Abstract
Frequency-dependent predation has been proposed as a general mechanism driving the phenotypic assortment of social groups via the ‘oddity effect’, which occurs when the presence of odd individuals in a group allows a predator to fixate on a single prey item, increasing the predator's attack-to-kill ratio. However, the generality of the oddity effect has been debated and, previously, there has not been an ecological assessment of the role of predation risk in driving the phenotypic assortment of social groups. Here, we compare the levels of body length assortment of social groups between populations of the Trinidadian guppy (Poecilia reticulata) that experience differences in predation risk. As predicted by the oddity effect hypothesis, we observe phenotypic assortment by body length to be greater under high predation risk. However, we found that a number of low-predation populations were also significantly assorted by body length, suggesting that other mechanisms may have a role to play.
Keywords: confusion effect, frequency-dependent selection, guppy, oddity effect, Poecilia reticulata
1. Introduction
Across a broad range of taxa, social groups are assorted by phenotypic traits such as body size, species and sex (see Krause & Ruxton (2002) for a review). A general mechanism proposed to drive this phenotypic assortment is frequency-dependent predation, with odd individuals in a group suffering an increased risk of predation due to the ‘oddity effect’ (Ohguchi 1978; Landeau & Terborgh 1986; Theodorakis 1989). The oddity effect occurs when the presence of odd individuals in a group allows a predator to fixate on a single prey item, overcoming the ‘confusion effect’ and increasing the predator's attack-to-kill ratio (Krakauer 1995; Tosh et al. 2006). The confusion effect occurs across taxonomic groups including fishes, mammals and reptiles (Neill & Cullen 1974; Ohguchi 1978; Landeau & Terborgh 1986; Theodorakis 1989; Schradin 2000) and previous work has generally shown that a predator's success is reduced when attacking homogeneous-looking groups (see Krause & Ruxton (2002) for a review). The oddity effect has been proposed as a major force driving evolution, so powerful that it can result in the convergence of phenotypic traits (Greenwood 1985) and may lead to the extinction of rare phenotypes or species from communities. For example, recent work on coral reef assemblages has demonstrated that rare species are preferentially taken by predators (Almany et al. 2007), which may lead to reduced species diversity in areas of high predation risk (Almany & Webster 2004). However, while the oddity effect has received a lot of attention in the literature, there is a paucity of studies evaluating the importance of this selective force in wild populations.
Much of the work on the role of the oddity effect in driving phenotypic assortment has used freshwater fishes, with some of the most convincing evidence coming from experimental work looking at predator–prey choice. For example, a number of studies have presented predators with groups of prey that differed in the abundance of different phenotypes, and demonstrated frequency-dependent predation with predators showing a preference for the rare prey type (Ohguchi 1978). The most commonly cited of these include an experiment by Theodorakis (1989) in which when predatory bass (Micropterus salmoides) were presented with shoals of minnows (bluntnose minnow, Pimephales notatus; fathead minnow, P. promelas; and stroneroller minnow, Campostoma anomalum) that were dominated by one size category, the minority size was taken more often than would be predicted by chance. In a similar study, Landeau & Terborgh (1986) showed that large mouth bass (M. salmoides) were more successful at predating shoals of minnows (Hybognathus nuchalis) when the shoals contained odd individuals (created by dying fish in blue to adjust the frequency of phenotypes in a group). However, these results are far from universal. For example, there are instances reported when predators show a preference for the common prey type (Fullick & Greenwood 1979) and where prey preference is independent of their frequency in a group (Fitzgibbon 1990).
In an attempt to tease apart the role of predation risk in driving phenotypic assortment, a number of studies have examined the behaviour of prey under predation threat. For example, Krause & Godin (1994) reported active preferences for conspecifics of a similar size, which increased in magnitude under predation threat. Other experiments have examined the behaviour of an odd fish in a shoal and suggest that odd individuals show more threat-sensitive behaviour under predation risk. For example, Peuhkuri (1997, 1998) found that the feeding activity of large fish (Gasterosteus aculeatus) was sensitive to the degree of assortment of the group, with large fish decreasing foraging when they were the odd phenotype in the group. By contrast, small fish showed no such sensitivity. In a similar study, Allan & Pitcher (1986) examined the response of mixed species shoals to a simulated predation attack, finding that the shoals segregated into single-species groups, which suggests that the oddity effect may have an important role to play. Once again, however, these results are not universal and Krause (1994), for example, found that simulated predation risk did not change group composition of fish shoals with regard to body length.
