico to the Arctic (Hebert 1995). Some North American lakes containing D. pulicaria have been invaded by D. lumholtzi (e.g. Schulze et al. 2006), and the overlap in their distribution may increase with future range expansion of D. lumholtzi. In North America, D. pulicaria coexists with several fish species (e.g. Hu & Tessier 1995). Our three D. pulicaria clones Whitford 1 (WF1), Laurence 9 (L9) and Warner 13 (WR13) were isolated from Gull Lake, Michigan, and were kindly provided by A. Tessier several months prior to the study. Daphnia pulicaria never forms helmets. Our D. pulicaria clones were slightly larger (mean body size of approx. 1.8–1.9 mm) than our D. lumholtzi clones.
All experiments were conducted in the laboratory (climate chambers) under constant conditions at a temperature of 20°C (±1°C) and fluorescent light (15 L : 9 D) in 32 l white polyethylene containers (39 cm height; 35 cm (top) and 31 cm (bottom) width). The animals of the two species were reared and synchronized in single-clone stocks (5 l glass beakers) in artificial medium based on ultrapure water, trace elements and phosphate buffer (Jeschke & Tollrian 2000). At the start of each experiment, adult females with broods (eggs or embryos) and females with filled ovaries were randomly chosen from each clone. We mixed the cohorts to provide a more continuous reproduction. To ensure identical starting conditions for both species, defined quantities of each adult cohort per clone were inoculated into each replicate. Each mesocosm was filled with 30 l of medium (20 l in the competition experiment with fish predation). Daphnia in cultures and in the experiments were fed in 2-day intervals with the green alga Scenedesmus obliquus. The food concentration was determined by the optical absorbance of the algae suspension at 800 nm in a photometer with a calibration curve for Scenedesmus relating optical density to the carbon content. During the initial growth period of the Daphnia populations (without sampling), only 0.4 mg C l−1 of Scenedesmus were fed every second day, to avoid settling of algae. When Daphnia had reached higher densities (visual inspection), algae at 0.6 mg C l−1 were added every 48 hours (until experimental conclusion). From that time on, 10 per cent of the entire volume per replicate was sampled every second day. Zooplankton was collected after gently mixing the mesocosm by filtering a 1 l sample of medium (100 μm mesh). By repeating this procedure, a total of 3 l (two in the competition experiment with fish predation) were sampled and pooled per day and mesocosm. Adding new medium restored the initial volume after each sampling. Daphnia were preserved in 70 per cent ethanol and counted later under a dissecting microscope. Adult females, juveniles (neonate and juvenile females) and males (all developmental stages) of each species were inspected. Population density curves were obtained and analysed based on the adult females only because they best reflect population growth under size-selective predation on adults. We determined the initial growth period and total experimental duration in the pilot experiments. These pre-experiments showed that approximately 40 days were optimal in the competition experiment to find the dominant species. The trends observed at that time did not reverse in longer lasting trials.
(b) Competition experiment without predators
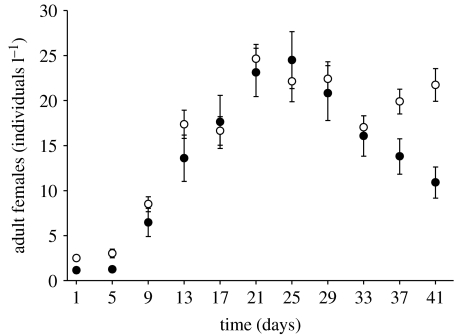
On the starting day, 20 adult females of each of the three different clones of D. lumholtzi (AR, TE and LA1) and D. pulicaria (WF1, L9 and WR13) were introduced into each of 10 replicates (starting density: four Daphnia l−1). The initial growth period for the Daphnia populations lasted 8 days. Sampling started on day 9 (corresponding to day 1 of the experiment in figure 1). Samples were taken on 2-day intervals over a period of 41 days. Every second sampling date was counted.
Figure 1.
Population dynamics of the invasive D. lumholtzi (filled circles) and the native D. pulicaria (open circles) over time (days) in the competition experiment without predators. After an initial growth period of 8 days, sampling started on day 1. The densities of adult females (individuals l−1) are shown per species as means (±s.e.) of the 10 competition replicates.
(c) Single-species growth experiment
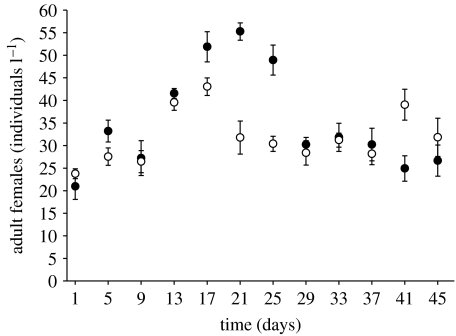
To determine whether competition had affected the population sizes, mesocosms were run with each species alone (D. lumholtzi and D. pulicaria) and the resulting densities were compared with the densities from the competition experiment without predators. The three clones per species were inoculated into six single-species replicates. The starting densities (two Daphnia l−1) were identical to the densities of each species in the competition treatment (see above). The food concentration was adjusted as described previously. The initial growth period for the Daphnia lasted 12 days. Sampling started on day 13 (equally to day 1 of the experiment in figure 2). Again, samples from every second sampling date were counted over a period of 45 days.
Figure 2.
Population dynamics of the invasive D. lumholtzi (filled circles) and the native D. pulicaria (open circles) over time (days) in the single-species growth experiment without predators. After an initial growth period of 12 days, sampling started on day 1. The densities of adult females (individuals l−1) are shown per species as means (±s.e.) of the six single-species replicates.