From a review of the literature, it is clear that experimental evidence for the oddity effect remains inconclusive. In much of the published literature, more attention has been given to the studies that report results in favour of the oddity effect (e.g. Landeau & Terborgh 1986; Theodorakis 1989), leading some authors to suggest that there is a ‘premature belief in the pervasiveness of the oddity effect in Nature’ (Krause & Ruxton 2002). Moreover, much of the work examining the role of predation risk in driving phenotypic assortment has been conducted under laboratory conditions and there is a need to evaluate the importance of this selective force in wild populations. In the current study, we examine the role of predation risk in driving phenotypic assortment by comparing the degree of body size assortment of social groups sampled from 10 wild populations of Trinidadian guppies (Poecilia reticulata) that experience differing levels of predation.
The guppy system in Trinidad offers a rare opportunity to compare populations that live under different ecological conditions, particularly that differ in predation risk (Magurran 2005). In the Northern mountain range, predatory fish assemblages change along an elevation gradient as waterfalls restrict the upstream movement of major predators, leaving headwaters relatively predator free (Magurran 2005). This allows for comparative studies of populations that differ in the predation risk they experience. Early pioneering work in Trinidad compared the degree of social behaviour between populations, providing compelling evidence for the role of predation risk in selecting for group living (Seghers 1974; Magurran & Seghers 1991). More recent work demonstrates that under high predation, groups are assorted based on body size and that this assortment is in part due to active choice (Croft et al. 2003), making the guppy particularly suited to the present study. In accordance with the oddity effect hypothesis, we predict that the populations living under high predation risk will form social groups that are more assorted by body length than the populations living under low predation risk. To our knowledge, this study provides a first and much needed ecological assessment of the role of predation risk in driving phenotypic assortment.
2. Material and methods
A total of 10 populations were sampled in the Northern mountain range of Trinidad, five that experience high predation risk and five that experience low predation risk (table 1). Areas of high predation were defined by the presence of the major guppy predators Crenicichla frenata, Aequidens pulcher and Hoplias malabaricus (Seghers 1974; Endler 1978; Magurran & Seghers 1990). Low-predation areas contained only the minor predators Rivulus hartii (known to prey preferentially on juveniles and small guppies; Seghers 1973; Rodd & Reznick 1991) and the freshwater prawn, Macrobrachium spp. Six of the rivers were sampled over a six-week period (May and June) in 2004 and the remaining four rivers were sampled over a six-week period (May and June) in 2008 (table 1). The sampling design was balanced in that the same number of high- and low-predation rivers were sampled in a given year. In addition to sampling naturally established populations, we used a previous transplant of guppies carried out by Haskins in 1957 (reported by Shaw et al. 1991; Magurran et al. 1992). Haskins transplanted approximately 200 adult guppies from the Arima River (high predation) to a previously guppy-free and low predation risk location in the upper Turure River. Previous work on the transplant population has shown that behaviour can be modified by selection in accordance with the reduction in predation risk (Magurran et al. 1992).
Table 1.
Rivers sampled in the study, with grid references, classification as high or low predation, the number shoals caught per river and the total number of fishes sampled per river.
| grid reference | ||||||
|---|---|---|---|---|---|---|
| river | predation | year sampled | N | W | no. of shoal captures | total fish captures |
| Turure | low | 2004 | 10°41′ | 61°10′ | 40 | 273 |
| Naranjo | low | 2004 | 10°41′ | 61°14′ | 35 | 446 |
| Paria | low | 2004 | 10°45′ | 61°16′ | 38 | 374 |
| Yarra | low | 2008 | 10°45′ | 61°20′ | 25 | 101 |
| Marianne | low | 2008 | 10°45′ | 61°17 | 29 | 114 |
| Arima | high | 2004 | 10°41′ | 61°17′ | 33 | 460 |
| Aripo | high | 2004 | 10°40′ | 61°14′ | 38 | 433 |
| Guanapo | high | 2004 | 10°40′ | 61°15′ | 33 | 348 |
| Tacarigua | high | 2008 | 10°41′ | 61°22′ | 30 | 302 |
| Quare | high | 2008 | 10°40′ | 61°12′ | 30 | 290 |
During sampling, guppy shoals (defined as a group of fish with less than four body lengths of distance between individuals; see Croft et al. 2003) were caught by two observers from each population using a beach seine net (190 ×115 cm; mesh size, 3 mm) between 10.00 h and 16.00 h. Shoals were considered for analysis only when both observers were satisfied that the entire shoal had been captured. To provide a representative sample of each population, a minimum of 25 shoals were captured from a minimum of five different pools in each of the rivers (the only exception to this was the Yara low-predation population where only one pool could be sampled), and from different locations in each pool. The body lengths (total length measured to the nearest mm) of all fish captured in each shoal were recorded. To prevent multiple captures of the same individuals, sampled shoals were not released back into the river until all shoals had been captured.
3. Analysis
As a measure of phenotypic assortment, we used the shoal coefficient of variation (COV) of body length, which was calculated for each shoal captured and we refer to this measure as the degree of absolute assortment. To determine whether shoals were more assorted than we would have expected if fish associated randomly with regard to body size, we calculated the expected assortment for each shoal assuming random interactions between individuals within a river. This was calculated using a constrained randomization test in which individuals from all captured shoals within a river were combined and shoals (consisting of the number of individuals in a natural shoal from the respective river) were then selected at random and the COV of body length calculated for each resampled shoal. Ten thousand random shoals were generated for each shoal captured and the average COV of body length calculated for each shoal as the value of expected assortment. Finally, to provide a single variable that allowed us to compare the degree of non-random assortment among rivers, we calculated the degree of relative assortment for each shoal as the proportion of iterations in the randomization that produced a COV of less than or equal to the observed COV. Given that the value of relative assortment is a proportion, we transformed the data using an arcsine square-root transformation.
To examine the degree of phenotypic assortment by body length within rivers, we used a one sample t-test for each river to compare the difference between the observed and expected values of assortment (absolute assortment minus expected assortment) to a value of 0 (i.e. the expected value assuming no assortment). We used a nested general linear model to assess whether shoal assortment differed between habitat types (high and low predation risk) and/or among rivers. Relative assortment was entered as our dependent variable and predation risk and river nested within predation risk were entered as our fixed effects. All statistical analyses were carried out in SPSS v. 14.0. Where appropriate, we used a Bonferroni correction to control for multiple testing.
4. Results
(a) Body length assortment within rivers
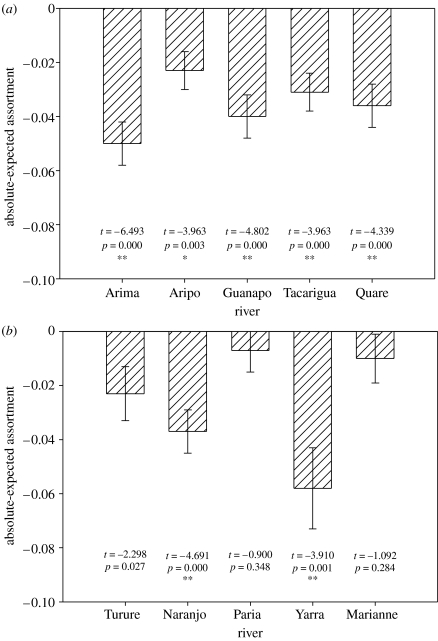
Significant body length assortment was observed in all five populations living under high predation risk (figure 1a). Interestingly, significant size assortment was also seen in two of the low-predation populations, Naranjo and Yara (figure 1b).
Figure 1.
Patterns of body length assortment (mean±(1 s.e.) absolute-expected assortment) within (a) high predation risk and (b) low predation risk rivers. Also shown are the results of one sample t-tests comparing the difference in assortment (absolute assortment−expected assortment) with the expected value of 0. Results are significant at *p<0.05 and **p<0.01 after Bonferroni corrections.