(d) Fish predation experiment
The set-up was analogous to the competition experiment without predators (see above), but fish-conditioned water was added in a circuit system to induce the defences in Daphnia. Two independent systems were used, each consisting of one 20 l aquarium that supported five mesocosms. Each mesocosm was filled with 20 l of medium only, to facilitate fish removal during the experiment. For the continuous kairomone supply, medium of the aquarium containing fishes was discharged into the mesocosms by a multichannel peristaltic pump. It was pumped back into the aquarium from the other side of each tank. Fish kairomone was supplied throughout the entire experiment. The flow-through rate was adjusted to 340 ml h−1 per mesocosm. The peristaltic pumps were started 1 day prior to the experiment. At the same time, each aquarium was filled with 15 l of aerated medium and stocked with 20 small Leucaspius delineatus (Heckel) (sunbleaks; Cyprinidae; length: 20–30 mm). Mortality caused a decline to 16 fishes per aquarium at the end of the experiment. We chose this relatively high concentration of predator cues to induce the morphological changes in Daphnia, because still little is known about the chemical compounds of the fish kairomone (Von Elert & Pohnert 2000). Five litres of the medium were renewed daily, and all fishes were fed daily with chironomids and different Daphnia species (Daphnia pulex and Daphnia magna).
At the starting day of the experiment, 15 adult females per clone of each species (D. lumholtzi and D. pulicaria) were inoculated into each of the 10 replicated mesocosms (starting density: 4.5 Daphnia l−1).
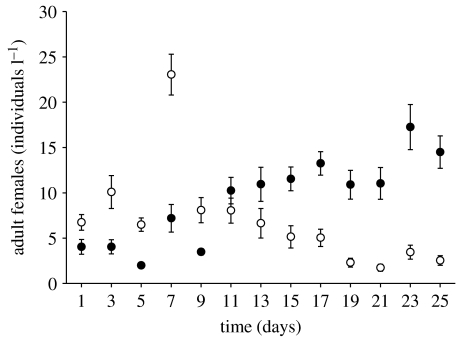
To simulate natural predation and selection, we allowed fishes (juvenile sunbleaks; length: 40–60 mm) to feed freely in the mesocosms for a limited time every second day. The fishes had been naive to both Daphnia species prior to the experiment. After the initial growth period for the Daphnia populations, vertebrate predation started on day 11 (corresponding to day 1 of the experiment in figure 3). Out of 15 fishes, 10 were randomly selected and placed individually into the containers. If a fish did not start feeding within the first few minutes, it was replaced. We adjusted the predation period following direct observation to allow for a significant predation impact but to avoid depletion of all Daphnia. Owing to low predation, we extended the period from 10 to 30 min from day 7. After each fish predation event, samples were taken over a period of 25 days. To monitor the impact of fish foraging, every sample was analysed.
Figure 3.
Population dynamics of the invasive D. lumholtzi (filled circles) and the native D. pulicaria (open circles) over time (days) in the fish predation experiment. After an initial growth period of 10 days, sampling started on day 1. The densities of adult females (individuals l−1) are shown per species as means (±s.e.) of the 10 mixed-species replicates. Prior to sampling, fish predation took place every second day. Note that the predation period was extended on day 7.
We measured Daphnia helmet length (HL; distance from the tip of the helmet to the upper edge of the compound eye), BL (from the upper edge of the compound eye to the base of the tail spine) and tail spine length (TL; from the base to the end of the tail spine) with a digital image analysis system (Analysis Pro; Münster, Germany). Gravid females from all 10 replicates per treatment were measured on (i) an early and (ii) a late sampling date. In the competition experiment without predators (control treatment), animals were measured on days 9 and 21 (figure 1). In the experiment with fish predation (fish treatment), daphnids were measured on day 7 (after four predation events; figure 3) and day 17 (after nine predation events; figure 3). Samples with none or one adult were excluded from the analysis (at date 1: two control replicates and one fish kairomone replicate in D. lumholtzi; at date 2: two fish kairomone replicates in D. pulicaria). Prior to analysis, we calculated the relative HL (HL/BL×100) and the relative TL (TL/BL×100) to compensate for size-dependent changes in trait lengths within both species. Nested ANOVAs, with 8–10 replicates per treatment as a random factor, were performed to identify treatment effects between induced (with fish kairomone/predation) and control (without predator cues) animals per species at each date. Data were tested for normal distribution and homogeneity of variances. In a few cases, variances were not homogeneous. Since non-parametric analyses with Mann–Whitney U-tests did not change significances, we present here (for simplicity) the results of the nested ANOVAs.
On the last sampling date of the fish predation experiment, additional 1 l samples were taken from each replicate, to assess the final clonal composition. Daphnia were placed in 2 ml Eppendorf tubes and frozen. For the analysis, samples were defrosted and all Daphnia (n) were identified to species under a dissecting microscope. Prior to cellulose acetate electrophoresis, individuals of the constitutively defended clone D. lumholtzi AR were removed (this was possible due to their higher helmets) because they are not distinguishable from the inducible clone TE based on the enzymes used. Random subsamples of 12 Daphnia per replicate were taken and analysed on cellulose acetate gels (Titan III; Helena Laboratories, USA), using a Tris–glycine buffer (3 g l−1 Trizma base, 14.4 g l−1 glycine; pH=8). The cellulose acetate electrophoresis was carried out following Hebert & Beaton (1989). In test runs, a variety of enzymes had been assayed electrophoretically to find optimal polymorphisms (allozyme patterns) for the differentiation of the various clones. Six different enzymes could be expressed, but only PGI (phosphoglucose isomerase) and PGM (phosphoglucomutase) were reliably resolved and polymorphic. A tandem stain for PGM and PGI was carried out (Harris & Hopkinson 1976). Five different allozyme patterns resulted for the electrophoretically analysed clones. On the gel of replicate 2, only 11 allozyme patterns could be clearly identified. After the clonal identification by electrophoresis, we subtracted the percentage (%) of the clone D. lumholtzi AR from the total Daphnia number in each 1 l replicate sample (100%). The remaining percentage then corresponded to the 12 electrophoretically examined individuals (subsample) per replicate. According to their occurrence in each subsample, the relative abundances of the five analysed clones were determined and the mean relative abundances (%) per clone were calculated. The proportions were arcsine transformed prior to analysis (Sokal & Rohlf 1995). A one-way ANOVA with a Tamhane post hoc test was performed to determine significant differences in the clonal abundance.
3. Results
(a) Competition experiment without predators
Temporal dynamics in all replicates were similar. Both Daphnia species increased in density until food limitation and competition became evident. The population peaks were reached between days 21 and 25 (approx. 47 total Daphnia l−1; figure 1). Subsequently, the populations of both species declined during the course of competition to a mean total abundance of approximately 35 Daphnia l−1 (days 29–41). Daphnia pulicaria was the superior competitor and increased in density again, whereas D. lumholtzi declined continuously. At the end of the experiment, D. lumholtzi had a significantly lower abundance than D. pulicaria (paired t-tests, all d.f.=9, day 29: p=0.704, day 33: p=0.700, day 37: p=0.052, day 41: p=0.002).
(b) Single-species growth experiment
In single-species growth, the populations of the two Daphnia species developed very similarly (figure 2). Both species reached distinctly higher numbers during the final experimental period of the single-species growth (figure 2) compared with the competition experiment (t-tests, last 4 days, D. lumholtzi: all d.f.=14, all p<0.05, D. pulicaria: all d.f. =14 except on last but 1 day: d.f.=7, all p<0.05; figure 1), indicating that each species was negatively affected by the presence of its competitor.
(c) Fish predation experiment
The sunbleak kairomone supply induced morphological changes in both Daphnia species. The difference in the relative HL of D. lumholtzi in the fish treatment was significantly higher at both dates compared with the control (table 1). The relative HL (fish kairomone induction) in D. lumholtzi did not differ between the two circuit systems (nested ANOVAs, date 1: F1,7=0.06, p=0.806, date 2: F1,8=1.98, p=0.194). The relative TL in D. lumholtzi, as well as in D. pulicaria, was significantly longer in the fish treatment compared with the control at both dates (table 1). In comparison with the control Daphnia, the BL in D. lumholtzi remained almost equal at the earlier date, despite the fish chemical supply. At the later date, when predation had acted nine times, the body size in the exotic species was significantly smaller with kairomones. Daphnia pulicaria in the fish treatment were significantly shorter than the control Daphnia at both dates (table 1). Without predators, D. pulicaria was significantly larger than D. lumholtzi at both dates (nested ANOVAs, date 1: F1,16=66.75, p<0.001, date 2: F1,18=77.37, p<0.001), but in the fish treatment, no significant BL difference between both species was observed on either date (nested ANOVAs, date 1: F1,17=0.92, p=0.346, date 2: F1,16=0.68, p=0.413).
Table 1.
Relative helmet length (HL) and relative tail spine length (TL; %; mean (±s.e.)) and absolute body length (BL; μm; mean (±s.e.)) of D. lumholtzi and D. pulicaria raised with (kairomone and predation) and without (control) chemical cues exuded by fishes. Adults of both Daphnia species were recorded at an early (1) and a late (2) sampling date of the experiment with (fish kairomone and predation) and without (control; competition) fish predation. n indicates the number of individual Daphnia pooled from 8 to 10 replicates per treatment. Each species was analysed for treatment effects using a nested ANOVA with 8–10 replicates per treatment as a random factor.
| D. lumholtzi | D. pulicaria | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| control (without fishes) | fish kairomone/predation | control (without fishes) | fish kairomone/predation | ||||||||
| trait | date | mean (±s.e.) | n | mean (±s.e.) | n | p-value | mean (±s.e.) | n | mean (±s.e.) | n | p-value |
| HL(%) | 1 | 10.80 (0.24) | 105 | 33.58 (1.01) | 93 | <0.001 | 3.70 (0.07) | 152 | 3.58 (0.05) | 214 | 0.173 |
| 2 | 10.19 (0.16) | 159 | 32.84 (0.58) | 197 | <0.001 | 4.77 (0.10) | 211 | 3.87 (0.13) | 57 | 0.008 | |
| TL(%) | 1 | 65.72 (1.11) | 105 | 91.22 (1.77) | 93 | <0.001 | 30.67 (0.34) | 152 | 37.26 (0.32) | 214 | <0.001 |
| 2 | 48.80 (0.76) | 159 | 90.35 (1.04) | 197 | <0.001 | 24.53 (0.29) | 211 | 35.55 (0.73) | 57 | <0.001 | |
| BL(μm) | 1 | 1520.57 (14.29) | 105 | 1530.53 (28.63) | 93 | 0.611 | 1879.80 (17.33) | 152 | 1589.73 (12.14) | 214 | <0.001 |
| 2 | 1578.49 (12.09) | 159 | 1448.64 (13.31) | 197 | <0.001 | 1865.89 (13.86) | 211 | 1478.62 (13.74) | 57 | <0.001 | |
Initially, the D. pulicaria population grew faster to a higher density than D. lumholtzi (figure 3). Daphnia pulicaria peaked after four predation events on day 7 (approx. 23 individuals l−1), and declined continuously thereafter. By contrast, D. lumholtzi steadily increased in abundance (from day 11) and reached a maximum density (approx. 17 females l−1) on day 23. The Daphnia populations (both species combined per replicate) had significantly lower densities under fish predation (figure 3) than without predators (t-tests, last 4 days: all d.f.=18, all p≤0.001; figure 2), indicating that predation was the dominant factor (not direct resource competition). At the end of the experiment, D. lumholtzi had a significantly higher abundance than D. pulicaria (paired t-tests, all d.f.=9, day 19: p=0.002, day 21 and 23: both p=0.001, day 25: p<0.001).