(b) Differences in assortment between high and low predation
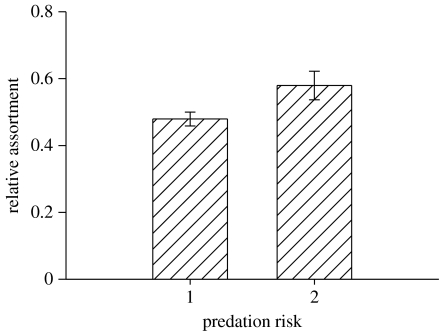
We found significant differences in relative assortment between high and low predation risk, with shoals under high predation being significantly more assorted than shoals under low predation (F1,325=10.283, p=0.001; see figure 2). There was also a significant difference in relative assortment among sites (F8,325=2.270, p=0.023; see figure 1). When we made a direct comparison between the degrees of relative assortment of the populations transplanted from high to low predation (Turure mean(±1 s.e.)=0.60±0.04) and the original founder population (Arima mean(±1 s.e.)=0.43±0.02), assortment was significantly reduced in the transplanted population living under low predation (t-test t75=2.64, p=0.010).
Figure 2.
Patterns of body length assortment across high and low predation risk rivers showing the grand mean (±1 s.e.) relative assortment. 1, high predation risk; 2, low predation risk.
5. Discussion
Our results demonstrate that predation risk is an important factor driving phenotypic assortment in wild populations. By comparing the levels of phenotypic assortment in populations that experience differences in predation risk, we were able to show that the magnitude of assortment, in comparison with what would be expected by random interactions, is greater under high predation risk than that under low predation risk, as predicted by the oddity effect hypothesis.
The comparative approach is a powerful tool to emphasize the role of ecological variables in driving population differences in behaviour. Some of the earliest and most significant applications of this approach investigated the role of predation risk in driving group living, with a number of studies finding that species living under high predation risk form larger groups (Crook 1965; Jarman 1974; Seghers 1974). In the current investigation, we extend this analysis to look at the composition of social groups that experience differences in predation risk. In accordance with the theoretical predictions stemming from the oddity effect hypothesis, under high predation, shoals of fishes were more assorted than shoals from low-predation rivers relative to what we would expect if interactions occurred at random. This result could be due to predators selectively removing odd individuals from shoals via frequency-dependent predation. However, given the dynamic nature of the fission–fusion system with individuals exchanging shoals over a time scale of minutes (Croft et al. 2003) and that previous work has shown that shoal assortment in guppies is in part driven by active choice (Croft et al. 2003), it is likely that the observed patterns are based on some sort of active choice of shoal membership based on body length. Future work comparing the shoal choice behaviour of individuals that experience different predation regimes would be greatly rewarding.
While the comparative approach provides a powerful tool, it does have its limitations (Clutton-Brock & Harvey 1979; Gould & Lewontin 1979); in particular, associations between ecological variables may confound the results of a comparative study. In guppy populations in the Northern mountain range of Trinidad, habitat differences in predation risk are often correlated with productivity (Grether et al. 2001; Reznick et al. 2001). High-predation rivers tend to have higher levels of productivity than low-predation rivers as the forest canopy is usually less dense in these habitats (Grether et al. 2001; Reznick et al. 2001). This, combined with the fact that low-predation rivers usually have a higher density of fishes, leads to higher competition between individuals for food under low predation, which is known to be a strong selective force in guppies (Reznick et al. 2001; Arendt & Reznick 2005). Competition for food has been proposed previously as a mechanism selecting for size-assortative shoaling in fishes (Ranta et al. 1994). However, the food competition hypothesis for body length assortment predicts that the assortment will be greater under intense competition for food, which, in the case of the current investigation, is more likely to occur in low-predation populations. In support of predation risk being the major driving force for phenotypic assortment, we see a reversal of this predicted pattern.