The clonal identification by electrophoresis revealed that the inducible clones (TE and LA1), not the constitutively defended clone D. lumholtzi AR, dominated under fish predation (table 2). The permanently defended clone AR occurred with a maximum of 6.8 per cent, less often than the two induced clones. The phenotypically plastic clone D. lumholtzi LA1 reached significantly higher abundances than the five other clones (ANOVA, F5,54=32.26, p<0.001; Tamhane post hoc tests, all p<0.001; table 2).
Table 2.
Relative abundance per replicate (%) and mean relative abundance (%) of the electrophoretically analysed clones of D. lumholtzi and D. pulicaria from the fish predation experiment. Per replicate, a random subsample of 12 individuals from the total Daphnia number in the 1 l sample (n) was evaluated by cellulose acetate electrophoresis. In replicate 2, only 11 allozyme patterns could be clearly identified. Note that D. lumholtzi AR is the fixed spine clone.
| percentage (%) of the clones | |||||||
|---|---|---|---|---|---|---|---|
| D. lumholtzi | D. pulicaria | ||||||
| replicate | n | AR | TE | LA1 | WF1 | L9 | WR13 |
| 1 | 63 | 4.8 | 7.9 | 79.4 | 0 | 7.9 | 0 |
| 2 | 168 | 1.2 | 9.0 | 89.8 | 0 | 0 | 0 |
| 3 | 136 | 0 | 8.3 | 66.7 | 0 | 25.0 | 0 |
| 4 | 160 | 0.6 | 8.3 | 91.1 | 0 | 0 | 0 |
| 5 | 208 | 0.5 | 41.5 | 58.0 | 0 | 0 | 0 |
| 6 | 44 | 6.8 | 7.8 | 77.7 | 0 | 7.8 | 0 |
| 7 | 31 | 3.2 | 0 | 72.6 | 16.1 | 0 | 8.1 |
| 8 | 26 | 0 | 0 | 100.0 | 0 | 0 | 0 |
| 9 | 52 | 0 | 8.3 | 91.7 | 0 | 0 | 0 |
| 10 | 35 | 0 | 0 | 16.7 | 41.7 | 16.7 | 25.0 |
| mean abundance (%) | 1.7 | 9.1 | 74.4 | 5.8 | 5.7 | 3.3 | |
4. Discussion
Our study suggests that the invasive D. lumholtzi is competitively inferior to the native D. pulicaria in the absence of fish predators but superior in their presence. The phenotypically plastic helmets and tail spines in D. lumholtzi protect against fish predation.
Competition for resources is one of the most important interactions an exotic species faces in a new environment. Superior competing native species will probably prevent the establishment of the alien species, whereas competitive superiority by the invader is more likely to favour its establishment in the novel community (Orians 1986). Previous studies found that high competitive ability permitted the invasions of introduced plant and animal species (Byers 2000; Hager 2004; Vilà & Weiner 2004). For example, Baker & Levinton (2003) reported that the invasive zebra mussel Dreissena polymorpha has a higher filtration efficiency than the native North American mussels and attributed the observed decline of native species to this competitive difference.
Most studies on the invasion of D. lumholtzi in North America concentrated on water temperature to explain its establishment (e.g. Havel et al. 1995; Work & Gophen 1999). Lennon et al. (2001) found that D. lumholtzi has a high temperature optimum between 20 and 30°C. They concluded that D. lumholtzi has a comparable intrinsic rate of increase to native Daphnia species between 20 and 25°C, but they did not test their results in direct competition between D. lumholtzi and native daphnids. Field enclosure competition experiments by Johnson & Havel (2001) indicated that at high densities D. lumholtzi suppressed the population growth rates of the smaller D. parvula during summer and autumn. In our study, a moderate temperature of 20°C was used to guarantee that D. lumholtzi has no thermal advantage over the North American D. pulicaria, and to exclude temperature as a relevant factor. Indeed, the expected competitive superiority of D. pulicaria over D. lumholtzi emerged in the absence of predators (figure 1). While the D. lumholtzi population declined in direct competition (figure 1), it had a significantly higher density in single-species growth (figure 2). Thus, competition between both species was responsible for the decline.
However, with fish predation, the dominance reversed (figure 3). The morphologically defended clones of D. lumholtzi now had a distinct advantage. If experiments had lasted longer, fishes might have completely extirpated the native D. pulicaria.
In response to predation, Daphnia exhibit various defensive changes of morphology, life history and behaviour evoked by chemical cues released from different predators (for a review, see Tollrian & Dodson 1999). In our study, D. lumholtzi responded to fish exudates by forming plastic defences (table 1). The inducible clones D. lumholtzi TE and LA1 produced long helmets and extended their tail spines when the fish kairomone was supplied. This is in accordance with the earlier results by Tollrian (1994) and Dzialowski et al. (2003; see figure 1 in the electronic supplementary material). However, the induced spines in our two inducible clones were still smaller than the constitutive features in the clone D. lumholtzi AR. The relative head length in the native D. pulicaria remained almost equal in both treatments (although significantly, but only 1% smaller in the fish treatment). The relative tail spine in D. pulicaria was distinctly longer in the fish treatment compared with the control (table 1). This is in accordance with the results by Dodson (1989), when he exposed D. pulicaria to fish cues. Tail spines also provide a defence against fish larvae in Daphnia (e.g. Jacobs 1967).
Could other factors have caused the dominance of D. lumholtzi under fish predation? Fish selectivity is not only influenced by prey defences but may also be influenced by prey visibility (O'Brien et al. 1976; Zaret 1980), which in turn is affected by prey body size (Brooks & Dodson 1965; Werner & Hall 1974). Without predator cues, D. pulicaria was larger than D. lumholtzi. Dodson (1989) found in D. pulicaria exposed to Lepomis kairomones an induced smaller BL compared with control animals. Also, in our experiment D. pulicaria became smaller in the presence of fish kairomones and both Daphnia species reached a similar body size of approximately 1.5 mm (table 1). This concurs with the data published by Hu & Tessier (1995), who found similar-sized (BL at maturity) D. pulicaria in Gull Lake where the native species cooccurs with fishes. Thus, our foraging fishes could not have selected their prey according to the differences in prey BL. To summarize, our results indicate that the advantage of D. lumholtzi under fish predation cannot be attributed to body size, but to its morphological defences against fishes. Likewise, Green (1967) observed in a field study that Alestes baremose fed selectively on non-helmeted prey, and concluded that helmeted forms of D. lumholtzi are at an advantage in the presence of fishes. Similarly, permanent spines (head and tail spines) in other zooplankton species have been shown to protect against fish predation (Zaret 1972; Barnhisel 1991).
The fishes in our study were large enough to, and obviously able to, consume both Daphnia species. Thus, the body enlargement by the spines cannot provide the main defensive effect for D. lumholtzi. In our predation experiments, we observed that the spined D. lumholtzi were attacked equally often in the beginning when the fishes were naive to this type of prey. However, D. lumholtzi were often expelled by the sunbleaks and generally survived these attacks (K. Engel 2003, personal observations). Thereafter, the fishes avoided attacking the spiny prey. A selective advantage at vertebrate predation has been shown in previous studies. Swaffar & O'Brien (1996) found that juvenile bluegill sunfish (20–35 mm) repelled more and consumed fewer D. lumholtzi than similar-sized D. magna. Similarly, Kolar & Wahl (1998) determined in juvenile Lepomis a selective advantage of D. lumholtzi over equal body-sized D. pulex. Capture efficiencies of bluegill (less than 50 mm) were lower and handling times were longer when feeding on D. lumholtzi compared with the native species. Larger Lepomis (more than 50 mm) consumed the exotic species but still had lower capture efficiencies compared with D. pulex.
The argument that inducible defences present a key factor for the invasion provides an alternative explanation for the observed correlation between the abundance of D. lumholtzi and water temperature (e.g. Lennon et al. 2001; Havel & Graham 2006). This correlation may be an artefact since the intensity of fish predation also is distinctly correlated with temperature (e.g. Gliwicz & Pijanowska 1989). Fish larvae and young fishes exert the highest predation pressure on zooplankton. Predation usually peaks in the summer when young fishes are abundant (Gliwicz & Pijanowska 1989). The seasonal appearance of D. lumholtzi during summer (e.g. Work & Gophen 1999) concurs with the period of the highest fish predation. Predators are common in invaded North American waters and the species interaction might change in favour of D. lumholtzi. The decline during autumn occurs when fish predation is relatively low again (Lienesch & Gophen 2001) and, following from our competition experiment, native cladocerans may be better competitors. Field enclosure experiments by De Mott (1983) demonstrated the competitive prowess of D. pulicaria against other native Daphnia species during late summer and autumn. However, we do not argue that the inducible defence is the only factor facilitating the invasion of D. lumholtzi in North America. Certainly, high temperatures can also have an influence, as invasions follow a match between attributes of the alien species and the invaded ecosystems (e.g. Shea & Chesson 2002; Facon et al. 2006). Current global climate change may favour range expansions of exotics (Dukes & Mooney 1999), as native species may be increasingly stressed by altered ecosystem properties and processes (e.g. elevated temperatures).
Under our fish predation conditions, the inducible, phenotypically plastic clone D. lumholtzi LA1 eventually dominated the five other clones (table 2). Although D. lumholtzi AR had the longest defence features, it did not gain an advantage. This constitutively defended clone shows a slower population growth rate and possibly is better adapted to higher fish densities. Thus, our results support the suggestion that phenotypic plasticity in invading species can be a crucial factor for their establishment in new regions (Agrawal 2001; Ghalambor et al. 2007). Phenotypically plastic species may adequately adapt to varying abiotic (e.g. climate) and biotic (e.g. competition and predation) conditions (reviewed in Tollrian & Harvell (1999) and Miner et al. (2005)). As a result, plasticity may distinctively alter interactions on population, community or ecosystem levels.