One of the strengths of the comparative approach is that it can be used to identify general ecological and evolutionary trends; however, it does this using correlational evidence that makes it difficult to invoke cause–effect relationships. More direct evidence for the link between predation risk and behaviour can be gained through transplant experiments in which predators and/or their prey are moved between habitats. In the current investigation, we make use of a historical transplant experiment in which fishes were moved from a high- to a low-predation habitat (see §2 for details). Previous work on the transplanted population has shown that behaviour has been modified by selection in accordance with the reduction in predation risk (Magurran et al. 1992). Our results show that shoal assortment by body length is reduced in the transplanted population, providing compelling support for the importance of predation risk in selecting for phenotypic assortment.
While our results provide strong support for the role of predation risk in driving the phenotypic assortment of social groups, we observed significant variation among rivers independent of predation risk, and two populations living under low predation risk had social groups that were significantly assorted by body size. Taken together, these results suggest that factors in addition to predation risk may contribute to assortative grouping by body size. Two alternative hypotheses have been proposed in the literature that may drive body length assortment of social groups under low predation risk. First, as discussed above, the avoidance of competition for food may select for assortative grouping by body size in these low predation risk populations with more intense competition for food. Furthermore, variation in productivity among rivers independent of predation risk may contribute to the among-river differences in body length assortment. Second, a mechanism that may contribute to the body length assortment is that proposed by the activity budget hypothesis (Conradt 1998; Ruckstuhl 1998). Individuals of a different size may have different activity budgets, leading some authors to propose the activity budget hypothesis to explain group assortment, particularly between mixed sexed groups where there is sexual dimorphism in body size (Conradt 1998; Ruckstuhl 1998). In fishes, optimal foraging rates and swimming speeds are size dependent (Beamish 1978; Hjelm & Persson 2001) and individuals in a group of others of dissimilar body length may be forced to travel and forage at suboptimal speeds, potentially incurring an energetic cost, which may contribute selection for group assortment by body size in shoaling fishes (Ruckstuhl 2007). Experimentally testing the importance of competition and activity synchrony as mechanisms driving phenotypic assortment in natural populations, particularly under low predation risk, provides an exciting challenge for future research.
In conclusion, our results provide compelling evidence for the role of predation risk and the oddity effect in driving phenotypic assortment by body size. However, our results also suggest that other factors may have an important role to play and highlights the need for future work on this phenomenon in wild populations.
Acknowledgments
This research adhered to the legal requirements of Trinidad and all institutional guidelines.
We thank Marc Botham, Mathew Edenbrow and Audie Hazenberg for their assistance in the field and Indar Ramnarine, Ronnie Hernandez and the Board of Asa Wright Nature Centre for their support in Trinidad. We also thank Jens Krause, Kathreen Jones, Nils Stenseth and two anonymous referees whose comments greatly improved this manuscript. Funding was provided to D.P.C. by the Fisheries Society of the British Isles and a research grant from the National Environmental Research Council UK (NE/E001181/1).
References