The effect of the invasive D. lumholtzi on higher trophic levels of the natural community may differ depending on the mechanism of its success. If thermal tolerance is the main mechanism, it may simply fill a vacant thermal niche during summer. In this case, the invasion might actually be a benefit for fish communities by providing food during times of scarcity. By contrast, if, as suggested by Tollrian (1994) and this study, a defence against fishes is an important mechanism, D. lumholtzi could replace native species and might have a negative effect on the fish populations, especially during times when young fishes require abundant food.
While synergies between biological invasions and global change (e.g. climate change) have been widely discussed in recent years (Dukes & Mooney 1999; reviewed by Chown & Gaston 2008), few studies have analysed the importance of phenotypic plasticity for the invasion process (e.g. Chown et al. 2007). Here, we showed a predator-mediated superiority of the invasive D. lumholtzi. We demonstrated that the inducible defence in D. lumholtzi can cause a switch in dominance. The formation of an effective phenotypically plastic defence against fish predation may represent a key adaptation for the successful invasion. Our work provides initial support for the relevance of phenotypic plasticity in defensive traits for successful invasions and cautions against monocausal explanations.
Acknowledgments
We thank W. Gabriel for encouragement and discussion, M. Kredler and R. Selmeier for their assistance in the laboratory, C. Laforsch for the permission to use his scanning electron micrograph and J. Jeschke and J. Havel for their comments on the manuscript. We also thank A. Magurran and two anonymous reviewers for their helpful comments and suggestions. This work was partially supported by a PhD grant from the Hanns Seidel Foundation.
Supplementary Material
The individual on the left was exposed to chemical cues exuded by fish (induced) and the individual on the right was not (control) (photo credit C. Laforsch).
References
- Agrawal A.A. Phenotypic plasticity in the interactions and evolution of species. Science. 2001;294:321–326. doi: 10.1126/science.1060701. doi:10.1126/science.1060701 [DOI] [PubMed] [Google Scholar]
- Baker S.M., Levinton J.S. Selective feeding by three native North American freshwater mussels implies food competition with zebra mussels. Hydrobiologia. 2003;505:97–105. doi:10.1023/B:HYDR.0000007298.52250.99 [Google Scholar]
- Barnhisel D.R. The caudal appendage of the cladoceran Bythotrephes cederstroemi as defense against young fish. J. Plankton Res. 1991;13:529–537. doi:10.1093/plankt/13.3.529 [Google Scholar]
- Benzie J.A.H. The systematics of Australian Daphnia (Cladocera: Daphniidae). Species descriptions and keys. Hydrobiologia. 1988;166:95–161. doi:10.1007/BF00028632 [Google Scholar]
- Brooks J.L., Dodson S.I. Predation, body size and composition of plankton. Science. 1965;150:28–35. doi: 10.1126/science.150.3692.28. doi:10.1126/science.150.3692.28 [DOI] [PubMed] [Google Scholar]
- Byers J.E. Competition between two estuarine snails: implications for invasions of exotic species. Ecology. 2000;81:1225–1239. doi:10.1890/0012-9658(2000)081[1225:CBTESI]2.0.CO;2 [Google Scholar]
- Chown S.L., Gaston K.J. Macrophysiology for a changing world. Proc. R. Soc. B. 2008;275:1469–1478. doi: 10.1098/rspb.2008.0137. doi:10.1098/rspb.2008.0137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chown S.L., Slabber S., McGeoch M.A., Janion C., Leinaas H.P. Phenotypic plasticity mediates climate change responses among invasive and indigenous arthropods. Proc. R. Soc. B. 2007;274:2531–2537. doi: 10.1098/rspb.2007.0772. doi:10.1098/rspb.2007.0772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Mott W.R. Seasonal succession in a natural Daphnia assemblage. Ecol. Monogr. 1983;53:321–340. doi:10.2307/1942534 [Google Scholar]
- Dodson S.I. The ecological role of chemical stimuli for the zooplankton: predator-induced morphology in Daphnia. Oecologia. 1989;78:361–367. doi: 10.1007/BF00379110. doi:10.1007/BF00379110 [DOI] [PubMed] [Google Scholar]
- Dukes J.S., Mooney H.A. Does global change increase the success of biological invaders? Trends Ecol. Evol. 1999;14:135–139. doi: 10.1016/s0169-5347(98)01554-7. doi:10.1016/S0169-5347(98)01554-7 [DOI] [PubMed] [Google Scholar]
- Dzialowski A.R., Lennon J.T., O'Brien W.J., Smith V.H. Predator-induced phenotypic plasticity in the exotic cladoceran Daphnia lumholtzi. Freshw. Biol. 2003;48:1593–1602. doi:10.1046/j1365-2427.2003.01111.x [Google Scholar]
- Facon B., Genton B.J., Shykoff J., Jarne P., Estoup A., David P. A general eco-evolutionary framework for understanding bioinvasions. Trends Ecol. Evol. 2006;21:130–135. doi: 10.1016/j.tree.2005.10.012. doi:10.1016/j.tree.2005.10.012 [DOI] [PubMed] [Google Scholar]
- Ghalambor C.K., McKay J.K., Carroll S.P., Reznick D.N. Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct. Ecol. 2007;21:394–407. doi:10.1111/j.1365-2435.2007.01283.x [Google Scholar]
- Gliwicz Z.M., Pijanowska J. The role of predation in zooplankton succession. In: Sommer U., editor. Plankton ecology: succession in plankton communities. Springer; Berlin, Germany: 1989. pp. 253–296. [Google Scholar]
- Green J. The distribution and variation of Daphnia lumholtzi (Crustacea: Cladocera) in relation to fish predation in Lake Albert, East Africa. J. Zool. Lond. 1967;151:181–197. [Google Scholar]
- Hager H.A. Competitive effect versus competitive response of invasive and native wetland plant species. Oecologia. 2004;139:140–149. doi: 10.1007/s00442-004-1494-6. doi:10.1007/s00442-004-1494-6 [DOI] [PubMed] [Google Scholar]
- Harris H., Hopkinson D.A. North-Holland; Amsterdam, The Netherlands: 1976. Handbook of enzyme electrophoresis in human genetics. [Google Scholar]
- Havel J.E., Graham J.L. Complementary population dynamics of exotic and native Daphnia in North American reservoir communities. Arch. Hydrobiol. 2006;167:245–264. doi:10.1127/0003-9136/2006/0167-0245 [Google Scholar]
- Havel J.E., Hebert P.D.N. Daphnia lumholtzi in North America: another exotic zooplankter. Limnol. Oceanogr. 1993;38:1823–1827. [Google Scholar]
- Havel J.E., Shurin J.B. Mechanisms, effects, and scales of dispersal in freshwater zooplankton. Limnol. Oceanogr. 2004;49:1229–1238. [Google Scholar]
- Havel J.E., Mabee W.R., Jones J.R. Invasion of the exotic cladoceran Daphnia lumholtzi into North American reservoirs. Can. J. Fish. Aquat. Sci. 1995;52:151–160. doi:10.1139/f95-015 [Google Scholar]
- Hebert, P. D. N. 1995 The Daphnia of North America: an illustrated Fauna CD-ROM, distributed by the author. Ontario, Canada: University of Guelph.
- Hebert P.D.N., Beaton M.J. Helena Laboratories; Beaumont, TX: 1989. Methodologies for allozyme analysis using cellulose acetate electrophoresis. [Google Scholar]
- Holzapfel A.M., Vinebrooke R.D. Environmental warming increases invasion potential of alpine lake communities by imported species. Global Change Biol. 2005;11:2009–2015. doi:10.1111/j.1365-2486.2005.01057.x [Google Scholar]
- Hu S.S., Tessier A.J. Seasonal succession and the strength of intra- and interspecific competition in a Daphnia assemblage. Ecology. 1995;76:2278–2294. doi:10.2307/1941702 [Google Scholar]
- Jacobs J. Untersuchungen zur Funktion und Evolution der Zyklomorphose bei Daphnia, mit besonderer Berücksichtigung der Selektion durch Fische. Arch. Hydrobiol. 1967;62:467–541. [Google Scholar]
- Jeschke J.M., Strayer D.L. Invasion success of vertebrates in Europe and North America. Proc. Natl Acad. Sci. USA. 2005;102:7198–7202. doi: 10.1073/pnas.0501271102. doi:10.1073/pnas.0501271102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeschke J.M., Tollrian R. Density-dependent effects of prey defences. Oecologia. 2000;123:391–396. doi: 10.1007/s004420051026. doi:10.1007/s004420051026 [DOI] [PubMed] [Google Scholar]
- Johnson J.L., Havel J.E. Competition between native and exotic Daphnia: in situ experiments. J. Plankton Res. 2001;23:373–387. doi:10.1093/plankt/23.4.373 [Google Scholar]
- Kaufman S.R., Smouse P.E. Comparing indigenous and introduced populations of Melaleuca quinquenervia (Cav.) Blake: response of seedlings to water and pH levels. Oecologia. 2001;127:487–494. doi: 10.1007/s004420000621. doi:10.1007/s004420000621 [DOI] [PubMed] [Google Scholar]
- Kolar C.S., Lodge D.M. Progress in invasion biology: predicting invaders. Trends Ecol. Evol. 2001;16:199–204. doi: 10.1016/s0169-5347(01)02101-2. doi:10.1016/S0169-5347(01)02101-2 [DOI] [PubMed] [Google Scholar]
- Kolar C.S., Wahl D.H. Daphnid morphology deters fish predators. Oecologia. 1998;116:556–564. doi: 10.1007/s004420050621. doi:10.1007/s004420050621 [DOI] [PubMed] [Google Scholar]
- Lennon J.T., Smith V.H., Williams K. Influence of temperature on exotic Daphnia lumholtzi and implications for invasion success. J. Plankton Res. 2001;23:425–434. doi:10.1093/plankt/23.4.425 [Google Scholar]
- Lienesch P.W., Gophen M. Predation by inland silversides on an exotic cladoceran, Daphnia lumholtzi, in Lake Texoma, U.S.A. J. Fish Biol. 2001;59:1249–1257. doi:10.1111/j.1095-8649.2001.tb00189.x [Google Scholar]
- Lodge D.M. Biological invasions: lessons for ecology. Trends Ecol. Evol. 1993;8:133–137. doi: 10.1016/0169-5347(93)90025-K. doi:10.1016/0169-5347(93)90025-K [DOI] [PubMed] [Google Scholar]
- Mack R.N., Simberloff D., Lonsdale W.M., Evans H., Clout M., Bazzaz F.A. Biotic invasions: causes, epidemiology, global consequences, and control. Ecol. Appl. 2000;10:689–710. doi:10.1890/1051-0761(2000)010[0689:BICEGC]2.0.CO;2 [Google Scholar]
- Millennium Ecosystem Assessment. World Resources Institute; Washington, DC: 2005. Ecosystems and human well-being: biodiversity synthesis. [Google Scholar]
- Miner B.G., Sultan S.E., Morgan S.G., Padilla D.K., Relyea R.A. Ecological consequences of phenotypic plasticity. Trends Ecol. Evol. 2005;20:685–692. doi: 10.1016/j.tree.2005.08.002. doi:10.1016/j.tree.2005.08.002 [DOI] [PubMed] [Google Scholar]
- O'Brien W.J., Slade N.A., Vinyard G.L. Apparent size as the determinant of prey selection by bluegill sunfish (Lepomis macrochirus) Ecology. 1976;57:1304–1310. doi:10.2307/1935055 [Google Scholar]
- Orians G.H. Site characteristics favoring invasions. In: Mooney H.A., Drake J.A., editors. Ecology of biological invasions of North America and Hawaii. Springer; New York, NY: 1986. pp. 133–148. [Google Scholar]
- Parker I.M., Rodriguez J., Loik M.E. An evolutionary approach to understanding the biology of invasions: local adaptation and general-purpose genotypes in the weed Verbascum thapsus. Conserv. Biol. 2003;17:59–72. doi:10.1046/j.1523-1739.2003.02019.x [Google Scholar]