- Allan J.R., Pitcher T.J. Species segregation during predator evasion in cyprinid fish shoals. Freshw. Biol. 1986;16:653–659. doi:10.1111/j.1365-2427.1986.tb01007.x [Google Scholar]
- Almany G.R., Webster M.S. Odd species out as predators reduce diversity of coral-reef fishes. Ecology. 2004;85:2933–2937. doi:10.1890/03-3150 [Google Scholar]
- Almany G.R., Peacock L.F., Syms C., McCormick M.I., Jones G.P. Predators target rare prey in coral reef fish assemblages. Oecologia. 2007;152:751–761. doi: 10.1007/s00442-007-0693-3. doi:10.1007/s00442-007-0693-3 [DOI] [PubMed] [Google Scholar]
- Arendt J.D., Reznick D.N. Evolution of juvenile growth rates in female guppies (Poecilia reticulata): predator regime or resource level? Proc. R. Soc. B. 2005;272:333–337. doi: 10.1098/rspb.2004.2899. doi:10.1098/rspb.2004.2899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beamish F.W.H. Swimming capacity. In: Hoar W.S., Randall J.D., editors. Fish physiology. vol. 7. Academic Press, Inc; New York, NY: 1978. pp. 101–187. [Google Scholar]
- Clutton-Brock T.H., Harvey P.H. Comparison and adaptation. Proc. R. Soc. Lond. B. 1979;205:547–565. doi: 10.1098/rspb.1979.0084. doi:10.1098/rspb.1979.0084 [DOI] [PubMed] [Google Scholar]
- Conradt L. Could asynchrony in activity between the sexes cause intersexual social segregation in ruminants? Proc. R. Soc. B. 1998;265:1359–1363. doi: 10.1098/rspb.1998.0442. doi:10.1098/rspb.1998.0442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft D.P., Arrowsmith B.J., Bielby J., Skinner K., White E., Couzin I.D., Magurran A.E., Ramnarine I., Krause J. Mechanisms underlying shoal composition in the Trinidadian guppy (Poecilia reticulata) Oikos. 2003;100:429–438. doi:10.1034/j.1600-0706.2003.12023.x [Google Scholar]
- Crook J.H. The adaptive significance of avian social organizations. Symp. Zool. Soc. Lond. 1965;14:181–218. [Google Scholar]
- Endler J.A. A predator's view of animal colour patterns. Evol. Biol. 1978;11:319–364. [Google Scholar]
- Fitzgibbon C.D. Mixed-species grouping in Thomson's and Grant's gazelles: the antipredator benefits. Anim. Behav. 1990;39:1116–1126. doi:10.1016/S0003-3472(05)80784-5 [Google Scholar]
- Fullick T.G., Greenwood J.J.D. Frequency dependent food selection in relation to two models. Am. Nat. 1979;113:762–765. doi:10.1086/283433 [Google Scholar]
- Gould S.J., Lewontin R.C. The Spandrels of San Marco and the Panglossian paradigm—a critique of the adaptationist programme. Proc. R. Soc. Lond. B. 1979;205:581–598. doi: 10.1098/rspb.1979.0086. doi:10.1098/rspb.1979.0086 [DOI] [PubMed] [Google Scholar]
- Greenwood J.J.D. Frequency-dependent selection by seed-predators. Oikos. 1985;44:195–210. doi:10.2307/3544062 [Google Scholar]
- Grether G.F., Millie D.F., Bryant M.J., Reznick D.N., Mayea W. Rain forest canopy cover, resource availability, and life history evolution in guppies. Ecology. 2001;82:1546–1559. doi:10.2307/2679799 [Google Scholar]
- Hjelm J., Persson L. Size-dependent attack rate and handling capacity: inter-cohort competition in a zooplanktivorous fish. Oikos. 2001;95:520–532. doi:10.1034/j.1600-0706.2001.950317.x [Google Scholar]
- Jarman P.J. The social organisation of antelope in relation to their ecology. Behaviour. 1974;48:215–267. doi:10.1163/156853974X00345 [Google Scholar]
- Krakauer D.C. Groups confuse predators by exploiting perceptual bottlenecks—a connectionist model of the confusion effect. Behav. Ecol. Sociobiol. 1995;36:421–429. doi:10.1007/BF00177338 [Google Scholar]
- Krause J. The influence of food competition and predation risk on size-assortative shoaling in juvenile chub (Leuciscus cephalus) Ethology. 1994;96:105–116. [Google Scholar]
- Krause J., Godin J.G.J. Shoal choice in the banded killifish (Fundulus diaphanus, Teleostei, Cyprinodontidae)—effects of predation risk, fish size, species composition and size of shoals. Ethology. 1994;98:128–136. [Google Scholar]
- Krause J., Ruxton G.D. Oxford University Press; Oxford, UK: 2002. Living in groups. [Google Scholar]
- Landeau L., Terborgh J. Oddity and the confusion effect in predation. Anim. Behav. 1986;34:1372–1380. doi:10.1016/S0003-3472(86)80208-1 [Google Scholar]
- Magurran A.E. Oxford Series in Ecology and Evolution. Oxford University Press; Oxford, UK: 2005. Evolutionary ecology: the Trinidadian guppy. [Google Scholar]
- Magurran A.E., Seghers B.H. Population differences in the schooling behaviour of newborn guppies, Poecilia reticulata. Ethology. 1990;84:334–342. [Google Scholar]
- Magurran A.E., Seghers B.H. Variation in schooling and aggression amongst guppy (Poecilia reticulata) populations in Trinidad. Behaviour. 1991;118:214–234. doi:10.1163/156853991X00292 [Google Scholar]
- Magurran A.E., Seghers B.H., Carvalho G.R., Shaw P.W. Behavioural consequences of an artificial introduction of guppies (Poecilia reticulata) in N. Trinidad: evidence for the evolution of antipredator behaviour in the wild. Proc. R. Soc. B. 1992;248:117–122. doi:10.1098/rspb.1992.0050 [Google Scholar]
- Neill S.R.S.J., Cullen J.M. Experiments on whether schooling by their prey affects the hunting behavoiur of cephalopods and fish predators. J. Zool. 1974;172:549–569. [Google Scholar]
- Ohguchi O. Experiments on the selection against colour oddity of water fleas by three-spined stickelbacks. Z. Tierpsychol. 1978;47:254–267. [Google Scholar]
- Peuhkuri N. Size-assortative shoaling in fish: the effect of oddity on foraging behaviour. Anim. Behav. 1997;54:271–278. doi: 10.1006/anbe.1996.0453. doi:10.1006/anbe.1996.0453 [DOI] [PubMed] [Google Scholar]
- Peuhkuri N. Shoal composition, body size and foraging in sticklebacks. Behav. Ecol. Sociobiol. 1998;43:333–337. doi:10.1007/s002650050499 [Google Scholar]
- Ranta E., Peuhkuri N., Laurila A. A theoretical exploration of antipredatory and foraging factors promoting phenotype-assorted fish schools. Ecoscience. 1994;1:99–106. [Google Scholar]
- Reznick D., Butler M.J., Rodd H. Life-history evolution in guppies. VII. The comparative ecology of high- and low-predation enviroments. Am. Nat. 2001;157:12–26. doi: 10.1086/318627. doi:10.1086/318627 [DOI] [PubMed] [Google Scholar]
- Rodd F.H., Reznick D.N. Life-history evolution in guppies. 3. The impact of prawn predation on guppy life histories. Oikos. 1991;62:13–19. doi:10.2307/3545440 [Google Scholar]
- Ruckstuhl K.E. Foraging behaviour and sexual segregation in bighorn sheep. Anim. Behav. 1998;56:99–106. doi: 10.1006/anbe.1998.0745. doi:10.1006/anbe.1998.0745 [DOI] [PubMed] [Google Scholar]
- Ruckstuhl K.E. Sexual segregation in vertebrates: proximate and ultimate causes. Integr. Comp. Biol. 2007;47:245–257. doi: 10.1093/icb/icm030. doi:10.1093/icb/icm030 [DOI] [PubMed] [Google Scholar]
- Schradin C. Confusion effect in a reptilian and a primate predator. Ethology. 2000;106:691–700. doi:10.1046/j.1439-0310.2000.00582.x [Google Scholar]
- Seghers, B. H. 1973 An analysis of geographic variation in the anti predator adaptations of the guppy, Poecilia reticulata PhD thesis, The University of British Columbia.
- Seghers B.H. Schooling behaviour in the guppy (Poecilia reticulata): an evolutionary response to predation. Evolution. 1974;28:486–489. doi: 10.1111/j.1558-5646.1974.tb00774.x. doi:10.2307/2407174 [DOI] [PubMed] [Google Scholar]
- Shaw P.W., Carvalho G.R., Magurran A.E., Seghers B.H. Population differentiation in Trinidadian guppies (Poecilia reticulata)—patterns and problems. J. Fish Biol. 1991;39:203–209. doi:10.1111/j.1095-8649.1991.tb05084.x [Google Scholar]
- Theodorakis C.W. Size segregation and the effects of oddity on predation risk in minnow schools. Anim. Behav. 1989;38:496–502. doi:10.1016/S0003-3472(89)80042-9 [Google Scholar]
- Tosh C.R., Jackson A.L., Ruxton G.D. The confusion effect in predatory neural networks. Am. Nat. 2006;167:E52–E65. doi: 10.1086/499413. doi:10.1086/499413 [DOI] [PubMed] [Google Scholar]