- Sakai A.K., et al. The population biology of invasive species. Annu. Rev. Ecol. Syst. 2001;32:305–332. doi:10.1146/annurev.ecolsys.32.081501.114037 [Google Scholar]
- Schulze P.C., Gillespie J.H., Womble J.R., Silen A.F. The effect of suspended sediments on Lake Texoma Daphnia: field distributions and in situ incubations. Freshw. Biol. 2006;51:1447–1457. doi:10.1111/j.1365-2427.2006.01579.x [Google Scholar]
- Sexton J.P., McKay J.K., Sala A. Plasticity and genetic diversity may allow saltcedar to invade cold climates in North America. Ecol. Appl. 2002;12:1652–1660. doi:10.1890/1051-0761(2002)012[1652:PAGDMA]2.0.CO;2 [Google Scholar]
- Shea K., Chesson P. Community ecology theory as a framework for biological invasions. Trends Ecol. Evol. 2002;17:170–176. doi:10.1016/S0169-5347(02)02495-3 [Google Scholar]
- Sokal R.R., Rohlf F.J. 3rd edn. Freeman; New York, NY: 1995. Biometry. [Google Scholar]
- Sorensen K.H., Sterner R.W. Extreme cyclomorphosis in Daphnia lumholtzi. Freshw. Biol. 1992;28:257–262. doi:10.1111/j.1365-2427.1992.tb00582.x [Google Scholar]
- Stachowicz J.J., Terwin J.R., Whitlatch R.B., Osman R.W. Linking climate change and biological invasions: ocean warming facilitates nonindigenous species invasions. Proc. Natl Acad. Sci. USA. 2002;99:15 497–15 500. doi: 10.1073/pnas.242437499. doi:10.1073/pnas.242437499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaffar S.M., O'Brien W.J. Spines of Daphnia lumholtzi create feeding difficulties for juvenile bluegill sunfish (Lepomis macrochirus) J. Plankton Res. 1996;18:1055–1061. doi:10.1093/plankt/18.6.1055 [Google Scholar]
- Tollrian R. Fish-kairomone induced morphological changes in Daphnia lumholtzi (Sars) Arch. Hydrobiol. 1994;130:69–75. [Google Scholar]
- Tollrian R., Dodson S.I. Inducible defenses in Cladocera. In: Tollrian R., Harvell C.D., editors. The ecology and evolution of inducible defenses. Princeton University Press; Princeton, NJ: 1999. pp. 177–202. [Google Scholar]
- Tollrian R., Harvell C.D. Princeton University Press; Princeton, NJ: 1999. The ecology and evolution of inducible defenses. [Google Scholar]
- Vilà M., Weiner J. Are invasive plants species better competitors than native plant species?—evidence from pair-wise experiments. Oikos. 2004;105:229–238. doi:10.1111/j.0030-1299.2004.12682.x [Google Scholar]
- Vitousek P.M., D'Antonio C.M., Loope L.L., Rejmánek M., Westbrooks R. Introduced species: a significant component of human-caused global change. New Zeal. J. Ecol. 1997;21:1–16. [Google Scholar]
- Von Elert E., Pohnert G. Predator specificity of kairomones in diel vertical migration of Daphnia: a chemical approach. Oikos. 2000;88:119–128. doi:10.1034/j.1600-0706.2000.880114.x [Google Scholar]
- Walther G.-R., Post E., Convey P., Menzel A., Parmesan C., Beebee T.J.C., Fromentin J.-M., Hoegh-Guldberg O., Bairlein F. Ecological responses to recent climate change. Nature. 2002;416:389–395. doi: 10.1038/416389a. doi:10.1038/416389a [DOI] [PubMed] [Google Scholar]
- Werner E.E., Hall D.J. Optimal foraging and the size selection of prey by the bluegill sunfish (Lepomis macrochirus) Ecology. 1974;55:1042–1052. doi:10.2307/1940354 [Google Scholar]
- Work K.A., Gophen M. Environmental variability and the population dynamics of the exotic Daphnia lumholtzi and native zooplankton in Lake Texoma, U.S.A. Hydrobiologia. 1999;405:11–23. doi:10.1023/A:1003742709605 [Google Scholar]
- Zaret T.M. Predator–prey interaction in a tropical lacustrine ecosystem. Ecology. 1972;53:248–257. doi:10.2307/1934078 [Google Scholar]
- Zaret T.M. Yale University Press; New Haven, CT: 1980. Predation and freshwater communities. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The individual on the left was exposed to chemical cues exuded by fish (induced) and the individual on the right was not (control) (photo credit C. Laforsch).